Abstract
Current guidelines for obesity treatment recommend reducing daily caloric intake for weight loss. However, long-term weight loss continues to be an issue in obesity management. Alternative weight loss strategies have increased in popularity, such as intermittent energy restriction (IER), a type of eating pattern with periods of fasting alternating with unrestricted eating. The effects of IER on weight loss, cardiovascular risk factors, inflammation, and appetite are not clear. The purpose of this systematic review was to analyze short- (<24 weeks) and long-term (≥24 weeks) effects of IER on anthropometric, cardiometabolic, inflammatory, and appetite outcomes in adults with overweight/obesity. PubMed, CINAHL, Embase, and PsycInfo were searched from inception to July 2020. Human randomized controlled trials (RCTs) on IER with participants with a body mass index ≥25 kg/m2 were included in this review. A total of 42 articles (reporting on 27 different RCTs) were included. In short-term studies, IER showed pre-to-post treatment improvements in eight of nine studies that assessed weight. Weight outcomes were sustained in the long-term. However, no significant long-term between group differences were observed in fat mass, other anthropometric, cardiometabolic, inflammatory, or appetite outcomes. Compared to continuous energy restriction (CER), IER showed no significant long-term differences in anthropometric, cardiometabolic, inflammatory, or appetite outcomes in included studies. More long-term studies are needed to assess the benefits of IER on health outcomes.
Keywords: intermittent fasting, obesity, weight loss
Guidelines for behavioral obesity treatment prescribe daily, continuous energy restriction (CER) by reducing baseline intake by 25-30% or prescribing a goal of consuming 1200–1800 kcals/day depending on baseline weight (Jensen et al., 2014). Behavioral interventions using CER produce an average loss of 5–10% of initial body weight over 6 months, along with clinically significant improvements in cardiometabolic risk factors and psychosocial functioning (Look AHEAD Research Group, 2013, 2014). However, in the absence of continuing care, adults on average regain one-third of their lost weight in the year following behavioral treatment, with increasing regain over time as well as a return in metabolic risk and psychosocial impairment (Wadden et al., 2012). Long-term weight loss is hindered by a complex interaction of behavioral, cognitive, and biological factors (Montesi et al., 2016). Given the potential health benefits of long-term weight loss, strategies that can effectively improve weight management are urgently needed.
Patients often view weight management as a constant and ongoing process (Metzgar et al., 2015). Many patients with obesity are unable to sustain the daily demands and burden of adhering to CER to prevent subsequent weight regain (Alhassan et al., 2008). Adherence to daily calorie restriction decreases over time (Dansinger et al., 2005; Moreira et al., 2011). Novel approaches are needed that minimize the constant burden of CER. Intermittent energy restriction (IER) protocols have been designed to address adherence issues by prescribing restriction during specific time frames, rather than daily. IER involves fast days (e.g., individuals consume 25% of energy needs), alternated with feast days (e.g., individuals are permitted to consume food ad libitum) (Varady & Hellerstein, 2007). During weight loss induction over 3 months, IER produces a loss of 4–8% of initial body weight and improvements in cardiometabolic parameters; these results are comparable to those achieved with CER (Harris et al., 2018; Trepanowski et al., 2017). IER could also mitigate biological changes that occur with weight loss that promote subsequent weight regain.
In addition, some have suggested that the benefits of IER arise not only from caloric restriction but also from “metabolic switching” between fed and fasting states (Anton et al., 2018; de Cabo & Mattson, 2019). Metabolic switching occurs when the body preferentially switches from utilization of glucose from glycogenolysis to fatty acid and fatty acid-derived ketones. This has been hypothesized to improve body composition and may offer benefits during weight loss by preserving muscle mass and function.
Previous systematic reviews on IER in participants with overweight/obesity primarily focus on short-term weight loss (Harris et al., 2018; Lima et al., 2020; Welton et al., 2020) and cardiometabolic outcomes (Cioffi et al., 2018; Harris et al., 2018) such as blood pressure, fasting glucose, fasting insulin, and lipoproteins. Prior systematic reviews primarily investigated randomized controlled trials (RCTs) with defined IER regimens in comparison to CER and/or control groups. Few RCTs were included and studies focused on short-term effects. Longer duration studies were needed to further investigate long-term effects of IER such as weight loss, cardiometabolic, and appetite outcomes.
The purpose of this systematic review was to examine short- (<24 weeks) and long-term (≥24 weeks) RCTs on IER regimens on weight loss, anthropometric, cardiometabolic, inflammation, and appetite factors in participants with overweight and/or obesity. We examined the effects of IER on these outcomes by examining various IER regimens when compared to a no intervention control group, CER, with or without exercise groups, and other active IER regimens.
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
Study Inclusion Criteria
Studies were included if they were: a RCT of any duration; had human participants with overweight/obesity defined by a body mass index (BMI) ≥ 25 kg/m2; contained an intervention arm using IER regimen for weight loss; peer-reviewed journal article; published in English; and assessed weight change. We included studies that compared an IER intervention arm to a control, defined as no intervention, or ad libitum dietary pattern, and/or CER and/or other forms of IER interventions that include exercise. Trials that assessed cardiometabolic, inflammatory markers, and appetite and/or hunger outcomes in addition to weight were also included. No specific inclusion criteria for IER intervention protocols were pre-determined. Secondary analyses of primary studies were also included.
Studies were excluded if they did not include participants with overweight/obesity. Studies were excluded if they examined religious or cultural fasting intervention arms. RCTs were excluded if participants studied had other underlying health conditions (e.g., type 1 diabetes), with the exception of type 2 diabetes mellitus or metabolic syndrome as these underlying conditions are often co-occurring with overweight/obesity. Abstracts, periodicals, reviews, chapters of books, manuals, theses, dissertations, manuals, magazines, reports, orals, posters, or articles not in English were excluded.
Search Strategy
A keyword search was performed in PubMed, CINAHL, Embase, and PsycInfo. Electronic databases were searched from inception to July 2, 2020. An initial search was conducted on PubMed to find relevant published studies and reviews on IER and obesity or weight loss. Relevant IER key terms were extracted, searched through PubMed, and combined to create the finalized search syntax (Supplementary Table 1).
Titles and abstracts were screened against the study selection criteria. Potentially relevant articles were retrieved and evaluated at full text. Reference lists of previously conducted systematic reviews and records eligible for full text screening were reviewed for relevant studies to ensure the inclusion of all eligible records.
Data Extraction
A standardized data extraction form was used to collect information on methods, study characteristics, and outcome variables. The following data were extracted from studies that met inclusion and exclusion criteria: author, year of publication, location intervention regimens, sample size, participant characteristics (e.g., number of participants, age, sex, race/ethnicity, BMI), variables assessed, time of assessment, outcomes, attrition, and follow-up data provided in secondary analyses if available. Outcomes assessed include anthropometric (weight, waist circumference, body fat percentage, fat mass, lean mass, fat-free mass [FFM], resting metabolic rate [RMR]), cardiometabolic (systolic and diastolic blood pressure, heart rate, fasting glucose, fasting insulin, hemoglobin A1c [HbA1c], homeostatic model assessment of insulin resistance [HOMA-IR], total triglycerides, total cholesterol, low density lipoprotein [LDL], high density lipoprotein [HDL]), inflammatory (high sensitivity c-reactive protein [hs-CRP], interleukin-6 [IL-6], interleukin-8 [IL-8], interleukin-10 [IL-10], interferon-gamma [IFN-γ], tumor necrosis factor-alpha [TNF-∝]), appetite (leptin, resistin, ghrelin, adiponectin, brain-derived neurotrophic factor [BDNF]), and hunger. Studies were classified as short-term if weight loss phase was <24 weeks. Studies were classified as long-term if there were longer-term weight loss results ≥24 weeks in duration. Follow-up data was analyzed separately as no intervention was provided during this period of time.
Study Quality Assessment
A risk of bias assessment was conducted using the Cochrane risk of bias tool for randomized trials on the primary articles of RCTs that met inclusion and exclusion criteria (Sterne et al., 2019). The following domains were assessed: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. Each domain was rated low, high, or some concerns of bias and an overall risk-of-bias judgement was determined. Two assessors independently rated each article and disagreements were reconciled by discussion.
Results
Study Characteristics
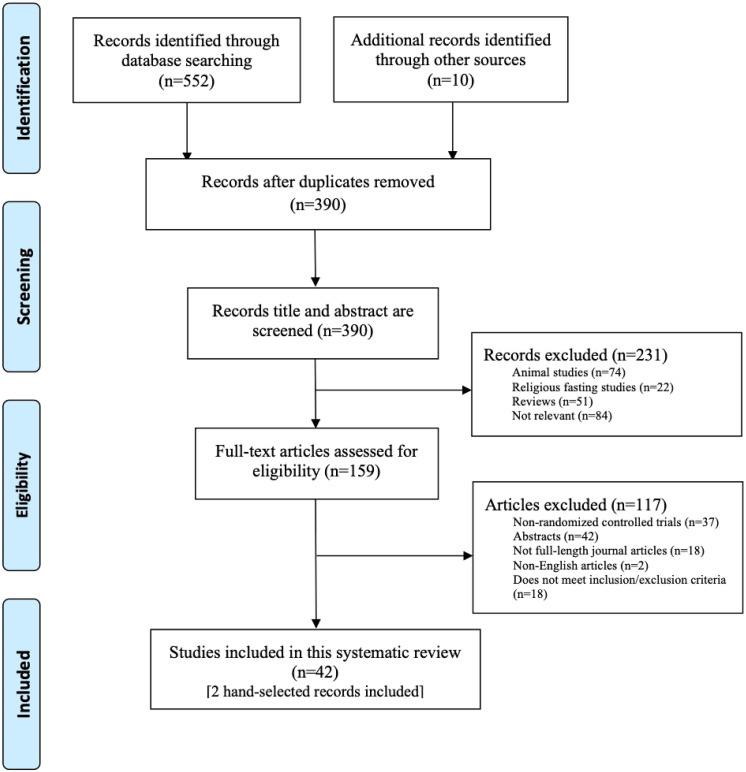
There were 552 records identified through the database search. Due to the small number of results in PsycInfo, only IER search terms were utilized to ensure inclusion of all studies that met our criteria. In total, 380 titles and abstracts were screened against inclusion and exclusion criteria after the removal of duplicates. Figure 1 summarizes the screening process and reasons for exclusion. A total of 42 articles were included in this systematic review with a total of 27 RCTs. There were 15 articles that published results from secondary analyses for eight of these RCTs. Common types of IER diets were alternate day fasting (ADF), 5:2 diet, week-on-week-off (WOWO) diet, and time restricted feeding (TRF; Table 1). Thirteen RCTs focused on a form of the ADF diet regimen, nine on a form of the 5:2 diet, three on a form of the WOWO diet, one on a form of TRF, and one RCT focused on a 6:1 (6 days of energy restriction with one fasting day) diet.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram.
Table 1.
Common Types of Intermittent Energy Restriction Diets.
| Type of Intermittent Energy Restriction Diet | Description |
|---|---|
| Alternate Day Fasting (ADF) | A type of eating pattern with alternating fasting (none or little energy intake) and feasting (full energy or ad libitum intake) days |
| 5:2 | A type of eating pattern with two consecutive or nonconsecutive fasting days and 5 days of full energy or ad libitum intake |
| Week-On-Week-Off (WOWO) | A type of eating pattern with alternating weeks of energy restriction and full energy or ad libitum intake |
| Time Restricted Feeding (TRF) | A type of eating pattern with a restricted ad libitum eating period throughout the day |
RCTs were conducted in eight different countries: United States (n = 9), Australia (n = 8), Germany (n = 2), Iran (n = 2), Norway (n = 2), United Kingdom (n = 2), Korea (n = 1), and New Zealand (n = 1; Table 2). RCTs ranged from a total duration of 7.5–104 weeks with a range of 22–332 total randomized participants. In short-term trials, the attrition for IER ranged from 0% to 37.5%. In long-term trials, the attrition for IER ranged from 4.1% to 58.5%. The mean age for each study ranged from 33.5 to 68.0 years. Most participants were female. Across the 27 RCTs, the mean BMI reported in each study ranged from 25.8 to 39.5 kg/m2 and weight from 71.1 to 114.0 kg at baseline. Study protocols differed in terms to diet, physical activity, provision of food, and behavioral support (Supplementary Table 2).
Table 2.
Characteristics of Randomized Controlled Trials.
| Randomized Controlled
Trial Author (year) |
Country | Duration (Follow-up) | Regimen | Matched Diets? (Yes/No) | N a | Attrition b , % | Baseline Characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | Sex, % Female | BMI, kg/m2 | Weight, kg | |||||||
| #1. Parvaresh et al. (2019)**, CA | Iran | 8 weeks | ADF | No | 35 c | 0.0 | 44.6 (9.08)SD | 40.0 | 31.1 (3.35)SD | 86.7 (10.65)SD |
| CER | 35 c | 2.9 | 46.4 (7.94)SD | 41.2 | 31.6 (3.82)SD | 84.2 (12.21)SD | ||||
| #2. Beaulieu et al. (2020)ITT | United Kingdom | 12 weeks | ADF | No | 24 | 25.0 | 35 (11)SD | 100.0 | 29.4 (2.5)SD | 81.2 (13.0)SD |
| CER | 22 | 9.1 | 34 (9)SD | 100.0 | 28.9 (2.3)SD | 78.6 (10.0)SD | ||||
| #3. Catenacci et al. (2016)CA | United States | 8 weeks (24 weeks) | ADF | No | 15 c | 6.7 | 39.6 (9.5)SD | 76.9 | 35.8 (3.7)SD | 94.7 (10.6)SD |
| CER | 14 c | 0.0 | 42.7 (7.9)SD | 75.0 | 39.5 (6.0)SD | 114.0 (20.0)SD | ||||
| #4. Coutinho et al. (2018)CA | Norway | 12 weeks | ADF | Yes | 18 c | 22.2 | 39.4 (11.0)SD | 71.4 | 35.6 (3.2)SD | 107.2 (13.6)SD |
| CER | 17 c | 17.6 | 39.1 (9.0)SD | 85.7 | 35.1 (4.2)SD | 97.5 (12.8)SD | ||||
| #5 a. Hutchison et al.
(2019)CA #5 b. Liu et al. (2019)2nd, CA |
Australia | 8 weeks | ADF with 100% energy requirements per week | Yes | 25 | 12.0 | 51.0 (2)SE | 100.0 | 31.2 (0.9)SE | 84.1 (2.8)SE |
| ADF with 70% energy requirements per week | 25 | 12.0 | 49.0 (2.0)SE | 100.0 | 32.4 (0.8)SE | 89.4 (2.8)SE | ||||
| CER70 | 26 | 7.7 | 51.0 (2.0)SE | 100.0 | 32.6 (1.0)SE | 88.4 (2.8)SE | ||||
| Control | 12 | 8.3 | 49.0 (3.0)SE | 100.0 | 30.9 (1.5)SE | 83.8 (4.8)SE | ||||
| #6. Bowen et al. (2018)CA | Australia | 16 weeks (8 weeks) | ADF | Unclear | 82 | 18.3 | 40.0 (8.3)SD | 81.7 | 35.7 (5.8)SD | 100.6 (19.6)SD |
| CER | 81 | 16.0 | 40.6 (8.8)SD | 80.2 | 35.5 (5.5)SD | 99.6 (15.6)SD | ||||
| #7. Razavi et al. (2020)*, CA, ∼ | Iran | 16 weeks | ADF | No | 40 c | 5 | 41.3 (8.65)SD | 40.0 | 31.3 (3.12)SD | 89.4 (7.72)SD |
| CER | 40 c | 7.5 | 43.1 (9.26)SD | 41.2 | 31.2 (3.95)SD | 87.1 (8.17)SD | ||||
| #8 a. Trepanowski et al.
(2017)ITT #8 b. Trepanowski et al. (2018)2nd, CA #8 c. Barnosky et al. (2017)X, 2nd, CA #8 d. Gabel et al. (2019)#, X, 2nd, CA #8 e. Miranda et al. (2018)X, 2nd, CA |
United States | 52 weeks | ADF | Yes | 34 | 38.2 | 44.0 (10.0)SD | 88.2 | 34.0 (4.0)SD | 95.0 (13.0)SD |
| CER | 35 | 28.6 | 43.0 (12.0)SD | 82.9 | 35.0 (4.0)SD | 101.0 (16.0)SD | ||||
| Control | 35 | 22.9 | 44.0 (11.0)SD | 77.1 | 34.0 (4.0)SD | 92.0 (16.0)SD | ||||
| #9. Varady et al. (2011)CA | United States | 12 weeks | ADF | No | 15 c | 13.3 | 47.0 (2.0)SE | 76.9 | 32.0 (2.0)SE | NR |
| CER | 15 c | 20.0 | 47.0 (3.0)SE | 83.3 | 32.0 (2.0)SE | NR | ||||
| Exercise | 15 c | 20.0 | 46.0 (3.0)SE | 83.3 | 33.0 (1.0)SE | NR | ||||
| Control | 15 c | 20.0 | 46.0 (3.0)SE | 83.3 | 32.0 (2.0)SE | NR | ||||
| #10 a. Bhutani et al.
(2013c)ITT #10 b. Bhutani et al. (2013a)2nd, ITT #10 c. Bhutani et al. (2013b)2nd, ITT |
United States | 12 weeks | ADF | Yes | 25 | 36.0 | 42.0 (2.0)SE | 96.0 | 35.0 (1.0)SE | 94.0 (3.0)SE |
| ADF with exercise | 18 | 11.1 | 45.0 (5.0)SE | 100.0 | 35.0 (1.0)SE | 91.0 (6.0)SE | ||||
| Exercise | 24 | 33.3 | 42.0 (2.0)SE | 95.8 | 35.0 (1.0)SE | 93.0 (2.0)SE | ||||
| Control | 16 | 0.0 | 49.0 (2.0)SE | 93.8 | 35.0 (1.0)SE | 93.0 (5.0)SE | ||||
| #11. Cho et al. (2019)CA, ^ | Korea | 8 weeks | ADF | Yes | 26 c | 26.9 | 33.5 (5.0)SD | 75.0 | 27.8 (3.4)SD | 74.6 (13.7)SD |
| ADF with exercise | 28c | 25 | 34.5 (5.7)SD | 44.4 | 28.0 (2.6)SD | 78.2 (14.5)SD | ||||
| Exercise | 24 c | 29.2 | 38.6 (8.2)SD | 44.4 | 26.9 (3.9)SD | 74.2 (13.2)SD | ||||
| Control | 22 c | 27.3 | 42.6 (10.6)SD | 66.6 | 25.8 (3.4)SD | 71.1 (11.7)SD | ||||
| #12. Hoddy et al. (2014)CA | United States | 8 weeks | ADF with small meal at lunch | Yes | 24 c | 16.7 | 46.0 (3.0)SE | 85.0 | 35.0 (1.0)SE | 94.0 (2.0)SE |
| ADF with small meal at dinner | 25 c | 24.0 | 45.0 (3.0)SE | 78.9 | 34.0 (1.0)SE | 97.0 (3.0)SE | ||||
| ADF with small meals throughout the day | 25 c | 20.0 | 46.0 (2.0)SE | 90.0 | 34.0 (1.0)SE | 90.0 (2.0)SE | ||||
| #13 a. Klempel et al.
(2013b)CA #13 b. Klempel et al. (2013c)CA #13 c. Klempel et al. (2013a)2nd, CA #13 d. Varady et al. (2015)X, 2nd, CA |
United States | 8 weeks | ADF with high fat diet | Yes | 17 c | 11.8 | 42.4 (3.0)SE | 100.0 | 35.3 (0.7)SE | 91.5 (2.6)SE |
| ADF with low fat diet | 18 c | 5.6 | 43.2 (2.3)SE | 100.0 | 35.5 (0.7)SE | 91.5 (2.9)SE | ||||
| #14. Antoni et al. (2018)CA | United Kingdom | Max duration of 36 weeks | 5:2 | Yes | 24 c | 37.5 | 42.0 (4.0)SE | 53.3 | 29.8 (0.9)SE | 88.8 (3.4)SE |
| CER | 24 c | 50.0 | 48.0 (3.0)SE | 50.0 | 30.8 (1.1)SE | 89.3 (4.5)SE | ||||
| #15 a. Carter et al.
(2018)+, ITT #15 b. Carter et al. (2019)+, 2nd, ITT |
Australia | 52 weeks (52 weeks) | 5:2 | Yes | 70 | 27.1 | 61.0 (9.0)SD | 55.7 | 35.0 (5.8)SD | 100.0 (19)SD |
| CER | 67 | 31.3 | 61.0 (9.2)SD | 56.7 | 37.0 (5.7)SD | 102.0 (17)SD | ||||
| #16. Carter et al. (2016)+, ITT (weight and HbA1c only) + CA | Australia | 12 weeks | 5:2 | No | 31 | 16.1 | 61.0 (7.5)SD | 54.8 | 35.0 (4.8)SD | 99.0 (16.0)SD |
| CER | 32 | 21.9 | 62.0 (9.1)SD | 50.0 | 36.0 (5.2)SD | 99.0 (15.0)SD | ||||
| #17. Conley et al. (2018)CA | Australia | 24 weeks | 5:2 | No | 12 c | 8.3 | 68.0 (2.7)SE | 0.0 | 33.4 (1.8)SE | 99.1 (7.9)SE |
| CER | 12 c | 0.0 | 67.1 (3.9)SE | 0.0 | 36.2 (4.3)SE | 107.3 (17.1)SE | ||||
| #18. Schübel et al. (2018)ITT | Germany | 24 weeks (26 weeks) | 5:2 | Yes | 49 | 4.1 | 49.4 (9.0)SD | 49.0 | 32.0 (3.8)SD | 96.4 (15.8)SD |
| CER | 49 | 8.2 | 50.5 (8.0)SD | 49.0 | 31.2 (4.0)SD | 92.5 (15.7)SD | ||||
| Control | 52 | 1.9 | 50.7 (7.1)SD | 52.0 | 31.1 (3.6)SD | 93.3 (13.3)SD | ||||
| #19 a. Sundfør et al.
(2018)ITT #19 b. Sundfør et al. (2018)2nd, CA |
Norway | 52 weeks | 5:2 | Yes | 54 | 7.4 | 49.9 (10.1)SD | 48.1 | 35.1 (3.9)SD | 108.6 (16.3)SD |
| CER | 58 | 5.2 | 47.5 (11.6)SD | 51.7 | 35.3 (3.5)SD | 107.5 (16.1)SD | ||||
| #20. Hirsh et al. (2019)CA | United States | 7.5 weeks | 5:2 | No | 10 | 0.0 | 43.4 (13.0)SD | 80.0 | 26.7 (1.9)SD | 76.3 (9.8)SD |
| Control | 12 | 0.0 | 39.0 (10.7)SD | 41.7 | 27.7 (3.1)SD | 79.4 (8.9)SD | ||||
| #21. Hottenrott et al. (2020)CA | Germany | 12 weeks | 5:2 with placebo | Yes | 20 | 10.0 | NR | NR | NR | NR |
| 5:2 with alkaline supplementation | 20 | 15.0 | NR | NR | NR | NR | ||||
| Control with placebo | Yes | 20 | 15.0 | NR | NR | NR | NR | |||
| Control with alkaline supplementation | 20 | 20.0 | NR | NR | NR | NR | ||||
| #22. Corley et al. (2018)+, CA | New Zealand | 12 weeks | 5:2 with consecutive fasting days | Yes | 19 c | 5.3 | 62 (44–77) | 38.9 | 36.6 (5.3)SD | 108.7 (20.4)SD |
| 5:2 with nonconsecutive fasting days | 22 c | 13.6 | 58 (42–74) | 42.1 | 36.8 (5.2)SD | 109.8 (20.3)SD | ||||
| #23. Byrne et al. (2018)ITT | Australia | 30/16 weeks (WOWO/CER) (24 weeks) | WOWO | Yes | 26 | 26.9 | 39.9 (9.2)SD | 0.0 | 34.6 (4.2)SD | 109.8 (14.1)SD |
| CER | 25 | 12.0 | 39.3 (6.6)SD | 0.0 | 34.4 (3.3)SD | 111.6 (10.0)SD | ||||
| #24. Keogh et al. (2014)CA | Australia | 52 weeks | WOWO | No | 39 c | 51.3 | 59.5 (8.7)SD | 100.0 | 33.1 (3.8)SD | 86.9 (14.1)SD |
| CER | 36 c | 52.8 | 60.8 (12.5)SD | 100.0 | 33.0 (7.5)SD | 90.2 (18.8)SD | ||||
| #25 a. Headland et al.
(2019a)ITT (weight
only)+CA #25 b. Headland et al. (2020)2nd, CA #25 c. Headland et al. (2019b)X, 2nd, CA |
Australia | 52 weeks (52 weeks) | 5:2 | Yes | 118 | 58.5 | 47.5 (14.5)SD | 77.1 | 32.7 (5.1)SD | 88.8 (14.9)SD |
| WOWO | 110 | 60.0 | 49.0 (13.2)SD | 85.5 | 33.3 (5.1)SD | 92.6 (17.1)SD | ||||
| CER | 104 | 49.0 | 51.7 (13.0)SD | 81.7 | 32.2 (4.0)SD | 88.2 (13.7)SD | ||||
| #26 a. Klempel et al.
(2012)CA #26 b. Kroeger et al. (2012)CA |
United States | 8 weeks | 6:1 with liquid meal replacements | Yes | 28 c | 7.1 | 47.0 (2.0)SE | 100.0 | 35.0 (1.0)SE | 95.0 (3.0)SE |
| 6:1 with food-based diet | 26 c | 15.4 | 48.0 (2.0)SE | 100.0 | 35.0 (1.0)SE | 94.0 (3.0)SE | ||||
| #27. Chow et al. (2020)CA | United States | 12 weeks | TRF | No | 13 c | 15.4 | 46.5 (12.4)SD | 81.8 | 33.8 (7.6)SD | 95.2 (22.6)SD |
| Control | 9 c | 0.0 | 44.2 (12.3)SD | 88.9 | 34.4 (7.8)SD | 100.9 (28.1)SD | ||||
Note. X = sub sample from parent study; 2nd = secondary analysis; ITT = intention-to-treat analysis; CA = completer’s analysis; SD = standard deviation; SE = standard error; NR = not reported; ADF = alternate day fasting; TRF = time restricted feeding; CER = continuous energy restriction; WOWO = week-on-week-off.
+ = participants with type 2 DM; * = participants with metabolic syndrome; # = participants characterized as insulin resistant; ^ = inclusion criteria BMI >23. ADF = participants randomized into a form of the alternate day fasting eating pattern; 5:2 = participants randomized into a form of the 5:2 eating pattern; WOWO = participants randomized into a form of the week on and week off eating pattern; Control = participants with no prescribed diet interventions, participants were informed to maintain their usual diet; 6:1 = 6 days of restricted or ad libitum intake with 1 day of fasting; TRF = time restricted fasting eating pattern.
arandomized.
bfrom randomized population to end of weight loss and/or weight maintenance phase, attrition for follow-up not included.
cbaseline data of completers only, baseline data of all randomized participants were not available.
Anthropometric Outcomes
Intermittent Energy Restriction Versus Control
There were significantly greater improvements in weight in IER relative to a no intervention control group in most studies in the short-term (8 of 9) and long-term (2 of 2; Table 3). Of the studies that found significant weight improvements in IER in the short-term, three studies scored low risk of bias, three studies scored some concerns, and two studies scored high risk of bias. Greater improvements in fat mass in IER were reported in three (#5 a; #11; #21) out of five RCTs over the short-term. However, no significant between group differences were found in the long-term. Over the short-term, one RCT (#27) showed significantly less improvement in lean mass compared to a control group but no significant long-term differences were observed. However, greater improvements in long-term body fat percentage in ADF compared to control was shown by one study (#8 e). No significant between group differences were observed in waist circumference or FFM over the short- or long-term, and no studies examined RMR.
Table 3.
Intermittent Energy Restriction Versus Control Anthropometric, Cardiometabolic, Inflammatory, and Appetite Outcomes.
| Short-Term a | Long-Term b | |||||
| Antdropometric | IER > Control c | IER < Control d | IER = Control e | IER > Control c | IER < Control d | IER = Control e |
| Weight loss | #5 a; #8 e; #9; #10 a, b, c; #11; #18; #21; #27 | #20 | #8 a, b, c, d, e; #18 #18* |
|||
| Waist circumference | #5 a; #10 a, b, c; #18 | #18 #18* |
||||
| Body fat percentage | #11; #27 | #8 | ||||
| Fat mass (kg) | #5 a; #11; #21 | #10 a, b, c; #27 | #8 a | |||
| Lean mass (kg) | #27 | #8 a, c, d | ||||
| Fat-free mass | #5 a; #10 a, b, c | #8 b | ||||
| Resting metabolic rate | ||||||
| Cardiometabolic | IER > Control c | IER < Control d | IER = Control e | IER > Control c | IER < control d | IER = control e |
| Systolic blood pressure | #5 a; #10 a, c; #18; #20; #27 | #8 a, d #18* |
||||
| Diastolic blood pressure | #5 a; #10 a, c; #18; #20; #27 | #8 a, d #18* |
||||
| Heart rate | #10 a, c; #20 | #8 a, d | ||||
| Fasting glucose | #8 e | #5 a; #10 a, c; #11; #18; #21; #27 | #8 a, b, d, e; #18 | |||
| Fasting insulin | #20 | #5 a | #8 e; #10 a, c; #11; #18; #27 | #8 a, b, d, e | #18 #18* |
|
| HbA1c | #27 | |||||
| HOMA-IR | #8 e; #20 | #5 a; #10 a, c; #11; #18; #27 | #8 a, b, e | |||
| Triglycerides | #9 | #5 a; #10 a, c; #11; #18; #27 | #8 a | #18 #18* |
||
| Total cholesterol | #5 a; #9; #10 a, c; #18; #20 | #8 a, d; #18 18* |
||||
| LDL | #9 | #5 a; #10 a, c; #11; #18; #20; #27 | #8 a | #18 #18* |
||
| HDL | #5 a; #9; #10 a, c; #11; #18; #20; #27 | #8 a | #18 #18* |
|||
| Inflammatory | IER > Control c | IER < Control d | IER = Control e | IER > Control c | IER < control d | IER = control e |
| hs-CRP | #5 a; #10 a; #11; #18 | #8 a, d; #18 #18* |
||||
| IL-6 | #8 e; #18 | #8 b, d, e | ||||
| IL-8 | #18 | |||||
| IL-10 | ||||||
| IFN-γ | #18 | |||||
| TNF-α | #8 e; #18 | #8 b, d, e | ||||
| Appetite | IER > Control c | IER < Control d | IER = Control e | IER > Control c | IER < Control d | IER = Control e |
| Leptin | #10 c | #18 | #8 b | #18 | ||
| Ghrelin | ||||||
| Resistin | #18 | #8 b; #18 | ||||
| BDNF | #18 | |||||
| Adiponectin | #8 e; #10 c; #18 | #8 b, e | ||||
Note. If study reported intention-to-treat, completer's, and/or subpopulation analysis, the intention-to-treat analysis was reported. IER = intermittent energy restriction; CER = continuous energy restriction; HbA1c = hemoglobin A1c; HOMA-IR = homeostatic model assessment of insulin resistance; LDL = low density lipoprotein; HDL = high density lipoprotein; hs-CRP = high sensitivity c-reactive protein; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IFN-γ = interferon-gamma; TNF-α = tumor necrosis factor-alpha; BDNF = brain-derived neurotropic factor.
*follow-up
a<24 weeks.
b≥24 weeks.
cintermittent energy restriction shows significantly greater improvement in comparison to control.
dintermittent fasting shows significantly less improvement in comparison to control.
eno significant between group differences.
Intermittent Energy Restriction versus Continuous Energy Restriction
Nine (#2; #3; #4; #6; #9; #16; #18; #19 b; #24) out of 13 studies found that relative to CER, IER resulted in similar short-term weight loss (Table 4). Four (#1; #5 a, b; #7; #23) out of 13 RCTs showed significantly greater improvements in weight in IER compared to CER over the short-term (Table 4). No significant between group differences in weight loss was observed over the long-term between IER and CER. Significantly greater weight improvements in IER than CER during follow-up were observed in one (#23) out of five RCTs.
Table 4.
Intermittent Energy Restriction versus Continuous Energy Restriction: Anthropometric, Cardiometabolic, Inflammatory, and Appetite Outcomes.
| Short-Term a | Long-Term b | |||||
| Weight loss | #1; #5 a, b; #7; #23 | #2; #3; #4; #6; #9; #16; #18; #19 b; #24 | #23f | #8 a, b, c, d; #14; #15 a; #17; #18;
#19 a; #25 a, c #3*; #15 b*; #18*; #24*; #25 b* |
||
| Waist circumference | #1; #7 | #2; #5 a, b; #18 | #14; #17; #18; #19 a;
#24 #18* |
|||
| Body fat percentage | #2; #16 | #15 a #15 b* |
||||
| Fat mass (kg) | #5 a; #7 | #2; #3; #4; #6; #16; #23 | #8 a, b, c, d; #14; #15 a; #25
a #3*; #15 b*; #23*; #25 b* |
|||
| Lean mass (kg) | #3 | #8 a, c, d; #25 a #3*; #25 b* |
||||
| Fat-free mass | #2; #4; #5 a; #6; #7; #16; #23 | #8 b; #14; #15 a #15 b*; #23* |
||||
| Resting metabolic rate | #23g | #2; #3; #4; #19 a | #14 #3* |
|||
| Cardiometabolic | IER > CER c | IER < CER d | IER = CER e | IER > CER c | IER < CER d | IER = CER e |
| Systolic blood pressure | #1 | #5 a; #8 a; #18 | #14 | #8 a, d; #17 #18* |
||
| Diastolic blood pressure | #1; #5 a; #6; #18 | #8 a, d; #14; #17; #19
a #18* |
||||
| Heart rate | #8 a, d; #19 a | |||||
| Fasting glucose | #1 | #18 | #3; #5 a; #6 | #8 a, b, d; #14; #17; #18;
#25a #3*; #15 b*; #18*; #25 b* |
||
| Fasting insulin | #1; #3; #5 a; #6; #18 | #8 a, b; #14; #18 #3*; #18* |
||||
| HbA1c | #16 | #15 a #15 b* |
||||
| HOMA-IR | #1; #5 a; #6; #18 | #8 a; #14; #18 #18* |
||||
| Triglycerides | #9 | #5 a; #6 | #1; #3; #18 | #8 a, d; #14; #15 a; #17; #18; #19 a;
#25 a #3*; #15 b*; #18*; #25 b* |
||
| Total cholesterol | #5 a | #1; #3; #9; #18 | #8 a, d; #14, #15 a; #17; #18; #19 a;
#25 a #15 b*; #18*; #25 b* |
|||
| LDL | #5 a | #1; #3; #6; #9; #18 | #8 a | #15 a, #17, #18, #19.a, #25
a #15 b*; #18*, #25b* |
||
| HDL | #5 a | #1; #3; #6; #9; #18 | #8 a, d; #14; #15 a; #17; #18; #19 a;
#25 a #15 b*; #18*; #25 b* |
|||
| Inflammatory | IER > CER c | IER < CER d | IER = CER e | IER > CER c | IER < CER d | IER = CER e |
| hs-CRP | #7 | #5 a; #6; #18 | #8 a, d; #18; #19 a #18* |
|||
| IL-6 | #6 b; #7; #18 | #8 b, d | ||||
| IL-8 | #18 | |||||
| IL-10 | #5 b | |||||
| IFN-γ | #18 | |||||
| TNF-α | #5 b; #7; #18 | #8 b, d | ||||
| Appetite | IER > CER c | IER < CER d | IER = CER e | IER > CER c | IER < CER d | IER = CER e |
| Leptin | #3; #18 | #8 b; #18 #3* |
||||
| Ghrelin | #3; #4 | #3* | ||||
| Resistin | #18* | #8 b; #18* | ||||
| BDNF | #3; #18 | #3* | ||||
| Adiponectin | #18 | #8 b | ||||
Note. If study reported intention-to-treat, completer's, and/or subpopulation analysis, the intention-to-treat analysis was reported. IER = intermittent energy restriction; CER = continuous energy restriction; HbA1c = hemoglobin A1c; HOMA-IR = homeostatic model assessment of insulin resistance; LDL = low density lipoprotein; HDL = high density lipoprotein; hs-CRP = high sensitivity c-reactive protein; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IFN-γ = interferon-gamma; TNF-α = tumor necrosis factor-alpha; BDNF = brain derived neurotropic factor.
^ = adjusted for fat mass and fat-free mass.
*follow-up
a< 24 weeks.
b≥ 24 weeks.
cintermittent energy restriction shows significantly greater improvement in comparison to continuous energy restriction.
dintermittent fasting shows significantly less improvement in comparison to continuous energy restriction.
eno significant between group differences.
Results for waist circumference and fat mass were mixed though most studies showed no benefit of IER relative to CER on changes in body fat percentage (#2; #16; #15 a), lean mass (#3; #8 a, c, d; #25 a), FFM (#2; #4; #5 a; #6; #7; #8 b; #14; #15 a; #16, #23), or RMR (#2; #3; #4; #14; #19 a) in the short- or long-term. Significantly greater improvements in waist circumference were observed in IER in two (#1; #7) out of five RCTs when compared to CER over the short-term. Two (#5 a; #7) out of eight RCTs showed greater improvements in fat mass in IER. However, no significant between group differences in waist circumference (#14; #17; #18; #19 a; #24) or fat mass (#8 a, b, c, d; #14; #15 a; #25 a) was observed over the long-term. One study (#23) observed greater IER improvement in RMR in comparison to CER after adjusting for FFM and fat mass over the short-term. No significant between group differences were found for waist circumference, body fat percentage, fat mass, lean mass, FFM, or RMR between IER and CER during follow-up. However, one study (#3) observed significantly greater improvements in lean mass percentage and fat mass percentage in IER when compared to CER during follow-up.
Intermittent Energy Restriction versus Exercise
Three (#9; #10 a, b, c; #11) studies that compared ADF to an exercise-alone intervention group found no between group differences in weight loss (#9; #10 a, b, c; #11), waist circumference (#10 a, b, c), body fat percentage (#11), fat mass (#10 a, b, c; #11), or FFM (#10 a, b, c). Two (#10 a, b, c; #11) RCTs that compared ADF with exercise with an ADF, exercise-alone, or control group found mixed results. One RCT (#10 a, b, c) found significantly greater improvement in weight, waist circumference, and fat mass in the ADF with exercise group than the ADF, exercise-alone, or control group. However, one study (#11) found no significant between group differences in weight, body fat percentage, and fat mass when ADF with exercise is compared with the ADF or exercise-alone group.
Comparisons of Different Types of Intermittent Energy Restriction Diets
Five RCTs (#5 a, b; #12; #13 a, b, c, d; #22; #26 a, b) examined modified IER diets over the short-term. No significant between group anthropometric differences were observed when comparing different mealtimes (#12), high- or low-fat diet (#13 a, b, c, d), or consecutive versus nonconsecutive fasting days (#22). When comparing an ADF group that provided 70% of energy needs and an ADF group providing 100% of energy needs, greater improvements in weight (#5 a, b), waist circumference (#5 b), and fat mass (#5 a, b) were observed in the ADF group that provided 70% of energy needs. No significant between group differences were found for FFM (#5 a). One study (#26 a, b) observed that a 6-day liquid diet with one fasting day had significantly greater improvement in weight (#26 a, b) and waist circumference (#26 b) than a 6-day food diet with one fasting day regimen. No significant between group differences were found in FM and FFM (#26 a, b).
Cardiometabolic
Intermittent Energy Restriction versus Control
Most studies found no significant differences in systolic and diastolic blood pressure (#5 a; 8 a, d; #10 a, c; #18; #20; #27), heart rate (#8 a, d; #10 a, c; #20), fasting glucose (#5 a; #8 a, b, d, e; #10 a, c; #11; #18; #21; #27), fasting insulin (#8 e; #10 a, c; #11; #18; #27), HbA1c (#27), triglycerides (#5 a; #10 a, c; #11; #18; #27), total cholesterol (#5 a; #8 a, d; #9; #10 a, c; #18; #20), LDL (#5 a; #10 a, c; #11; #18; #20; #27), and HDL (#5 a; #9; #10 a, c; #11; #18; #20; #27) outcomes when IER is compared to a no-intervention control group in the short- or long-term (Table 3). One (#8 e) out of seven RCTs found greater improvement in fasting glucose when compared to a control group over the short-term but no long-term effects were observed. Significantly greater improvement in fasting insulin was observed in one (#20) out of seven RCTs in IER when compared to a control group over the short-term but one (#5 a) RCT found significantly less improvement in fasting insulin in IER. Over the long-term, one (#8 a, b, c, d, e) out of two RCTs found significantly greater fasting insulin improvements in IER. Two (#8 e; #20) out of six RCTs found significantly greater improvement in HOMA-IR in IER when compared to a control group over the short-term but no significant between group differences are observed in the long-term. One (#9) out of six RCTs in the short-run and one (#8 a) out of two RCTs in the long-run found significantly greater improvement in triglycerides in IER when compared to control. One (#9) out of seven RCTs found significantly greater improvement in IER LDL outcomes in the short-term and one (#8 a) out of two RCTs found significant improvements over the long-term. Greater improvement in HDL was observed in one (#8 a) out of two RCTs when compared to a control group in the long-term. No significant between group differences are observed in systolic or diastolic blood pressure, heart rate, HbA1c, and total cholesterol over the short- or long-term. One study examined follow-up outcomes and found no significant between group differences in systolic or diastolic blood pressure, fasting glucose, fasting insulin, triglycerides, total cholesterol, LDL, or HDL outcomes.
Intermittent Energy Restriction versus Continuous Energy Restriction
Most studies found that IER, relative to CER, resulted in similar changes in systolic (#5 a; #8 a, d; #17; #18) and diastolic (#1; #5 a; #6; #8 a, d; #14; #17; #18; #19 a) blood pressure, heart rate (#8 a, d; #19 a), fasting glucose (#3; #5 a; #6; #8 a, b, d; #14; #17; #18; #25 a), fasting insulin (#1; #3; #5 a; #6; #8 a, b; #14; #18), HbA1e (#15 a; #16), HOMA-IR (#1; #5 a; #6; #8 a; #14; #18), triglycerides (#1; #3; #8 a, d; #14; #15 a; #17; #18; #19 a; #25 a), total cholesterol (#1; #3; #8 a, d; #9; #14; #15 a; #17; #18; #19 a; #25 a), LDL (#1; #3; #6; #9; #15 a; #17; #18; #19 a; #25 a), and HDL (#1; #3; #6; #8 a, d; #9; #14; #15 a; #17; #18; #19 a; #25 a) (Table 4). One (#1) out of four RCTs showed significantly greater improvement in systolic blood pressure IER when compared to CER over the short-term. Over the long-term, one (#14) out of three RCTs showed significantly greater systolic blood pressure improvements. IER showed significantly greater improvements in fasting glucose in one (#1) of five RCTs when compared to CER over the short-term. However, (#18) found significantly less improvements in IER when compared to CER over the short-term and no long-term effects were observed. One (#9) out of six RCTs showed significantly greater improvements in triglycerides in IER when compared to CER over the short-term. However, two (#5 a; #6) RCTs showed significantly less improvement in IER when compared to CER but no long-term effects were observed. One (#5 a) of five RCTs found significantly greater improvements in total cholesterol in IER when compared to CER over the short-term with no long-term effects. One (#5 a) out of six RCTs showed significantly less improvement in HDL in IER when compared to CER over the short-term. One (#5 a) out of six RCTs showed significantly greater improvements in LDL in IER when compared to CER over the short-term. Over the long-term, one (#8 a) out of six RCTs found greater LDL improvements in IER. No between group differences were observed in diastolic blood pressure, heart rate, fasting insulin, HbA1c, and HOMA-IR over the short- or long-term. No significant between group differences were observed for follow-up.
Intermittent Energy Restriction versus Exercise
Mixed results were observed in triglycerides, HDL, and LDL between three RCTs (#9; #10 a, b, c; #11) that compared ADF to an exercise-alone intervention group. When comparing ADF with exercise and an ADF, exercise-alone, or control over the short-term, mixed results were observed for HDL outcomes. One (#9) out of three RCTs found significantly greater improvement in triglycerides and one study (#11) found significantly less improvement in ADF when compared to an exercise-alone group. One study (#9) also found significantly greater improvement in LDL and significantly less improvement in HDL in a ADF group when compared to an exercise-alone group. No significant between group differences were observed in systolic or diastolic blood pressure, heart rate, fasting glucose, fasting insulin, HOMA-IR, or total cholesterol. Two (#10 a, c; #11) of two RCTs found significantly greater HDL improvements in ADF with exercise group when compared to a control group. One (#10 a, c) of two RCTs also found greater improvements in HDL when ADF with exercise is compared with an ADF or exercise-alone group. No significant between group differences were found in systolic or diastolic blood pressure, heart rate, fasting glucose, fasting insulin, HOMA-IR, triglycerides, total cholesterol, or LDL between an ADF with exercise group and an ADF, exercise-alone, or control group.
Comparisons of Different Types of Intermittent Energy Restriction Diets
Differences in cardiometabolic outcomes were observed between modified IER regimens. One study (#12) found greater improvements in heart rate in an ADF diet that consumed a fasting meal at lunch in comparison to consuming small meals throughout the fasting day. No significant between group differences between an ADF regimen with a small meal at lunch, dinner, or throughout the fasting day were observed in systolic and diastolic blood pressure, fasting glucose, fasting insulin, HOMA-IR, triglycerides, total cholesterol, HDL, or LDL outcomes. Another study (#22) found significantly less improvement in total cholesterol in a consecutive fasting 5:2 diet compared to a nonconsecutive fasting 5:2 diet. However, significantly greater improvements in LDL were observed in consecutive fasting days in comparison to nonconsecutive fasting days. No between group differences were observed in systolic and diastolic blood pressure, fasting glucose, HbA1c, triglycerides, or HDL outcomes. No between group differences were observed when comparing a high- and low-diet regimen (#13 a, b, c). When comparing an ADF group that provided 70% of energy needs and an ADF group that provided 100% of energy needs, greater improvements in fasting glucose, HbA1c, triglycerides, total cholesterol, and LDL were observed in the ADF group provided with 70% of energy needs. No significant between group differences were observed in systolic or diastolic blood pressure and HDL outcomes (#5 a). One study (#26 a, b) found significantly greater improvements in heart rate, total cholesterol, and LDL outcomes in a 6-day liquid diet with 1 day fasting group in comparison to a 6-day food diet with 1 day fasting group. However, no significant between group differences were found in systolic and diastolic blood pressure, fasting glucose, fasting insulin, triglycerides, and HDL outcomes.
Inflammatory
Of the 42 studies reviewed, 13 examined the effects of IER on inflammatory markers. Ten (#5 a; #6; #7; #8 a, d; #10 a; #11; #18; #19 a) of the 13 studies examined the effect of intermittent fasting on hs-CRP (Table 3 and Table 4). Of the 10 studies, only one study (#7) reported between group differences. The study found that adherence to the ADF diet led to a significant reduction in serum hs-CRP concentrations (mg/L) from baseline, compared to the CER diet (−2.06 ± 1.18 vs. −0.97 ± 0.82; p = 0.03) in the short-term. There were no between group differences found for IL-6, IL-8, IL-10, IFN- γ, or TNF-α across studies comparing IER and CER in the short- and long-term. No between group differences were observed when comparing IER and a no intervention control group.
Appetite
Intermittent Energy Restriction versus Control
Few studies assessed hormones and appetite outcomes (Table 3). Mixed results were reported for leptin. One (#10 c) out of two RCTs found greater improvements in IER when compared to a control group in the short-term. Long-term effects were observed in one (#8 b) of two studies. Studies reported no significant between group differences for resistin, BDNF, and adiponectin.
Two (#5 a; #18) of four studies found significantly less energy intake in IER group in comparison to a control group. However, two studies (#10 b; #11) found no significant between group differences in energy intake in the short-term. One study (#5 a) measured hunger and reported lower hunger scores during IER feed day in comparison to control during the first week and greater hunger scores during IER fast days in comparison to control during the last week of the study.
Intermittent Energy Restriction versus Continuous Energy Restriction
No significant between group differences in leptin (#3; #8 b; #18), ghrelin (#3; #4), resistin (#8 b), BDNF (#3; #18), and adiponectin (#8 b; #18) were reported between IER and CER in the short- or long-term (Table 4). One study (#3) found significantly less improvement in BDNF in IER group when compared to CER group during follow-up.
Mixed results were reported for energy intake in the short-term and most studies report no significant between group differences in the long-term. One (#5 a) out of six RCTs (#3; #4; #5 a; #8 c; #18; #19 b) reported significantly lower energy intake in IER group in comparison to CER group in the short-term. However, one study (#3) reported significantly greater energy intake in IER group than CER group. One study (#14) out of three RCTs (#14; #17; #24) found significantly lower energy intake in IER group than a CER group in the long-term. Most studies examined reported no significant between group differences in hunger (#2; #4), satisfaction (#16), fullness (#2; #16), and desire to eat (#2; #4) in the short-term and only one study (#19 a) examined hunger in the long-term. Two (#2; #4) of three studies reported no significant between group differences for hunger. However, one study (#5 a) reported significantly greater hunger scores during a fast day in comparison to CER during baseline and significantly less hunger scores during a feed day in comparison to CER. One study (#19 a) reported significantly greater scores for the question “I have often felt hungry while on the diet” in the IER group in comparison to the CER group.
Intermittent Energy Restriction versus Exercise
Greater improvements in leptin outcomes were observed when comparing ADF with exercise to ADF, exercise, or control group and no other significant differences were observed for energy intake or appetite outcomes (#10 c). Greater improvements for leptin were observed in the ADF with exercise group when compared to an ADF, exercise-alone, or control group but no significant between group differences were observed for adiponectin (#10 c). No significant between group differences in leptin or adiponectin outcomes between ADF and exercise-alone intervention group (#10 c). The same RCT (#10 b) found no significant between group differences in energy intake between ADF and exercise, ADF with exercise and ADF, exercise-alone, or control. No significant between group differences were found in hunger, fullness, and satisfaction when comparing ADF with exercise with ADF group in the short-term.
Comparisons of Different Types of Intermittent Energy Restriction Diets
Few studies examined hormones and appetite outcomes between different types if IER diets with varying outcomes. No significant between group differences were reported when comparing a high- and low-fat ADF regimen for leptin, resistin, and adiponectin outcomes (#13 c). Greater increase in fullness and satisfaction were observed over time in the low-fat ADF diet in comparison to the high-fat ADF diet (#13 c). In a study comparing consecutive and nonconsecutive fasting days in a 5:2 diet, no significant between group differences was reported for energy intake (#22). Another study comparing a6-day liquid diet with 1 day fasting group with a 6-day food diet with 1 day fasting group found no significant between group differences for leptin (#26 a, b) or adiponectin (#26 a).
Risk of Bias Assessment
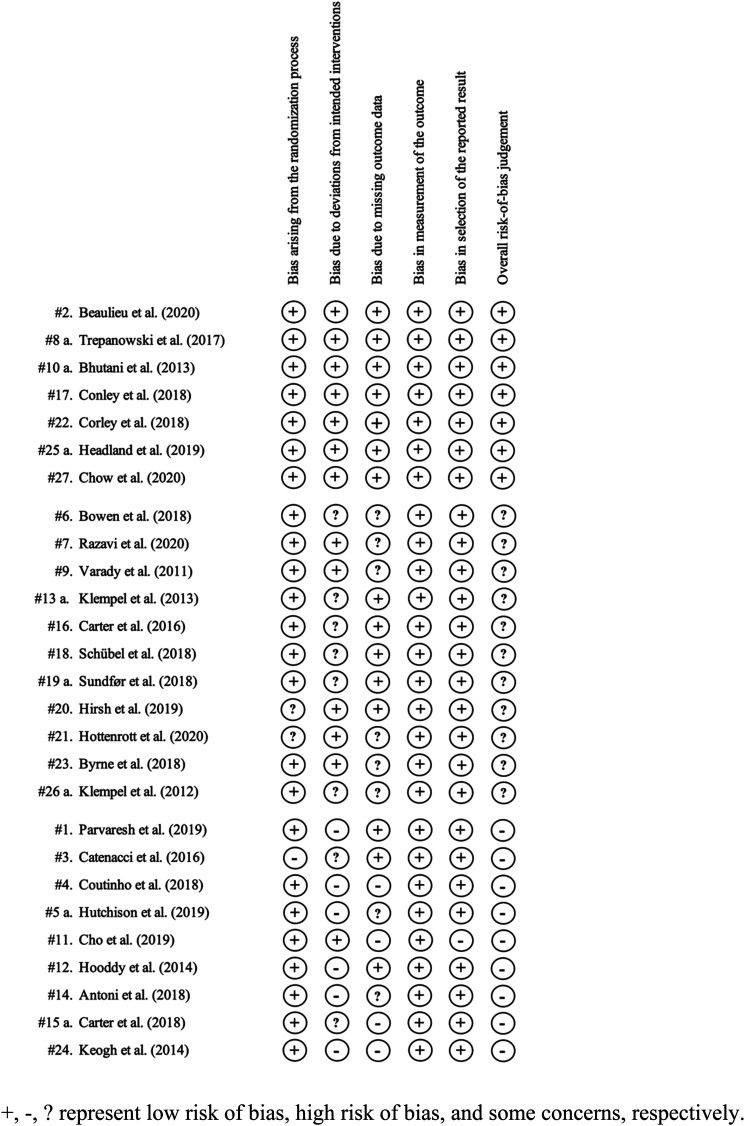
Of the 27 RCTs, seven studies scored low risk of bias, 11 scored some concerns, and nine studies scored high risk of bias (Figure 2). Most studies scored low risk of bias in bias arising from the randomization process, measurement of the outcome, and selection of the reported results. Two studies (#20; #21) scored some concerns in the randomization process due to limited information on randomization and allocation methods reported in the research article. One study (#3) scored high risk of bias in randomization due to weight differences in baseline characteristics of the participants. One study (#11) scored high risk of bias in the selection of the reported result as only weight outcomes of individuals who have agreed to complete a sterol examination were reported. Eight studies (#3; #6; #13 a; #15 a; #16; #18; #19a; #26 a) scored some concerns and six studies (#1; #4; #5 a; #12; #14; #24) scored high risk of bias due to deviations from intended interventions. All participants were aware of their assigned intervention due to the nature of IER regimens. Studies scored some concerns or high risk of bias due to the number of participants excluded from the final analysis, non-adherence to the intended diet, and/or type of analysis used to estimate outcomes if participants adhered to the diet. Eight studies (#5 a; #6; #7; #9; #14; #21; #23; #26 a) scored some concerns and 4 studies (#4; #11; #15 a; #24) scored high risk of bias due to missing outcome data based on high attrition and reasonings for participant drop-out related to the intervention.
Figure 2.
Risk of Bias Assessment.
Discussion
The aim of this systematic review was to examine short- and long-term effects of IER in participants with overweight and/or obesity. Short-term benefits of IER observed include improvements in weight and fat mass. However, long-term benefits were observed for weight only. When compared to a no-intervention control group, IER resulted in positive short-term effects in weight and fat mass. Few studies reported positive outcomes for glucose, insulin, HOMA-IR, triglycerides, LDL, and leptin that were sustained in the long-term. No significant differences in inflammatory markers were found. Few studies observed significant differences between CER and IER in anthropometric, cardiometabolic, inflammatory, energy intake, and appetite outcomes. This suggests similar benefits of CER and IER interventions for participants with overweight obesity.
Out of the 27 RCTs included in our review, 19 RCTs were also studied in previous systematic reviews. Previous systematic reviews found significant weight loss when comparing IER with a control group and no significant difference when comparing IER with CER. Harris et al. (2018) found significant weight loss and fat mass reduction in IER when compared to a control group. Our results also indicate continuous weight loss over the short- and long-term with improvements in fat mass in the short-term. However, more research is needed for long-term effects of IER in fat mass. Harris et al., 2018 also found no significant improvements in cardiometabolic outcomes. Previous systematic reviews found no significant differences in weight loss between IER and CER regimens (Cioffi et al., 2018; Harris et al., 2018; Lima et al., 2020) and significant improvement in fasting insulin outcomes (Cioffi et al., 2018; Harris et al., 2018). Our results also show no significant differences in both the short- and long-term in weight loss. However, improvements in fasting insulin were not observed in the short- or long-term. Similarly, anthropometric and other cardiometabolic outcomes in IER were not significantly different from CER (Cioffi et al., 2018; Harris et al., 2018).
Adherence to daily calorie restriction decreases over time (Dansinger et al., 2005; Moreira et al., 2011). Barriers to dietary adherence are behavioral fatigue and feelings of deprivation (Bray & Wadden, 2015; Forman & Butryn, 2015). IER protocols have been designed to address adherence issues by prescribing restriction on select days, rather than daily. However, we found little evidence that overall, IER is easier to adhere to than CER. CER and IER resulted in similar weight loss when isocaloric energy deficits were prescribed. Attrition rates were variable in the reviewed studies, ranging from 0.0% to 58.5% in IER intervention groups. More research is needed to address the sustainability of IER. Additionally, results suggest that IER may be beneficial for a certain subset population as positive outcomes, such as weight loss, is evident in IER regimens. However, characteristics of this subset have yet to be identified. Further studies are needed to identify characteristics of participants who may benefit from IER rather than CER. This may include certain genetic, environmental, or behavioral factors that contribute to greater success in utilizing IER for weight loss for individuals with overweight obesity.
“Metabolic switching” is hypothesized to improve body composition during weight loss in IER. Lean muscle mass may be preserved as the body switches from utilization of glucose to fatty acid and fatty acid-derived ketones for metabolism (Anton et al., 2018). Our findings indicate significant reductions in weight and fat mass with no significant reductions in lean mass or FFM in the short-term in IER when compared to a control group. This suggests preservation of lean or FFM with reductions in fat mass over a short-term IER regimen in individuals with overweight and/or obesity. Chow et al. 2020 found significant reductions in lean mass in their TRE group. However, this was the only study that indicated significant reductions in lean mass or FFM in an IER regimen. When comparing IER and CER, no significant fat mass, lean mass, or FFM differences were found. This suggests similar outcomes in body composition in IER and CER in individuals with overweight obesity.
Few studies assessed appetite outcomes and more studies are needed to examine the safety in individuals with certain medical conditions such as type 2 diabetes. Previous studies reported concerns of binge eating as a result of switching between fasting and feed states in IER. Only one study measured binge eating among participants and found a significant decrease overtime in IER and CER groups with no significant between group differences (Beaulieu et al., 2020). Assessing appetite and hunger may further help assess the feasibility and effects of IER regimens. IER may not be recommended for individuals with a history of binge eating disorder and more research is needed to assess eating behaviors of participants during IER. IER does not seem to produce severe negative physical adverse effects with reports of mild symptoms such as headaches, dizziness, fatigue, and nausea in several studies (Conley et al., 2018; Hirsh et al., 2019; Schübel et al., 2018; Sundfør et al., 2018), suggesting the relative safety of IER for weight loss in individuals with overweight obesity.
The strengths of this review include a comprehensive synthesis of RCTs on various IER regimens in individuals with overweight obesity. Anthropometric, cardiometabolic, inflammatory, and appetite outcomes were examined to assess the short- and long-term benefits of IER. Limitations include the heterogeneity of IER regimens and protocols and potential for publication bias. Authors of studies were not contacted for additional information and a selective number of databases were utilized due to resource constraints.
Conclusion
In conclusion, we reviewed a total of 42 articles that reported on 27 RCTs assessing IER for weight loss. We found that IER showed pre-to-post treatment improvements in 8 of 9 studies that assessed weight. However, compared to CER, IER showed no significant long-term differences in anthropometric, cardiometabolic, inflammatory, or appetite outcomes in included studies. More long-term studies are needed assess the benefits of IER on health outcomes.
Supplemental Material
Supplemental Material, sj-pdf-1-brn-10.1177_10998004221078079 for Intermittent Energy Restriction for Weight Loss: A Systematic Review of Cardiometabolic, Inflammatory and Appetite Outcomes by Xueting Wei, Ashley Cooper, Irene Lee, Christine A. Cernoch, Ginny Huntoon, Brandi Hodek, Hanna Christian and Ariana M. Chao in Biological Research For Nursing
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AMC reports grants from Eli Lilly and Company and WW International, Inc., outside the submitted work. The other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: XW was supported by the Penn Undergraduate Research Mentorship program of the Center of Undergraduate Research and Fellowships at the University of Pennsylvania. AMC was supported, in part, by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR017209.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Ariana M. Chao https://orcid.org/0000-0001-5633-8973
References
- *indicates an article included in the systematic review synthesis. The number in the parathesis represents the article in the results and lowercase letters indicate a primary or secondary article. Numbers with no letter and the letter ‘a’ represent a primary article. Other letters indicate secondary articles. [Google Scholar]
- Alhassan S., Kim S., Bersamin A., King A. C., Gardner C. D. (2008). Dietary adherence and weight loss success among overweight women: Results from the A TO Z weight loss study. International Journal of Obesity, 32(6), 985–991. 10.1038/ijo.2008.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (14)Antoni R., Johnston K. L., Collins A. L., Robertson M. D. (2018). Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. British Journal of Nutrition, 119(5), 507–516. 10.1017/S0007114517003890 [DOI] [PubMed] [Google Scholar]
- Anton S. D., Moehl K., Donahoo W. T., Marosi K., Lee S. A., Mainous A. G., III, Leeuwenburgh C., Mattson M. P. (2018). Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity, 26(2), 254–268. 10.1002/oby.22065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (8 c)Barnosky A., Kroeger C. M., Trepanowski J. F., Klempel M. C., Bhutani S., Hoddy K. K., Gabel K., Shapses S. A., Varady K. A. (2017). Effect of alternate day fasting on markers of bone metabolism: An exploratory analysis of a 6–month randomized controlled trial. Nutrition and Healthy Aging, 4(3), 255–263. 10.3233/NHA-170031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (2)Beaulieu K., Casanova N., Oustric P., Turicchi J., Gibbons C., Hopkins M., Varady K., Blundell J., Finlayson G. (2020). Matched weight loss through intermittent or continuous energy restriction does not lead to compensatory increases in appetite and eating behavior in a randomized controlled trial in women with overweight and obesity. The Journal of Nutrition, 150(3), 623–633. 10.1093/jn/nxz296 [DOI] [PubMed] [Google Scholar]
- * (10 b)Bhutani S., Klempel M. C., Kroeger C. M., Aggour E., Calvo Y., Trepanowski J. F., Hoddy K. K., Varady K. A. (2013. a). Effect of exercising while fasting on eating behaviors and food intake. Journal of the International Society of Sports Nutrition, 10(1), 50–58. 10.1186/1550-2783-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (10 c)Bhutani S., Klempel M. C., Kroeger C. M., Trepanowski J. F., Phillips S. A., Norkeviciute E., Varady K. A. (2013. b). Alternate day fasting with or without exercise: Effects on endothelial function and adipokines in obese humans. e-SPEN Journal, 8(5), e205–e209. 10.1016/j.clnme.2013.07.005 [DOI] [Google Scholar]
- * (10 a)Bhutani S., Klempel M. C., Kroeger C. M., Trepanowski J. F., Varady K. A. (2013. c). Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity, 21(7), 1370–1379. 10.1002/oby.20353 [DOI] [PubMed] [Google Scholar]
- * (6)Bowen J., Brindal E., James-Martin G., Noakes M. (2018). Randomized trial of a high protein, partial meal replacement program with or without alternate day fasting: similar effects on weight loss, retention status, nutritional, metabolic, and behavioral outcomes. Nutrients, 10(9), 1145. 10.3390/nu10091145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., Wadden T. A. (2015). Improving long‐term weight loss maintenance: Can we do it? Obesity, 23(1), 2–3. 10.1002/oby.20964 [DOI] [PubMed] [Google Scholar]
- * (23)Byrne N. M., Sainsbury A., King N., Hills A., Wood R. (2018). Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. International Journal of Obesity, 42(2), 129–138. 10.1038/ijo.2017.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (15 b)Carter S., Clifton P., Keogh J. (2016). The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Research and Clinical Practice, 122, 106–112. 10.1016/j.diabres.2016.10.010 [DOI] [PubMed] [Google Scholar]
- * (15 a)Carter S., Clifton P. M., Keogh J. B. (2018). Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: A randomized noninferiority trial. JAMA Network Open, 1(3), Article e180756–e180756. 10.1001/jamanetworkopen.2018.0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (16)Carter S., Clifton P., Keogh J. (2019). The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Research and Clinical Practice, 151, 11–19. 10.1016/j.diabres.2019.03.022 [DOI] [PubMed] [Google Scholar]
- * (3)Catenacci V. A., Pan Z., Ostendorf D., Brannon S., Gozansky W. S., Mattson M. P., Martin B., MacLean P. S., Melanson E. L., Troy Donahoo W. (2016). A randomized pilot study comparing zero‐calorie alternate‐day fasting to daily caloric restriction in adults with obesity. Obesity, 24(9), 1874–1883. 10.1002/oby.21581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (11)Cho A.-R., Moon J.-Y., Kim S., An K.-Y., Oh M., Jeon J. Y., Jung D.-H., Choi M. H., Lee J.-W. (2019). Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism, 93, 52–60. 10.1016/j.metabol.2019.01.002 [DOI] [PubMed] [Google Scholar]
- * (27)Chow L. S., Manoogian E. N., Alvear A., Fleischer J. G., Thor H., Dietsche K., Wang Q., Hodges J. S., Esch N., Malaeb S., Harindhanavudhi T., Nair K. S., Panda S., Mashek D. G. (2020). Time‐restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity, 28(5), 860–869. 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi I., Evangelista A., Ponzo V., Ciccone G., Soldati L., Santarpia L., Contaldo F., Pasanisi F., Ghigo E., Bo S. (2018). Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Journal of Translational Medicine, 16(1), 371. 10.1186/s12967-018-1748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (17)Conley M., Le Fevre L., Haywood C., Proietto J. (2018). Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutrition & Dietetics, 75(1), 65–72. 10.1111/1747-0080.12372 [DOI] [PubMed] [Google Scholar]
- * (22)Corley B., Carroll R., Hall R., Weatherall M., Parry‐Strong A., Krebs J. D. (2018). Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabetic Medicine, 35(5), 588–594. 10.1111/dme.13595 [DOI] [PubMed] [Google Scholar]
- * (4)Coutinho S. R., Halset E. H., Gåsbakk S., Rehfeld J. F., Kulseng B., Truby H., Martins C. (2018). Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clinical Nutrition, 37(3), 815–823. 10.1016/j.clnu.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Dansinger M. L., Gleason J. A., Griffith J. L., Selker H. P., Schaefer E. J. (2005). Comparison of the Atkins, Ornish, weight watchers, and zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA, 293(1), 43–53. 10.1001/jama.293.1.43 [DOI] [PubMed] [Google Scholar]
- de Cabo R., Mattson M. P. (2019). Effects of intermittent fasting on health, aging, and disease. New England Journal of Medicine, 381(26), 2541–2551. 10.1056/nejmra1905136 [DOI] [PubMed] [Google Scholar]
- Forman E. M., Butryn M. L. (2015). A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite, 84, 171–180. 10.1016/j.appet.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (8 d)Gabel K., Kroeger C. M., Trepanowski J. F., Hoddy K. K., Cienfuegos S., Kalam F., Varady K. A. (2019). Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity (Silver Spring), 27(9), 1443–1450. 10.1002/oby.22564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L., Hamilton S., Azevedo L. B., Olajide J., De Brún C., Waller G., Whittaker V., Sharp T., Lean M., Hankey C. (2018). Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database of Systematic Reviews and Implementation Reports, 16(2), 507–547. 10.11124/JBISRIR-2016-003248 [DOI] [PubMed] [Google Scholar]
- * (25 a)Headland M. L., Clifton P. M., Keogh J. B. (2019. a). Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. International Journal of Obesity, 43(10), 2028–2036. 10.1038/s41366-018-0247-2 [DOI] [PubMed] [Google Scholar]
- * (25 c)Headland M. L., Clifton P. M., Keogh J. B. (2019. b). Effects of weight loss on FGF-21 in human subjects: An exploratory study. International Journal of Environmental Research and Public Health, 16(23), 4877. 10.3390/ijerph16234877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (25 b)Headland M. L., Clifton P. M., Keogh J. B. (2020). Impact of intermittent vs. continuous energy restriction on weight and cardiometabolic factors: A 12-month follow-up. International Journal of Obesity, 44(6), 1236–1242. 10.1038/s41366-020-0525-7 [DOI] [PubMed] [Google Scholar]
- * (20)Hirsh S. P., Pons M., Joyal S. V., Swick A. G. (2019). Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study. Journal of Nutritional Science, 8, e11. 10.1017/jns.2019.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (12)Hoddy K. K., Kroeger C. M., Trepanowski J. F., Barnosky A., Bhutani S., Varady K. A. (2014). Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity, 22(12), 2524–2531. 10.1002/oby.20909 [DOI] [PubMed] [Google Scholar]
- * (21)Hottenrott K., Werner T., Hottenrott L., Meyer T. P., Vormann J. (2020). Exercise training, intermittent fasting and alkaline supplementation as an effective strategy for body weight loss: A 12-week placebo-controlled double-blind intervention with overweight subjects. Life, 10(5), 74. 10.3390/life10050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (5 a)Hutchison A. T., Liu B., Wood R. E., Vincent A. D., Thompson C. H., O’Callaghan N. J., Wittert G. A., Heilbronn L. K. (2019). Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity, 27(1), 50–58. 10.1002/oby.22345 [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Ryan D. H., Apovian C. M., Ard J. D., Comuzzie A. G., Donato K. A., Hu F. B., Hubbard V. S., Jakicic J. M., Kushner R. F., Loria C. M., Millen B. E., Nonas C. A., Pi-Sunyer F. X., Stevens J., Stevens V. J., Wadden T. A., Wolfe B. M., Yanovski S. Z. (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. Journal of the American College of Cardiology, 63(25), 2985–3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- * (24)Keogh J. B., Pedersen E, Petersen K. S., Clifton P. M. (2014). Effects of intermittent compared to continuous energy restriction on short‐term weight loss and long‐term weight loss maintenance. Clinical Obesity, 4(3), 150–156. 10.1111/cob.12052 [DOI] [PubMed] [Google Scholar]
- * (26 a)Klempel M. C., Kroeger C. M., Bhutani S., Trepanowski J. F., Varady K. A. (2012). Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutrition Journal, 11(1), 98. 10.1186/1475-2891-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (13 c)Klempel M. C., Kroeger C. M., Norkeviciute E., Goslawski M., Phillips S. A., Varady K. A. (2013. a). Benefit of a low-fat over high-fat diet on vascular health during alternate day fasting. Nutrition & Diabetes, 3(5), e71–e71. 10.1038/nutd.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (13 a)Klempel M. C., Kroeger C. M., Varady K. A. (2013. b). Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism, 62(1), 137–143. 10.1016/j.metabol.2012.07.002 [DOI] [PubMed] [Google Scholar]
- * (13 b)Klempel M. C., Kroeger C. M., Varady K. A. (2013. c). Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. European Journal of Clinical Nutrition, 67(7), 783–785. 10.1038/ejcn.2013.83 [DOI] [PubMed] [Google Scholar]
- * (26 b)Kroeger C. M., Klempel M. C., Bhutani S., Trepanowski J. F., Tangney C. C., Varady K. A. (2012). Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutrition & Metabolism, 9(1), 98. 10.1186/1743-7075-9-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C. H. R., Oliveira I. K. F., Frota K. M. G., Carvalho C. M. R. G., Paiva A. A., Campelo V., Martins M. C. C. (2020). Impact of intermittent fasting on body weight in overweight and obese individuals. Revista da Associação Médica Brasileira, 66(2), 222–226. 10.1590/1806-9282.66.2.222 [DOI] [PubMed] [Google Scholar]
- * (5 b)Liu B., Hutchison A. T., Thompson C. H., Lange K., Heilbronn L. K. (2019). Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obesity Research & Clinical Practice, 13(4), 408–415. 10.1016/j.orcp.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group (2013). Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New England Journal of Medicine, 369(2), 145–154. 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group (2014). Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: The Look AHEAD trial. Diabetes Care, 37(6), 1544–1553. 10.2337/dc13-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzgar C. J., Preston A. G., Miller D. L., Nickols-Richardson S. M. (2015). Facilitators and barriers to weight loss and weight loss maintenance: A qualitative exploration. Journal of Human Nutrition and Dietetics, 28(6), 593–603. 10.1111/jhn.12273 [DOI] [PubMed] [Google Scholar]
- * (8 e)Miranda E. R., Fuller K. N. Z., Perkins R. K., Kroeger C. M., Trepanowski J. F., Varady K. A., Haus J. M. (2018). Endogenous secretory RAGE increases with improvements in body composition and is associated with markers of adipocyte health. Nutrition, Metabolism and Cardiovascular Diseases, 28(11), 1155–1165. 10.1016/j.numecd.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesi L., El Ghoch M., Brodosi L., Calugi S., Marchesini G., Dalle Grave R. (2016). Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 9(1), 37–46. 10.2147/DMSO.S89836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira E. A., Most M., Howard J., Ravussin E. (2011). Dietary adherence to long‐term controlled feeding in a calorie‐restriction study in overweight men and women. Nutrition in Clinical Practice, 26(3), 309–315. 10.1177/0884533611405992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., Shamseer L., Tetzlaff J. M., Moher D. (2021). Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. Journal of Clinical Epidemiology, 134, 103–112. 10.1016/j.jclinepi.2021.02.003 [DOI] [PubMed] [Google Scholar]
- * (1)Parvaresh A., Razavi R., Abbasi B., Yaghoobloo K., Hassanzadeh A., Mohammadifard N., Safavi S. M., Hadi A., Clark C. C. T. (2019). Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complementary Therapies in Medicine, 47, 102187. 10.1016/j.ctim.2019.08.021 [DOI] [PubMed] [Google Scholar]
- * (7)Razavi R., Parvaresh A., Abbasi B., Yaghoobloo K., Hassanzadeh A., Mohammadifard N., Clark C. C., Safavi S. M. (2020). The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. International Journal for Vitamin and Nutrition Research, 91(3-4): 242–250. 10.1024/0300-9831/a000623 [DOI] [PubMed] [Google Scholar]
- * (18)Schübel R., Nattenmüller J., Sookthai D., Nonnenmacher T., Graf M. E., Riedl L., Schlett C. L., Von Stackelberg O., Johnson T., Nabers D. (2018). Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. The American Journal of Clinical Nutrition, 108(5), 933–945. 10.1093/ajcn/nqy196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., Cates C. J., Cheng H.-Y., Corbette M. S., Eldridge S. M., Hernán M. A., Hopewell S., Hróbiartsson A., Junqueira D. R., Jüni P., Kirkham J. J., Lasserson T., Li T., McAleenan A., … Higgins J. P. T. (2019) RoB: A revised tool for assessing risk of bias in randomized trials. BMJ 366, 14898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- * (19 a)Sundfør T., Svendsen M., Tonstad S. (2018). Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutrition, Metabolism and Cardiovascular Diseases, 28(7), 698–706. 10.1016/j.numecd.2018.03.009 [DOI] [PubMed] [Google Scholar]
- * (19 b)Sundfør T., Tonstad S., Svendsen M. (2019). Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. European Journal of Clinical Nutrition, 73(7), 1006–1014. 10.1038/s41430-018-0370-0 [DOI] [PubMed] [Google Scholar]
- * (8 a)Trepanowski J. F., Kroeger C. M., Barnosky A., Klempel M. C., Bhutani S., Hoddy K. K., Gabel K., Freels S., Rigdon J., Rood J, Ravussin E., Varady K. A. (2017). Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. JAMA Internal Medicine, 177(7), 930–938. 10.1001/jamainternmed.2017.0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (8 b)Trepanowski J. F., Kroeger C. M., Barnosky A., Klempel M., Bhutani S., Hoddy K. K., Rood J., Ravussin E., Varady K. A. (2018). Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clinical Nutrition, 37(6 Pt A), 1871–1878. 10.1016/j.clnu.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (9)Varady K. A., Bhutani S., Klempel M. C., Kroeger C. M. (2011). Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids in Health and Disease, 10(1), 119. 10.1186/1476-511X-10-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * (13 d)Varady K. A., Dam V. T., Klempel M. C., Horne M., Cruz R., Kroeger C. M., Santosa S. (2015). Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Scientific Reports, 5(1), 7561. 10.1038/srep07561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady K. A., Hellerstein M. K. (2007). Alternate-day fasting and chronic disease prevention: A review of human and animal trials. The American Journal of Clinical Nutrition, 86(1), 7–13. 10.1093/ajcn/86.1.7 [DOI] [PubMed] [Google Scholar]
- Wadden T. A., Webb V. L., Moran C. H., Bailer B. A. (2012). Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation, 125(9), 1157–1170. 10.1161/CIRCULATIONAHA.111.039453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton S., Minty R., O’Driscoll T., Wilms H., Poirier D., Madden S., Kelly L. (2020). Intermittent fasting and weight loss: Systematic review. Canadian Family Physician, 66(2), 117–125. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-brn-10.1177_10998004221078079 for Intermittent Energy Restriction for Weight Loss: A Systematic Review of Cardiometabolic, Inflammatory and Appetite Outcomes by Xueting Wei, Ashley Cooper, Irene Lee, Christine A. Cernoch, Ginny Huntoon, Brandi Hodek, Hanna Christian and Ariana M. Chao in Biological Research For Nursing