Abstract
Diabetes is a life-threatening and debilitating disease with pathological hallmarks, including glucose intolerance and insulin resistance. Plant compounds are a source of novel and effective therapeutics, and the flavonoid (−)-epicatechin, common to popular foods worldwide, has been shown to improve carbohydrate metabolism in both clinical studies and preclinical models. We hypothesized that (−)-epicatechin would alleviate thermoneutral housing-induced glucose intolerance. Male rats were housed at either thermoneutral (30 °C) or room temperature (24 °C) for 16 weeks and gavaged with either 1 mg/kg body weight or vehicle for the last 15 days before sacrifice. Rats housed at thermoneutrality had a significantly elevated serum glucose area under the curve (p < 0.05) and reduced glucose-mediated insulin secretion. In contrast, rats at thermoneutrality treated with (−)-epicatechin had improved glucose tolerance and increased insulin secretion (p < 0.05). Insulin tolerance tests revealed no differences in insulin sensitivity in any of the four groups. Pancreatic immunohistochemistry staining showed significantly greater islet insulin positive cells in animals housed at thermoneutrality. In conclusion, (−)-epicatechin improved carbohydrate tolerance via increased insulin secretion in response to glucose challenge without a change in insulin sensitivity.
Key words: (−)-epicatechin, Theobroma cacao, Sterculiaceae, insulin secretion, glucose tolerance
Introduction
Diabetes, characterized by elevated glucose secondary to a combination of insulin resistance and decreased insulin secretion, is a serious worldwide health problem 1 . This disease has an excess impact on low- and middle-income countries and is associated with a significant increase in cardiovascular and microvascular complications and premature mortality 1 .
Investigating medicinal plants for therapeutic potential has led to medications such as metformin (from Galega officinalis ), the most commonly prescribed medication for diabetes around the world. Diabetes and its complications pose a global threat to health, and innovations using insights from medicinal plants may provide inexpensive and effective treatment options. Flavonoids are secondary compounds found in plants that are largely responsible for flower and plant color. They are among the most diverse bioactive plant compounds in human-consumed foods. Flavonoids, and flavonoid-rich foods, have been shown to promote insulin secretion, potentiate insulin-stimulated glucose uptake, and moderate glucose-stimulated insulin secretion via the P13K/Akt/GLUT2 pathway 2 , 3 , 4 , 5 . Of these compounds, (−)-epicatechin (EPICAT), found primarily in chocolate ( Theobroma cacao L. Sterculiaceae), is highly bioactive and well studied for its support of vasodilation, cardiovascular health, mitochondrial function, and antioxidant activity 6 , 7 , 8 , 9 .
EPICAT has shown efficacy in the treatment of metabolic conditions, including diabetes 10 , 11 , 12 , 13 , 14 . In a clinical trial in healthy subjects, EPICAT supplementation decreased fasting insulin and improved insulin sensitivity, but did not impact fasting blood glucose concentrations 10 . Insulin sensitivity improvements, as well as pancreatic β -cell function, improved erythrocyte function, and beneficial impacts on blood pressure parameters under stress, were also observed in clinical trials with dark chocolate 15 , 16 containing EPICAT 11 . In vivo studies further report that EPICAT alone, cacao liquor or cocoa extract treatment, resulted in lower glucose concentrations 12 , 13 , 14 and alleviation of oxidative stress, cellular damage, and mitochondrial dysfunction in a model of myocardial infarction 17 , 18 , 19 . Most clinical trials have focused on dark chocolate and cocoa mixtures. These mixtures contain EPICAT, but detailed studies on this compound in isolation are still needed to determine specific responses. In tandem, animal models are crucial to further elucidate EPICATʼs bioactive mechanisms. Rat models of diabetes and metabolic syndrome do not precisely mimic human diabetes, type 2 diabetes, but can offer mechanistic insights in response to intervention.
Thermoneutrality (TN) refers to an environmental temperature where caloric intake is not used to maintain body temperature homeostasis. Human TN is between 14.8 °C and 24 °C 20 , thus animal research environments are kept at room temperature (RT), which is comfortable to human workers. However, TN for rats is much higher at 30 °C 21 , 22 , prompting debate regarding optimal housing temperature for rodent research of interest to human physiology 23 , 24 . Several recent studies in mice have shown that TN housing is well suited for translational comparison to humans, including similar energy expenditure to basal metabolic rate ratio 23 , a cardiovascular profile more aligned with that of humans 25 , induction of high-fat diet-induced diabetes 26 , 27 , and general differences in cardiovascular parameters of both rats and mice as compared with RT housing 28 , 29 . These studies highlight that TN is likely the most translational environment for rodent biomedical research; however, little is known regarding the impact of TN on rat carbohydrate physiology.
We hypothesized that housing male Wistar rats at their TN temperature (30 °C) would result in impaired glucose tolerance and insulin resistance, verifying a model of metabolic syndrome and diabetes in which to test potential therapeutics. Further, we hypothesized that EPICAT treatment would improve metabolic profiles.
Results
Metabolic parameters
Body temperature was taken superficially, and an elevated temperature was achieved in those housed at thermoneutral conditions compared with those housed at room temperature (30.4 ± 0.1 °C vs. 27.4 ± 0.1 °C; p < 0.001). Biometric parameters of the animals were assessed at 1 week and 16 weeks of the study. Rat weight was significantly lower in those housed at TN compared with those at RT after 16 weeks (p < 0.05) ( Table 1 ). There was an effect approaching significance on the interaction of housing temperature, EPICAT, and time (p < 0.08) ( Table 1 ), with those at TN treated with EPICAT having a higher weight at 16 weeks than those at RT ( Table 1 ). There was a significant interaction effect of EPICAT and time (p < 0.05) ( Table 1 ), as well as EPICAT, time, and TN (p < 0.05) ( Table 1 ) on fasting glucose concentrations, resulting in lower concentrations in animals treated with EPICAT at RT compared with TN after 16 weeks. There were also significant effects of both EPICAT and TN on fasting insulin concentrations (p < 0.001 for both) ( Table 1 ) as well as a significant interaction of time and TN (p < 0.05) ( Table 1 ). Animals treated with EPICAT had higher insulin concentrations compared with those given vehicle, and insulin was elevated to a greater degree in those at RT (p < 0.001) ( Table 1 ). Animals housed at TN consumed significantly less food after 16 weeks than those housed at RT (p < 0.001) ( Table 1 ). There was also a significant interaction between temperature and EPICAT on food consumption, resulting in those treated with EPICAT at TN consuming more than those treated with vehicle (p < 0.05) ( Table 1 ).
Table 1 Animal weight, fasting glucose and insulin concentrations, and food consumption at 1 and 16 weeks of treatment. Animals in each housing condition are shown in the 1-week table. The 16-week table shows animal groups after separating them into vehicle or EPICAT-treated groups.
| a P < 0.05 temperature, b EPICAT, c time × temperature, d time × EPICAT, e time × EPICAT × temperature effects; † p < 0.08 time × temperature × EPICAT. Data were analyzed with a mixed-effects model and/or repeated measures two or three-way ANOVA and data are reported as the mean ± standard error of the mean (SEM), n = 8. | ||||
| 1 Week | ||||
|---|---|---|---|---|
| Housing | RT | TN | ||
| Weight a, † (g) | 136.7 ± 3.5 | 121.2 ± 1.8 | ||
| Glucose d, e (mg · dL −1 ) | 85.9 ± 1.7 | 85.4 ± 2.3 | ||
| Insulin a, b, c (µg · mL −1 ) | 0.671 ± 0.084 | 0.611 ± 0.113 | ||
| 16 Weeks | ||||
| Housing | RT | TN | ||
| Treatment | -EPICAT | +EPICAT | -EPICAT | +EPICAT |
| Weight a, † (g) | 571.0 ± 13.2 | 571.6 ± 22.6 | 508.3 ± 23.7 | 557.6 ± 23.7 |
| Glucose d, e (mg · dL −1 ) | 70.4 ± 2.6 | 66.8 ± 1.9 | 67.2 ± 2.3 | 70.9 ± 3.7 |
| Insulin a, b, c (µg · mL −1 ) | 1.142 ± 0.173 | 2.094 ± 0.186 | 0.886 ± 0.236 | 1.035 ± 0.127 |
| Food consumption per animal a, e (g) | 22.82 ± 0.65 | 21.65 ± 0.45 | 15.24 ± 1.11 | 18.35 ± 0.71 |
(−)-Epicatechin treatment resulted in lower glucose area under the curve during intraperitoneal glucose tolerance test
Fasted animals were injected with glucose, and glucose concentrations were measured at timed intervals during a 2-h period. We observed a significant effect of EPICAT (p < 0.01), TN (p < 0.05), and interaction of time and TN on glucose area under the curve (AUC) (p < 0.01) ( Fig. 1 ). Specifically, animals treated with EPICAT showed a significantly less glucose AUC after 16 weeks compared with other groups (p < 0.01) ( Fig. 1 ). Glucose concentrations at timepoints 15, 30, 45, and 60 min were significantly different (p < 0.05 for all) ( Fig. 1 ), and animals at RT treated with EPICAT had significantly lower glucose concentrations compared to either RT or TN animals (p < 0.05 for all) ( Fig. 1 ). TN animals treated with EPICAT had a lower glucose AUC than those treated with vehicle ( Fig. 1 ).
Fig. 1.
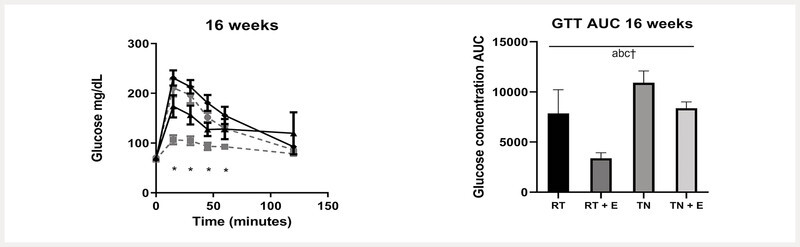
Intraperitoneal glucose tolerance test (IP-GTT). Fasted animals were injected with glucose; glucose concentrations were measured at time intervals during a 2-h period at 16 weeks. a P < 0.05 temperature, b EPICAT, c time × temperature, d time × EPICAT, e time × EPICAT × temperature effects; † p < 0.07 time × EPICAT. Data were analyzed with a mixed-effects model and/or repeated measures three-way ANOVA ± SEM, n = 8.
Insulin concentrations during intraperitoneal glucose tolerance test under thermoneutrality and (−)-epicatechin-treated conditions
Plasma insulin concentrations and AUC were assessed during the intraperitoneal glucose tolerance test (IP-GTT) at 1 week ( Table 2 ) and 16 weeks ( Fig. 2 ). No differences were observed after 1 week of TN exposure for glucose or insulin with IP-GTT ( Table 2 ). After 16 weeks of the study, there was a significant effect of TN housing on insulin AUC, resulting in an elevated AUC in those animals (p < 0.05) ( Fig. 2 ). We also observed a significantly greater AUC of 253.5 ± 63.5 in EPICAT-treated animals at TN compared with 110.1 ± 20.8 in EPICAT-treated animals at RT ( Fig. 2 ).
Table 2 Glucose concentrations for the IP-GTT group (mg/dL) ( a ), concurrent insulin concentrations (ng/mL) during an IP-GTT ( b ), and glucose concentrations during an IP-ITT ( c ) at 1 week of the study. Results at 16 weeks of the study for IP-GTT and IP-ITT for all groups are presented in Figs. 1 – 3 .
| Group | 0 min | 15 min | 30 min | 45 min | 60 min | 120 min | AUC |
|---|---|---|---|---|---|---|---|
| a P < 0.05 temperature, b EPICAT, c time × temperature, d time × EPICAT, e time × EPICAT × temperature effects; † p < 0.08 time × temperature × EPICAT, three-way ANOVA, mean ± SEM, n = 8. | |||||||
| a Glucose concentrations for the IP-GTT group (mg/dL). | |||||||
| RT | 85.4 ± 2.3 | 180.3 ± 6.5 | 125.8 ± 5.1 | 101.6 ± 2.7 | 102.1 ± 3.3 | 85.6 ± 2.9 | 3546.6 ± 283.2 |
| TN | 85.9 ± 1.7 | 206.9 ± 10.3 | 125.4 ± 4.6 | 92.5 ± 4.7 | 98.8 ± 4.3 | 85.2 ± 2.2 | 3615.0 ± 361.8 |
| b IP-GTT concurrent insulin concentrations (ng/mL). | |||||||
| RT | 0.671 ± 0.085 | 2.134 ± 0.268 | 1.161 ± 0.110 | 0.843 ± 0.099 | 0.682 ± 0.105 | 0.927 ± 0.099 | 45.8 ± 8.6 |
| TN | 0.611 ± 0.113 | 2.350 ± 0.360 | 1.170 ± 0.188 | 0.944 ± 0.194 | 0.844 ± 0.109 | 0.752 ± 0.102 | 62.1 ± 15.2 |
| c IP-ITT glucose concentrations (mg/dL). | |||||||
| RT | 88.2 ± 3.5 | 69.0 ± 2.6 | 51.7 ± 2.6 | 41.7 ± 2.3 | 40.8 ± 3.4 | 62.0 ± 5.1 | 4737.7 ± 511.7 |
| TN | 85.2 ± 2.5 | 64.4 ± 4.0 | 42.2 ± 2.4 | 36.2 ± 2.3 | 34.7 ± 2.0 | 61.7 ± 4.3 | 4936.4 ± 329.6 |
Fig. 2.
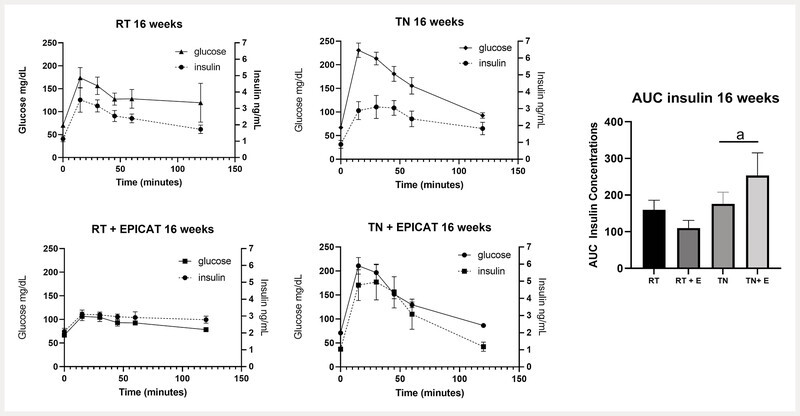
Concurrent insulin concentrations during IP-GTT. Plasma insulin concentrations were measured during IP-GTT, and area under the curve (AUC) was determined for each group at 16 weeks. a P < 0.05 for effect of temperature, two-way ANOVA or mixed-effects model. Data are presented as the mean ± SEM, n = 8.
Insulin tolerance was not impacted by either thermoneutrality housing or (−)-epicatechin treatment
Fasted animals were injected with insulin, and glucose concentrations were measured at time intervals during a 2-h period. There were no significant effects or interactions of TN housing or EPICAT treatment on insulin sensitivity after 16 weeks ( Fig. 3 ).
Fig. 3.
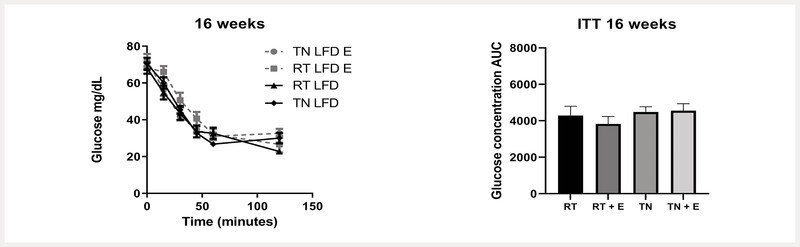
Intraperitoneal insulin tolerance test (IP-ITT). Fasted animals were injected with insulin; glucose concentrations were measured at time intervals during a 2-h period at 16 weeks. a P < 0.05 temperature, b EPICAT, c time × temperature, d time × EPICAT, e time × EPICAT × temperature effects; † p < 0.07 time × EPICAT. Data were analyzed with a mixed-effects model and/or repeated measures three-way ANOVA. Data are presented as the mean ± SEM, n = 8.
Thermoneutrality housing increased islet insulin-positive cells and pancreata of (−)-epicatechin-treated animals had less insulin
Insulin positive cells per islet were analyzed as a percentage of total islet area ( Fig. 4 a ). Animals housed at TN had significantly greater insulin-positive cells per islet than those housed at RT (p < 0.05) ( Fig. 4 a ). EPICAT-treated animals had significantly less insulin present in the pancreas than vehicle-treated animals, regardless of housing temperature, as expressed as insulin per total tissue area (p < 0.01) ( Fig. 4 b ). There was no difference between control animalsʼ amount of pancreatic insulin ( Fig. 4 b ).
Fig. 4.
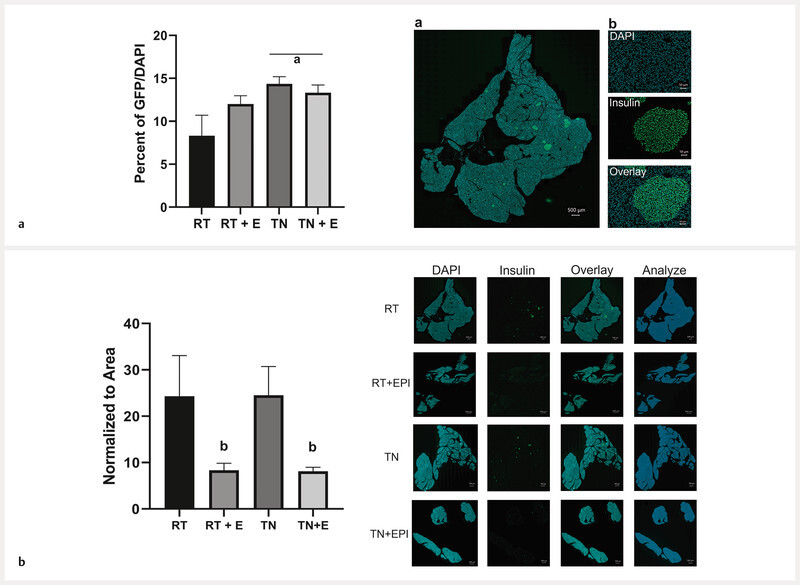
MATLAB analysis of islet insulin-positive cells ( a ), and pancreas stained for insulin ( b ). Animal pancreases were preserved in paraffin and sacrificed after 16 weeks, then stained for insulin using immunohistochemistry. Insulin-positive cells/DAPI per islet were quantified as a percentage of area analyzed ( a ). Stitched image of whole pancreatic tissue section ( a b). Representation of insulin-positive islet cell analysis taken from the whole tissue section ( a b) including cell nuclei stained with DAPI, insulin stained with GFP, and the overlay of both together. Insulin was quantified per total tissue area by analyzing the insulin antibody fluorescent signal and normalizing to that of the entire tissue area ( b ). Results of the two-way ANOVA test are depicted as effect of a p < 0.05 temperature, b EPICAT, and interaction of c EPICAT × temperature. Data were analyzed with a two-way ANOVA and presented as the mean ± SEM, n = 7 – 8.
Discussion
In this study, we observed a glucose-lowering response to EPICAT treatment in both RT and TN rats. Further, animals housed at TN conditions showed significantly worse glucose tolerance and lower insulin secretion in response to IP-GTT. Animals treated with EPICAT showed higher fasting insulin concentrations and improved glucose tolerance compared with controls. EPICAT treatment significantly stimulated insulin secretion in those housed at TN. Fasting insulin concentrations were higher in the RT EPICAT-treated rodents, whereas insulin secretion was not different with IP-GTT, likely related to the absence of a significant spike in glucose with IP-GTT. In contrast to prior reports 11 , we observed that insulin tolerance was not impacted by either housing temperature or EPICAT treatment. Taken together, these findings are consistent with a primary effect of insulin secretion as the mediator of the glucose-lowering effect of EPICAT. These data build upon reported studies on EPICATʼs glucose-lowering bioactivity 10 , 12 , 13 , 14 , 30 .
Glucose intolerance was present in our animals housed at TN, as measured by IP-GTT. Concurrent measurements of insulin secretion with the IP-GTT suggest that insulin secreted by TN-housed animals is inadequate, as we observed glucose intolerance without a change in insulin sensitivity. Treating animals housed at both RT and TN conditions with EPICAT resulted in improved glucose tolerance, most dramatically in animals housed at RT, aligning with previous reports 10 , 12 , 13 , 14 , 31 .
To address impacts of housing temperature and EPICAT treatment on insulin secretion, we investigated pancreatic islets. The amount of insulin per islet was significantly elevated in those housed at TN conditions, regardless of EPICAT treatment, consistent with pancreatic compensation in the context of impaired glucose tolerance in these animals. Impaired or compensatory insulin secretion and production is common in metabolic syndrome and diabetes 32 . Of interest, EPICAT treatment resulted in significantly less islet area per total pancreas regardless of housing environment. Taken together, these pancreatic analyses may point to EPICATʼs ability to augment efficiency of glucose-mediated insulin secretion. Previous reports on islet compensation show a larger islet area with less insulin staining per islet 33 . Our observation warrants further study as we noted improved insulin secretion without detecting a change in insulin staining per islet with EPICAT. The finding of augmented insulin secretion with 15 days of EPICAT treatment is most consistent with improved glucose-mediated insulin response. Our study shows that EPICAT improved insulin secretion in tandem with a glucose challenge, improving the match of insulin supply with glucose demand. Overall, these results agree with in vitro studies showing that EPICAT stimulates insulin secretion 34 , 35 , 36 .
There are several mechanisms potentially explaining our results that warrant future investigation. For example, EPICAT is reported to increase circulating glucagon like peptide − 1 (GLP-1) plasma concentrations 37 , an incretin that augments glucose-mediated insulin secretion. Other reports state that EPCIAT can act on cellular signaling in the beta cell via the CaMKII pathway upstream of insulin secretion 35 , and/or the stimulation of tissue-localized mitochondrial respiration (to augment beta cell glucose sensing) 38 . Most notably, EPICAT may be acting on various forms of nitric oxide synthase (NOS). Inducible/inflammatory NOS (iNOS) blunts insulin secretion. Elevated pancreatic iNOS, induced by inflammatory processes, suppresses insulin secretion 39 , 40 , 41 . EPICAT has been shown to decrease excess iNOS 42 , 43 , resulting in improved insulin secretion 43 . Endothelial NOS (eNOS) is activated by insulin signaling through the PI3K/Akt/eNOS cascade 44 ; many studies show that EPICAT stimulates eNOS 7 , 9 , 45 , 46 . In light of this previous work, our data could be explained by a model by which EPICAT may stimulate eNOS or suppress pancreatic iNOS, resulting in robust insulin secretion in response to glucose concentrations further normalizing postprandial carbohydrate dynamics. However, we showed no effect of EPICAT on insulin per islet, consistent with EPICAT acting on the secretion process itself in response to glucose rather than on β -cell function or adaptation. Future studies will address these and other mechanisms.
Intriguingly, insulin sensitivity in the intraperitoneal insulin tolerance test (IP-ITT) was unaffected by either housing temperature or EPICAT treatment. Although a study reported similar data in a clinical trial 47 , this is unexpected in light of the significant effects of both variables on glucose tolerance and islet insulin, and also is in contrast to other previous clinical studies 10 , 48 , 49 . We interpret these results as pointing to adaptation of glucose handling independent of the insulin signaling cascade involving cellular glucose uptake and/or β -cell function. EPICAT clearly modulates insulin secretion and perhaps glucose uptake at the cellular level, independent of impacts on insulin sensitivity.
We observed divergent impacts of EPICAT on fasting glucose concentrations, resulting in lower concentrations in animals housed at RT and higher concentrations in those housed at TN. As there were significant interactions between EPICAT, time, and temperature on this parameter, it follows that the interplay of the variables is impacting glucose concentrations. Although TN housing caused lower fasting insulin concentrations overall, EPICAT treatment resulted in higher concentrations compared with vehicle controls, agreeing with our other data showing more efficient insulin production and improved glucose tolerance.
This study has several limitations. Although our dosage and duration of EPICAT treatment was devised based on previous successful studies 12 , 50 , 51 , 15 days of this compound may not be enough time to impact insulin secretion and insulin action in animals housed at TN. Other reports have addressed bioavailability by administering EPICAT in a mixture 10 , 48 , 49 , and we used DMSO and a saline solution for solubility in oral gavage. This may have affected bioavailability, although we do report significant impacts on glucose tolerance and insulin secretion and related efficiency. Despite this, our dosage of 1 mg/kg body weight may also have been too low to perturb certain metabolic aspects in TN animals; dose responses will be considered in future studies. Also, to definitively conclude EPICATʼs mechanism of action in insulin secretion requires further study, especially in different animal models to verify broad bioactivity. Cellular signaling upstream of insulin secretion and glucose uptake may also be important to further define EPICATʼs antidiabetic bioactivity. Although this thermoneutral animal model has been ideal for the purpose of this work, additional studies are planned. Interestingly, we showed that animals housed at their thermoneutral temperature show metabolic dysfunction. This has been reported in previous reports for rodents 23 , 24 , 26 , 27 , and is one of the reasons this housing is an emerging method towards aligning rodent models with human metabolism. The physiology behind these unique metabolic responses to thermoneutral housing is a subject of ongoing investigation in our laboratory and others.
In conclusion, we show that EPICAT lowers glucose and improves insulin response to a glucose load, with a likely modulation of pancreatic insulin production and secretion. In addition, our study also shows that animals housed at TN consume less food, agreeing with other reports 52 . Investigating EPICAT with the use of IP-GTT concurrent insulin concentrations and islet insulin quantification is a somewhat unique approach to ascertaining botanical antidiabetic activity. Although we are not the first to report that EPICAT stimulates insulin secretion, our study adds to the existing data in the field by utilizing a thermoneutral animal model. Housing rats at their thermoneutral temperatures provides a novel animal paradigm of metabolic dysfunction easy to establish and prime for screening specific bioactivity from natural products and functional foods. Our results have broad implications for human health, as diabetes is prevalent around the world 1 . Importantly, EPICAT is already sold and consumed as a supplement as well as a component of highly popular foods, making it immediately applicable in several forms. Future studies will focus on a mechanistic evaluation of EPICATʼs impact on islet function and insulin secretion.
Methods and Materials
Reagents
Pharmaceutical grade glucose and PBS, and EDTA are from Sigma-Aldrich. DMSO, sodium chloride, and bovine serum albumin were purchased from Fisher Scientific. Insulin (Humulin R) was manufactured by Eli Lilly and Company. EPICAT was procured from Cayman Chemical. Unconjugated FLEX Polyclonal Guinea Pig Anti-Insulin antibody was used at a 1 : 5 dilution in 2% Normal Donkey Serum in 1 × PBST. Alexa Fluor 488 AffiniPure Donkey Anti-Guinea Pig IgG (H+L) was diluted 1 : 500 in 2% NDS/PBST. DAPI was diluted 1 : 1000 in 1 × PBST. Prolong Gold Antifade Mountant was purchased from Thermo Fisher Scientific.
In vivo experiments
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the RMR VA Medical Center, protocol number 2CD2013R, approved July 14, 2020. Animals (male Wistar rats, 5 weeks old, procured from Charles River Laboratories, Inc.), kept at 2 animals per cage, were housed at either RT (22 °C) or TN (29 – 30 °C) from the time they arrived at the facility. As quarantine is 1 week, all measurements were taken 1 week after initial housing. Animals were fed a customized diet containing 13% kcal fat (LFD) (Envigo [Teklad]) for 16 weeks, and randomized into 4 groups, with n = 8 in each group: RT + vehicle, RT + EPICAT, TN + vehicle, and TN + EPICAT. EPICAT solution was provided at 1 mg/kg body weight by diluting the specific dosage from a 15 mg/mL stock in 50 : 50 DMSO : PBS (vehicle) up to 0.046 mL for a final DMSO dosage of 1.58% of the solution. For a final gavage volume of 1.5 mL total, the dosage or vehicle was diluted in 1.454 mL of PBS. During the final 15 days, animals were gavaged test solutions once per day before noon. Blood (approximately 50 µL) from the tail vein was collected in 0.5 M EDTA and spun at 12 000 g for 10 min at 4 °C. Plasma was extracted and stored at − 80 °C. Fasting blood (6 h), also from the tail vein, was taken at the beginning and end of the study and fed blood from the tail vein was taken biweekly for glucose and insulin concentrations. Body weight and food consumption were measured weekly. Endpoint parameters were taken at sacrifice, and all animals were euthanized in the morning following ad libitum food consumption. Euthanasia was conducted via isoflurane gas administration, along with consistent oxygen. This method of euthanasia complies with IACUC requirements and was formally approved in the protocol listed above.
Insulin and glucose intraperitoneal tolerance tests
Insulin tolerance testing (ITT) was done at 1 and 16 weeks of the study, following a 6-hour fast, by interperitoneal injection of 1 U · kg −1 body weight of insulin 53 . Blood glucose concentrations were sampled at 0, 15, 30, 45, 60, and 120 min post-injection. Glucose tolerance testing (GTT) followed the same protocol using 1.5 g · kg −1 body weight of glucose, injected intraperitoneally, and was separated from ITT testing by 4 days. Fasting glucose and insulin concentrations were taken as the 0-minute blood collection during GTT. Baseline concentrations were subtracted for AUC analyses.
Pancreatic staining
The pancreas was collected from each animal after sacrifice and processed and analyzed the same way for each animal. Each pancreas was fixed for 24 hours in 10% formalin, then rinsed 3 times in 70% EtOH. These tissue samples were then processed in paraffin overnight using a Histocore Pearl instrument and embedded in paraffin using a Tissue Tek II instrument (Sakara Finetek). Each tissue mold was then sectioned into 5 µm sections using a microtome and positioned on positively charged slides. The slides were incubated overnight at 65 °C. Slides were incubated in xylene 2 times for 10 min each, 100% EtOH 2 times for 10 min each, 95% EtOH for 3 min, 70% EtOH for 3 min, 50% EtOH for 3 min, rinsed in DI H 2 O twice, and rehydrated in 1 × PBST for 10 min. Each tissue section was blocked using 2% NDS in 1 × PBST for 30 min at RT. After removing the blocking solution, unconjugated FLEX Polyclonal Guinea Pig Anti-Insulin antibody (1 : 5) was applied and incubated overnight in 4 °C covered with parafilm. The next day, the parafilm was removed and each slide was washed in 1 × PBST 4 times for 5 min each. Alexa Fluor 488 AffiniPure Donkey Anti-Guinea Pig IgG (H+L) (1 : 500) was applied and incubated for 1 h at RT, protected from light and covered with parafilm. Parafilm was removed and slides washed again 4 times in 1 × PBST for 5 min each. DAPI was applied to each tissue section and incubated for 15 min at RT, protected from light. The slides were washed 2 times in 1 × PBST and mounted using Prolong Gold mounting solution and a glass coverslip. This protocol is very similar to that previously described 54 . Each tissue section was imaged using a Keyence BZ-X800 fluorescence microscope with a DAPI and FITC lasers and 20 × zoom lens. Animals treated with EPICAT were compared with pooled controls of animals housed at RT and TN to allow for a robust statistical analysis.
Islet parameter assessment
A custom Matlab M-file was written to analyze the number of islets per section of pancreas as assessed by DAPI staining. The software reads an image into Matlab as an RGB image and then is made into a binary image based on fluorescence intensity. Otsuʼs method was used to choose a threshold of pixel intensity that minimizes the intraclass variance of the thresholded black and white pixels. The “binarization” uses a 256-bin image histogram to compute Otsuʼs threshold. The code begins by removing intensity artifacts from the green layer of the image (insulin positive) by eliminating items less than 15 pixels in size. The code then fills in any gaps within identified bodies larger than 15 pixels. This step is used to account for intensity gaps in the islet and helps to accurately measure islet perimeter and area. The Matlab function “bwboundaries” is then employed to find all boundaries in the image and aids in distinguishing true islets from artifacts by utilizing islet morphology. The area and perimeter of the islets are determined as part of the “bwboundaries” function. These two parameters are used in a metric calculation to determine islet morphology based on a sliding scale where 1 represents an islet and 0 represents an artifact in the image. The metric uses a threshold of 0.98 to identify true islets. Finally, the islets are evaluated by another threshold that measures size and islet morphology metric score concurrently and islets that do not meet a user define value are removed. The remaining islets are exported to an Excel file with the total islet count, area of each islet, and metric score listed.
Fluorescence imaging assessment
A second custom Matlab M-file was used to identify the FITC-positive (insulin) regions in the pancreas section (DAPI positive). The script asks the user to define an area of background fluorescence on the green layer (insulin) in the image. The mean intensity of this defined region is then used to background subtract the entire green layer; values less than zero are assigned a zero value. The user than selects the entire slice of tissue in the blue layer (DAPI) and a binary mask is generated. The binary mask is then applied to the green layer of the image. Values inside the region of interest retain their fluorescence intensity and items outside are assigned a zero value. The insulin layer was then binarized into pixels with fluorescence intensity (1) or areas without (0). The insulin-positive areas were summated and divided by the area of the user-defined slice and multiplied by 100. The percentage of insulin-positive area to DAPI-positive area was exported to the same Excel file.
Statistical analysis
To analyze data with time/dose along with EPICAT and temperature, we employed a repeated measures ANOVA, along with a mixed-effects model. For data without a time or dose component, we employed a two-way ANOVA for the variable temperature and EPICAT. Tukeyʼs post hoc analyses were conducted within ANOVA tests. A p value of less than 0.05 was used as the cutoff for statistical significance in all tests. A p value of equal or less than 0.08 was considered indicative of data trends approaching significance and thus reported.
Contributorsʼ Statement
ACK and JEBR generated ideas, wrote the manuscript, housed the project and provided oversight. ACK, JHC, LAK, SEH helped with writing the manuscript, conducted experiments, generated data and performed data analysis. MMH, GBP, and DGR generated data and performed data analysis.
Acknowledgements
The authors wish to thank Ms. Teri Armstrong, Ms. Melissa Blatzer, and Mr. Jeremy Rahkola for their technical assistance with the experiments described herein. The authors also wish to acknowledge Dr. Lori Sussel and Ms. Bryce Hopwood of the Barbara Davis Center for Diabetes Basic and Translational Research Division Islet Microscopy Core, sponsored by Diabetes Research Center (NIDDK P30-DK116073), for pancreatic insulin staining and processing. Experimental schematic created with BioRender.com. The authors wish to acknowledge the following funding sources: NIH/NCRR CCTSI UL1 RR025780, VA Merit (J. E. B. R. BX002046; CX001532), R01 DK124344-01A1 (J. E. B. R.), R01-AG066 562 (J. E. B. R.), VA CDA2 (A. C. K. BX003185), Denver Research Institute, NIDDK P30-DK116073 (J. E. B. R., C. K., D. R.) and the Ludeman Family Center for Womenʼs Health Research at the University of Colorado Anschutz Medical Campus Junior Faculty Research Development Grant (A. C. K.).
Conflict of Interest The authors declare that they have no conflict of interest.
Dedicated to Professor Dr. A. Douglas Kinghorn on the occasion of his 75th birthday.
References
- 1.WHO Diabetes fact sheet. News Room 2021Accessed November 5, 2021 at:https://www.who.int/news-room/fact-sheets/detail/diabetes
- 2.Santangelo C, Zicari A, Mandosi E, Scazzocchio B, Mari E, Morano S, Masella R. Could gestational diabetes mellitus be managed through dietary bioactive compounds? Current knowledge and future perspectives. Br J Nutr. 2016;115:1129–1144. doi: 10.1017/S0007114516000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh Y S, Jun H S. Role of bioactive food components in diabetes prevention: Effects on Beta-cell function and preservation. Nutr Metab Insights. 2014;7:51–59. doi: 10.4137/NMI.S13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanhineva K, Torronen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkanen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin M A, Ramos S. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells. 2021;10:1474. doi: 10.3390/cells10061474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller A, Hull S E, Elajaili H, Johnston A, Knaub L A, Chun J H, Walker L, Nozik-Grayck E, Reusch J EB. (−)-Epicatechin modulates mitochondrial redox in vascular cell models of oxidative stress. Oxid Med Cell Longev. 2020;2020:6.392629E6. doi: 10.1155/2020/6392629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galleano M, Bernatova I, Puzserova A, Balis P, Sestakova N, Pechanova O, Fraga C G. (−)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life. 2013;65:710–715. doi: 10.1002/iub.1185. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe K, Tamura Y, Lanaspa M A, Miyazaki M, Suzuki N, Sato W, Maeshima Y, Schreiner G F, Villarreal F J, Johnson R J, Nakagawa T. Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am J Physiol Renal Physiol. 2012;303:F1264–F1274. doi: 10.1152/ajprenal.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litterio M C, Vazquez Prieto M A, Adamo A M, Elesgaray R, Oteiza P I, Galleano M, Fraga C G. (−)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J Nutr Biochem. 2015;26:745–751. doi: 10.1016/j.jnutbio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Dower J I, Geleijnse J M, Gijsbers L, Zock P L, Kromhout D, Hollman P C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr. 2015;101:914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- 11.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Salmean G, Ortiz-Vilchis P, Vacaseydel C M, Garduno-Siciliano L, Chamorro-Cevallos G, Meaney E, Villafana S, Villarreal F, Ceballos G, Ramirez-Sanchez I. Effects of (−)-epicatechin on a diet-induced rat model of cardiometabolic risk factors. Eur J Pharmacol. 2014;728:24–30. doi: 10.1016/j.ejphar.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 13.Ruzaidi A, Amin I, Nawalyah A G, Hamid M, Faizul H A. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol. 2005;98:55–60. doi: 10.1016/j.jep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–355. doi: 10.1016/j.nut.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Radosinska J, Horvathova M, Frimmel K, Muchova J, Vidosovicova M, Vazan R, Bernatova I. Acute dark chocolate ingestion is beneficial for hemodynamics via enhancement of erythrocyte deformability in healthy humans. Nutr Res. 2017;39:69–75. doi: 10.1016/j.nutres.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Regecova V, Jurkovicova J, Babjakova J, Bernatova I. The effect of a single dose of dark chocolate on cardiovascular parameters and their reactivity to mental stress. J Am Coll Nutr. 2020;39:414–421. doi: 10.1080/07315724.2019.1662341. [DOI] [PubMed] [Google Scholar]
- 17.Prince P S. A biochemical, electrocardiographic, electrophoretic, histopathological and in vitro study on the protective effects of (−)epicatechin in isoproterenol-induced myocardial infarcted rats . Eur J Pharmacol. 2011;671:95–101. doi: 10.1016/j.ejphar.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Prince P S. (−) Epicatechin prevents alterations in lysosomal glycohydrolases, cathepsins and reduces myocardial infarct size in isoproterenol-induced myocardial infarcted rats. Eur J Pharmacol. 2013;706:63–69. doi: 10.1016/j.ejphar.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Stanely Mainzen Prince P. (−) Epicatechin attenuates mitochondrial damage by enhancing mitochondrial multi-marker enzymes, adenosine triphosphate and lowering calcium in isoproterenol induced myocardial infarcted rats. Food Chem Toxicol. 2013;53:409–416. doi: 10.1016/j.fct.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Kingma B R, Frijns A J, Schellen L, van Marken Lichtenbelt W D. Beyond the classic thermoneutral zone: Including thermal comfort. Temperature (Austin) 2014;1:142–149. doi: 10.4161/temp.29702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanovsky A A, Ivanov A I, Shimansky Y P. Selected contribution: Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol (1985) 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 22.Poole S, Stephenson J D. Body temperature regulation and thermoneutrality in rats. Q J Exp Physiol Cogn Med Sci. 1977;62:143–149. doi: 10.1113/expphysiol.1977.sp002384. [DOI] [PubMed] [Google Scholar]
- 23.Fischer A W, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab. 2018;7:161–170. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keijer J, Li M, Speakman J R. What is the best housing temperature to translate mouse experiments to humans? Mol Metab. 2019;25:168–176. doi: 10.1016/j.molmet.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overton J M. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 2010;34:S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 26.Stemmer K, Kotzbeck P, Zani F, Bauer M, Neff C, Muller T D, Pfluger P T, Seeley R J, Divanovic S. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int J Obes (Lond) 2015;39:791–797. doi: 10.1038/ijo.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganeshan K, Chawla A. Warming the use to model human diseases. Nat Rev Endocrinol. 2017;13:458–465. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swoap S J, Overton J M, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol. 2004;287:R391–R396. doi: 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 29.Maloney S K, Fuller A, Mitchell D, Gordon C, Overton J M. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 2014;29:413–420. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez-Salmean G, Meaney E, Lanaspa M A, Cicerchi C, Johnson R J, Dugar S, Taub P, Ramirez-Sanchez I, Villarreal F, Schreiner G, Ceballos G. A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects . Int J Cardiol. 2016;223:500–506. doi: 10.1016/j.ijcard.2016.08.158. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez-Salmean G, Ortiz-Vilchis P, Vacaseydel C M, Rubio-Gayosso I, Meaney E, Villarreal F, Ramirez-Sanchez I, Ceballos G. Acute effects of an oral supplement of (−)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct. 2014;5:521–527. doi: 10.1039/c3fo60416k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahler R J. The relationship between the hyperplastic pancreatic islet and insulin insensitivity in obesity. Acta Diabetol Lat. 1981;18:1–17. doi: 10.1007/BF02056101. [DOI] [PubMed] [Google Scholar]
- 33.Huang H H, Novikova L, Williams S J, Smirnova I V, Stehno-Bittel L. Low insulin content of large islet population is present in situ and in isolated islets . Islets. 2011;3:6–13. doi: 10.4161/isl.3.1.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitner B F, Ray J D, Kener K B, Herring J A, Tueller J A, Johnson D K, Tellez Freitas C M, Fausnacht D W, Allen M E, Thomson A H, Weber K S, McMillan R P, Hulver M W, Brown D A, Tessem J S, Neilson A P. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in beta-cells and skeletal muscle cells. J Nutr Biochem. 2018;62:95–107. doi: 10.1016/j.jnutbio.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang K, Chan C B. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharmacol Sin. 2018;39:893–902. doi: 10.1038/aps.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hii C S, Howell S L. Effects of epicatechin on rat islets of Langerhans. Diabetes. 1984;33:291–296. doi: 10.2337/diab.33.3.291. [DOI] [PubMed] [Google Scholar]
- 37.Cremonini E, Daveri E, Mastaloudis A, Oteiza P I. (−)-Epicatechin and anthocyanins modulate GLP-1 metabolism: Evidence from C57BL/6J mice and GLUTag cells. J Nutr. 2021;151:1497–1506. doi: 10.1093/jn/nxab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowley T J, 4th, Bitner B F, Ray J D, Lathen D R, Smithson A T, Dallon B W, Plowman C J, Bikman B T, Hansen J M, Dorenkott M R, Goodrich K M, Ye L, OʼKeefe S F, Neilson A P, Tessem J S. Monomeric cocoa catechins enhance beta-cell function by increasing mitochondrial respiration. J Nutr Biochem. 2017;49:30–41. doi: 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Mussa B M, Srivastava A, Mohammed A K, Verberne A JM. Nitric oxide interacts with cholinoceptors to modulate insulin secretion by pancreatic beta cells. Pflugers Arch. 2020;472:1469–1480. doi: 10.1007/s00424-020-02443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahraki Z S, Karbalaei N, Nemati M. Improving effect of combined inorganic nitrate and nitric oxide synthase inhibitor on pancreatic oxidative stress and impaired insulin secretion in streptozotocin induced-diabetic rats. J Diabetes Metab Disord. 2020;19:353–362. doi: 10.1007/s40200-020-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahadoran Z, Mirmiran P, Ghasemi A. Role of nitric oxide in insulin secretion and glucose metabolism. Trends Endocrinol Metab. 2020;31:118–130. doi: 10.1016/j.tem.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed R H, Karam R A, Amer M G. Epicatechin attenuates doxorubicin-induced brain toxicity: Critical role of TNF-alpha, iNOS and NF-kappaB. Brain Res Bull. 2011;86:22–28. doi: 10.1016/j.brainresbull.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Kim M J, Ryu G R, Kang J H, Sim S S, Min D S, Rhie D J, Yoon S H, Hahn S J, Jeong I K, Hong K J, Kim M S, Jo Y H. Inhibitory effects of epicatechin on interleukin-1beta-induced inducible nitric oxide synthase expression in RINm5F cells and rat pancreatic islets by down-regulation of NF-kappaB activation. Biochem Pharmacol. 2004;68:1775–1785. doi: 10.1016/j.bcp.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Gheibi S, Samsonov A P, Gheibi S, Vazquez A B, Kashfi K. Regulation of carbohydrate metabolism by nitric oxide and hydrogen sulfide: Implications in diabetes. Biochem Pharmacol. 2020;176:113819. doi: 10.1016/j.bcp.2020.113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taub P R, Ramirez-Sanchez I, Ciaraldi T P, Perkins G, Murphy A N, Naviaux R, Hogan M, Maisel A S, Henry R R, Ceballos G, Villarreal F. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: Effects of epicatechin rich cocoa. Clin Transl Sci. 2012;5:43–47. doi: 10.1111/j.1752-8062.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stote K S, Clevidence B A, Novotny J A, Henderson T, Radecki S V, Baer D J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur J Clin Nutr. 2012;66:1153–1159. doi: 10.1038/ejcn.2012.101. [DOI] [PubMed] [Google Scholar]
- 48.Davison K, Coates A M, Buckley J D, Howe P R. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32:1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 49.Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara M C, Marini C, Ferri C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60:794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez-Sanchez I, Taub P R, Ciaraldi T P, Nogueira L, Coe T, Perkins G, Hogan M, Maisel A S, Henry R R, Ceballos G, Villarreal F. (−)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int J Cardiol. 2013;168:3982–3990. doi: 10.1016/j.ijcard.2013.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez-Sanchez I, De los Santos S, Gonzalez-Basurto S, Canto P, Mendoza-Lorenzo P, Palma-Flores C, Ceballos-Reyes G, Villarreal F, Zentella-Dehesa A, Coral-Vazquez R. (−)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic delta-sarcoglycan null mouse striated muscle. FEBS J. 2014;281:5567–5580. doi: 10.1111/febs.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaiyala K J, Morton G J, Thaler J P, Meek T H, Tylee T, Ogimoto K, Wisse B E. Acutely decreased thermoregulatory energy expenditure or decreased activity energy expenditure both acutely reduce food intake in mice. PLoS One. 2012;7:e41473. doi: 10.1371/journal.pone.0041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller A C, He K, Briallantes A M, Kennelly E J. A characterized saponin-rich fraction of Momordica charantia shows antidiabetic activity in C57BLK/6 mice fed a high fat diet . Phytomedicine Plus. 2021;1:100134. [Google Scholar]
- 54.Ramirez D G, Abenojar E, Hernandez C, Lorberbaum D S, Papazian L A, Passman S, Pham V, Exner A A, Benninger R KP. Contrast-enhanced ultrasound with sub-micron sized contrast agents detects insulitis in mouse models of type1 diabetes. Nat Commun. 2020;11:2238. doi: 10.1038/s41467-020-15957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


