Abstract
1-Hydroxy-2-naphthoate (compound I) is a metabolite of the phenanthrene-degradative pathway in Nocardioides sp. strain KP7. This singly hydroxylated aromatic compound is cleaved by 1-hydroxy-2-naphthoate dioxygenase. In this study, the structure of the ring cleavage product generated by the action of homogeneous 1-hydroxy-2-naphthoate dioxygenase was determined upon separation by high-performance liquid chromatography at pH 2.5 by using nuclear magnetic resonance (NMR) and mass spectroscopic techniques. The ring cleavage product at this pH existed in equilibrium between two forms, 2-oxo-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate (compound III) and 2,2-dihydroxy-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate (compound IV). After the pH of the solution was raised to 7.5, the structure of the major species became (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (compound II; common name, trans-2′-carboxybenzalpyruvate), which was in equilibrium with compound III. Direct monitoring of the enzymatic formation of the ring cleavage product by 1H-NMR in a deuterated potassium phosphate buffer (pH 7.5) detected only compound II as a product, and the proton on carbon 3 of compound II was not exchanged with deuterium. Thus, compound II is likely to be the first stable product of dioxygenation of 1-hydroxy-2-naphthoate.
Biodegradation of polyaromatic hydrocarbons (PAHs) attracts much attention because they are contaminants frequently found in soils, aquifers, and sediments (2). Phenanthrene is a PAH which has often been used as a model for the biodegradation of PAHs (5). Two routes for phenanthrene degradation by bacteria have been described (1, 4, 8–10, 13). In one route, phenanthrene is converted via 1-hydroxy-2-naphthoate to 1,2-dihydroxynaphthalene, which is ring-cleaved by 1,2-dihydroxynaphthalene dioxygenase and further transformed to salicylate. In the other route, phenanthrene is transformed to 1-hydroxy-2-naphthoate, which is ring cleaved by 1-hydroxy-2-naphthoate dioxygenase and further converted to o-phthalate.
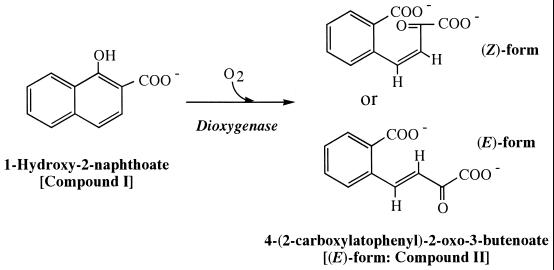
The ring cleavage dioxygenases have an essential role in the decomposition of singly and doubly hydroxylated aromatic compounds (6, 7). Several ring cleavage dioxygenases have been purified and characterized, but few chemical structures of dioxygenase ring cleavage products have been characterized. The product of ring cleavage of 1,2-dihydroxynaphthalene by 1,2-dihydroxynaphthalene dioxygenase has been determined by nuclear magnetic resonance (NMR) analysis to be 2-hydroxychromene-2-carboxylate, which is further transformed by 2-hydroxychromene-2-carboxylate isomerase to trans-2′-hydroxybenzalpyruvate [IUPAC name, (E)-4-(2-hydroxylatophenyl)-2-oxo-3-butenoate] (3). In phenanthrene degradation, the aromatic ring of 1-hydroxy-2-naphthoate is cleaved by 1-hydroxy-2-naphthoate dioxygenase (10). The product of ring cleavage of 1-hydroxy-2-naphthoate by 1-hydroxy-2-naphthoate dioxygenase exhibits a high level of absorption at 300 nm, which is consistent with the presence of double bonds in a substituent conjugated with an aromatic ring (1, 14). Since pyruvate and 2-carboxybenzaldehyde have been detected as pathway intermediates, the chemical structure of the product of ring cleavage of 1-hydroxy-2-naphthoate by 1-hydroxy-2-naphthoate dioxygenase was suggested to be 2′-carboxybenzalpyruvate [IUPAC name, 4-(2-carboxylatophenyl)-2-oxo-3-butenoate] (1, 8, 9, 14) (Fig. 1). However, the geometrical structure of the ring cleavage product has not been investigated.
FIG. 1.
Ring cleavage reaction of 1-hydroxy-2-naphthoate catalyzed by 1-hydroxy-2-naphthoate dioxygenase. The ring cleavage product is expected to be (Z)- or (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (1, 8, 9, 14).
In the present study, we enzymatically produced the ring cleavage product of 1-hydroxy-2-naphthoate by using 1-hydroxy-2-naphthoate dioxygenase, and the geometrical structure of the ring cleavage product of 1-hydroxy-2-naphthoate was determined by NMR and fast atom bombardment-mass spectrometry (FAB-MS) analyses.
MATERIALS AND METHODS
Chemical.
1-Hydroxy-2-naphthoic acid was purchased from Tokyo Organic Chemicals (Tokyo, Japan).
Purification of 1-hydroxy-2-naphthoate dioxygenase.
The pMKT290 plasmid is a derivative of pUC18 carrying the structural gene for 1-hydroxy-2-naphthoate dioxygenase from Nocardioides sp. strain KP7 (10, 11). Cells of Escherichia coli JM109 containing pMKT290 were cultivated in 10 liters of L broth containing 50 μg of ampicillin ml−1 (10). Cells from the overnight culture were pelleted by centrifugation at 10,000 × g for 20 min at 4°C, resuspended in 65 ml of a 10 mM Tris-H2SO4 buffer (pH 7.5), and disrupted by passage through a precooled French pressure cell (Ohtake Works, Tokyo, Japan) at a pressure of 800 kg/cm2. The cell extract was centrifuged at 27,700 × g for 30 min at 4°C, and the supernatant was recentrifuged at 250,000 × g for 60 min at 4°C. The supernatant was filtered through a Sterivex-HV filter (0.45-μm pore size; Millipore, Bedford, Mass.), and loaded into an anion-exchange column (TSKgel DEAE-5PW, 21.5 mm [inside diameter {i.d.}] by 150 mm; Tosoh, Tokyo, Japan) fitted to a high-performance liquid chromatography (HPLC) system (Tosoh). The protein was eluted by a linear gradient of 0 to 0.5 M Na2SO4 in 300 ml of a 10 mM Tris-H2SO4 buffer (pH 7.5) at a flow rate of 5 ml min−1. The eluate was collected in 5-ml fractions on ice. Ammonium sulfate was added to the pooled fractions containing the 1-hydroxy-2-naphthoate dioxygenase activity to achieve a final concentration of 0.6 M at 4°C, and proteins which precipitated were removed by centrifugation at 27,700 × g for 30 min at 4°C. The 1-hydroxy-2-naphthoate dioxygenase activity was recovered in the supernatant. The supernatant was filtered through a Millex-GV filter (0.45-μm pore size; Millipore) and loaded into a hydrophobic-interaction column (TSKgel Phenyl-5PW, 21.5 mm [i.d.] by 150 mm; Tosoh) pre-equilibrated with a 10 mM Tris-H2SO4 buffer (pH 7.5) containing 0.6 M ammonium sulfate. Proteins were eluted from the column by a linear gradient from 0.6 to 0 M ammonium sulfate in 60 ml of a 10 mM Tris-H2SO4 buffer (pH 7.5) at a flow rate of 1 ml min−1. Ammonium sulfate was added to the pooled fractions containing the 1-hydroxy-2-naphthoate dioxygenase activity to achieve a final concentration of 2 M at 4°C, and precipitated proteins were dissolved in 20 ml of a 10 mM Tris-H2SO4 buffer (pH 8.0) at 4°C. The dissolved proteins were loaded on an anion-exchange column (Mono Q HR5/5, 5 mm [i.d.] by 50 mm; Pharmacia, Uppsala, Sweden) fitted to an HPLC system (Tosoh). Finally, purified protein was eluted by a linear gradient of 0 to 0.5 M Na2SO4 in 60 ml of a 10 mM Tris-H2SO4 buffer (pH 8.0) at a flow rate of 1 ml min−1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in a premade polyacrylamide slab gel (Multigel Gel 4/20; Dai-ichi Pure Chemicals, Tokyo, Japan), as described previously (10).
Purification and identification of the ring cleavage product.
Purified recombinant 1-hydroxy-2-naphthoate dioxygenase (2.7 mg) was added to 1,600 ml of a 10 mM Tris-HCl buffer (pH 7.5) containing 150 μM 1-hydroxy-2-naphthoate. The progression and completion of the reaction at room temperature were monitored spectrophotometrically by measuring A300 (1, 10, 14). After acidification of the reaction mixture with approximately 2 ml of 10 N HCl to pH 2.5, the reaction mixture was extracted with 800 ml of chloroform and then with 800 ml of ethyl acetate. The chloroform and ethyl acetate phases were combined, and organic solvents were removed in vacuo. The dried sample was dissolved in 10 ml of 10% aqueous methanol containing 0.1% (vol/vol) H3PO4 and injected onto a C18 column (CapcellPak-C18 type SG120, 4.6 mm [i.d.] by 250 mm; Shiseido, Tokyo, Japan) fitted to an HPLC system (Tosoh) at 25°C. The elution profile was monitored at 280 nm. The ring cleavage product was eluted by a linear solvent gradient of 10 to 90% (vol/vol) methanol in 15 ml of ultrapure water containing 0.1% (vol/vol) H3PO4 at a flow rate of 1 ml min−1. The reaction product was eluted at 50% (vol/vol) methanol. Methanol was removed from the purified fraction in vacuo, the dried sample was dissolved in D2O, and its pH was adjusted with DCl or NaOD.
UV spectroscopy of the purified reaction product.
UV spectra of the purified reaction product at different pH values were measured by using a UV spectrophotometer (UV2000; Shimadzu, Kyoto, Japan) at 25°C. The purified sample was dissolved in a 50 mM potassium phosphate buffer (pH 7.5) or a 50 mM potassium phosphate buffer acidified by 10 N HCl to pH 2.5.
NMR and MS.
All NMR spectra were acquired on a Unity 500 spectrometer (Varian, Palo Alto, Calif.) at 30°C. Two-dimensional experiments such as H,H-correlated spectrometry (COSY), heteronuclear single-quantum coherence spectrometry (HSQC), and heteronuclear multibond correlation spectrometry (HMBC) were used for structure determination of the product. FAB-MS was done with a JMS-SX102 mass spectrometer (JEOL, Tokyo, Japan).
Monitoring of enzymatic reaction by 1H-NMR.
Transformation of 1-hydroxy-2-naphthoate in the reaction catalyzed by 1-hydroxy-2-naphthoate dioxygenase was monitored by 1H-NMR for 180 min at 30°C. Each data set was obtained after 32 scans. The reaction was carried out in 700 μl of 50 mM deuterated potassium phosphate buffer (pH 7.5) containing 0.56 mM 1-hydroxy-2-naphthoate and 0.5 mg of 1-hydroxy-2-naphthoate dioxygenase.
RESULTS AND DISCUSSION
Purification of 1-hydroxy-2-naphthoate dioxygenase from E. coli.
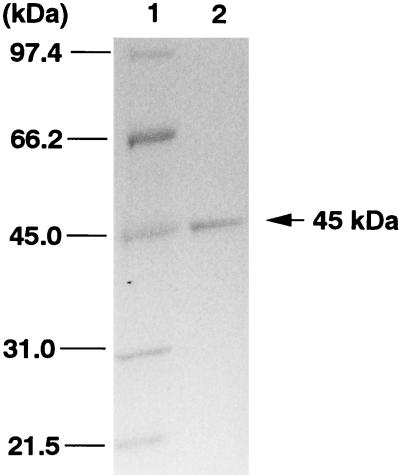
An extract of E. coli JM109(pMKT290) contained 1-hydroxy-2-naphthoate dioxygenase activity at 12 μmol min−1 mg of protein−1. This enzyme was purified from extracts of E. coli JM109(pMKT290) as shown in Table 1. The enzyme activity was eluted from the first anion-exchange column (DEAE column) by Na2SO4 at 0.11 M, and the activity was eluted from the second anion-exchange column (Mono Q column) by Na2SO4 at 0.2 M. The enzyme activity was broadly eluted from a phenyl column. After the second anion-exchange chromatography, the purified enzyme gave a single protein band at 45 kDa by SDS-PAGE (Fig. 2). The specific activity of the purified enzyme was 1.6 mmol min−1 mg of protein−1. The transformation of 1-hydroxy-2-naphthoate by the purified enzyme was spectrophotometrically examined, and the spectrum of 1-hydroxy-2-naphthoate changed immediately to one showing a maximal absorption at 300 nm (data not shown). Thus, it was concluded that the purified enzyme exhibits 1-hydroxy-2-naphthoate dioxygenase activity (1, 10, 14).
TABLE 1.
Purification of 1-hydroxy-2-naphthoate dioxygenase from E. coli containing pMKT290
| Step | Vol (ml) | Protein concn (mg ml−1) | Total amt of protein (mg) | Sp act (μmol min−1 mg of protein−1) | Total activity (μmol min−1) | Yield (%) |
|---|---|---|---|---|---|---|
| Cell extract | 65 | 17.2 | 1,118 | 12 | 13,304 | 100 |
| First anion exchangea | 10 | 8.0 | 80 | 86 | 6,880 | 52 |
| Ammonium sulfate precipitationb | 20 | 0.5 | 10 | 599 | 5,990 | 45 |
| Second anion exchangec | 2 | 1.7 | 3.4 | 1,567 | 5,328 | 40 |
Protein eluted on a TSKgel DEAE-5PW column (Tosoh).
Protein dissolved in a 10 mM Tris-H2SO4 buffer (pH 8.0) after precipitation by 2 M ammonium sulfate.
Protein eluted on a Mono Q HR5/5 column (Pharmacia).
FIG. 2.
SDS-PAGE of purified 1-hydroxy-2-naphthoate dioxygenase (45 kDa) from extracts of E. coli JM109(pMKT290). Lanes: 1, molecular mass markers; 2, pooled active fractions after the second anion-exchange column chromatography step (Mono Q column).
Chemical structures of the ring cleavage products of 1-hydroxy-2-naphthoate.
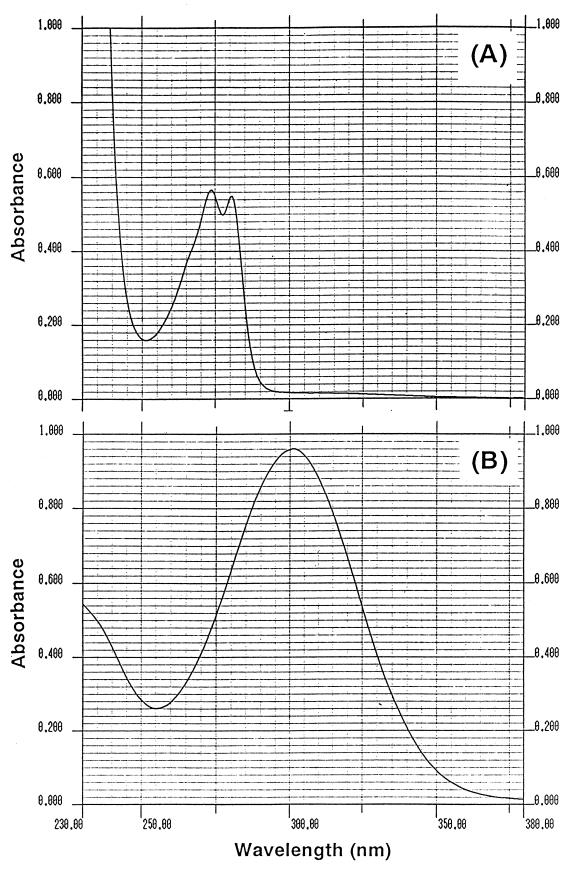
The enzymatically prepared ring cleavage product of 1-hydroxy-2-naphthoate was purified by reverse-phase HPLC. The yield of the purified ring cleavage product was 70%, and the purity of the purified product was 94.4% by HPLC analysis. UV spectra of the purified product at pH 2.5 and 7.5 are shown in Fig. 3A and B, respectively. The UV spectrum at pH 7.5 showed a high level of absorption at 300 nm; however, the spectrum at pH 2.5 showed two absorptions at 274 and 281 nm (Fig. 3A and B). These results indicate that the reaction product has different forms at two pH values.
FIG. 3.
UV spectra of the purified ring cleavage product at pH 2.5 (in a 50 mM potassium phosphate buffer acidified by 10 N HCl) (A) and pH 7.5 (in a 50 mM potassium phosphate buffer) (B).
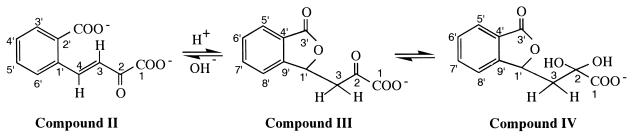
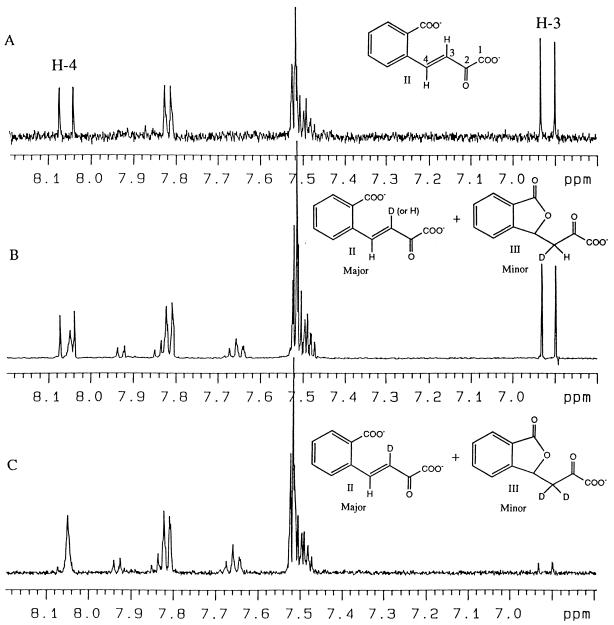
The 1H- and 13C-NMR spectra of the product were recorded as described in Materials and Methods. The assignment of 1H- and 13C-NMR signals of the ring cleavage product at two different pH values is shown in Table 2. The initial pH of the purified material was 2.5. At this pH, two molecular species, 2-oxo-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate (compound III in Table 2 and Fig. 4) and 2,2-dihydroxy-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate (compound IV in Table 2 and Fig. 4), were resolved by 1H-NMR, 13C-NMR, and other two-dimensional NMR analyses including COSY, HSQC, and HMBC. These structures were supported by the negative FAB-mass spectrum at pH 2.5 showing molecular ions at m/z values of 219 and 237 (M-H)−. When the solution was neutralized to pH 7.5 with NaOD, compound IV was not detected and compound III became a minor species. Instead, (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (compound II in Table 2, Fig. 1, and Fig. 4) was revealed as a major species. The (E) form of compound II was deduced from the coupling constant of (3J3-4) 16.4 Hz (Table 2). The negative FAB-mass spectrum of this compound showed a molecular ion at an m/z of 219 (M-H)−. Thus, the ring cleavage product of 1-hydroxy-2-naphthoate took at least three different structures at different pH values. Two forms, compound III and compound IV, were in equilibrium under acidic conditions, while at neutral pH, compound III and compound II were in equilibrium, the latter compound being the major species (Fig. 5B).
TABLE 2.
NMR spectra for the products recorded in D2Oa
| Compound | Position | 13C δ | 1H δb |
|---|---|---|---|
| II (pH 7.5) | 1 | 172.83 | |
| 2 | 198.00 | ||
| 3 | 124.53 | 6.91 (d,16.4) | |
| 4 | 148.99 | 8.05 (d,16.4) | |
| 1′ | 131.40 | ||
| 2′ | 142.03 | ||
| 3′ | 128.22 | 7.51 (m) | |
| 4′ | 131.99 | 7.50 (m) | |
| 5′ | 130.04 | 7.49 (m) | |
| 6′ | 128.03 | 7.81 (d, 7.1) | |
| 2′-C⩵O | 177.81 | ||
| III (pH 2.5) | 1 | 168.69 | |
| 2 | 201.44 | ||
| 3 | 43.67 | 3.60 (dd,18.3,4.4) | |
| 3.40 (dd,18.3,7.9) | |||
| 1′ | 78.65 | 6.10 (dd,7.9,4.4) | |
| 3′ | 173.96 | ||
| 4′ | 149.91 | ||
| 5′ | 125.94 | 7.93 (d,6.6) | |
| 6′ | 130.23 | 7.65 (t,7.6) | |
| 7′ | 135.86 | 7.82 (t,7.6) | |
| 8′ | 122.91 | 7.64 (d,7.6) | |
| 9′ | 125.27 | ||
| IV (pH 2.5) | 1 | 174.15 | |
| 2 | 93.84 | ||
| 3 | 43.5 | 2.69 (dd,15.1,2.7) | |
| 2.36 (dd,15.1,9.3) | |||
| 1′ | 79.65 | 5.85 (dd, 9.3,2.7) | |
| 3′ | 175.05 | ||
| 4′ | 150.53 | ||
| 5′ | 125.85 | 7.91 (d,7.6) | |
| 6′ | 130.11 | 7.65 (t,7.6) | |
| 7′ | 135.72 | 7.83 (t,7.6) | |
| 8′ | 123.01 | 7.64 (d, 7.6) | |
| 9′ | 125.00 |
pH was adjusted with DCl and NaOD. Trimethylsilylpropionic acid sodium salt was used as an external stadard.
Multiplicity (J), in hertz.
FIG. 4.
Structures of the ring cleavage products identified by NMR and FAB-MS analyses. In a solution at pH 2.5, compounds III and IV were revealed. When the pH of the solution was raised to pH 7.5, compounds II and III were revealed, with compound II being the major species. Compound II, (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (common name, trans-2′-carboxybenzalpyruvate); compound III, 2-oxo-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate; compound IV, 2,2-dihydroxy-3-(3-oxo-1,3-dihydro-1-isobenzofuranyl)propanoate.
FIG. 5.
1H-NMR signals of the freshly prepared ring cleavage product of 1-hydroxy-2-naphthoate (pH 7.5) (A), the ring cleavage product neutralized for 1 h at pH 7.5 after its purification at pH 2.5 (B), and the same sample after incubation in deuterated buffer for two more weeks at pH 7.5 (C).
Direct monitoring of the formation of the reaction product by 1H-NMR.
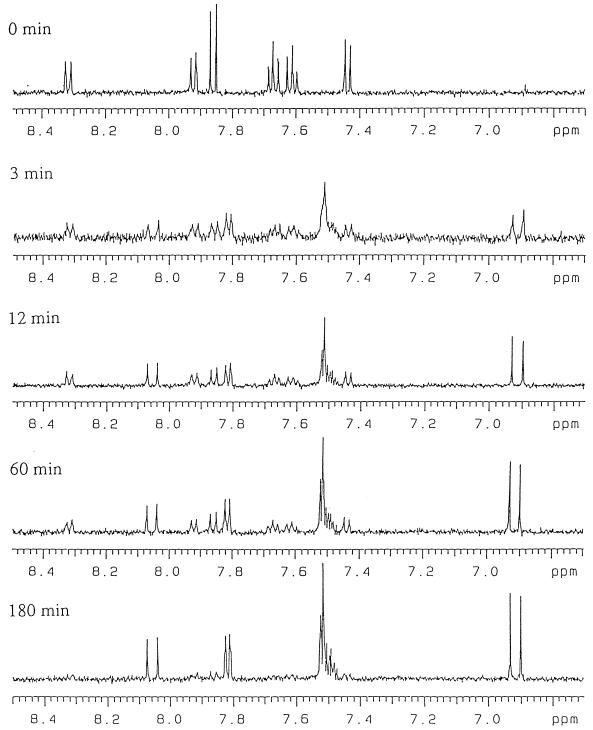
The transformation of 1-hydroxy-2-naphthoate to its ring cleavage product in a reaction catalyzed by 1-hydroxy-2-naphthoate dioxygenase was monitored in real time by 1H-NMR. The intensities of the signals corresponding to 1-hydroxy-2-naphthoate decreased as the reaction progressed. As shown in Fig. 6, a single product, (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (compound II), was formed and was stable for at least 180 min under the experimental conditions. In this reaction, 95% of the substrate (1-hydroxy-2-naphthoate; compound I) was converted to the reaction product (compound II). Thus, compound II is either the primary product of the reaction catalyzed by 1-hydroxy-2-naphthoate dioxygenase or a stable derivative of a hypothetical primary product.
FIG. 6.
Time course of the 1-hydroxy-2-naphthoate dioxygenase reaction monitored by 1H-NMR at pH 7.5 in 50 mM deuterated potassium phosphate buffer.
Deuterium exchange of the ring cleavage product.
The 1H-NMR spectrum of compound II (prepared as described above) is presented in Fig. 5A. In Fig. 5B, the 1H-NMR spectrum of the mixture of compound II and compound III obtained after purification of the ring cleavage product at pH 2.5 and neutralization to pH 7.5 is presented. The H4 signals in compound II (δ, 8.05 ppm) are different between Fig. 5A and B. In Fig. 5A, H4 is a doublet, and in Fig. 5B a broad single peak has appeared inside the double peak for H4. This result indicates that the proton at position 3 of compound II was partly displaced by deuterium during the acidification-neutralization cycle (Fig. 5B). The sample whose spectrum is shown in Fig. 5B was kept in deuterated buffer at pH 7.5 for two more weeks at room temperature in the dark, and a 1H-NMR spectrum was again recorded (Fig. 5C). The spectrum in Fig. 5C indicates that the proton at position 3 was exchangeable and was completely displaced by deuterium after 2 weeks.
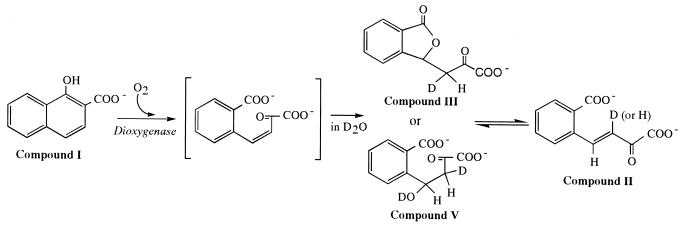
From the structure of 1-hydroxy-2-naphthoate (compound I), it is reasonable to assume that the primary product is (Z)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (Fig. 1), which is subsequently transformed into its (E) isomer (compound II) by a spontaneous or enzyme-catalyzed reaction. A model for (Z)-(E) nonenzymatic isomerization reactions in D2O is depicted in Fig. 7. If the reaction in Fig. 7 occurs, the proton at position 3 of compound II should be exchanged. In fact, the transformation of compound III to compound II in the deuterated buffer displaced the proton at position 3 of the product (Fig. 5B). However, the proton at position 3 of compound II formed by the enzymatic reaction in the deuterated buffer was not displaced by deuterium (Fig. 5A and 6). From these observations, we eliminated the spontaneous nonenzymatic reactions following the enzymatic reaction with dioxygenase as shown in Fig. 7 from the possible (Z)-(E) isomerization mechanisms of the ring cleavage product. Probably, the (Z)-(E) isomerization reaction is a part of the catalytic mechanism of 1-hydroxy-2-naphthoate dioxygenase.
FIG. 7.
Possible mechanisms of spontaneous isomerization of a hypothetical primary product [(Z)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate] to (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (compound II) in D2O. Compounds III and V are hypothetical intermediates in the nonenzymatic conversion from (Z)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate to (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate.
The chemical structures of the three different ring cleavage products have been determined. In the study of Eaton and Chapman (3), the product was isolated without changing the pH value, while in the study of Junker et al. (12), the product was isolated at pH 1.5. It is advisable to isolate such products without acidification, as this treatment may provoke the isomerization demonstrated in this study.
ACKNOWLEDGMENTS
We gratefully acknowledge S. Miyachi for encouragement, Y. Itazawa for technical assistance, and F. Nishida for mass spectrometry operation.
This study was supported by grants from New Energy and Industrial Technology Development Organization (Japan).
REFERENCES
- 1.Barnsley E A. Phthalate pathway of phenanthrene metabolism: formation of 2′-carboxybenzalpyruvate. J Bacteriol. 1983;154:113–117. doi: 10.1128/jb.154.1.113-117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerniglia C E, Heitkamp M A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi U, editor. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 41–68. [Google Scholar]
- 3.Eaton R W, Chapman P J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J Bacteriol. 1992;174:7542–7554. doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans W C, Fernley H N, Griffiths E. Oxidative metabolism of phenanthrene and anthracene by soil pseudomonads. Biochem J. 1965;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker; 1984. pp. 181–252. [Google Scholar]
- 6.Harayama S, Kok M, Neidle E L. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 7.Harayama S, Timmis K N. Aerobic biodegradation of aromatic hydrocarbons by bacteria. In: Sigel H, Sigel A, editors. Metal irons in biological systems. New York, N.Y: Marcel Dekker Inc.; 1992. pp. 99–156. [Google Scholar]
- 8.Iwabuchi T, Harayama S. Biochemical and genetic characterization of 2-carboxybenzaldehyde dehydrogenase, an enzyme involved in phenanthrene degradation by Nocardioides sp. strain KP7. J Bacteriol. 1997;179:6488–6494. doi: 10.1128/jb.179.20.6488-6494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabuchi T, Harayama S. Biochemical and genetic characterization of trans-2′-carboxybenzalpyruvate hydratase-aldolase from a phenanthrene-degrading Nocardioides strain. J Bacteriol. 1998;180:945–949. doi: 10.1128/jb.180.4.945-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwabuchi T, Harayama S. Biochemical and molecular characterization of 1-hydroxy-2-naphthoate dioxygenase from Nocardioides sp. KP7. J Biol Chem. 1998;273:8332–8336. doi: 10.1074/jbc.273.14.8332. [DOI] [PubMed] [Google Scholar]
- 11.Iwabuchi T, Yamauchi-Inomata Y, Katsuta A, Harayama S. Isolation and characterization of marine Nocardioides capable of growing and degrading phenanthrene at 42°C. J Mar Biotechnol. 1998;6:86–90. [Google Scholar]
- 12.Junker F, Field J A, Bangerter F, Ramsteiner K, Kohler H-P, Joannou C L, Mason J R, Leisinger T, Cook A M. Oxygenation and spontaneous deamination of 2-aminobenzenesulphonic acid in Alcaligenes sp. O-1 with subsequent meta ring cleavage and spontaneous desulphonation to 2-hydroxymuconic acid. Biochem J. 1994;300:429–436. doi: 10.1042/bj3000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyohara H, Nagao K, Nomi R. Degradation of phenanthrene through o-phthalate by an Aeromonas sp. Agric Biol Chem. 1976;40:1075–1082. [Google Scholar]
- 14.Kiyohara H, Nagao K. Enzymatic conversion of 1-hydroxy-2-naphthoate in phenanthrene-grown Aeromonas sp. S45P1. Agric Biol Chem. 1977;41:705–707. [Google Scholar]