Abstract
The World Health Organization declared coronavirus disease 2019 (COVID-19) a global pandemic in March 2020. Several vaccines have been developed to overcome the COVID-19 pandemic, and messenger RNA vaccines, commonly known as mRNA vaccines, were the first COVID-19 vaccines to be authorized in Korea. With the worldwide increase in vaccinations, reports of adverse reactions are increasing. However, to the best of our knowledge, there have been no reports of eosinophilic gastroenteritis (EGE) following mRNA vaccination. Here, we present the first case of EGE in a patient who received a second dose of the mRNA vaccine, BNT162b2 (Pfizer-BioNTech). A previously healthy 34-year-old woman presented to the emergency department with generalized abdominal pain for the preceding 2 weeks. She had received a second dose of the mRNA COVID-19 vaccine 2 weeks prior. Subserosal EGE was diagnosed, oral prednisolone was administered, and she recovered completely.
Keywords: Coronavirus, COVID-19, COVID-19 Vaccines, Eosinophilia
Graphical Abstract
INTRODUCTION
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was detected in December 2019, followed by an outbreak first reported in Wuhan, China.1 The World Health Organization named the infection due to SARS-CoV-2 as coronavirus disease 2019 (COVID-19) in February 2020 and declared COVID-19 to be a global pandemic in March 2020. Several vaccines have been developed to overcome the COVID-19 pandemic, and messenger RNA vaccines, commonly known as mRNA vaccines, are the first COVID-19 vaccines that were authorized and licensed in the Republic of Korea. Currently, two mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) are available.2 With increased vaccinations worldwide, reports of adverse reactions to these vaccines are increasing. However, to the best of our knowledge, there have been no reports of eosinophilic gastroenteritis (EGE) following mRNA vaccination. Here, we present the first case of EGE in a patient who received a second dose of the mRNA vaccine, BNT162b2 (Pfizer-BioNTech).
CASE DESCRIPTION
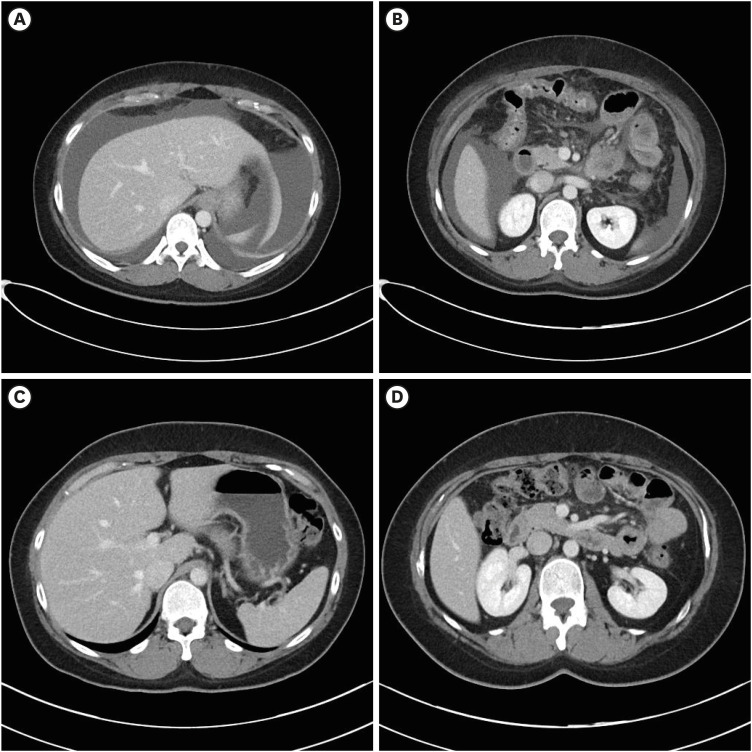
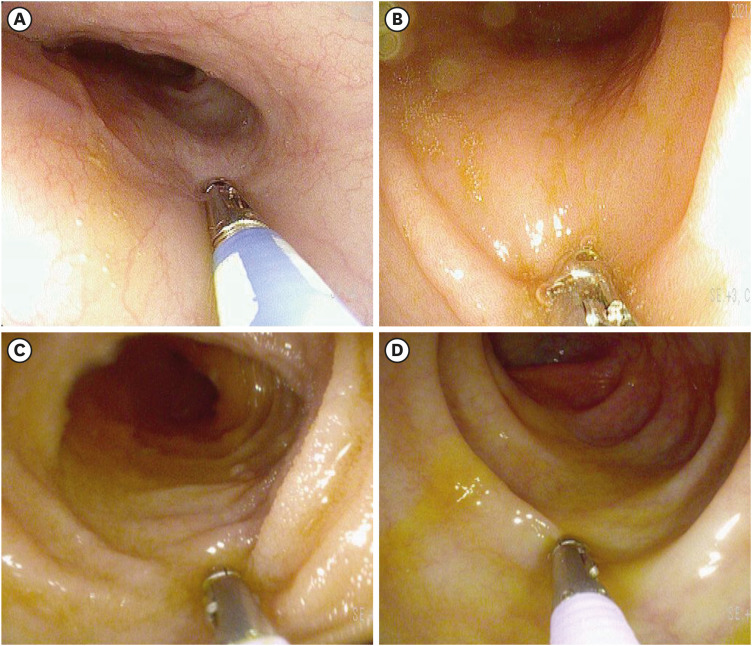
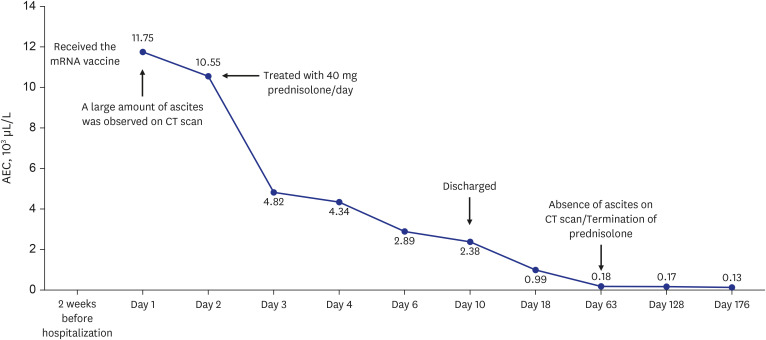
A previously healthy 34-year-old woman presented to the emergency department of Dong-A University Hospital with abdominal pain, abdominal distention, nausea, diarrhea, and dyspnea that had started within the preceding 2 weeks on 22 October 2021. She reported that she had not experienced any recent fever, chills, changes in bowel habits, joint swelling, or skin rashes. She had received the second dose of BNT162b2 vaccine 2 weeks prior, and the symptoms had started on the day after vaccination. She had experienced mild generalized aches after the first dose of BNT162b2 vaccine, but the symptoms had improved after a few days, and she had not experienced any severe adverse effects. However, after the second dose of BNT162b2 vaccine, the symptoms were different from those that she had experienced after the first dose. She visited an outpatient clinic for these symptoms 3 days after the second vaccination, but the symptoms progressively worsened. She had experienced allergic rhinitis as a child but this had improved since becoming an adult and she was not taking any treatment. She had no other medical history of note. She reported no history of illicit drug use or alcohol consumption and was not taking any medications, supplements, or herbal compounds. She had no history of liver disease, heart disease, or recent travel. The patient’s temperature was 36.7°C, heart rate was 86 beats/min, respiratory rate was 20 breaths/min, blood pressure was 100/60 mmHg, and oxygen saturation in room air was 98%. Abdominal tenderness but no rebound pain was observed. Cardiopulmonary examination results were normal. Clinical neurological examination results were normal. Blood tests revealed leukocytosis (2,351/μL) with an elevated eosinophil count (50.0%; 1,176/μL). C-reactive protein level was slightly elevated (2.77 mg/L). There was no elevation in hepatobiliary and pancreatic enzyme levels. Fecal test results were negative for occult blood and multiple enteric pathogens. Parasitological examination of her stool and blood samples yielded negative results. Serum immunoglobulin E (IgE) levels were elevated at 2,577 lU/mL (normal < 200 IU/mL). Rheumatological index, serum protein electrophoresis, and immunostaining were performed to exclude other systemic diseases that were negative. To rule out blood diseases that present with hypereosinophilia, a peripheral blood smear, FIP1L1-PDGFRa fusion gene qualitative test, bone marrow biopsy, and chromosome analysis were performed, all of which yielded negative results. Computed tomography revealed extensive small bowel wall edema and a large amount of ascites in the abdominal cavity. (Fig. 1A and B) Diagnostic paracentesis revealed that the ascitic fluid was suggested as exudate with a low serum albumin-ascitic gradient. Ascitic fluid revealed an amber fluid with protein level 4.4 g/dL, albumin 2.6 g/dL, lactate dehydrogenase 282 mg/dL, and white blood count of 7,900/mL with remarkable eosinophilia of 90%, without cytological signs of malignancy. Laboratory testing of ascitic fluid for bacterial culture and tuberculosis yielded negative results. Esophagoduodenoscopy and colonoscopy revealed unremarkable findings (Fig. 2). Multiple biopsies were performed at the esophagus, stomach, duodenum, terminal ileum, and colon. The impressive finding was subserosal EGE, presenting as isolated eosinophilic ascites without eosinophilic infiltration in the mucosal or muscular layer of the gastrointestinal (GI) tract. The patient was treated with 40 mg prednisolone per day. Following treatment, her abdominal pain disappeared within a few days and the ascites disappeared within 1 week. She was discharged 10 days after admission. The prednisolone dose was gradually reduced until it was discontinued after 2 months. A computed tomography scan obtained 2 months later showed that the ascites had completely disappeared (Fig. 1C and D). All blood tests, including her eosinophil count on a peripheral blood test and serum IgE were also normal. A follow-up examination 6 months after discharge had similar findings (Fig. 3). She did not receive a third dose of vaccine due to the adverse effects that had occurred after the second dose.
Fig. 1. Abdominal computed tomography scan. (A) Abdominal computed tomography scan demonstrated large amount of ascites in the abdominal cavity. (B) Abdominal computed tomography scan demonstrated intestinal wall edema thickening in the small bowel. (C) Six months later after discharge, abdominal computed tomography scan demonstrated ascites had disappeared and resolved in the abdominal cavity. (D) Six months later after discharge, abdominal computed tomography scan demonstrated resolved state of intestinal wall edema thickening in the small bowel.
Fig. 2. Esophagoduodenoscopy and colonoscopy. (A) Esophagoduodenoscopy demonstrated normal mucosal findings of the esophagus and (B) stomach. (C) Colonoscopy demonstrated normal mucosal findings of the ileum and (D) colon.
Fig. 3. Absolute eosinophil count over treatment course.
AEC = absolute eosinophilic count, CT = computed tomography.
Ethics statement
Informed consent for publication of clinical data was obtained from the case patient.
DISCUSSION
EGE is a rare inflammatory disease characterized by eosinophilic infiltration of the GI tract. The disease includes three types: mucosal, muscular, and subserosal, depending on the depth of the eosinophilic infiltration. Subserosal EGE is the rarest type, presenting either with isolated eosinophilic ascites or eosinophilic ascites in combination with symptoms characteristic of mucosal or muscular EGE.3,4 The pathophysiology of this disease is unclear. Several epidemiologic and clinical studies suggest an allergic component in 50–80% of patients with concomitant atopic conditions.3,5,6 Previous investigations support the important role of eosinophils, Th2 cytokines (e.g., interleukin [IL]-3, IL-4, IL-5, IL-13), and eotaxin as important factors in the pathogenesis of EGE.7,8 The diagnosis of EGE is based on the presence of eosinophilic infiltration of the GI tract on biopsy or eosinophilic ascitic fluid, absence of other causes of intestinal eosinophilia, and lack of involvement of other organs. There is no established defined cut-off value for the number of eosinophils/high-power field (HPF) to diagnose EGE, but there is a movement to establish more formal diagnostic criteria in EGE. The following cut-off values for the number of eosinophils/HPF have been suggested: ≥ 30 eosinophils per HPF in stomach/duodenum, > 56 per HPF in ileum, > 100 per HPF in right colon, > 84 per HPF in transverse/descending colon, and > 64 per HPF in rectosigmoid colon.9 Other diseases (e.g., infection, inflammatory bowel disease, autoimmune diseases, connection tissue diseases, and lymphoproliferative malignancies) in which GI symptoms are associated with peripheral eosinophilia can be distinguished from EGE based on the clinical presentation, laboratory findings, and biopsies of the GI tract.10 Previous studies have reported that drugs act as an allergen and induce secondary EGE. Gold salts,11 azathioprine,12 clofazimine,13 gemfibrozil,14 enalapril,15 carbamazepine,16 and nivolumab17 have been reported to induce EGE, and co-trimoxazole has been reported to induce eosinophilic ascites similar to this case.18 Reports of adverse reactions from COVID-19 vaccines are increasing. In some studies, eosinophilia or eosinophilic reactions were reported,19,20,21 but some of them were from other forms of the vaccine, not the mRNA vaccine,22,23 and there were no cases of eosinophilic gastroenteritis. The relationship between COVID-19 vaccines and EGE is not yet clearly understood. The pathophysiology of EGE is related to the excessive production of Th2 cytokines and eotaxins in response to various stimuli. COVID-19 seems to be one of these stimuli, as there is a reported case of eosinophilic responses to SARS-CoV-2 infection and COVID-19 vaccination.24 Therefore, it may be inferred that mRNA COVID-19 vaccines can stimulate excessive production of Th2 cytokines and eotaxins, which leads to hypereosinophilia and eosinophilic infiltration of the GI tract, leading to EGE.
Treatment includes elemental diet and medical therapy using anti-allergic agents. Steroids are the mainstay of therapy, including oral prednisolone (20–40 mg/day). After symptom improvement was achieved, the initial dose was tapered off. In this case, after rapid symptom improvement, the dose was tapered for two months. Most subserosal EGE respond quickly to steroids.3 In addition, treatments for EGE, such as mast cell inhibitors, leukotriene receptor antagonists, and anti-IL-5, have been suggested, but they have not yet been established due to limited data.10
We presented here a case of COVID-19 vaccine-associated hypereosinophilia and eosinophilic gastroenteritis presenting as eosinophilic ascites in a patient receiving a second dose of BNT162b2 (Pfizer-BioNTech). Rapid resolution of the associated symptoms was achieved with prednisolone. To the best of our knowledge, this is the first case report of subserosal eosinophilic gastroenteritis caused by the mRNA COVID-19 vaccine.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee JY, Lee JH.
- Writing - original draft: Lee JY.
- Writing - review & editing: Lee JY, Lee JH.
References
- 1.Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31(1):54–58. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian XQ, Chen X, Chen SL. Eosinophilic gastroenteritis with abdominal pain and ascites: A case report. World J Clin Cases. 2021;9(17):4238–4243. doi: 10.12998/wjcc.v9.i17.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff SC, Ulmer FA. Eosinophils and allergic diseases of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22(3):455–479. doi: 10.1016/j.bpg.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Yun MY, Cho YU, Park IS, Choi SK, Kim SJ, Shin SH, et al. Eosinophilic gastroenteritis presenting as small bowel obstruction: a case report and review of the literature. World J Gastroenterol. 2007;13(11):1758–1760. doi: 10.3748/wjg.v13.i11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168(5):2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 8.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125(5):1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257–268. doi: 10.1016/j.gtc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Li Y. Eosinophilic gastroenteritis: a state-of-the-art review. J Gastroenterol Hepatol. 2017;32(1):64–72. doi: 10.1111/jgh.13463. [DOI] [PubMed] [Google Scholar]
- 11.Michet CJ, Jr, Rakela J, Luthra HS. Auranofin-associated colitis and eosinophilia. Mayo Clin Proc. 1987;62(2):142–144. doi: 10.1016/s0025-6196(12)61885-0. [DOI] [PubMed] [Google Scholar]
- 12.Riedel RR, Schmitt A, de Jonge JP, Hartmann A. Gastrointestinal type 1 hypersensitivity to azathioprine. Klin Wochenschr. 1990;68(1):50–52. doi: 10.1007/BF01648893. [DOI] [PubMed] [Google Scholar]
- 13.Ravi S, Holubka J, Veneri R, Youn K, Khatib R. Clofazimine-induced eosinophilic gastroenteritis in AIDS. Am J Gastroenterol. 1993;88(4):612–613. [PubMed] [Google Scholar]
- 14.Lee JY, Medellin MV, Tumpkin C. Allergic reaction to gemfibrozil manifesting as eosinophilic gastroenteritis. South Med J. 2000;93(8):807–808. [PubMed] [Google Scholar]
- 15.Barak N, Hart J, Sitrin MD. Enalapril-induced eosinophilic gastroenteritis. J Clin Gastroenterol. 2001;33(2):157–158. doi: 10.1097/00004836-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Shakeer VK, Devi SR, Chettupuzha AP, Mustafa CP, Sandesh K, Kumar SK, et al. Carbamazepine-induced eosinophilic enteritis. Indian J Gastroenterol. 2002;21(3):114–115. [PubMed] [Google Scholar]
- 17.Tomiyasu H, Komori T, Ishida Y, Otsuka A, Kabashima K. Eosinophilic gastroenteritis in a melanoma patient treated with nivolumab. J Dermatol. 2021;48(10):E486–E487. doi: 10.1111/1346-8138.16036. [DOI] [PubMed] [Google Scholar]
- 18.Wienand B, Sanner B, Liersch M. Eosinophilic gastroenteritis as an allergic reaction to a trimethoprim-sulfonamide preparation. Dtsch Med Wochenschr. 1991;116(10):371–374. doi: 10.1055/s-2008-1063622. [DOI] [PubMed] [Google Scholar]
- 19.de Las Vecillas L, López J, Morchón E, Rodriguez F, Drake M, Martino M. Viral-like reaction or hypersensitivity? Erythema multiforme minor reaction and moderate eosinophilia after the Pfizer-BioNTech BNT162b2 (mRNA-Based) SARS-CoV-2 vaccine. J Investig Allergol Clin Immunol. 2021;32(1):77–78. doi: 10.18176/jiaci.0757. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka K, Katayama K, Kaji Y, Tawara J, Ohira Y. Non-episodic angioedema with eosinophilia after BNT162b2 mRNA COVID-19 vaccination. QJM. 2021;114(10):745–746. doi: 10.1093/qjmed/hcab245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Montjoye L, Marot L, Baeck M. Eosinophilic cellulitis after BNT162b2 mRNA Covid-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(1):e26–e28. doi: 10.1111/jdv.17685. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor T, O’Callaghan-Maher M, Ryan P, Gibson G. Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID-19 vaccine. JAAD Case Rep. 2022;20:14–16. doi: 10.1016/j.jdcr.2021.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaikati J, Ghanem A, El Bahtimi R, Helou J, Tomb R. Eosinophilic panniculitis: a new side effect of Sinopharm COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(5):e337–e339. doi: 10.1111/jdv.17920. [DOI] [PubMed] [Google Scholar]
- 24.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]