Abstract
Non-small cell lung cancer (NSCLC) is a malignant tumor with a high morbidity and mortality rate that is a threat to human health. With the development of molecular targeted research, breakthroughs have been made on the molecular mechanism of lung cancer. The echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene is one of the most important pathogenic driver genes of NSCLC discovered thus far. Four generations of targeted drugs for EML4-ALK have been developed, with patients benefiting significantly from these drugs. Therefore, EML4-ALK has become a research hotspot in NSCLC. The aim of the present study is to introduce the current research progress of EML4-ALK and its association with NSCLC.
Keywords: EML4-ALK, non-small cell lung cancer, fusion gene, targeted therapy
1. Introduction
According to the latest data released by the International Agency for Research on Cancer, the incidence of lung cancer is increasing annually (1); it is one of the most common malignant tumors, accounting for 15% of global cancer diagnoses, with a 10-year survival rate of just 5% (1–3). Lung cancer is the second most common cancer type worldwide and the malignant tumor with the highest mortality rate; it is also associated with poor survival following the initial diagnosis (1). Possibly due to the popularization of diagnostic imaging technology and the improvement in the awareness of physical examinations, more patients with lung cancer are being diagnosed at an early stage; however, a number of them are young (4).
With the emergence of technologies such as fluorescence in situ hybridization (FISH) and next-generation sequencing (NGS), tumor diagnosis and treatment have entered the molecular field, and tumor gene screening has become a routine diagnostic and treatment method (5–7). Non-small cell lung cancer (NSCLC) is the main pathological type of lung cancer; in recent years, breakthroughs have been made in the research on targeted genes for NSCLC (8). Among them, the echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene is the most important pathogenic gene of NSCLC discovered so far (9). Targeted drug therapy for EML4-ALK has achieved marked curative effects, bringing a glimmer of hope to patients with NSCLC (9,10).
2. EML4-ALK fusion gene
The EML4-ALK fusion gene was first reported by Soda et al (11), after amplification of a 3926-bp DNA fragment in the tumor tissue of a patient with lung adenocarcinoma, which encoded a protein composed of 1,059 amino acids, the fusion protein EML4-ALK (11). In the follow-up experiments by Soda et al (12), the implantation of the EML4-ALK gene into normal lung cells was shown to induce carcinogenesis, suggesting that EML4-ALK has an oncogenic effect.
Suprenant et al (13) was the first to discover a substance that binds to tubulin and is involved in mediating mitosis, the echinoderms microtubule-associated protein (EMAP; also known as EML). To date, a total of 6 human-expressed EML family members (EML1-6) have been found (14,15), and EML4 is the homologous protein that expresses the most representative EMAP characteristics (16). EML4 is composed of an N-terminal basic domain, a hydrophobic motif in EML proteins (HELP) and a C-terminal tryptophan-aspartic acid repeats (WD) (17). The base domain of the N-terminal is an a-helical-coiled base region that contains a coiled region that promotes trimerization oligomerization, namely that of the trimerization domain (15). The initial study by Soda et al (11) found that the construction of EML4 cells with basal domain deletion did not induce tumorigenesis in nude mice, while HELP and WD deletion did, which indicated that the basal domain in EML4 played a key role in inducing tumorigenesis.
ALK is an insulin receptor subfamily of the receptor tyrosine kinase family (18), originally identified in anaplastic large cell lymphoma (19). ALK is normally expressed during the embryonic period and is involved in the regulation and development of the nervous system (20,21). ALK is mainly composed of a tyrosine kinase domain and a transmembrane domain (22–24). Under normal circumstances, following the activation of ALK by exogenous ligands, two ALK monomers are phosphorylated to form an ALK dimer with kinase activity to participate in cellular regulation (23–25).
The EML4 and ALK genes are located on p21 and p23 of human chromosome 2, respectively, and are ~10 Mb apart (26); the orientation of their gene sequences is reversed on the short arm of chromosome 2 (27). The essence of EML4-ALK is a translocation fusion caused by an intra-arm interchange, and one of the genes needs to be reversed during gene fusion (27,28). In the EML4-ALK fusion gene, the EML4 gene fragments all contain the basal domain, and ALK contains the kinase region (29). The fusion gene of EML4 and ALK can encode a fusion protein with tumorigenic activity, namely the EML4-ALK fusion protein (30). The fusion protein can directly form an ALK dimer without the activation of an exogenous ligand, thereby activating ALK and its downstream RAS/ERK/STAT3/mTOR and other signaling pathways. Finally, through the promotion of cell proliferation and invasion, and the inhibition of apoptosis, it leads to the occurrence of NSCLC. The RAS/ERK signaling pathway is associated with cell proliferation, and the mTOR and STAT3 pathways are associated with cell survival and apoptosis (31,32). Studies have shown that the HELP domain on EML4 is necessary for the specific activation of RAS, and the EML4-ALK fusion protein can promote the upregulation of RAS and the phosphorylation of ERK. The EML4-ALK fusion protein activates and upregulates the expression of STAT3, and the overexpression of STAT3 promotes the phosphorylation level of mTOR and promotes the tumor anti-apoptotic ability by activating mTOR signaling (Fig. 1) (33–37).
Figure 1.
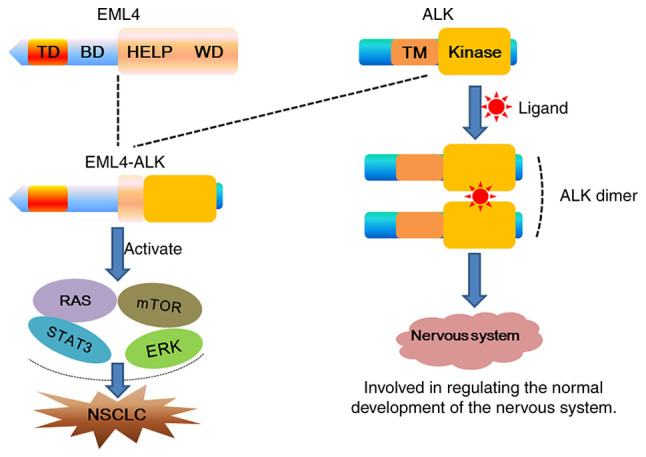
Schematic diagram of the EML4-ALK fusion gene. During embryonic development, ligands activate ALK to form ALK dimers, which are involved in regulating the growth and development of the nervous system. When the EML4-ALK fusion gene is formed, it can be directly activated without the need for ligands, and abnormally activate downstream signaling pathways, promote cell proliferation and invasion, and inhibit apoptosis, ultimately leading to the occurrence of NSCLC. TD, trimerization domain; BD, basic domain; HELP, hydrophobic motif in EML proteins; WD, tryptophan-aspartic acid repeats; TM, transmembrane domain; NSCLC, non-small cell lung cancer; EML4, echinoderm microtubule-associated protein-like 4; ALK, anaplastic lymphoma kinase.
To date, several variants of the EML4-ALK fusion gene have been identified (38), and the differences among variants mainly depend on the different truncation sites in the WD region of EML4, which form EML4 gene cleavage fragments of different lengths (39). In a previous study, these EML4 fragments of different lengths were inserted into exon 20 of the ALK gene to form different fusion genotypes (40). Currently the most common EML4-ALK fusion genotypes in NSCLC are as follows: EML4-ALK V1 (exon 13 of EML4 fused to exon 20 of ALK; 33%), EML4-ALK V2 (exon 20 of EML4 fused to exon 20 of ALK; 10%), EML4-ALK V3 a/b (exon 6 of EML4 is fused to exon 20 of ALK; 29%) (Fig. 2) (41–43), and different fusion genotypes have different tyrosine kinase activities (44). Further research on the differences in biological behavior among variants of the EML4-ALK fusion gene is required (45).
Figure 2.
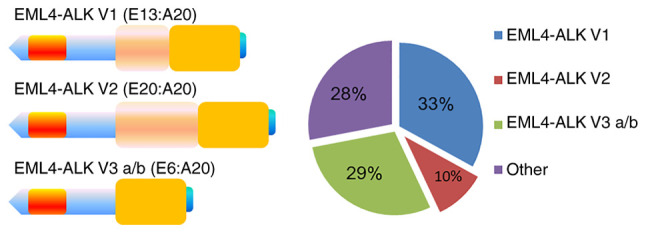
Three most important variants of EML4-ALK. EML4-ALK V1, EML4-ALK V2 and EML4-ALK V3 a/b are formed by inserting exon 13 (E13), exon 20 (E20) and exon 6 (E6) of EML4, respectively, into exon 20 (A20) of ALK. EML4, echinoderm microtubule-associated protein-like 4; ALK, anaplastic lymphoma kinase.
3. Clinical features of EML4-ALK in NSCLC
Lung cancer is classified into two histological groups: SCLC (~17.3%) and NSCLC (~82.7%) (46–48), of which lung adenocarcinoma and lung squamous cell carcinoma are the major subgroups (48). Since the EML4-ALK fusion gene was discovered in lung adenocarcinoma in 2007, it has been considered to be a characteristic gene of lung cancer; however, in recent years, it has gradually been detected in other types of cancer, such as thyroid cancer, gastric stromal tumors and leiomyoma (11,49–51). According to a previous study, the oncogenic fusion of EML4-ALK is present in 3–5% of NSCLC (27). One study found that EML4-ALK fusion gene positivity occurred mostly in female patients with NSCLC who did not smoke or smoked infrequently (52), and the positive detection rate was higher in patients with NSCLC without an epidermal growth factor receptor (EGFR) or KRAS gene mutation (53). Male patients with lung cancer with a long-term history of smoking exhibited a particularly low detection rate for the EML4-ALK fusion gene (54). At present, the EML4-ALK fusion gene is a routine gene mutation test for patients with NSCLC.
4. EML4-ALK detection method
At present, the commonly used EML4-ALK fusion gene detection methods in clinical diagnosis and treatment, as well as laboratory research, mainly include immunohistochemistry (IHC), FISH, reverse transcription PCR (RT-PCR) and NGS (55). IHC is the simplest, cheapest and most commonly used detection method, and is widely used in hospitals and laboratories (56). IHC uses antigen-antibody reactions to detect whether the EML4-ALK fusion protein is produced in tumor tissues (56). However, due to the low expression level of the EML4-ALK fusion protein in NSCLC lung tissue, this method has low sensitivity and cannot distinguish between fusion types (57).
FISH is a relatively specific and sensitive method that uses fluorescence-labeled specific nucleic acid probes to hybridize with targeted DNA or RNA in cells to generate fluorescent signals (58,59). EML4-ALK fusion gene detection is performed through the fluorescent labeling of EML4 and ALK, and subsequent observation of the positional relationship of the two fluorescent signals to determine whether the chromosome is translocated, so as to determine whether the EML4-ALK fusion gene exists in the tumor tissue; however, this method also fails to distinguish between fusion variant types (60). RT-PCR can distinguish between different types of EML4-ALK fusion genes by designing primers for different fusion variants (60). RT-PCR is characterized by rapid diagnosis and high sensitivity. The quality requirement for the extracted RNA and the positive detection rate of EML4-ALK for fresh tumor specimens are high, but most tumor specimens are fixed in neutral formaldehyde, resulting in RNA degradation and reduced sensitivity (60,61). NGS has revolutionized traditional sequencing, as it can sequence hundreds of thousands to millions of DNA molecules at once, which renders the detailed and comprehensive analysis of the transcriptome and genome of a species possible (62). NGS has a high degree of specificity and sensitivity, and can detect various known and unknown fusion gene types, but the procedure is complicated, the technical difficulty is high and the detection standards are not uniform (63,64) (Table I).
Table I.
Comparison of echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase fusion gene detection methods.
| Parameter | IHC | FISH | RT-PCR | NGS |
|---|---|---|---|---|
| Time | Fast | Fast | Slow | Slow |
| Expense | Cheap | Expensive | Expensive | Expensive |
| Specificity | Relatively high | High | High | High |
| Sensitivity | Relatively high | Relatively high | High | High |
| Variant classification | No | No | Yes | Yes |
| Application range | Most extensive | Extensive | Extensive | Not extensive |
| Operation difficulty | Simple | Complex | Simple | Complex |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction; NGS, next-generation sequencing.
5. Targeted therapy for EML4-ALK
In recent years, breakthroughs have been made in targeted therapy technology, and a variety of targeted therapy drugs for EML4-ALK have been developed (65). Crizotinib, approved for marketing in 2011, was the first drug to target the EML4-ALK fusion gene (66). Crizotinib is an orally active aminopyridine-derived small molecule competitive inhibitor (10). The study showed that, in patients with advanced EML4-ALK fusion gene-positive NSCLC, the objective response rate (ORR; 53%) and progression-free survival (PFS) time (8.5 months) of patients receiving crizotinib were significantly higher than those of patients receiving standard platinum-based chemotherapy (67,68). The results showed that targeted therapy with crizotinib was more effective than traditional standard chemotherapy and did not increase the number of serious adverse reactions (69,70). However, when used as a first-line treatment regimen, resistance to crizotinib often develops at varying degrees within 1 year of treatment (71). As a means to overcome resistance to crizotinib, second-generation EML4-ALK-targeted drugs, such as ceritinib (72), brigatinib (73) and alectinib (74), as well as the third-generation targeted drug lorlatinib, have been developed (75), and the fourth-generation targeted drug repotrectinib (TPX-0005) has been undergoing phase I/II clinical trials (76,77). The American Society of Clinical Oncology performed a phase II study on the efficacy of a new generation of targeted drugs in patients with ALK rearrangement-positive advanced NSCLC who progressed after EML4-ALK targeted therapy. The results showed that the new generation of targeted drugs could significantly improve the ORR (77.8%) and PFS time (10.7 months) of patients (78), and at the same time exhibit good efficacy in patients with intracranial metastasis or in other NSCLC patients with mutations in genes such as ROS1 (10,79,80). However, following the long-term use of targeted therapy, acquired resistance inevitably occurs, which affects the therapeutic effect (81). There is currently evidence that different EML4-ALK fusion gene variants have varying degrees of sensitivity to targeted drugs in NSCLC (39). A previous study analyzed 77 tumor biopsies from patients with EML4-ALK V1 and EML4-ALK V3 fusion genes and found that resistance mutations were more common in V3 than in V1 (57 vs. 30%; P=0.023) (82). Therefore, variant typing of the EML4-ALK fusion gene is necessary.
The acquired resistance mechanisms of EML4-ALK discovered in the present study mainly included the following: i) Secondary mutation of the kinase domain (83); the secondary gene mutation in the ALK kinase domain leads to a change in the spatial conformation of the binding region of the kinase and the drug, which increases the binding force of the kinase and ATP, thereby affecting the binding of the drug and the kinase, leading to drug resistance (84). A gene mutation was detected in ~30% of patients with resistance to a first-generation targeted drug, resulting in a point mutation of a glycine residue located in the ATP-binding region to valine. The mutation rate following second-generation drug resistance exceeded 50%, resulting in the mutation of glycine residues to arginine (84). ii) Activation of alternative signaling pathways (85); when the ALK signaling pathway is inhibited by targeted drugs, other tumor-promoting signaling pathway proteins, such as EGFR and KIT, are abnormally activated and continue to promote tumor cell proliferation (86). iii) Epithelial-mesenchymal transition; the transformation of tumor epithelial cells to mesenchymal cells increases the ability of tumor cells to invade and metastasize (87). In patients with NSCLC EML4-ALK-targeted drug resistance, the expression of the mesenchymal marker vimentin was increased, and that of the epithelial marker E-cadherin was decreased, suggesting that epithelial-mesenchymal transition may be involved in the drug resistance response (88–90). In order to overcome the drug resistance of tumor cells, it is currently possible to strengthen the combination of EML4-ALK-targeted and other antitumor drugs, such as the EGFR inhibitor erlotinib, cyclin-dependent kinase inhibitor, and riboxy and heat shock protein 90 inhibitors (91–93). Combined use of these drugs can synergistically enhance antitumor activity and inhibit ALK kinase activity (94).
6. Summary and outlook
The EML4-ALK fusion gene is one of the important tumor driver genes discovered in NSCLC in recent years, and it is an important molecular target affecting the diagnosis and treatment of NSCLC. In particular, the detection rate of the EML4-ALK fusion gene is higher in patients with NSCLC who are young, non-smoking, females without EGFR and other gene mutations. Although the current detection technologies have their own shortcomings, they can meet the needs of current clinical diagnosis and treatment. Most patients with NSCLC with EML4-ALK fusion gene positivity can benefit significantly from molecular targeted therapy, but drug resistance is an important factor that plagues current targeted therapy. It is believed that with the successful development of a new generation of EML4-ALK-targeted drugs and the elucidation of the drug resistance mechanism, the survival of patients with NSCLC with EML4-ALK fusion gene mutation will be further improved.
Acknowledgements
Not applicable.
Funding Statement
Funding: Not applicable.
Availability of data and materials
Not applicable.
Authors' contributions
YuL, YaL and JJW designed the theme of the review. YuL, YaL, XS and JJW retrieved the relevant literature. XS wrote and reviewed the article. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5; doi: 10.1002/ijc.33588. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 3.Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85:8. doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Li D, Liang D, He Y. Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci Rep. 2021;11:6817. doi: 10.1038/s41598-021-86203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamard C, Mignard X, Pecuchet N, Mathiot N, Blons H, Laurent-Puig P, Leroy K, Lupo A, Chapron J, Giraud F, et al. IHC, FISH, CISH, NGS in non-small cell lung cancer: What changes in the biomarker era? Rev Pneumol Clin. 2018;74:327–338. doi: 10.1016/j.pneumo.2018.09.013. (In French) [DOI] [PubMed] [Google Scholar]
- 6.Lim AS, Lim TH. Fluorescence in situ hybridization on tissue sections. Methods Mol Biol. 2017;1541:119–125. doi: 10.1007/978-1-4939-6703-2_11. [DOI] [PubMed] [Google Scholar]
- 7.Morganti S, Tarantino P, Ferraro E, D'Amico P, Duso BA, Curigliano G. Next generation sequencing (NGS): A revolutionary technology in pharmacogenomics and personalized medicine in cancer. Adv Exp Med Biol. 2019;1168:9–30. doi: 10.1007/978-3-030-24100-1_2. [DOI] [PubMed] [Google Scholar]
- 8.Gao S, Zhang G, Lian Y, Yan L, Gao H. Exploration and analysis of the value of tumor-marker joint detection in the pathological type of lung cancer. Cell Mol Biol (Noisy-le-grand) 2020;66:93–97. doi: 10.14715/cmb/2020.66.6.17. [DOI] [PubMed] [Google Scholar]
- 9.Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis F, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Heigener DF, Reck M. Crizotinib. Recent Results Cancer Res. 2018;211:57–65. doi: 10.1007/978-3-319-91442-8_4. [DOI] [PubMed] [Google Scholar]
- 11.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 12.Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, Haruta H, Hamada T, Yamashita Y, Ishikawa Y, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci USA. 2008;105:19893–19897. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suprenant KA, Dean K, McKee J, Hake S. EMAP, an echinoderm microtubule-associated protein found in microtubule-ribosome complexes. J Cell Sci. 1993;104:445–450. doi: 10.1242/jcs.104.2.445. [DOI] [PubMed] [Google Scholar]
- 14.Fry AM, O'Regan L, Montgomery J, Adib R, Bayliss R. EML proteins in microtubule regulation and human disease. Biochem Soc Trans. 2016;44:1281–1288. doi: 10.1042/BST20160125. [DOI] [PubMed] [Google Scholar]
- 15.Richards MW, O'Regan L, Roth D, Montgomery JM, Straube A, Fry AM, Bayliss R. Microtubule association of EML proteins and the EML4-ALK variant 3 oncoprotein require an N-terminal trimerization domain. Biochem J. 2015;467:529–536. doi: 10.1042/BJ20150039. [DOI] [PubMed] [Google Scholar]
- 16.Richards MW, Law EW, Rennalls LP, Busacca S, O'Regan L, Fry AM, Fennell DA, Bayliss R. Crystal structure of EML1 reveals the basis for Hsp90 dependence of oncogenic EML4-ALK by disruption of an atypical β-propeller domain. Proc Natl Acad Sci USA. 2014;111:5195–5200. doi: 10.1073/pnas.1322892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mano H. The EML4-ALK oncogene: Targeting an essential growth driver in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91:193–201. doi: 10.2183/pjab.91.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tulpule A, Guan J, Neel DS, Allegakoen HR, Lin YP, Brown D, Chou YT, Heslin A, Chatterjee N, Perati S, et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell. 2021;184:2649–2664.e18. doi: 10.1016/j.cell.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladanyi M, Cavalchire G, Morris SW, Downing J, Filippa DA. Reverse transcriptase polymerase chain reaction for the Ki-1 anaplastic large cell lymphoma-associated t(2;5) translocation in Hodgkin's disease. Am J Pathol. 1994;145:1296–1300. [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley SP, Clary DO, Copie V, Lefcort F. Anaplastic lymphoma kinase is dynamically expressed on subsets of motor neurons and in the peripheral nervous system. J Comp Neurol. 2006;495:202–212. doi: 10.1002/cne.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 22.Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC) Mol Cancer. 2018;17:52. doi: 10.1186/s12943-018-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016;27((Suppl 3)):iii4–iii15. doi: 10.1093/annonc/mdw301. [DOI] [PubMed] [Google Scholar]
- 24.Roskoski R., Jr Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol Res. 2013;68:68–94. doi: 10.1016/j.phrs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Bennasroune A, Mazot P, Boutterin MC, Vigny M. Activation of the orphan receptor tyrosine kinase ALK by zinc. Biochem Biophys Res Commun. 2010;398:702–706. doi: 10.1016/j.bbrc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M, University of Hong Kong Lung Cancer Study Group The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 27.Vašíková A. EML4-ALK fusion gene in patients with lung carcinoma: Biology, diagnostics and targeted therapy. Klin Onkol. 2012;25:434–439. [PubMed] [Google Scholar]
- 28.Kodama T, Motoi N, Ninomiya H, Sakamoto H, Kitada K, Tsukaguchi T, Satoh Y, Nomura K, Nagano H, Ishii N, et al. A novel mechanism of EML4-ALK rearrangement mediated by chromothripsis in a patient-derived cell line. J Thorac Oncol. 2014;9:1638–1646. doi: 10.1097/JTO.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 29.Yang T, Liu H, Chen J. EML4-ALK fusion gene in lung cancer and its biological function. Zhongguo Fei Ai Za Zhi. 2012;15:112–116. doi: 10.3779/j.issn.1009-3419.2012.02.09. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayliss R, Choi J, Fennell DA, Fry AM, Richards MW. Molecular mechanisms that underpin EML4-ALK driven cancers and their response to targeted drugs. Cell Mol Life Sci. 2016;73:1209–1224. doi: 10.1007/s00018-015-2117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson FM, Petricoin Iii EF, Van Laere SJ, Bertucci F, Chu K, Fernandez SV, Mu Z, Alpaugh K, Pei J, Circo R, et al. Presence of anaplastic lymphoma kinase in inflammatory breast cancer. Springerplus. 2013;2:497. doi: 10.1186/2193-1801-2-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuitty E, Zhang W, Hendrickson H, Tio FO, Jagirdar J, Olsen R, Cagle PT. Lung adenocarcinoma biomarker incidence in Hispanic versus non-Hispanic white patients. Arch Pathol Lab Med. 2014;138:390–394. doi: 10.5858/arpa.2013-0225-OA. [DOI] [PubMed] [Google Scholar]
- 33.Sampson J, Richards MW, Choi J, Fry AM, Bayliss R. Phase-separated foci of EML4-ALK facilitate signalling and depend upon an active kinase conformation. EMBO Rep. 2021;22:e53693. doi: 10.15252/embr.202153693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Li Y, Zhang H, Shi R, Zhang Z, Liu H, Chen J. EML4-ALK-mediated activation of the JAK2-STAT pathway is critical for non-small cell lung cancer transformation. BMC Pulm Med. 2021;21:190. doi: 10.1186/s12890-021-01553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Li G, Zhao L, Pan F, Qiang J, Han S. Blocking the PI3K pathway enhances the efficacy of ALK-targeted therapy in EML4-ALK-positive nonsmall-cell lung cancer. Tumour Biol. 2014;35:9759–9767. doi: 10.1007/s13277-014-2252-y. [DOI] [PubMed] [Google Scholar]
- 36.Takezawa K, Okamoto I, Nishio K, Jänne PA, Nakagawa K. Role of ERK-BIM and STAT3-survivin signaling pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive lung cancer. Clin Cancer Res. 2011;17:2140–2148. doi: 10.1158/1078-0432.CCR-10-2798. [DOI] [PubMed] [Google Scholar]
- 37.Ducray SP, Natarajan K, Garland GD, Turner SD, Egger G. The transcriptional roles of ALK fusion proteins in tumorigenesis. Cancers (Basel) 2019;11:1074. doi: 10.3390/cancers11081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao H, Shi L, Zhou A, Li H, Gai F, Huang Z, Che N, Liu Z. Distribution of EML4-ALK fusion variants and clinical outcomes in patients with resected non-small cell lung cancer. Lung Cancer. 2020;149:154–161. doi: 10.1016/j.lungcan.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, Meder L, Lovly CM, Heukamp LC, Pao W, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–4690. doi: 10.1158/1078-0432.CCR-11-3260. [DOI] [PubMed] [Google Scholar]
- 40.Maus MK, Stephens C, Zeger G, Grimminger PP, Huang E. Identification of novel variant of EML4-ALK fusion gene in NSCLC: Potential benefits of the RT-PCR method. Int J Biomed Sci. 2012;8:1–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Maus MK, Desai SJ, Beckett LA, Stephens C, Huang E, Hsiang J, Zeger G, Danenberg KD, Astrow SH, Gandara DR. Large-scale screening and molecular characterization of EML4-ALK fusion variants in archival non-small-cell lung cancer tumor specimens using quantitative reverse transcription polymerase chain reaction assays. J Thorac Oncol. 2014;9:18–25. doi: 10.1097/JTO.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 42.Cha YJ, Kim HR, Shim HS. Clinical outcomes in ALK-rearranged lung adenocarcinomas according to ALK fusion variants. J Transl Med. 2016;14:296. doi: 10.1186/s12967-016-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SS, Nagasaka M, Zhu VW, Ou SI. Going beneath the tip of the iceberg. Identifying and understanding EML4-ALK variants and TP53 mutations to optimize treatment of ALK fusion positive (ALK+) NSCLC. Lung Cancer. 2021;158:126–136. doi: 10.1016/j.lungcan.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Qin Z, Sun H, Yue M, Pan X, Chen L, Feng X, Yan X, Zhu X, Ji H. Phase separation of EML4-ALK in firing downstream signaling and promoting lung tumorigenesis. Cell Discov. 2021;7:33. doi: 10.1038/s41421-021-00270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel M, Malhotra J, Jabbour SK. Examining EML4-ALK variants in the clinical setting: The next frontier? J Thorac Dis. 2018;10((Suppl 33)):S4104–S4107. doi: 10.21037/jtd.2018.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider F, Dacic S. Histopathologic and molecular approach to staging of multiple lung nodules. Transl Lung Cancer Res. 2017;6:540–549. doi: 10.21037/tlcr.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panico F, Rizzi F, Fabbri LM, Bettuzzi S, Luppi F. Clusterin (CLU) and lung cancer. Adv Cancer Res. 2009;105:63–76. doi: 10.1016/S0065-230X(09)05004-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Wu L, Xu Y, Zhang B, Wu X, Wang Y, Pang Z. Trends in the incidence rate of lung cancer by histological type and gender in Sichuan, China, 1995–2015: A single-center retrospective study. Thorac Cancer. 2018;9:532–541. doi: 10.1111/1759-7714.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–1780. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aydemirli MD, van Eendenburg JDH, van Wezel T, Oosting J, Corver WE, Kapiteijn E, Morreau H. Targeting EML4-ALK gene fusion variant 3 in thyroid cancer. Endocr Relat Cancer. 2021;28:377–389. doi: 10.1530/ERC-20-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akimoto E, Tokunaga M, Sato R, Yoshida A, Naito Y, Yamashita R, Kinoshita T, Kuwata T. Gastric mesenchymal tumor with smooth muscle differentiation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion. Pathol Int. 2021;71:707–711. doi: 10.1111/pin.13154. [DOI] [PubMed] [Google Scholar]
- 52.Ferrara MG, Di Noia V, D'Argento E, Vita E, Damiano P, Cannella A, Ribelli M, Pilotto S, Milella M, Tortora G, Bria E. Oncogene-addicted non-small-cell lung cancer: Treatment opportunities and future perspectives. Cancers (Basel) 2020;12:1196. doi: 10.3390/cancers12051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohba T, Toyokawa G, Osoegawa A, Hirai F, Yamaguchi M, Taguchi K, Seto T, Takenoyama M, Ichinose Y, Sugio K. Mutations of the EGFR, K-ras, EML4-ALK, and BRAF genes in resected pathological stage I lung adenocarcinoma. Surg Today. 2016;46:1091–1098. doi: 10.1007/s00595-015-1295-z. [DOI] [PubMed] [Google Scholar]
- 54.Guo Y, Ma J, Lyu X, Liu H, Wei B, Zhao J, Fu S, Ding L, Zhang J. Non-small cell lung cancer with EML4-ALK translocation in Chinese male never-smokers is characterized with early-onset. BMC Cancer. 2014;14:834. doi: 10.1186/1471-2407-14-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin C, Shi X, Yang S, Zhao J, He Q, Jin Y, Yu X. Comparison of ALK detection by FISH, IHC and NGS to predict benefit from crizotinib in advanced non-small-cell lung cancer. Lung Cancer. 2019;131:62–68. doi: 10.1016/j.lungcan.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Teixidó C, Karachaliou N, Peg V, Gimenez-Capitan A, Rosell R. Concordance of IHC, FISH and RT-PCR for EML4-ALK rearrangements. Transl Lung Cancer Res. 2014;3:70–74. doi: 10.3978/j.issn.2218-6751.2014.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pekar-Zlotin M, Hirsch FR, Soussan-Gutman L, Ilouze M, Dvir A, Boyle T, Wynes M, Miller VA, Lipson D, Palmer GA, et al. Fluorescence in situ hybridization, immunohistochemistry, and next-generation sequencing for detection of EML4-ALK rearrangement in lung cancer. Oncologist. 2015;20:316–322. doi: 10.1634/theoncologist.2014-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayani J, Squire JA. Fluorescence in situ hybridization (FISH) Curr Protoc Cell Biol. 2004 doi: 10.1002/0471143030.cb2204s23. Chapter 22: Unit 22.4. [DOI] [PubMed] [Google Scholar]
- 59.Querido E, Dekakra-Bellili L, Chartrand P. RNA fluorescence in situ hybridization for high-content screening. Methods. 2017;126:149–155. doi: 10.1016/j.ymeth.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Liu L, Zhan P, Zhou X, Song Y, Zhou X, Yu L, Wang J. Detection of EML4-ALK in lung adenocarcinoma using pleural effusion with FISH, IHC, and RT-PCR methods. PLoS One. 2015;10:e0117032. doi: 10.1371/journal.pone.0117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Zhang J, Gao G, Li X, Zhao C, He Y, Su C, Zhang S, Chen X, Zhang J, et al. EML4-ALK fusion detected by RT-PCR confers similar response to crizotinib as detected by FISH in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2015;10:1546–1552. doi: 10.1097/JTO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 62.Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed. 2013;98:236–238. doi: 10.1136/archdischild-2013-304340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hume S, Nelson TN, Speevak M, McCready E, Agatep R, Feilotter H, Parboosingh J, Stavropoulos DJ, Taylor S, Stockley TL, Canadian College of Medical Geneticists (CCMG) CCMG practice guideline: Laboratory guidelines for next-generation sequencing. J Med Genet. 2019;56:792–800. doi: 10.1136/jmedgenet-2019-106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, Temple-Smolkin RL, Voelkerding KV, Nikiforova MN. Guidelines for validation of next-generation sequencing-based oncology panels: A joint consensus recommendation of the association for molecular pathology and college of American pathologists. J Mol Diagn. 2017;19:341–365. doi: 10.1016/j.jmoldx.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma PC. Personalized targeted therapy in advanced non-small cell lung cancer. Cleve Clin J Med. 2012;79((Electronic Suppl 1)):eS56–eS60. doi: 10.3949/ccjm.79.s2.12. [DOI] [PubMed] [Google Scholar]
- 66.Fallet V, Toper C, Antoine M, Cadranel J, Wislez M. Management of crizotinib, a new individualized treatment. Bull Cancer. 2012;99:787–791. doi: 10.1684/bdc.2012.1604. (In French) [DOI] [PubMed] [Google Scholar]
- 67.Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, Liu M, Yuan Y. ALK inhibitors in the treatment of ALK positive NSCLC. Front Oncol. 2019;8:557. doi: 10.3389/fonc.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cameron LB, Hitchen N, Chandran E, Morris T, Manser R, Solomon BJ, Jordan V. Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer. Cochrane Database Syst Rev. 2022;1:CD013453. doi: 10.1002/14651858.CD013453.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Q, Hu H, Jiang F, Guo CY, Yang XW, Liu X, Kuang YK. Meta-analysis of incidence and risk of severe adverse events and fatal adverse events with crizotinib monotherapy in patients with ALK-positive NSCLC. Oncotarget. 2017;8:75372–75380. doi: 10.18632/oncotarget.18536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 71.Casaluce F, Sgambato A, Sacco PC, Palazzolo G, Maione P, Rossi A, Ciardiello F, Gridelli C. Resistance to crizotinib in advanced non-small cell lung cancer (NSCLC) with ALK rearrangement: Mechanisms, treatment strategies and new targeted therapies. Curr Clin Pharmacol. 2016;11:77–87. doi: 10.2174/1574884711666160502124134. [DOI] [PubMed] [Google Scholar]
- 72.Dhillon S, Clark M. Ceritinib: First global approval. Drugs. 2014;74:1285–1291. doi: 10.1007/s40265-014-0200-1. [DOI] [PubMed] [Google Scholar]
- 73.Spencer SA, Riley AC, Matthew A, Di Pasqua AJ. Brigatinib: Novel ALK inhibitor for non-small-cell lung cancer. Ann Pharmacother. 2019;53:621–626. doi: 10.1177/1060028018824578. [DOI] [PubMed] [Google Scholar]
- 74.Herden M, Waller CF. Alectinib. Recent Results Cancer Res. 2018;211:247–256. doi: 10.1007/978-3-319-91442-8_17. [DOI] [PubMed] [Google Scholar]
- 75.Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, Riely GJ, Ou SI, Clancy JS, Li S, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.2019.37.15_suppl.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yun MR, Kim DH, Kim SY, Joo HS, Lee YW, Choi HM, Park CW, Heo SG, Kang HN, Lee SS, et al. Repotrectinib exhibits potent antitumor activity in treatment-naïve and solvent-front-mutant ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2020;26:3287–3295. doi: 10.1158/1078-0432.CCR-19-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, Zhu VW, Ahn MJ, Camidge DR, Nguyen J, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 78.Revannasiddaiah S, Thakur P, Bhardwaj B, Susheela SP, Madabhavi I. Pulmonary adenocarcinoma: Implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J Thorac Dis. 2014;6((Suppl 5)):S502–S525. doi: 10.3978/j.issn.2072-1439.2014.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu Z, Wang X, Luo Y, Wei J, Zeng Z, Xiong Q, Cai J, Liu A. EGFR (p. G719A+L747V)/EML4-ALK co-alterations in lung adenocarcinoma with leptomeningeal metastasis responding to afatinib treatment: A case report. Onco Targets Ther. 2021;14:2823–2828. doi: 10.2147/OTT.S294635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rybarczyk-Kasiuchnicz A, Ramlau R, Stencel K. Treatment of brain metastases of non-small cell lung carcinoma. Int J Mol Sci. 2021;22:593. doi: 10.3390/ijms22020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada K, Araki M, Sakashita T, Ma B, Kanada R, Yanagitani N, Horiike A, Koike S, Oh-Hara T, Watanabe K, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine. 2019;41:105–119. doi: 10.1016/j.ebiom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, Jessop NA, Jiang GY, Le LP, Gowen K, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36:1199–1206. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dagogo-Jack I, Shaw AT. Crizotinib resistance: Implications for therapeutic strategies. Ann Oncol. 2016;27((Suppl 3)):iii42–iii50. doi: 10.1093/annonc/mdw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kunimasa K, Hirotsu Y, Kukita Y, Ueda Y, Sato Y, Kimura M, Otsuka T, Hamamoto Y, Tamiya M, Inoue T, et al. EML4-ALK fusion variant.3 and co-occurrent PIK3CA E542K mutation exhibiting primary resistance to three generations of ALK inhibitors. Cancer Genet. 2021:256–257. 131–135. doi: 10.1016/j.cancergen.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Kwon JH, Kim KJ, Sung JH, Suh KJ, Lee JY, Kim JW, Kim SH, Lee JO, Kim JW, Kim YJ, et al. Afatinib overcomes pemetrexed-acquired resistance in non-small cell lung cancer cells harboring an EML4-ALK rearrangement. Cells. 2019;8:1538. doi: 10.3390/cells8121538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 88.Shen J, Meng Y, Wang K, Gao M, Du J, Wang J, Li Z, Zuo D, Wu Y. EML4-ALK G1202R mutation induces EMT and confers resistance to ceritinib in NSCLC cells via activation of STAT3/Slug signaling. Cell Signal. 2022;92:110264. doi: 10.1016/j.cellsig.2022.110264. [DOI] [PubMed] [Google Scholar]
- 89.Guo F, Liu X, Qing Q, Sang Y, Feng C, Li X, Jiang L, Su P, Wang Y. EML4-ALK induces epithelial-mesenchymal transition consistent with cancer stem cell properties in H1299 non-small cell lung cancer cells. Biochem Biophys Res Commun. 2015;459:398–404. doi: 10.1016/j.bbrc.2015.02.114. [DOI] [PubMed] [Google Scholar]
- 90.Voena C, Varesio LM, Zhang L, Menotti M, Poggio T, Panizza E, Wang Q, Minero VG, Fagoonee S, Compagno M, et al. Oncogenic ALK regulates EMT in non-small cell lung carcinoma through repression of the epithelial splicing regulatory protein 1. Oncotarget. 2016;7:33316–33330. doi: 10.18632/oncotarget.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Mello RA, Liu DJ, Aguiar PN, Tadokoro H. EGFR and EML4-ALK updated therapies in non-small cell lung cancer. Recent Pat Anticancer Drug Discov. 2016;11:393–400. doi: 10.2174/1574892811666160803090944. [DOI] [PubMed] [Google Scholar]
- 92.Guo J, Shi J, Yao M, Jin Y, Liu D, Liu W, Wang K, Jiang D. A rare double ALK fusion variant EML4-ALK and CDK15-ALK in lung adenocarcinoma and response to crizotinib: A case report. Medicine (Baltimore) 2020;99:e22631. doi: 10.1097/MD.0000000000022631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laszlo A, Thotala D, Hallahan DE. Membrane phospholipids, EML4-ALK, and Hsp90 as novel targets in lung cancer treatment. Cancer J. 2013;19:238–246. doi: 10.1097/PPO.0b013e31829a68eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) Lung Cancer. 2019;137:113–122. doi: 10.1016/j.lungcan.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


