Abstract
Purpose
We assessed prenatal detection rates of congenital heart disease (CHD) and associations between maternal serum biomarkers and non-chromosomal CHD in singleton pregnancies.
Materials and Methods
This study was conducted as a secondary analysis of data obtained during a multicenter prospective cohort study that investigated the cost-effectiveness of prenatal testing for fetal aneuploidy. We analyzed the prenatal detection rate and accuracy for CHD screening via ultrasound during the second trimester, as well as associations between serum biomarkers and CHDs, in singleton newborns without chromosomal abnormalities.
Results
Among 6715 women, 142 (2.1%) newborns were born with CHDs, of which 67 (1.0%) newborns had major CHDs. The prenatal detection rate for all CHDs and major CHDs were 34.5% and 58.2%, respectively. After excluding isolated ventricular septal defects, the detection rate for critical CHDs was 85.9%. Women with low pregnancy-associated plasma protein A (PAPP-A) (<0.4 multiples of the median, MOM) face increased risks of non-chromosomal CHDs [adjusted odds ratio (aOR) 2.76; 95% confidence interval (CI) 1.36–5.13] and major CHDs (aOR 7.30; 95% CI 3.18–15.59), compared to those without CHDs. A higher inhibin A level (≥2.5 MOM; aOR 4.84; 95% CI 1.42–12.46) was associated with non-chromosomal major CHDs.
Conclusion
Ultrasonography performed during the second trimester by obstetricians detected over 85% of critical CHDs. Low maternal serum PAPP-A or high inhibin-A was associated with non-chromosomal CHDs. These results may contribute to an improvement in prenatal diagnosis of CHDs.
Keywords: Congenital heart disease, prenatal diagnosis ultrasonic, second-trimester screening, pregnancy-associated plasma protein-A, inhibin A
INTRODUCTION
A major purpose of fetal sonographic evaluation is to detect congenital abnormalities to optimize counseling and care of the fetus. Congenital heart disease (CHD) is the most common birth defect (4 to 13/1000 live births) with significant morbidity, and it is considered to be the leading cause of infant death among congenital anomalies. Therefore, screening during the second trimester for CHD via ultrasonography is very important.1 Prenatal diagnosis of critical CHD prior to planned neonatal cardiac surgery could reduce the risk of death from cardiovascular compromise.2 In addition, the importance of interdisciplinary parental counseling for prenatally diagnosed congenital disease has been emphasized, especially for CHD.3,4 A recent study reported a significant increase in the possibility of delivery after severe CHD has been diagnosed prenatally and addressed via a multidisciplinary approach.4
Many risk factors have been identified for CHD, including family history, teratogen exposure, known aneuploidy, and maternal diabetes, and more than 90% of cases occur in low-risk patients.5 The guidelines for fetal cardiac screening during second-trimester anatomical ultrasound were developed by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) to improve antenatal detection rates and standardize fetal cardiac screening examinations.6 A nationwide study in Denmark demonstrated that mortality from major CHDs decreased significantly when general prenatal heart screening was conducted rather than selective prenatal heart screening.7 Recent evidence suggests an association between abnormal placentation and CHDs in fetuses.8,9 Preeclampsia and CHDs have been found to exhibit positive associations with reduced levels of angiogenic biomarkers and increased levels of anti-angiogenic biomarkers in maternal serum or umbilical cord blood, with low placental weight, with abnormal umbilical cord insertions, and with abnormal placental perfusion assessed by uterine artery pulsatile index.8,9 However, further studies are required to elucidate the association between abnormal placentation markers and CHDs and to investigate an appropriate ultrasonographic screening protocol of the fetal heart during the second trimester.
Second-trimester prenatal screening had been performed by obstetricians in secondary or tertiary centers in South Korea. The goal of the present analysis was to evaluate detection rates and accuracy of ultrasound for CHD in the second trimester and associations for maternal serum biomarkers with risk of CHD without chromosomal abnormalities.
MATERIALS AND METHODS
Study population
This study was designed as a secondary analysis of a multicenter prospective cohort study in South Korea carried out from December 2016 to April 2018 at 12 different secondary or tertiary healthcare centers. The aforementioned study had assessed the cost-effectiveness of prenatal testing in women who were screened for fetal aneuploidy. The protocol of this prospective observational cohort study has been published.10 Pregnant women (<24 weeks) who had received counselling for fetal aneuploidy testing were invited to enroll in the original study. The participants were stratified according to the risk of fetal chromosomal abnormalities based on previous pregnancy outcomes, maternal demographic findings, maternal serum screening (MSS) biomarkers, and fetal ultrasonographic findings, including fetal nuchal translucency (NT), sonographic soft markers, and any suspicious congenital anomalies. The pregnancy outcomes were recorded, including gestational age at delivery, neonatal birth weight, the presence of congenital anomalies, and postnatal cytogenetics.
This secondary analysis included women with singleton pregnancies who underwent ultrasonography screening during the second trimester. Women with spontaneous abortions, terminations, fetal death in utero, and follow-up loss were excluded. However, patients with terminations or those lost to follow-up after a prenatal diagnosis of major congenital anomaly were included in the final analysis.
Neonatal outcomes and congenital anomalies
Newborn outcomes were determined via physical examination at birth or through any form of genetic testing and imaging. Newborns with a normal physical examination were considered to be euploid in the absence of genetic testing. Congenital anomalies were all considered as congenital disease as defined in the Korean Standard Classification of Disease, 7th edition (KCD-7, codes Q00–Q99), which have been modified from the ICD-10. CHDs were confirmed via postnatal echocardiography after birth to newborns suspected of having CHD based on prenatal ultrasound or postnatal diagnosis. CHD requiring surgeries or regular follow-up, such as hypoplastic left heart syndrome, atrioventricular septal defect (VSD), double outlet right ventricle, double inlet ventricle, tetralogy of Fallot, transposition of the great arteries, situs inversus, Ebstein anomaly, pulmonary or aortic valve stenosis or atresia, mitral or tricuspid valve stenosis or atresia, abnormal pulmonary venous return, interrupted aortic arch, coarctation of the aorta, ectopia cordis, rhabdomyoma, cardiomegaly, and VSD were defined as the major CHDs in this study. Isolated atrial septal defects were excluded from consideration for major CHDs, and patent ductus arteriosus and persistent foramen ovale were not considered as CHDs.
Prenatal sonographic markers for congenital defect
Information was requested for all pregnant women regarding NT ultrasound, aneuploidy screening, and diagnostic testing for chromosomal abnormalities. For fetal NT, the measurements performed between 11 and 13+6 gestational weeks were selected. Sonographic soft markers for CHDs, included choroid plexus cyst, echogenic bowel, echogenic intracardiac focus (EIF), short long bone, short or absence of nasal bone, increased nuchal fold, pyelectasis, single umbilical artery, and persistent right umbilical vein (PRUV).
Fetal aneuploidy screening, and diagnostic testing
The screening tests for fetal aneuploidy included MSS or cell free deoxyribonucleic acid (cfDNA) screening. MSS included the Dual test [pregnancy associated plasma protein-A (PAPP-A), total or free beta human chorionic gonadotropin (hCG)], Triple test [alpha fetoprotein (AFP), unconjugated estriol (uE3), total hCG], Quad test (AFP, total hCG, uE3, inhibin A), integrated test, and sequential test. The cfDNA screening analyzed for trisomies 21, 18, and 13 and sex chromosomal abnormality. The prenatal diagnostic tests included chorionic villus sampling and amniocentesis. When the screening test showed a positive result, counseling was performed on further evaluations.
Statistical analysis
The detection rate for CHD was calculated as the number of CHDs suspected prenatally (live-born or terminated or follow-up loss) divided by sum of CHD suspected prenatally and CHD not diagnosed in prenatal ultrasonogram. We calculated the sensitivity, specificity, positive prediction value, and negative predictive value of prenatal screening ultrasound examinations in the second trimester for all CHDs.
After excluding chromosomal abnormalities, we compared maternal baseline characteristics, sonographic findings, and MSS according to the severity of CHDs. We categorized the maternal serum biomarkers according to multiples of the median (MOM) values for each of the markers as ≤2.5, ≤5.0, ≥95.0, and ≥97.5th percentiles. For each biomarker’s cut-off, the associations between maternal serum biomarkers and non-chromosomal CHD were presented as odds ratios from logistic regression analysis before and after adjustment for confounders.
We used a chi-squared test for categorical variables and analysis of variance with Bonferroni correction for continuous variables. A p value less than 0.05 was considered significant including for multiple testing. Statistical analysis was conducted using R package software (version 4.1.0; The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
The Institutional Review Boards of each participating institution, including College of Medicine, at the Catholic University of Korea (KC16ONMI0989), approved the original study of the KPDS, as was previously stated.10 We followed the ethical standards for human experimentation provided by the Declaration of Helsinki and obtained written consents from the participants.
RESULTS
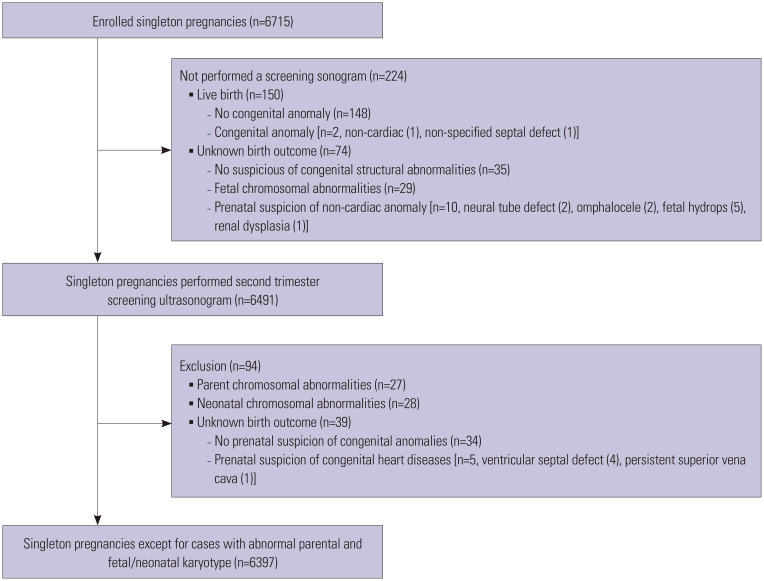
A total of 6715 singleton pregnant women was enrolled in this original study. Among them, 6491 women underwent screening sonogram in the second trimester. After further excluding 95 women [27 parental chromosomal abnormalities, 28 neonatal chromosomal abnormalities, 5 unknown birth outcomes with suspicion of CHDs (4 VSD, 1 left superior vena cava), and 35 follow-up loss], a total of 6397 women was included in the final analysis (Fig. 1).
Fig. 1. Flow chart of study population.
A CHD was identified in 142 cases (2.2%), and 67 newborns (1.1%) were considered to have major CHDs postnatally. The detection rate of any CHDs was 34.5% [95% confidence interval (CI) 26.7–42.9], and that of major CHDs was 58.2% (95% CI 45.5–70.2) for a second trimester screening ultrasonogram. After excluding isolated VSD (n=32), the detection rate of critical CHDs reached 85.7% (95% CI 69.7–95.2). The sensitivity, specificity, and positive and negative predictive values for major CHDs in women who underwent screening ultrasonogram in the second trimester are listed in Table 1. Detection rates for mitral stenosis (0%, 95% CI 0–97.5%) and pulmonary stenosis (PS) (25.0%, 95% CI 0.6–80.6) were poor. The accompanying chromosomal abnormalities, prenatal suspicion, and birth outcomes for major CHD except isolated VSD are summarized in Supplementary Table 1 (only online).
Table 1. Detection Rates of CHDs by a Second Trimester Screening Ultrasonogram.
| CHD | Fetus with CHD (n) | CHD detected prenatally (n) | Sensitivity [% (95% CI)] | Specificity [% (95% CI)] | PPV [% (95% CI)] | NPV [% (95% CI)] | |
|---|---|---|---|---|---|---|---|
| All CHD (any suspicious) | 142 | 49 | 34.5 (26.7–42.9) | 98.8 (98.5–99.0) | 38.9 (30.3–48.0) | 98.5 (98.2–98.8) | |
| Major CHD | 67 | 39 | 58.2 (45.5–70.2) | 99.2 (99.0–99.4) | 44.8 (34.1–55.9) | 99.6 (99.4–99.7) | |
| Isolated VSD | 32 | 9 | 28.1 (13.7–46.7) | 99.3 (99.1–99.5) | 17.0 (0.8–29.8) | 99.6 (99.5–99.8) | |
| Critical CHD | 35 | 29 | 85.7 (69.7–95.2) | 100.0 (99.9–100.0) | 90.9 (75.7–98.1) | 99.9 (99.8–100.0) | |
| Hypoplastic left heart syndrome | 3 | 3 | 100.0 (29.2–100.0) | 100.0 (99.9–100.0) | 100.0 (29.2–100.0) | 100.0 (99.9–100.0) | |
| Atrioventricular septal defect | 5 | 4 | 80.0 (28.4–99.5) | 100.0 (99.9–100.0) | 100.0 (39.8–100.0) | 100.0 (99.9–100.0) | |
| Double outlet right ventricle | 4 | 4 | 100.0 (39.8–100.0) | 100.0 (99.9–100.0) | 100.0 (39.8–100.0) | 100.0 (99.9–100.0) | |
| Pulmonary atresia | 2 | 2 | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | |
| Tetralogy of Fallot | 5 | 4 | 80.0 (28.4–99.5) | 100.0 (99.9–100.0) | 100.0 (39.8–100.0) | 100.0 (99.9–100.0) | |
| Pulmonary stenosis | 4 | 1 | 25.0 (0.6–80.6) | 100.0 (99.9–100.0) | 33.3 (0.8–90.6) | 100.0 (99.9–100.0) | |
| Transposition of the great arteries | 2 | 2 | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | |
| Situs inversus | 2 | 2 | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | |
| Abnormal pulmonary venous return | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Aortopulmonary septal defect | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Interrupted aortic arch | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Ebstein anomaly | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Ectopic cordis | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Rhabdomyoma | 1 | 1 | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | 100.0 (25.0–100.0) | 100.0 (99.9–100.0) | |
| Mitral stenosis | 1 | 0 | 0 (0–97.5) | 100.0 (99.9–100.0) | Not available | 100.0 (99.9–100.0) | |
| Cardiomegaly | 2 | 2 | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | 100.0 (15.8–100.0) | 100.0 (99.9–100.0) | |
CHD, congenital heart disease; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; VSD, ventricular septal defect.
The maternal baseline characteristics of the women with no confirmed neonatal chromosomal abnormality are presented in Table 2. Women with non-chromosomal CHDs had a lower gestational age at delivery, birthweight, and higher body mass index than those in the non-cardiac defect group. Women with non-chromosomal CHDs had significantly higher rates of a low education level, lower house income, overt diabetes, gestational diabetes, and epilepsy, compared to the non-cardiac defect group (all p<0.05), but there was no difference between major CHDs and the control group (all p>0.05). Women with maternal CHDs had a higher rate of neonatal major CHDs than the control group. Preterm birth (20.2% vs. 6.3%, p<0.001) and placental abruption (3.1% vs. 0.4%, p<0.001) occurred more frequently in the CHDs group than in the control group. However, these differences were not found between the control group and the major CHD group (all p>0.05).
Table 2. The Maternal Baseline Characteristics of Women with or without CHD of Their Fetus in the Study Population.
| Characteristics | No CHD (n=6267) | Any CHD (n=130) | p value | Minor CHD (n=71) | Major CHD (n=59) | p value* | |
|---|---|---|---|---|---|---|---|
| Maternal age, yr | 33.6±3.9 | 33.8±3.8 | 0.634 | 34.5±3.9 | 32.9±3.5 | 0.045c | |
| Gestational age, at delivery, wk | 38.9±1.7 | 37.2±4.3 | <0.001 | 37.6±3.3 | 36.7±5.3 | <0.001a,b | |
| Cesarean section | 2743 (43.9) | 72 (55.4) | 0.012 | 41 (57.7) | 31 (52.5) | 0.028 | |
| Birth weight, g | 3177.8±458.5 | 2926.9±895.7 | 0.002 | 3025.0±806.8 | 2795.5±995.5 | <0.001a,b | |
| Gestational age, at sonogram, wk | 21.7±1.7 | 21.5±1.9 | 0.200 | 21.5±1.6 | 21.5±2.2 | 0.440 | |
| Assisted reproductive technique | 760 (12.2) | 14 (10.9) | 0.772 | 8 (11.4) | 6 (10.3) | 0.896 | |
| Nulliparity | 3812 (60.8) | 74 (56.9) | 0.417 | 36 (49.3) | 38 (64.4) | 0.187 | |
| BMI at pre-pregnancy, kg/m2 | 21.4±3.0 | 21.9±3.5 | 0.104 | 22.0±3.6 | 21.7±3.5 | 0.232 | |
| Married | 6096 (97.3) | 125 (96.2) | 0.608 | 69 (97.2) | 56 (94.9) | 0.539 | |
| Low education level† | 447 (7.1) | 18 (13.8) | 0.006 | 13 (18.3) | 5 (8.5) | 0.001a | |
| Low family income level‡ | 640 (10.2) | 26 (20.0) | 0.001 | 16 (22.5) | 10 (16.9) | 0.001a | |
| Maternal medical disease | |||||||
| Overt diabetes | 90 (1.4) | 6 (4.6) | 0.010 | 4 (5.6) | 2 (3.4) | 0.007a | |
| GDM | 397 (6.3) | 20 (15.4) | <0.001 | 15 (21.1) | 5 (8.5) | <0.001a,c | |
| Hypertension | 86 (1.4) | 4 (3.1) | 0.209 | 2 (2.8) | 2 (3.4) | 0.254 | |
| Epilepsy | 11 (0.2) | 2 (1.5) | 0.015 | 2 (2.8) | 0 (0.0) | <0.001a,c | |
| CHD | 21 (0.3) | 3 (2.3) | 0.004 | 1 (1.4) | 2 (3.4) | <0.001b | |
| Pregnancy outcome | |||||||
| Preterm birth | 395 (6.3) | 26 (20.2) | <0.001 | 18 (25.4) | 8 (13.8) | <0.001a,c | |
| Fetal growth restriction§ | 216 (3.5) | 9 (7.0) | 0.054 | 3 (4.3) | 6 (10.3) | 0.017b | |
| Placenta abruptio | 28 (0.4) | 4 (3.1) | <0.001 | 3 (4.3) | 1 (1.7) | <0.001a | |
CHD, congenital heart disease; BMI, body mass index; GDM, gestational diabetes mellitus.
The data are presented as a mean±standard deviation or number (%).
*Tukey post-hoc analysis was used to assess the following: a. significant differences between No CHD and Minor CHD, b. significant differences between No CHD and Major CHD, and c. significant differences between Minor CHD and Major CHD; †Low education level was defined as not having graduated from high school; ‡Low household income was defined as a monthly income of less than 3 million Korean won; §Fetal growth restriction was defined as birth weight less than the 5th percentile.
Table 3 shows the sonographic findings and MSS according to individual CHDs. A thickened NT (≥2.5 mm and ≥3.0 mm) and an increased risk of neural tube defect (NTD) in MSS were significantly higher in women with any and with major CHDs, compared to women without CHDs. Sonographic soft markers including EIF (4.6% vs. 0.5%), pyelectasis (4.6%, vs. 1.7%), and single umbilical artery (3.8% vs. 0.4%) were more frequently observed in women with any CHDs than in the control group (all p<0.05, respectively). Women with major CHD had higher frequency of the increased nuchal fold (3.4% vs. 0.7%), pyelectasis (6.8% vs. 1.7%), single umbilical artery (5.1% vs. 0.4%), and PRUV (1.7% vs. 0.1%) than women in the control group (all p<0.05). EIF in minor CHDs (8.5%) was significantly higher than that in the control group (0.5%, p<0.001), and there were no significant differences between the major CHD group and the control group (0.0% vs. 0.5%, p>0.05).
Table 3. The Sonographic Soft Markers and Maternal Serum Screening Markers according to the Presence of CHDs in the Study Population.
| Characteristics | No CHD (n=6267) | Any CHD (n=130) | p value | Minor CHD (n=71) | Major CHD (n=59) | p value* | |
|---|---|---|---|---|---|---|---|
| Nuchal translucency (n=6011) | |||||||
| ≥2.5 mm | 203/5890 (3.4) | 10/121 (8.3) | 0.010 | 3/67 (4.5) | 7/54 (13.0) | 0.001b | |
| ≥3.0 mm | 74/5890 (1.3) | 6/121 (5.0) | 0.002 | 2/67 (3.0) | 4/54 (7.4) | 0.002b | |
| ≥3.5 mm | 38/5890 (0.6) | 2/121 (1.7) | 0.433 | 0 (0) | 2/54 (3.7) | 0.018b,c | |
| Maternal serum marker | |||||||
| High risk for down syndrome (n=5078) | 411/4974 (8.3) | 13/104 (12.5) | 0.172 | 7/58 (12.1) | 6/45 (13.0) | 0.298 | |
| High risk for edward syndrome (n=4883) | 26/4782 (0.5) | 1/101 (1.0) | 0.549 | 0 (0) | 1/45 (2.2) | 0.273 | |
| High risk for NTD (n=5454) | 32/5347 (0.6) | 5/107 (4.7) | <0.001 | 1/62 (1.6) | 4/45 (8.9) | <0.001b,c | |
| Sonographics soft marker | |||||||
| Any soft marker | 324 (5.2) | 21 (16.2) | <0.001 | 12 (16.9) | 9 (15.3) | <0.001a,b | |
| Choroid plexus cyst | 111 (1.8) | 2 (1.6) | 0.922 | 2 (2.9) | 0 (0) | 0.463 | |
| Echogenic intracardiac foci | 33 (0.5) | 6 (4.6) | <0.001 | 6 (8.5) | 0 (0.0) | <0.001a,c | |
| Nuchal fold | 46 (0.7) | 2 (1.5) | 0.590 | 0 (0) | 2 (3.4) | 0.048b | |
| Pyelectasis | 105 (1.7) | 6 (4.6) | 0.028 | 2 (2.8) | 4 (6.8) | 0.009b | |
| Single umbilical artery | 25 (0.4) | 5 (3.8) | <0.001 | 2 (2.8) | 3 (5.1) | <0.001a,b | |
| Persistent right umbilical vein | 7 (0.1) | 1 (0.8) | 0.398 | 0 (0) | 1(1.7) | 0.003b,c | |
| Echogenic bowel | 43 (0.7) | 1 (0.8) | 0.853 | 0 (0) | 1 (1.7) | 0.505 | |
| Absence or short nasal bone | 5 (0.1) | 0 (0.0) | 1.000 | 0 (0) | 0 (0) | 0.949 | |
| Short long bone | 11 (0.2) | 0 (0.0) | 1.000 | 0 (0) | 0 (0) | 0.892 | |
CHD, congenital heart disease; NTD, neural tube defect.
Data are presented as numbers (%).
*Tukey post-hoc analysis was used to assess the following: a. significant differences between No CHD and Minor CHD, b. significant differences between No CHD and Major CHD, and c. significant differences between Minor CHD and Major CHD.
Correlations between the maternal serum biomarkers and non-chromosomal CHD are presented in Table 4. Women with CHD had significantly lower PAPP-A, compared to those in the non-cardiac defect group, yielding an adjusted odds ratio (aOR) of 5.21 (95% CI 2.34–10.37) for the less than 2.5th percentile (0.311 MOM), 2.92 (95% CI 1.43–5.42) for less than the 5th percentile (0.399 MOM), and 2.76 (95% CI 1.36–5.13) for less than 0.4 MOM, respectively. Also, women with a lower level of first trimester PAPP-A had also a higher rate of major CHDs [aOR 12.77 (95% CI 4.94–29.23) for less than 2.5th percentile, aOR 7.91 (95% CI 3.40–16.95) for less than 5th percentile, and aOR 7.30 (95% CI 3.15–15.59) for less than 0.4 MOM, respectively]. In the second trimester, a high maternal serum AFP more than the 97.5th percentile was associated with increased risks of CHDs [aOR 2.80 (95% CI 1.07–6.07)] and major CHDs [aOR 4.51 (95% CI 1.33–11.47)]. Women with major CHDs had significantly higher inhibin-A levels, compared to those in the non-cardiac defect group, yielding an aOR of 4.48 (95% CI 1.32–11.53) for more than the 97.5th percentile (2.460 MOM) and 4.84 (95% CI 1.42–12.46) for more than 2.5 MOM, respectively. Other maternal serum analytes were not associated with non-chromosomal CHDs.
Table 4. Correlations Between Maternal Serum Analytes and CHDs in the Study Population.
| Maternal serum analyte (percentile) | No CHD (n=6267) | All CHD (n=130) | Odds ratio (95% CI) | Major CHD (n=59) | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||
| PAPP-A | 4002 | 82 | 32 | |||||
| ≤2.5 (0.311 MOM) | 90 (2.2) | 9 (11.0) | 5.36 (2.34–10.52) | 5.21 (2.34–10.37) | 7 (21.9) | 12.17 (4.75–27.47) | 12.77 (4.94–29.23) | |
| ≤5.0 (0.399 MOM) | 191 (4.8) | 11 (13.4) | 3.09 (1.53–5.69) | 2.92 (1.43–5.42) | 9 (28.1) | 7.81 (3.38–16.56) | 7.91 (3.40–16.95) | |
| ≤0.4 MOM | 204 (5.1) | 11 (13.4) | 2.88 (1.43–5.31) | 2.76 (1.36–5.13) | 9 (28.1) | 7.29 (3.16–15.43) | 7.30 (3.15–15.59) | |
| AFP | 5363 | 108 | 45 | |||||
| ≥95.0 (1.737 MOM) | 257 (4.8) | 8 (7.4) | 1.59 (0.70–3.10) | 1.44 (0.6–2.94)* | 4 (8.9) | 1.45 (0.35–4.01) | 2.05 (0.61–5.18)* | |
| ≥97.5 (1.977 MOM) | 123 (2.3) | 7 (6.5) | 2.95 (1.22–6.05) | 2.80 (1.07–6.07) | 4 (8.9) | 3.09 (0.74–8.65) | 4.51 (1.33–11.47) | |
| ≥2.0 MOM | 117 (2.2) | 7 (6.5) | 3.11 (1.29–6.37) | 2.95 (1.13–6.40) | 4 (8.9) | 3.25 (0.78–9.11) | 4.75 (1.40–12.11) | |
| ≥2.5 MOM | 34 (0.6) | 5 (4.6) | 7.61 (2.57–18.20) | 6.76 (1.95–17.89) | 4 (8.9) | 11.14 (2.61–32.58) | 16.93 (4.84–45.79) | |
| Inhibin A | 4739 | 98 | 41 | |||||
| ≥95.0 (2.067 MOM) | 220 (4.6) | 6 (6.1) | 1.34 (0.52–2.84) | 1.34 (0.52–2.88) | 4 (9.8) | 2.22 (0.66–5.60) | 2.32 (0.69–5.90)* | |
| ≥97.5 (2.460 MOM) | 107 (2.3) | 4 (4.1) | 1.84 (0.56–4.51) | 0.75 (0.52–4.33) | 4 (9.8) | 4.68 (1.38–11.93) | 4.48 (1.32–11.53) | |
| ≥2.5 MOM | 100 (2.1) | 4 (4.1) | 1.97 (0.60–4.84) | 1.89 (0.57–4.70) | 4 (9.8) | 5.02 (1.48–12.81) | 4.84 (1.42–12.46) | |
CHD, congenital heart disease; PAPP-A, pregnancy associated plasma protein-A; MOM, multiples of the median; AFP, alpha fetoprotein; CI, confidence interval.
Data are presented as numbers (%).
*Adjusted for maternal age, increased nuchal translucency more than 25 mm, and any soft marker.
In women with fetal isolated VSD or PS, low serum PAPP-A (≤0.4 MOM, 28.6% vs. 5.1%), high AFP (≥2.5 MOM, 6.9% vs. 0.6%), and high inhibin-A (≥2.5 MOM, 11.5% vs. 2.1%) were associated with an increased risk of fetal CHD, yielding ORs of 7.45 (95% CI 2.63–18.53), 11.61 (95% CI 1.83–40.92), and 6.05 (95% CI 1.42–17.75), respectively. Also, in women with major fetal CHDs, except isolated VSD or PS, low serum PAPP-A (≤0.4 MOM, 27.3% vs. 5.1%) and high AFP (≥2.5 MOM, 12.5% vs. 0.6%) were associated with an increased risk of fetal CHD, yielding ORs of 6.98 (95% CI 1.52–24.34) and 22.39 (95% CI 3.43–84.31), respectively. However, in women with major fetal CHDs excluding isolated VSD and PS, high maternal serum inhibin-A (≥2.5 MOM, 6.7% vs. 2.1%, p=0.745) was not associated with the risk of fetal CHD.
DISCUSSION
This study found significant associations of both lower maternal serum PAPP-A in the first trimester and higher maternal serum AFP in second trimester with an increased risk of non-chromosomal CHD. Lower serum PAPP-A or higher AFP, as well as higher inhibin-A, were significantly more common in women with fetal major non-chromosomal CHD than those without fetal CHDs.
The development processes of the heart and placenta share the same regulatory pathways, and placental dysfunction is considered to be a contributor to the development of CHD.11,12 PAPP-A is an angiogenic biomarker produced mainly in placental trophoblasts, and low PAPP-A is associated with chromosomal anomalies.13,14 Previous studies have indicated that low maternal serum PAPP-A is associated with an increased risk of fetal CHD.15,16 This study also found that women with low PAPP-A (≤0.4 MOM) in the first trimester had an increased risk of fetal non-chromosomal CHDs [aOR 2.76 (95% CI 1.36–5.13)], and even a greater risk of major fetal CHDs [aOR 7.30 (95% CI 3.15–15.59)]. More than one-fourth (28.1%) of major CHDs occurred in women with low PAPP-A levels less than 0.4 MOM.
AFP is a major protein in fetal serum, and it increases in maternal serum for women with fetal integument defects.17 Data on the association between maternal serum AFP and fetal CHD, however, are discrepant across studies.18,19,20 In this study, women with a high AFP (>2.5 MOM, that is, cutoff for risk of NTD) had six-fold higher odds of CHD and 16-fold higher odds of major CHDs even without fetal integument defects. This finding is consistent with the study of Jelliffe-Pawlowski, et al.19 We suspect that women with high serum AFP (≥2.5 MOM) may need detailed ultrasound examination not only for NTDs but also for CHDs.
Inhibin A is a glycoprotein produced by the placenta and is associated with fetal Down syndrome.21 Previous studies have reported that an increase of inhibin A is associated with adverse pregnancy outcomes, such as preeclampsia, fetal growth restriction, and preterm birth.22,23 In contrast, there is limited information on the association between congenital malformation and an increased level of inhibin-A.23 This study found an association between maternal serum inhibin-A in the second trimester with major fetal CHDs, but not with overall fetal cardiac defects. Women with high inhibin-A (≥2.5 MOM) had a 4.8-fold (95% CI 1.42–12.46) increased risk of major non-chromosomal fetal CHDs. Since several studies have suggested shared pathways for development of the heart and placenta, it seems that increased levels of inhibin-A show a significant association with CHDs, although the causes and consequences are still unclear.24,25,26 In addition, severe isolated fetal CHDs have been found to be more likely associated with placental vascular malperfusion, which were consistent with the result of this study.27,28
In this study, prenatal detection rates of any CHD and major CHDs were 34.5% and 58.2%, respectively. After excluding isolated VSD, the detection rate of major CHD reached 85.7%. PS (25%) and isolated VSD (28.1%) showed relatively low detection rates. However, associations between maternal serum markers with fetal CHDs were consistent, even in women with fetal PS or isolated VSD. Although isolated PS is a relatively common form of CHD, with a prevalence of about 0.7 per 1000 live births,29 the prenatal diagnosis of valvar PS has been reported to be as low as 3.2%.30,31 Since severe cyanosis and hypoxia may develop in affected infants, the prenatal detection of PS is important to provide optimal care in a tertiary care hospital by administering prostaglandins to prevent closure of the ductus arteriosus in the neonatal period until definitive treatment has been provided. The addition of a color Doppler evaluation of the fetal heart during routine second-trimester sonography in low-risk patients had been reported to improve the detection rates for valvular abnormalities including PS.32,33 Our findings can be used to improve prenatal detection of isolated VSD and PS in a low-risk population. In women with low PAPP-A, high AFP, or high inhibin-A, even if major heart abnormalities are not found on detailed sonography in the second trimester, efforts might be continued to find CHDs including isolated VSD or PS using an additional ultrasonogram with or without color Doppler.
Different pathophysiologic pathways of fetal PS show various ultrasonographic presentations in the early mid-trimester, resulting in some cases of missed diagnoses in mid-trimester ultrasonography.34 Although there was no case of fetal aortic stenosis in this study, the prenatal detection of aortic stenosis is very difficult in the second trimester due to a relatively normal four-chamber view, and about 20% of critical aortic sternosis cases are diagnosed in the third trimester due to progressive left ventricular dysfunction and other physiologic aberrations, suggestive of hemodynamically significant obstruction.35,36 Since some valvular abnormalities cannot be detected until at least 30 gestational weeks, the fetal echocardiography guidelines in Japan suggest another fetal heart screening at approximately 30 gestational weeks with the routine use of color Doppler.37 When women have abnormal serum screening markers, a detailed ultrasonogram in the third trimester needs to be discussed to improve prenatal detection of CHD, especially for those with a valvular disease.
Our study has some limitations. There were cases lost to follow up and cases where prenatal diagnosis could not be validated, such as those terminated after diagnosis without an autopsy. Another limitation is that the sample size of our study is relatively small. A further large-scale prospective study is needed to evaluate clinical applications and to assess risks according to the type of CHDs. However, our study has the strength in its multicenter prospective nature. To our knowledge, this is the first study to show an association between maternal serum inhibin A and major CHD. Fetuses with CHDs are more vulnerable to a hypoxic environment, so there is increased importance in antenatal and postnatal development for CHD fetuses with placental malperfusion.38,39 This study can contribute to improving prenatal detection of CHD by selecting high risk mothers with maternal serum markers for aneuploidy.
In conclusion, this study suggests that screening ultrasonogram performed at second trimester is useful in the prenatal diagnosis of critical CHDs and that abnormal maternal serum biomarkers of aneuploidy are associated with non-chromosomal CHDs. A detailed obstetric ultrasound or fetal echocardiography can be useful to detect major CHDs in women with low PAPP-A (≤0.4 MOM) or with high AFP or inhibin A (≥2.5 MOM) during second serum screening for aneuploidy. Prospective large-scale studies may be required to verify the predictive value of abnormal biomarkers in MSS and further detailed fetal heart screening in women with abnormal MSS.
ACKNOWLEDGEMENTS
We thank pregnant women who participated in the KPDS study, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1336).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hyun Sun Ko, Jeong Ha Wie, and Hyun Mee Ryu.
- Data curation: Jeong Ha Wie and Hyun Sun Ko.
- Formal analysis: Jeong Ha Wie and Hyun Sun Ko.
- Funding acquisition: Hyun Mee Ryu.
- Investigation: Soo Hyun Kim, You Jung Han, Hee Young Cho, Mi-Young Lee, Jin Hoon Chung, Seung Mi Lee, Hye Yeon Boo, Geum Joon Cho, Han-Sung Kwon, and Hyun Sun Ko.
- Methodology: Jeong Ha Wie, Hyun Sun Ko, and Hyun Mee Ryu.
- Resources: Hyun Mee Ryu and Hyun Sun Ko.
- Supervision: Moon Young Kim, Jin Hoon Chung, Joon Ho Lee, Byoung Jae Kim, Soo-young Oh, and Mi Hye Park.
- Writing—original draft: Jeong Ha Wie and Hyun Sun Ko.
- Writing—revise & editing: Soo-young Oh and Hyun Mee Ryu.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Combined Abnormalities of Critical Congenital Heart Defects in the Study Population
References
- 1.Ko HS, Kim DJ, Chung Y, Wie JH, Choi SK, Park IY, et al. A national cohort study evaluating infant and fetal mortality caused by birth defects in Korea. BMJ Open. 2017;7:e017963. doi: 10.1136/bmjopen-2017-017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland BJ, Myers JA, Woods CR., Jr Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol. 2015;45:631–638. doi: 10.1002/uog.14882. [DOI] [PubMed] [Google Scholar]
- 3.Ko HS, We JS, Kim YH, Park IY, Lee Y, Lee GS, et al. Significance of multidisciplinary counseling on prospective parents with fetus of congenital disease. Korean J Obstet Gynecol. 2010;53:700–706. [Google Scholar]
- 4.Kim ST, Song J, Huh J, Kang IS, Yang JH, Jun TG, et al. The effect of multidisciplinary approach on the birth rate of fetuses with prenatally diagnosed congenital heart disease. J Korean Med Sci. 2019;34:e170. doi: 10.3346/jkms.2019.34.e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clur SA, Van Brussel PM, Mathijssen IB, Pajkrt E, Ottenkamp J, Bilardo CM. Audit of 10 years of referrals for fetal echocardiography. Prenat Diagn. 2011;31:1134–1140. doi: 10.1002/pd.2847. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho JS, Allan LD, Chaoui R, Copel JA, DeVore GR, Hecher K, et al. ISUOG practice guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41:348–359. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- 7.Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, et al. Mortality and morbidity of major congenital heart disease related to general prenatal screening for malformations. Int J Cardiol. 2019;290:93–99. doi: 10.1016/j.ijcard.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Snoep MC, Aliasi M, van der Meeren LE, Jongbloed MRM, DeRuiter MC, Haak MC. Placenta morphology and biomarkers in pregnancies with congenital heart disease-A systematic review. Placenta. 2021;112:189–196. doi: 10.1016/j.placenta.2021.07.297. [DOI] [PubMed] [Google Scholar]
- 9.Binder J, Carta S, Carvalho JS, Kalafat E, Khalil A, Thilaganathan B. Evidence for uteroplacental malperfusion in fetuses with major congenital heart defects. PLoS One. 2020;15:e0226741. doi: 10.1371/journal.pone.0226741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Lee SM, Jun JK, Han YJ, Kim MH, Shim JY, et al. Prospective observations study protocol to investigate cost-effectiveness of various prenatal test strategies after the introduction of noninvasive prenatal testing. BMC Pregnancy Childbirth. 2018;18:307. doi: 10.1186/s12884-018-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney JA, Cnota JF, Jones HN. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol. 2018;9:1045. doi: 10.3389/fphys.2018.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Falco M, Cobellis L, Giraldi D, Mastrogiacomo A, Perna A, Colacurci N, et al. Expression and distribution of notch protein members in human placenta throughout pregnancy. Placenta. 2007;28:118–126. doi: 10.1016/j.placenta.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Sun IY, Overgaard MT, Oxvig C, Giudice LC. Pregnancy-associated plasma protein A proteolytic activity is associated with the human placental trophoblast cell membrane. J Clin Endocrinol Metab. 2002;87:5235–5240. doi: 10.1210/jc.2002-020561. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaides KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn. 2011;31:7–15. doi: 10.1002/pd.2637. [DOI] [PubMed] [Google Scholar]
- 15.Fantasia I, Kasapoglu D, Kasapoglu T, Syngelaki A, Akolekar R, Nicolaides KH. Fetal major cardiac defects and placental dysfunction at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;51:194–198. doi: 10.1002/uog.18839. [DOI] [PubMed] [Google Scholar]
- 16.Llurba E, Syngelaki A, Sánchez O, Carreras E, Cabero L, Nicolaides KH. Maternal serum placental growth factor at 11-13 weeks’ gestation and fetal cardiac defects. Ultrasound Obstet Gynecol. 2013;42:169–174. doi: 10.1002/uog.12346. [DOI] [PubMed] [Google Scholar]
- 17.Milunsky A, Alpert E, Neff RK, Frigoletto FD., Jr Prenatal diagnosis of neural tube defects. IV. Maternal serum alpha-fetoprotein screening. Obstet Gynecol. 1980;55:60–66. [PubMed] [Google Scholar]
- 18.Albar H, MacDonald KM, Harman CR, Evans JA, Chodirker BN. Elevated MSAFP levels and congenital heart defects: lack of an association. Am J Med Genet. 1994;49:337–340. doi: 10.1002/ajmg.1320490319. [DOI] [PubMed] [Google Scholar]
- 19.Jelliffe-Pawlowski L, Baer R, Moon-Grady AJ, Currier RJ. Second trimester serum predictors of congenital heart defects in pregnancies without chromosomal or neural tube defects. Prenat Diagn. 2011;31:466–472. doi: 10.1002/pd.2720. [DOI] [PubMed] [Google Scholar]
- 20.Jelliffe-Pawlowski LL, Walton-Haynes L, Currier RJ. Identification of second trimester screen positive pregnancies at increased risk for congenital heart defects. Prenat Diagn. 2009;29:570–577. doi: 10.1002/pd.2239. [DOI] [PubMed] [Google Scholar]
- 21.Aitken DA, Wallace EM, Crossley JA, Swanston IA, van Pareren Y, van Maarle M, et al. Dimeric inhibin A as a marker for Down’s syndrome in early pregnancy. N Engl J Med. 1996;334:1231–1236. doi: 10.1056/NEJM199605093341904. [DOI] [PubMed] [Google Scholar]
- 22.Singnoi W, Wanapirak C, Sekararithi R, Tongsong T. A cohort study of the association between maternal serum Inhibin-A and adverse pregnancy outcomes: a population-based study. BMC Pregnancy Childbirth. 2019;19:124. doi: 10.1186/s12884-019-2266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue CY, Zhang CY, Ni YH, Ying CM. Are serum levels of inhibin A in second trimester predictors of adverse pregnancy outcome? PLoS One. 2020;15:e0232634. doi: 10.1371/journal.pone.0232634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee S, Chung JI, King DA, D’amato G, Paik DT, Duan A, et al. Endothelial deletion of Ino80 disrupts coronary angiogenesis and causes congenital heart disease. Nat Commun. 2018;9:368. doi: 10.1038/s41467-017-02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, et al. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature. 2018;555:463–468. doi: 10.1038/nature26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones HN, Olbrych SK, Smith KL, Cnota JF, Habli M, Ramos-Gonzales O, et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta. 2015;36:1078–1086. doi: 10.1016/j.placenta.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozcan T, Kikano S, Plummer S, Strainic J, Ravishankar S. The association of fetal congenital cardiac defects and placental vascular malperfusion. Pediatr Dev Pathol. 2021;24:187–192. doi: 10.1177/1093526620986497. [DOI] [PubMed] [Google Scholar]
- 28.Miremberg H, Gindes L, Schreiber L, Raucher Sternfeld A, Bar J, Kovo M. The association between severe fetal congenital heart defects and placental vascular malperfusion lesions. Prenat Diagn. 2019;39:962–967. doi: 10.1002/pd.5515. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 30.Marek J, Tomek V, Skovránek J, Povysilová V, Samánek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart. 2011;97:124–130. doi: 10.1136/hrt.2010.206623. [DOI] [PubMed] [Google Scholar]
- 31.Hornberger LK, Sanders SP, Rein AJ, Spevak PJ, Parness IA, Colan SD. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus. A longitudinal study. Circulation. 1995;92:1531–1538. doi: 10.1161/01.cir.92.6.1531. [DOI] [PubMed] [Google Scholar]
- 32.Nadel AS. Addition of color Doppler to the routine obstetric sonographic survey aids in the detection of pulmonic stenosis. Fetal Diagn Ther. 2010;28:175–179. doi: 10.1159/000318192. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Cai AL, Ren WD, Guo YJ, Zhang DY, Sun W, et al. Identification of fetal cardiac anatomy and hemodynamics: a novel enhanced screening protocol. BMC Pregnancy Childbirth. 2016;16:145. doi: 10.1186/s12884-016-0933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronshtein M, Blumenfeld Z, Khoury A, Gover A. Diverse outcome following early prenatal diagnosis of pulmonary stenosis. Ultrasound Obstet Gynecol. 2017;49:213–218. doi: 10.1002/uog.17332. [DOI] [PubMed] [Google Scholar]
- 35.Yagel S, Weissman A, Rotstein Z, Manor M, Hegesh J, Anteby E, et al. Congenital heart defects: natural course and in utero development. Circulation. 1997;96:550–555. doi: 10.1161/01.cir.96.2.550. [DOI] [PubMed] [Google Scholar]
- 36.Freud LR, Moon-Grady A, Escobar-Diaz MC, Gotteiner NL, Young LT, McElhinney DB, et al. Low rate of prenatal diagnosis among neonates with critical aortic stenosis: insight into the natural history in utero. Ultrasound Obstet Gynecol. 2015;45:326–332. doi: 10.1002/uog.14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satomi G. Guidelines for fetal echocardiography. Pediatr Int. 2015;57:1–21. doi: 10.1111/ped.12467. [DOI] [PubMed] [Google Scholar]
- 38.Andescavage N, Yarish A, Donofrio M, Bulas D, Evangelou I, Vezina G, et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta. 2015;36:1024–1030. doi: 10.1016/j.placenta.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leon RL, Mir IN, Herrera CL, Sharma K, Spong CY, Twickler DM, et al. Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes. Pediatr Res. 2022;91:787–794. doi: 10.1038/s41390-021-01521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combined Abnormalities of Critical Congenital Heart Defects in the Study Population