Abstract
Background:
Preventive therapy among patients with established atherosclerotic cardiovascular disease (ASCVD) is generally underused. Whether new guideline recommendations and a focus on implementation have improved the use of high-intensity statins is unknown.
Objectives:
To evaluate the patterns and predictors of statin use among patients with ASCVD.
Methods:
In this retrospective cohort study, pharmacy and medical claims data from a commercial health plan were queried for patients with established ASCVD between 31st January 2018 and 31st January 2019. Statin use on an index date of 31st January 2019 was evaluated, as was 12-month adherence and discontinuation. Multivariable logistic regression was used to determine independent associations with varying intensity statin use.
Results:
Of the 601 934 patients with established ASCVD, 41.7% were female, mean age 67.5±13.3. Overall, 22.5% of the cohort were on a high-intensity statin, 27.6% were on low- or moderate-intensity statin, and 49.9% were not on any statin. In multivariable analysis, younger patients, females and those with higher Charlson comorbidity were less likely to be prescribed any statin. Among statin users, females, older patients and those with peripheral artery disease were less likely to be on a high-intensity formulation, while a cardiology encounter in the prior year increased the odds. The majority of high-intensity stain users achieved high levels of adherence.
Conclusions:
Substantial underuse of statins persists in a large, insured and contemporary cohort of patients with ASCVD from the United States. In particular, concerning gaps in appropriate statin use remain among younger patients, women and those with non-coronary ASCVD.
Keywords: statins, prevention, predictors, secondary prevention, atherosclerosis
CONDENSED ABSTRACT
It is unknown whether new guidelines and a focus on implementation has addressed underuse of statin therapy among patients with ASCVD. Of 601 934 patients with ASCVD on the 31st January 2019, 22.5% were on high-intensity statin, 27.6% were on low- or moderate intensity statin and 49.9% were not on any statin. Younger patients, females and those with higher Charlson comorbidity were less likely to be prescribed any statin. Among statin users, females, older patients and those with PAD were less likely to be on a high-intensity formulation. Concerning gaps persist among high risk patients with ASCVD in the US.
INTRODUCTION
Almost 1 in 2 Americans will develop a clinical manifestation of atherosclerotic cardiovascular disease (ASCVD) in their lifetime(1). Once established, the risk of sustaining a subsequent ASCVD event such as a myocardial infarction, stroke, limb loss or cardiovascular death is at least 10% per year(2). Statins can reduce the risk of ASCVD events by approximately 30%(3) with an additional 15% reduction with high-intensity compared to low or moderate-intensity regimens(4,5).
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines included a Class I recommendation for high-intensity statin use among patients with established ASCVD who were less than 75 years of age(6). While the release of the guideline resulted in a demonstrable increase in the prescription of high-intensity statins, a number of studies showed the rate of use had plateaued in 2017 with approximately 30–50% of eligible patients ultimately being treated(7–13). Since then, an update to the guidelines has broadened the high-intensity statin recommendation to class II for those over 75 years of age with ASCVD(14), with the ACC/AHA publicly calling for the need to improve implementation of their guidelines(15). As prices have decreased with generic availability of high-intensity statins, more potential barriers to optimal statin use have been identified(16–19), and a number of initiatives, including performance and quality measures such as HEDIS (Healthcare Effectiveness Data and Information Set), have been described to improve the adoption of statins more generally(16–19). However, the impact of these developments on contemporary high-intensity statin use, particularly in the context of broader availability of non-statin alternatives such as ezetimibe and Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors, is unknown. Since statins are inexpensive, well tolerated, and have the most robust evidence of improving clinical outcomes of all the lipid lowering therapies, continued efforts to understand and address underuse is a high priority.
In a large national commercial health plan dataset, we sought to evaluate the patterns and predictors of high-intensity statin use among patients with established ASCVD. In particular, we aimed to understand utilization patterns among key subgroups (e.g. age strata, ASCVD phenotype) as well as temporal changes in both individual statin intensity prescription and subsequent adherence.
METHODS
The New England Institutional Review Board provided an exemption from informed consent given the use of a limited, de-identified dataset. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Data Source
Eligible patients were identified via a computerized query within the HealthCore Integrated Research Environment (HIRE); a repository of longitudinal claims which comprise medical, pharmacy and laboratory data from a large commercial health plan. The population within HIRE is geographically diverse and considered representative of the commercially insured population in the United States.
Study population
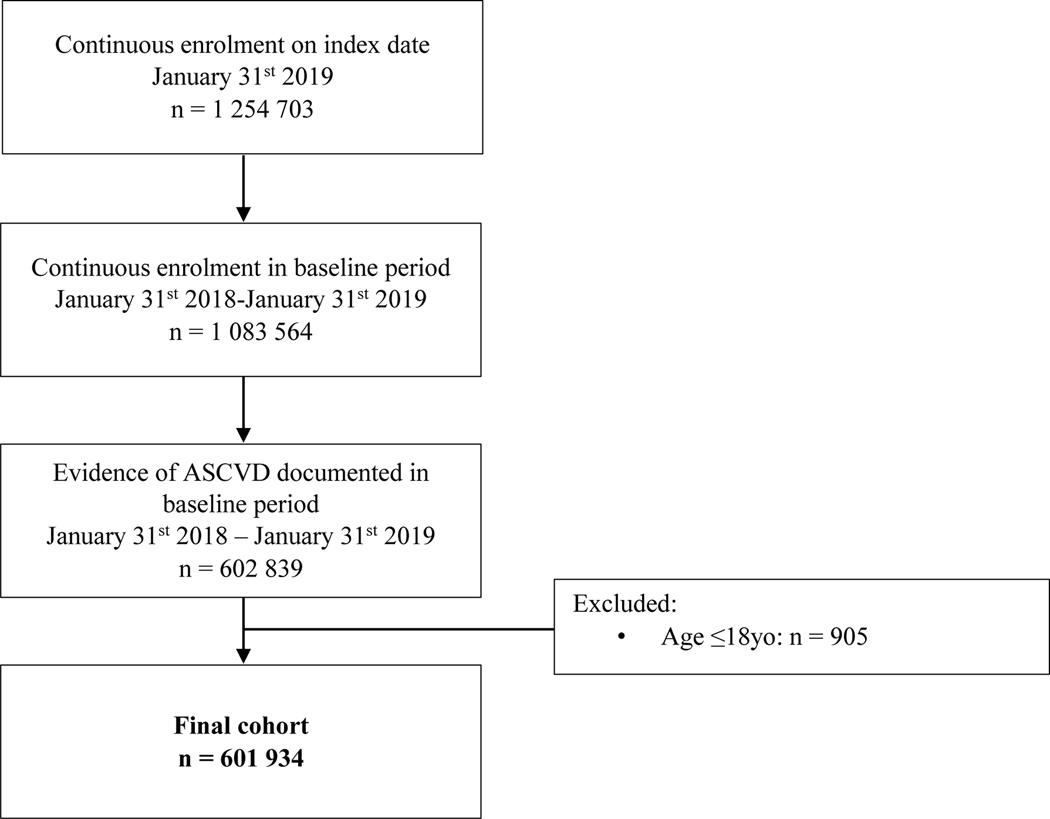
An index date of the 31st January 2019 was established with a 1-year baseline period from 31st January 2018 used to determine cohort eligibility. International Classification of Diseases, Ninth/Tenth Revisions, Clinical Modification (ICD‐9/10‐CM), Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes were used to identify patients with ASCVD. Validated code lists are available in the supplementary appendix. In brief, ASCVD was defined as those with a diagnosis or procedure code representative of coronary artery disease [CAD] (e.g. obstructive coronary atherosclerosis, prior myocardial infarction, prior percutaneous coronary intervention or coronary artery bypass grafts), peripheral arterial disease [PAD] (e.g. vascular claudication, prior percutaneous or open revascularization or amputation from poor circulation) or cerebrovascular disease (e.g. carotid atherosclerosis, ischemic stroke or prior percutaneous or open revascularization). To be included, patients were required to be ≥18 years of age at the index date and to have one year of continuous medical eligibility in a health plan prior to the index date.
Follow up
Pharmacy claims were evaluated for a 12-month period of follow up from the index date of 31st January 2019.
Variables
Comorbid conditions were defined by the presence of encounters with corresponding ICD10‐CM diagnosis codes in the baseline period for dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, obesity, chronic kidney disease stage 4 or 5, and depression. Charlson comorbidity score was calculated as previously described. Outpatient visits were defined by the presence of an encounter with a service location of outpatient in the baseline period with further breakdown by cardiology and primary care professional specialties. Non-statin LDL-C lowering therapy was defined as either ezetimibe and/or PCSK-9 inhibitor.
Outcomes of interest
The primary outcome of interest was a prescription fill for a high-intensity statin (atorvastatin 40–80mg or rosuvastatin 20–40mg) that commenced on, or covered, ± 30 days of the index date. Additional outcomes of interest were the prescription of any statin on the index date, the proportion of statin users continuing vs. discontinuing, and the proportion of days covered (PDC) by statin prescriptions over the course of a year as a measure of adherence. Continuous prescription was defined by the presence of a prescription with 0 days without treatment. The proportion of days covered (PDC) was defined as the number of days covered by a prescription divided by 365 days with high levels of adherence defined as ≥75% PDC. Where there were multiple values for LDL-C, the most recent value was used. Age strata were defined as ‘younger’ (<45 years), ‘middle’ (45–74 years) and ‘older’ (≥75 years) with those <75 years of age representing a key subgroup as a class I indication for high-intensity statin use.
Statistical analysis
Categorical variables are presented as frequencies and percentages and continuous variables are presented as means and standard deviations or medians and quartile 1, quartile 3, as appropriate. Univariable comparisons between mutually exclusive groups were made statistically with student’s t-test or chi-square. Comparisons between non-mutually exclusive groups were made descriptively. A binomial logistic regression model was generated to identify independent predictors of a) statin use and b) high-intensity statin use, with the following clinically-relevant covariates entered into the model: age stratum, sex, ASCVD type, cardiovascular risk factors, medical comorbidities (Charlson comorbidity score of ≥3, CKD stage ≥4, depression), care patterns (LDL-C checked within 12 months and baseline non-statin LDL-C lowering therapy) and the presence of cardiology outpatient visit and primary care outpatient visit in the prior 12 months.
RESULTS
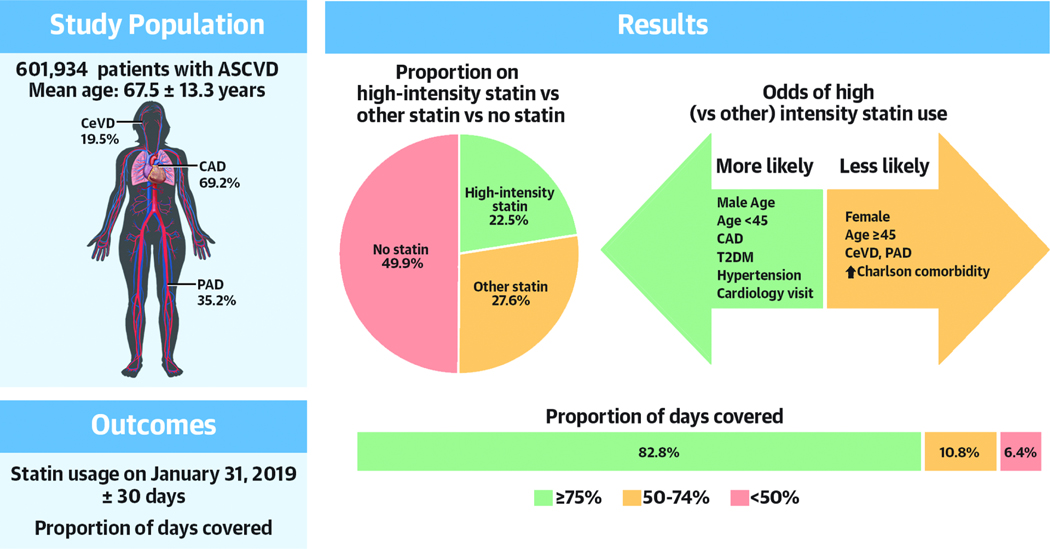
We identified 1 083 564 patients with a prior diagnosis of ASCVD and 12 months of continuous enrollment, of which 601 934 had an ASCVD diagnosis in the 12 month baseline period and were ≥18 years of age. The mean age was 67.5±13.3 years with 41.7% being female. Geographically 27.1% were from the Midwest, 16.7% from the Northeast, 31.2% from the South and 25.0% from the West. CAD was present in 69.2%, cerebrovascular disease in 19.5% and PAD in 35.2%. Polyvascular disease (the presence of two or more of CAD, cerebrovascular disease or PAD) was present in 19.8%.
Characteristics of statin users and non-users
Of the 601 934 patients on the index date, 22.5% were on a high-intensity statin, 27.6% were on some other statin and 49.9% were not on a statin. Cohort characteristics by statin use are presented in Table 1. Compared to those not on a statin, statin users tended to be older, more likely to be male and more likely to have CAD. In particular (Figure 2), only 43.2% of females were on a statin compared to 55.1% of males, and while similar rates of statin use were observed between the middle and older age strata (52.3% vs. 49.3%), younger patients (<45 years) were far less likely to be prescribed a statin (22.4%). Statin users had a higher proportion of ASCVD risk factors that was particularly marked for dyslipidemia and T2DM. Ezetimibe and PCSK-9 inhibitor use was higher among high intensity statin users (6.9%) compared with non-users (4.0%) and non-high statin users (4.0%). Non-users had similar attendance at primary care in the prior 12 months (81.3% vs. 80.6% [high-intensity] or 82.1% [non-high] but were less frequently seen by cardiologists compared to statin users (66.8% vs. 79.2% [high-intensity] or 71.4% [non-high]).
Table 1.
Cohort Characteristics by Statin Use
| Overall (N = 601,934 [100%]) | High-Intensity Statin (n = 135,569 [22.5%]) | Other Statin (n = 166,231 [27.6%]) | No Statin (n = 300,134 [49.9%]) | P Value | |
|---|---|---|---|---|---|
|
| |||||
| Age, y | 67.5 ± 13.3 | 65.9 ± 11.1 | 70.0 ± 11.9 | 66.9 ± 14.7 | <0.0001 |
| <45 (younger) | 23,801 (4.0) | 3,017 (2.2) | 2,313 (1.4) | 18,471 (6.1) | <0.0001 |
| 45–74 (middle) | 383,630 (63.7) | 100,247 (73.9) | 100,365 (60.4) | 183,018 (61.0) | <0.0001 |
| ≥75 (older) | 194,503 (32.3) | 32,305 (23.8) | 63,553 (38.2) | 98,645 (32.9) | <0.0001 |
| Sex | <0.0001 | ||||
| Female | 250,875 (41.7) | 39,576 (29.2) | 68,778 (41.4) | 142,521 (47.5) | |
| Male | 351,059 (58.3) | 95,993 (70.8) | 97,453 (58.6) | 157,613 (52.5) | |
| Cardiovascular history | |||||
| CAD | 416,349 (69.2) | 114,115 (84.2) | 115,218 (69.3) | 187,016 (62.3) | <0.0001 |
| CeVD | 117,202 (19.5) | 25,843 (19.1) | 34,046 (20.5) | 57,313 (19.1) | <0.0001 |
| PAD | 211,616 (35.2) | 35,269 (26.0) | 58,811 (35.4) | 117,536 (39.2) | <0.0001 |
| ASCVD risk factors | |||||
| Dyslipidemia | 474,823 (78.9) | 123,762 (91.3) | 150,393 (90.5) | 200,668 (66.9) | <0.0001 |
| T2DM | 219,188 (36.4) | 55,717 (41.1) | 68,047 (40.9) | 95,424 (31.8) | <0.0001 |
| Hypertension | 493,050 (81.9) | 118,245 (87.2) | 144,239 (86.8) | 230,566 (76.8) | <0.0001 |
| Obesity | 109,334 (18.2) | 27,009 (19.9) | 29,782 (17.9) | 52,543 (17.5) | <0.0001 |
| Medical comorbidities | |||||
| Charlson comorbidity score ≥3 | 157,451 (26.2) | 32,879 (24.3) | 44,205 (26.6) | 80,367 (26.8) | <0.0001 |
| CKD ≥stage 4 | 16,392 (2.7) | 3,545 (2.6) | 4,663 (2.8) | 8,184 (2.7) | 0.006 |
| Depression | 101,990 (16.9) | 21,218 (15.6) | 26,223 (15.8) | 54,549 (18.2) | <0.0001 |
| Care patterns | |||||
| LDL-C checked last 12 months | 153,305 (25.5) | 38,395 (28.3) | 44,262 (26.6) | 70,648 (23.5) | <0.0001 |
| Ezetimibe/PCSK-9 inhibitor | 27,855 (4.6) | 9,408 (6.9) | 6,597 (4.0) | 11,850 (4.0) | <0.0001 |
| Outpatient visits | 25 (13–44) | 23 (12–42) | 26 (14–45) | 25 (13–45) | <0.0001 |
| Cardiology visit | 426,788 (70.9) | 107,360 (79.2) | 118,717 (71.4) | 200,711 (66.8) | <0.0001 |
| PCP visit | 489,589 (81.3) | 109,261 (80.6) | 136,477 (82.1) | 243,851 (81.3) | <0.0001 |
Values are mean ± SD, n (%), or median IQR.
ASCVD = atherosclerotic cardiovascular disease; CAD = coronary arterydisease; CKD = chronic kidneydisease; LDL-C = low-density lipoprotein cholesterol; PAD = peripheral artery disease; PCP = primary care physician; PCSK-9 = proprotein convertase subtilisin/kexin type 9; PDC = proportion of days covered; T2DM = type 2 diabetes mellitus.
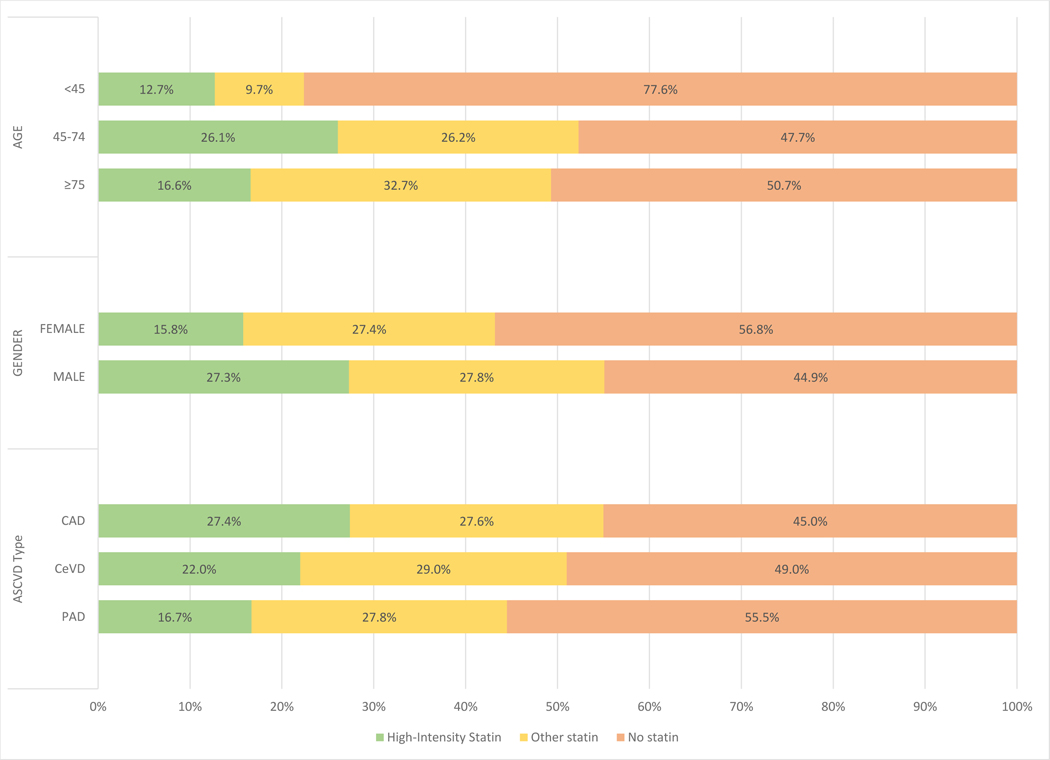
Figure 2. Statin use among key subgroups.
Patients presented by baseline statin use (high-intensity = green; other statin = yellow, and no statin = orange) within key subgroups of age, gender and ASCVD phenotype.
CAD - coronary artery disease; CeVD – cerebrovascular disease; PAD – peripheral artery disease;
Characteristics of high-intensity statin users
High-intensity statin use was lowest among the poles of age; least frequent among younger patients (12.7%), followed by older patients (16.6%) followed by middle-aged (26.1%) (Figure 2). Compared with the overall cohort, users of a high-intensity statin were less likely to be female (29.2 vs. 41.7%) or have a history of PAD (26.0 vs. 35.2%) (Central Illustration). Rates of cardiovascular risk factors were higher among those using high-intensity statins although medical comorbidity burden was similar. LDL-C levels were only available in 22.8% (137174/601934) of patients. Of those, only 28.9% (39764/137174) had levels ≤70mg/dL with the majority of those on a high intensity statin (24786/39764).
Central illustration. Statin use in 601934 ASCVD patients on 31st January 2019.
Proportion on high-intensity statin vs. other statin vs. no statin. Odds of high (vs. other) intensity statin use. Proportion of days covered among users of high-intensity statins.
Independent predictors of any statin use
‘Middle’-aged and ‘older’ patients were over twice as likely as ‘younger’ patients to be on a statin despite having premature ASCVD (Table 2). Females were 30% less likely to be treated with a statin compared to males (OR 0.70, 95%CI 0.69–0.71). Compared with CAD, those with cerebrovascular disease were 20% less likely to receive a statin (OR 0.78, 95%CI 0.76–0.79) and those with PAD almost half as likely (OR 0.55, 95%CI 0.55–0.56). Dyslipidemia was significantly more prevalent among statin users (OR 4.23, 95%CI 4.17–4.29), as was a diagnosis of either T2DM (OR 1.47, 95%CI 1.45–1.49) or hypertension (OR 1.39, 95%CI 1.37–1.41). A high Charlson comorbidity score reduced the odds of statin prescription (OR 0.72, 95%CI 0.71–0.73), as did the presence of depression (OR 0.93, 95%CI 0.92–0.95).
Table 2.
Independent predictors of a) any statin use and b) high-intensity statin use
| Any statin vs. no statin |
High intensity vs. other statin |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | ||||||
| <45 (younger) | Ref | Ref | ||||
| 45–74 (middle) | 2.66 | 2.58 – 2.75 | <0.0001 | 0.83 | 0.79 – 0.87 | <0.0001 |
| ≥75 (older) | 2.09 | 2.02 – 2.15 | <0.0001 | 0.44 | 0.42 – 0.46 | <0.0001 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 0.70 | 0.69 – 0.71 | <0.0001 | 0.68 | 0.68 – 0.69 | <0.0001 |
| Cardiovascular history | ||||||
| CAD | Ref | Ref | ||||
| CeVD | 0.78 | 0.76 – 0.79 | <0.0001 | 0.65 | 0.63 – 0.66 | <0.0001 |
| PAD | 0.55 | 0.55 – 0.56 | <0.0001 | 0.43 | 0.42 – 0.43 | <0.0001 |
| ASCVD Risk factors | ||||||
| Dyslipidemia | 4.23 | 4.17 – 4.29 | <0.0001 | 1.03 | 1.01 – 1.05 | 0.013 |
| T2DM | 1.47 | 1.45 – 1.49 | <0.0001 | 1.12 | 1.10 – 1.14 | <0.0001 |
| Hypertension | 1.39 | 1.37 – 1.41 | <0.0001 | 1.13 | 1.11 – 1.15 | <0.0001 |
| Obesity | 0.99 | 0.98 – 1.01 | 0.20 | 1.00 | 0.98 – 1.02 | 0.96 |
| Medical comorbidities | ||||||
| Charlson comorbidity ≥3 | 0.72 | 0.71 – 0.73 | <0.0001 | 0.95 | 0.93 – 0.97 | <0.0001 |
| CKD ≥ Stage 4 | 1.03 | 1.02 – 1.05 | 0.0006 | 1.04 | 1.02 – 1.06 | <0.0001 |
| Depression | 0.93 | 0.92 – 0.95 | <0.0001 | 1.10 | 1.08 – 1.12 | <0.0001 |
| Care patterns: | ||||||
| LDL-C checked last 12months | 1.09 | 1.08 – 1.11 | <0.0001 | 0.91 | 0.90 – 0.93 | <0.0001 |
| Ezetimibe/PCSK-9i | 0.89 | 0.87 – 0.92 | <0.0001 | 1.44 | 1.40 – 1.49 | <0.0001 |
| Outpatient visits | ||||||
| Cardiology visit PCP visit | 1.06 | 1.04 – 1.07 | <0.0001 | 1.21 | 1.19 – 1.23 | <0.0001 |
| PCP visit | 0.91 | 0.89 – 0.92 | <0.001 | 0.97 | 0.96 – 0.99 | 0.0016 |
Independent predictors of high intensity statin use among statin users
While middle-aged and older patients were more likely to be on a statin overall, among statin users they were less likely to be on a high-intensity formulation compared to the younger age stratum (Table 2). In contrast to men, women were not only less likely to be on a statin overall, but were also less likely to be on a high-intensity formulation (OR 0.68, 95%CI 0.68–0.69). Patients with PAD were less than half as likely as CAD patients to be on a high-intensity statin (OR 0.43, 95%CI 0.42–0.43). The odds of high-intensity statin use were modestly increased among those with hypertension (OR 1.13, 95%CI 1.11–1.15) and T2DM (OR 1.12, 95%CI 1.10–1.14). Use of other non-statin LDL-C lowering agents increased the likelihood that a patient was also on a high intensity statin (OR 1.44, 95%CI 1.40–1.49), as did visiting a cardiologist in the prior 12 months (OR 1.21, 95%CI 1.19–1.23).
Patterns of high-intensity statin use among patients < 75 years of age
In the key subgroup of patients < 75 years old, high-intensity statin users were less commonly women (26.2 vs. 38.9%), more likely to have obesity (22.5 vs. 11.6%) less often had PAD (23.0 vs. 35.5%) or cerebrovascular disease (16.5 vs. 27.2%) and generally had lower medical comorbidity burden (Charlson score ≥ 3, 19.8 vs. 38.5%; CKD ≥ 4, 31.8 vs. 5.2%) compared with those ≥ 75 years old (Supplementary Table 1). Those < 75 years old on high-intensity statins attended fewer outpatient appointments (median 21 vs. 30) reflected in lower proportions having seen a cardiologist in the last 12 months (78.3 vs. 82.1%) and a primary care physician over the same period (79.7 vs. 83.4%).
Proportion of days covered by high-intensity statins
Over 80% of high-intensity statin users at index were able to achieve high levels of adherence (Table 3). Compared with the low adherence group (<50% PDC), the high adherence group had a greater proportion of older patients (24.3 vs. 18.4%) and a lower proportion of younger patients (1.9 vs. 3.9%). Among the least adherent group (PDC <50%), there was a higher prevalence of CKD (8.7 vs. 6.7%) and depression (18.0 vs. 15.0%) compared with the most adherent group.
Table 3.
High-intensity statin users by proportion of days covered.
| Proportion of days covered | |||
|---|---|---|---|
|
|
|||
| <50% | 50–74% | ≥75% | |
| n = 8 051 (6.4%) | n = 13 673 (10.8%) n | n = 104 679 (82.8%) | |
|
| |||
| Age, mean±SD | 63.4±11.2 | 64.3±11.3 | 66.2±11.0 |
| <45 (younger) | 315 (3.9) | 428 (3.1) | 2 029 (1.9) |
| 45–74 (middle) | 6 254 (77.7) | 10 487 (76.7) | 77 196 (73.7) |
| ≥75 (older) | 1 482 (18.4) | 2 758 (20.2) | 25 454 (24.3) |
|
| |||
| Sex | |||
| Female | 2 575 (32.0) | 4 204 (30.8) | 29 694 (28.4) |
| Male | 5 476 (68.0) | 9 469 (69.3) | 74 985 (71.6) |
|
| |||
| Cardiovascular history | |||
| CAD | 6 507 (80.8) | 11 311 (82.7) | 88 878 (84.9) |
| CeVD | 1 543 (19.2) | 2 583 (18.9) | 19 648 (18.8) |
| PAD | 2 208 (27.4) | 3 725 (27.2) | 26 501 (25.3) |
|
| |||
| ASCVD Risk factors | |||
| Dyslipidemia | 7 276 (90.4) | 12 553 (91.8) | 95 694 (91.4) |
| T2DM | 3 302 (41.0) | 5 868 (42.9) | 42 615 (40.7) |
| Hypertension | 6 915 (85.9) | 11 899 (87.0) | 91 362 (87.3) |
| Obesity | 1 683 (20.9) | 2 852 (20.9) | 20 676 (19.8) |
|
| |||
| Medical comorbidities | |||
| Charlson comorbidity ≥3 | 2 058 (25.6) | 3 382 (24.7) | 24 472 (23.4) |
| CKD ≥ Stage 4 | 701 (8.7) | 1 023 (7.5) | 6 961 (6.7) |
| Depression | 1 445 (18.0) | 2 349 (17.2) | 15 691 (15.0) |
|
| |||
| Care patterns: | |||
| LDL-C checked last 12months | 2 461 (30.6) | 4 245 (31.1) | 29 164 (27.9) |
| Baseline non-statin LLT | 511 (6.4) | 1 007 (7.4) | 7 340 (7.0) |
|
| |||
| Outpatient visits, median [Q1-Q3] | 22 [11–42] | 23 [12–41] | 23 [12–41] |
| Cardiology visit | 6 322 (78.5) | 10 607 (77.6) | 83 151 (79.4) |
| PCP visit | 6 540 (81.2) | 11 020 (80.6) | 84 041 (80.3) |
CAD - coronary artery disease; CeVD – cerebrovascular disease; CKD – chronic kidney disease; LDL-C – low-density lipoprotein cholesterol; PAD – peripheral artery disease; PCP – primary care physician;
Trajectory of statin use
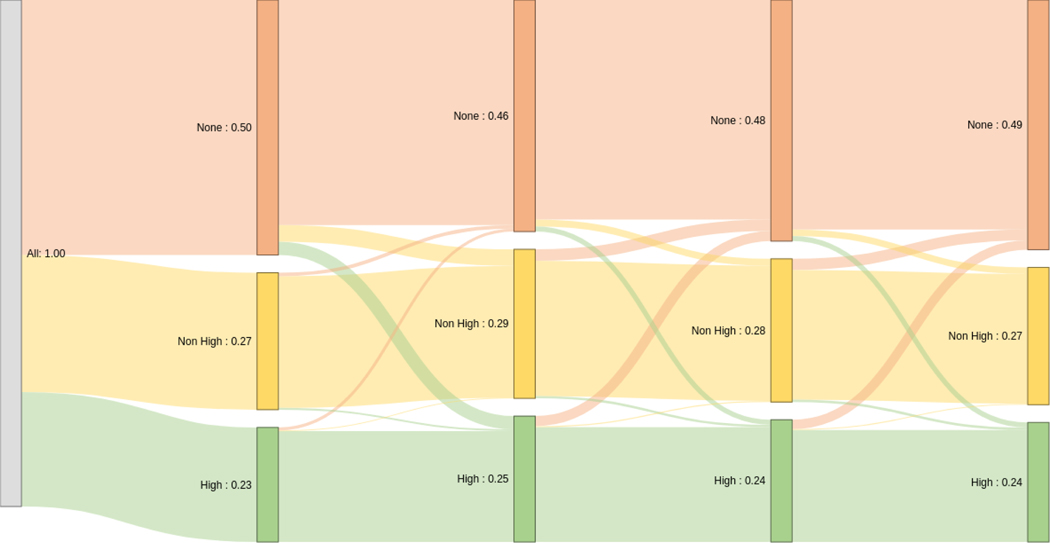
Compared to the index date, there was essentially no difference in the final proportions of patients on high-intensity statin, non-high-intensity statin or no statin therapy at 12 months (Figure 3). In total only 19.1% of patients underwent some form of change in statin therapy however almost half of these (9.2%) were related to down-titration or discontinuation. Of the patients on no statin at index, 83.6% (n=253 459) were still not on any statin at follow up with the vast majority (81.3%, n=246 253) receiving no prescription for any statin over a 12 month period. Of the remaining 16.4% (n=49 607) on no statin at index, 9.2% (n=27 767) ended up on a non-high intensity statin and 7.2% (n=21 840) were on a high-intensity statin at follow-up.
Figure 3. Sankey diagram illustrating the dynamics of statin use in follow-up.
The proportion of patients receiving high-intensity statins are shaded green, those receiving non-high-intensity statins in yellow and no statin in orange. The black numbers represent the shaded proportion as a percentage of the overall population. The vertical lines represent 3-month intervals.
Of the patients on non-high-intensity statin at index, 81.7% (n=133 043) remained on a similar formulation at follow up with only 326 of those patients undergoing some form of unsuccessful up-titration in between. At 12 months, only 3.8% (n=6 305) of the non-high intensity group at index were commenced and remained on high-intensity statin at follow up while 15.2% (n=24 740) were no longer receiving a prescription for any statin.
Of the patients on high-intensity statin at index, 83.7% (n=113 876) remained on a high-intensity statin at follow-up with 202 patients undergoing some period of down-titration in the interim. At 12 months, 1.7% (n=2 358) were on a non-high-intensity statin at 12 months while 14.6% (n=19 878) were on no statin at all, of whom 276 were transiently on a non-high-intensity statin.
Continuation and discontinuation of high-intensity statin
Individuals in the oldest stratum tended to be more likely to discontinue their high-intensity statin, as did women (Table 4). Patients with PAD more commonly discontinued their high-intensity statin while the reverse was true for those with T2DM. Those discontinuing high-intensity statins in follow up had a higher burden of medical comorbidities.
Table 4.
Persistence of high-intensity statin use in follow-up.
| High intensity statin use | ||
|---|---|---|
|
|
||
| Continued | Discontinued | |
| n = 126 403 (93.3) | n = 9 166 (6.7) | |
|
| ||
| Age, mean±SD | 65.8±11.1 | 67.4±11.8 |
| <45 (younger) | 2 772 (2.2) | 245 (2.7) |
| 45–74 (middle) | 93 937 (74.3) | 6 310 (68.8) |
| ≥75 (older) | 29 694 (23.5) | 2 611 (28.5) |
|
| ||
| Sex | ||
| Female | 36 473 (28.9) | 3 103 (33.9) |
| Male | 89 930 (71.2) | 6 063 (66.2) |
|
| ||
| Cardiovascular history | ||
| CAD | 106 696 (84.4) | 7 419 (80.9) |
| CeVD | 23 774 (18.8) | 2 069 (22.6) |
| PAD | 32 434 (25.7) | 2 835 (30.9) |
|
| ||
| ASCVD Risk factors | ||
| Dyslipidemia | 115 523 (91.4) | 8 239 (89.9) |
| T2DM | 51 785 (50.0) | 3 932 (42.9) |
| Hypertension | 110 176 (87.2) | 8 069 (88.0) |
| Obesity | 25 211 (19.9) | 1 798 (19.6) |
|
| ||
| Medical comorbidities | ||
| Charlson comorbidity ≥ 3 | 29 912 (23.7) | 2 967 (32.4) |
| CKD ≥ Stage 4 | 8 685 (6.9) | 1 067 (11.6) |
| Depression | 19 485 (15.4) | 1 733 (18.9) |
|
| ||
| Care patterns: | ||
| LDL-C checked last 12months | 35 870 (28.4) | 2 525 (27.6) |
| Baseline non-statin LLT | 8 858 (7.0) | 550 (6.0) |
|
| ||
| Outpatient visits, median [Q1-Q3] | 23 [12–41] | 27 [14–50] |
| Cardiology visit | 100 080 (79.2) | 7 280 (79.4) |
| PCP visit | 101 601 (80.4) | 7 660 (83.6) |
DISCUSSION
This multicenter analysis of over 600 000 patients with established ASCVD represents one of the largest contemporary analyses of statin prescribing habits in the United States. The study has a number of important findings. First, despite having established ASCVD, barely half of our population were on a statin and less than a quarter overall were on a high-intensity formulation. Second, younger patients and females were less likely to receive any form of statin therapy. Third, among statin users, women and those with non-CAD ASCVD were less likely to be on a high-intensity formulation whereas those seeing a cardiologist were more likely. Finally, multiple opportunities for care improvement exist; among the no-statin cohort almost two-thirds had seen a cardiologist in the prior 12 months, and among those with the lowest levels of adherence, over 80% had seen their PCP or cardiologist in the prior 12 months.
Our finding that only 50.1% of patients were using a statin is lower than other comparable studies which have reported usage rates between 60 and 70% among patients with established ASCVD. The reasons underlying the lower usage rates observed here are likely multifactorial, but most probably relate to three key differences. First, most other studies reporting statin use among ASCVD patients have evaluated prescription fill rates within 30 days of an index event whereas our cohort deliberately included patients with any qualifying ASCVD encounter in the prior 12 months. Not only is prescription highest around an acute event, but discontinuation increases over time (20); thus, our estimates are likely to be a more representative snapshot of a general ASCVD population rather than an incident ASCVD cohort. Second, some studies have relied on self-reported medication use which would over-estimate ‘use’ when compared to prescription fill(12). Thirdly, our use of a 30-day prescription window from a single index date is a relatively strict definition of use and when relaxed to ‘any use’ over 365 days, approaches a more comparable 66%. However, notwithstanding differences in study population or outcome measure, our data add to the compelling narrative that statin use remains suboptimal. Low rates of statin use have been repeatedly shown to associate with higher mortality(21), even after adjustment for the presence of any healthy adherer effect(11). Most importantly, the randomized trial evidence showing reductions in death and major vascular events makes understanding and addressing underuse a public health priority.
Despite guideline recommendations, less than half of our statin users and only 22.5% overall were on a high-intensity formulation. These figures suggest that despite early uptake in high-intensity statin use following the 2013 guidelines, there has been limited progress over the last 2–3 years. This is of particular concern as our cohort ought to reflect a comparably well-treated population with high rates of adherence and excellent access to care evidenced by over two-thirds having seen a cardiologist and over three-quarters having seen a PCP within the prior 12 months. The finding that only 19% of this cohort underwent some form of statin-related medication change over a 12 month period is consistent with data from the GOULD registry which showed that only 17.1% of patients with ASCVD underwent intensification of lipid lowering therapy in a 2 year period(22). The lack of medication changes together with evidence that over 70% of this cohort’s LDL-C were not to target supports the presence of significant clinician inertia (23). Some of the contributing factors may include a lack of knowledge of guidelines and the role of high-intensity statins(17), discordance between guideline knowledge and physician prescribing habits(24), a lack of confidence navigating perceived statin-intolerance(25) as well as contrarian beliefs about the role of statins(26). Given prior studies have previously documented evidence of widespread practice and provider-level variation in the use of high-intensity statins(27,28), multifaceted interventions harnessing educational outreach as well as audit-and-feedback are likely to be needed to overcome patient, clinician and system-level barriers. The development of novel models of care elevating the role of nurses and pharmacists in multidisciplinary care(29) as well as the potential for harnessing remote and digital management platforms(30), in addition to interventions aimed at patients (31) offers hope, although data supporting their capacity for dissemination at scale is awaited.
In particular, the low rates of discontinuation suggest the need for a renewed focus on shifting the perceptions of those who have already discontinued, largely on the account of intolerance, and to begin the lengthy process of rechallenge(32). A number of studies have shown patients previously considered intolerant are able to resume statin use(25,33–35). While optimism surrounds the emergence of a number of non-statin alternatives for lipid lowering(36), these agents remain costly and ought to be added to baseline statin therapy as most were studied. Thus in order to achieve patient-level treatment goals and cost-effective lipid-lowering, greater use of high-intensity statins has both individual and population-level implications.
Our study included over 20 000 patients less than 45 years of age which represents one of the largest cohorts to evaluate statin prescribing in those with very premature ASCVD. Our concerning finding that only 22.4% of ‘younger’ patients were using a statin likely reflects a knowledge gap around premature ASCVD contributing to underuse at both the patient and clinician level. It is clear that ‘younger’ patients are less likely to use statins despite established and premature ASCVD. At the patient level there may be a mismatch between actual and perceived future risk of a recurrent event – either overly optimistic or fatalistic - which could lead to suboptimal behavioral choices including statin discontinuation and non-adherence(18,37–40). Further, given the inverse association between age and online information seeking(41,42), younger patients may be more likely to acquire the prevailing belief from the media that statins are harmful and thus may be more likely to refuse treatment or discontinue(43). At the clinician level, a lower burden of traditional risk factors(44,45) may lead to the misconception that the need for secondary preventive therapies is negated or mitigated. This phenomenon is likely especially pertinent among individuals further out from their index event.
This data adds to the growing body of literature shining light on treatment disparity by sex. Women in our cohort were not only undertreated with statins, but even those using a statin were significantly less likely to be receiving a high intensity formulation and had higher rates of discontinuation. While variation in ASCVD risk factor profile, greater medical comorbidity burden or older age are frequently cited as reasons for less aggressive secondary preventive care among women, after adjustment for these factors women remained half as likely to be on a high intensity statin compared to men. Our study also confirmed greater rates of high-intensity statin discontinuation among women(13). With no evidence of heterogeneity in efficacy by gender(46), ongoing work must not only address misperceptions and barriers to the prescription of high-intensity statins in women(47), but also further understand (and address) differences in tolerability which may relate to sex-based variation in statin metabolism(48).
Our study confirms that patients with PAD continue to represent a neglected ASCVD subgroup with respect to statin use. PAD has long been recognized as a high risk ASCVD manifestation for both cardiovascular and non-cardiovascular mortality, and yet relative under-treatment has been documented for over 20 years(49) with fewer than 20% receiving a statin as recently as 2013(7). In more contemporary data from 2017, Colantonio et al. evaluated a harmonized Medicare and MarketScan cohort of over 900 000 US patients with established ASCVD and found between 33.9 and 56.0% of patients with PAD were on some dose and formulation of statin with between 15.6 and 29.1% of those reporting high intensity use(50). This is remarkably consistent with our own finding that 44.5% of PAD patients received a statin, of which only 37.5% received a high-intensity formulation, reaffirming not only the persistent gap in PAD care but also a lack of progress. For a disease affecting >200 million worldwide that carries a high risk of mortality and morbidity, and for which statins have proven efficacy, (and high intensity statins incremental benefit(51)), the lack of guideline(14,52) adoption is of ongoing concern.
There are limitations to our study. Administrative claims data are subject to misclassification; we attempted to mitigate this through the use of validated code lists. Coding for myalgia and muscle aches is generally poor in administrative claims data and thus parsing out statin intolerance as a contributor to statin non-prescription was not possible. Additionally there may be use of statins that is not captured through billing, with relatively inexpensive prescriptions being paid with cash. This is unlikely a major factor in the era of electronic prescriptions. Our study evaluated prescription dispensing and thus the impact of primary non-adherence to a written prescription cannot be determined. The study included only privately insured participants, and thus does not include uninsured patients. However as one of the larger insurers with approximately 37 million fully insured members, Anthem/ HealthCore represents a large portion of U.S. healthcare recipients. Furthermore the described gaps are likely to be even larger in uninsured populations.
CONCLUSION
Our study of over 600 000 patients from the United States with established atherosclerotic cardiovascular disease reveals substantial underuse of statins and their high intensity formulations that is likely a major contributor to preventable death and disability. In particular, gaps in the care of younger patients, women, and those with non-coronary ASCVD show no signs of improving when compared to prior literature. Multifaceted intervention must be refined and implemented to address barriers to guideline-recommended care.
Supplementary Material
Figure 1. CONSORT Diagram.
Progressive identification of eligible participants based on inclusion criteria to generate final analytic cohort of 601 934.
CLINICAL PERSPECTIVES.
Competency in Systems-Based Practice:
Statin medications, particularly high-intensity formulations, are underutilized among patients with established atherosclerosis in the U.S. Younger patients, women, and those with comorbidities are less likely to take statin therapy, while statin users who are older, female, or have peripheral artery disease are less likely to receive high-intensity formulations.
Translational Outlook:
Auditing, feedback and benchmarking of high-intensity statin therapy (rather than simply the use of any statin drug) may be needed promote more frequent use.
ACKNOWLEDGEMENTS
This analysis was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award U18FD005292. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
ABBREVIATIONS
- ASCVD
atherosclerotic cardiovascular disease
- CAD
coronary artery disease
- CKD
chronic kidney disease
- HEDIS
Healthcare Effectiveness Data and Information Set
- LDL-C
low-density lipoprotein cholesterol
- PAD
peripheral artery disease
- PCP
primary care physician
- PCSK-9
Proprotein convertase subtilisin/kexin type 9
- PDC
proportion of days covered
- T2DM
Type 2 diabetes mellitus
Footnotes
Disclosures:
AJN: Grants from Diabetes Australia and the Royal Australasian College of Physicians.
KH Employee of HealthCore, subsidiary of Anthem
SS Employee of HealthCore, subsidiary of Anthem
ZE Previous employee of HealthCore
MJC: Employee of HealthCore, subsidiary of Anthem
MGN: Supported by NIH training grant T-32-HL069749.
SBC: Supported by US FDA grant U18FD005292 and CTTI membership fees https://www.ctti-clinicaltrials.org/membership/annual-membership-feesclinicaltrials.org/membership/annual-membership-fees.
KG: Employee of HealthCore
NJP: Grants from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Regeneron, Sanofi, Verily Life Sciences. Consulting fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Novo Nordisk.
CBG: Research grants and consulting/speaker fees from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceutica Products, L.P., and Pfizer, research grants from AKROS, Apple, AstraZeneca, Daichii-Sankyo, US Food & Drug Administration, GlaxoSmithKline, Medtronic Foundation, and Novartis Pharmaceutical Company, consulting/speaker fees from Abbvie, Bayer Corp US, Boston Scientific Corp, CeleCor Therapeutics, Correvio, Espero BioPharma, Medscape, Medtronic Inc., Merck, National Institutes of Health, NovoNordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics
Of 601 934 pts with ASCVD on 01.31.2019, 22.5% were on high-intensity statin, 27.6% were on low- or moderate intensity statin & 49.9% were not on any statin. Concerning gaps in high-intensity statin use must be addressed among females, older pts & those with non-CAD ASCVD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lloyd-Jones DM, Leip EP, Larson MG et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–8. [DOI] [PubMed] [Google Scholar]
- 2.Lindh M, Banefelt J, Fox KM et al. Cardiovascular event rates in a high atherosclerotic cardiovascular disease risk population: estimates from Swedish population-based register data. Eur Heart J Qual Care Clin Outcomes 2019;5:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins R, Reith C, Emberson J et al. Interpretanttion of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Braunwald E, McCabe CH et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]
- 5.LaRosa JC, Grundy SM, Waters DD et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Knowlton KU, May HT et al. Temporal changes in statin prescription and intensity at discharge and impact on outcomes in patients with newly diagnosed atherosclerotic cardiovascular disease-Real-world experience within a large integrated health care system: The IMPRES study. J Clin Lipidol 2018;12:1008–1018 e1. [DOI] [PubMed] [Google Scholar]
- 8.Bellows BK, Olsen CJ, Voelker J, Wander C. Antihyperlipidemic Medication Treatment Patterns and Statin Adherence Among Patients with ASCVD in a Managed Care Plan After Release of the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol. J Manag Care Spec Pharm 2016;22:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamprecht DG Jr., Shaw PB, King JB, Hogan KN, Olson KL. Trends in high-intensity statin use and low-density lipoprotein cholesterol control among patients enrolled in a clinical pharmacy cardiac risk service. J Clin Lipidol 2018;12:999–1007. [DOI] [PubMed] [Google Scholar]
- 10.Pokharel Y, Tang F, Jones PG et al. Adoption of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guideline in Cardiology Practices Nationwide. JAMA Cardiol 2017;2:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association Between Intensity of Statin Therapy and Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiol 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- 12.Salami JA, Warraich H, Valero-Elizondo J et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol 2017;2:56–65. [DOI] [PubMed] [Google Scholar]
- 13.Yao X, Shah ND, Gersh BJ, Lopez-Jimenez F, Noseworthy PA. Assessment of Trends in Statin Therapy for Secondary Prevention of Atherosclerotic Cardiovascular Disease in US Adults From 2007 to 2016. JAMA Netw Open 2020;3:e2025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan WV, Pearson TA, Bennett GC et al. ACC/AHA Special Report: Clinical Practice Guideline Implementation Strategies: A Summary of Systematic Reviews by the NHLBI Implementation Science Work Group: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;69:1076–1092. [DOI] [PubMed] [Google Scholar]
- 16.Bradley CK, Wang TY, Li S et al. Patient-Reported Reasons for Declining or Discontinuing Statin Therapy: Insights From the PALM Registry. J Am Heart Assoc 2019;8:e011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowenstern A, Li S, Navar AM et al. Does clinician-reported lipid guideline adoption translate to guideline-adherent care? An evaluation of the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J 2018;200:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowenstern A, Li S, Virani SS et al. Beliefs, risk perceptions, and lipid management among patients with and without diabetes: Results from the PALM registry. Am Heart J 2020;225:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowenstern A, Navar AM, Li S et al. Association of Clinician Knowledge and Statin Beliefs With Statin Therapy Use and Lipid Levels (A Survey of US Practice in the PALM Registry). Am J Cardiol 2019;123:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang WT, Hellkamp A, Doll JA et al. Lipid Testing and Statin Dosing After Acute Myocardial Infarction. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol 2014;78:684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon CP, de Lemos JA, Rosenson RS et al. Use of Lipid-Lowering Therapies Over 2 Years in GOULD, a Registry of Patients With Atherosclerotic Cardiovascular Disease in the US. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aujoulat I, Jacquemin P, Rietzschel E et al. Factors associated with clinical inertia: an integrative review. Adv Med Educ Pract 2014;5:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virani SS, Pokharel Y, Steinberg L et al. Provider understanding of the 2013 ACC/AHA cholesterol guideline. J Clin Lipidol 2016;10:497–504 e4. [DOI] [PubMed] [Google Scholar]
- 25.Wood FA, Howard JP, Finegold JA et al. N-of-1 Trial of a Statin, Placebo, or No Treatment to Assess Side Effects. N Engl J Med 2020;383:2182–2184. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs FD, Banach M, Mikhailidis DP, Malhotra A, Capewell S. Is statin-modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med 2016;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanna MG, Navar AM, Wang TY et al. Practice-level variation in statin use and low-density lipoprotein cholesterol control in the United States: Results from the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J 2019;214:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virani SS, Kennedy KF, Akeroyd JM et al. Variation in Lipid-Lowering Therapy Use in Patients With Low-Density Lipoprotein Cholesterol >/=190 mg/dL: Insights From the National Cardiovascular Data Registry-Practice Innovation and Clinical Excellence Registry. Circ Cardiovasc Qual Outcomes 2018;11:e004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daly CJ, Verrall K, Jacobs DM. Impact of Community Pharmacist Interventions With Managed Care to Improve Medication Adherence. J Pharm Pract 2021;34:694–702. [DOI] [PubMed] [Google Scholar]
- 30.Scirica BM, Cannon CP, Fisher NDL et al. Digital Care Transformation: Interim Report From the First 5000 Patients Enrolled in a Remote Algorithm-Based Cardiovascular Risk Management Program to Improve Lipid and Hypertension Control. Circulation 2021;143:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paruchuri K, Finneran P, Marston NA et al. Outcomes of a smartphone-based application with live health-coaching post-percutaneous coronary intervention. EBioMedicine 2021;72:103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson AJ, Pagidipati NJ, Granger CB. The SAMSON trial: Using a Placebo to Improve Medication Tolerability. Eur Heart J Cardiovasc Pharmacother 2021;(in press). [DOI] [PubMed] [Google Scholar]
- 33.Herrett E, Williamson E, Brack K et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ 2021;372:n135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriarty PM, Jacobson TA, Bruckert E et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol 2014;8:554–561. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Stroes E, Dent-Acosta RE et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016;315:1580–90. [DOI] [PubMed] [Google Scholar]
- 36.Hegele RA, Tsimikas S. Lipid-Lowering Agents. Circ Res 2019;124:386–404. [DOI] [PubMed] [Google Scholar]
- 37.Abshire DA, Lennie TA, Moser DK, Mudd-Martin GT. Perceptions Related to Cardiovascular Disease Risk in Caucasian College Males. Am J Mens Health 2016;10:NP136-NP144. [DOI] [PubMed] [Google Scholar]
- 38.Nanna MG, Navar AM, Zakroysky P et al. Association of Patient Perceptions of Cardiovascular Risk and Beliefs on Statin Drugs With Racial Differences in Statin Use: Insights From the Patient and Provider Assessment of Lipid Management Registry. JAMA Cardiol 2018;3:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navar AM, Wang TY, Li S et al. Patient-Perceived Versus Actual Risk of Cardiovascular Disease and Associated Willingness to Consider and Use Prevention Therapy. Circ Cardiovasc Qual Outcomes 2021;14:e006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy 2010;3:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs W, Amuta AO, Jeon KC. Health information seeking in the digital age: An analysis of health information seeking behavior among US adults. Cogent Social Sciences 2017;3:1302785. [Google Scholar]
- 42.Tennant B, Stellefson M, Dodd V et al. eHealth literacy and Web 2.0 health information seeking behaviors among baby boomers and older adults. J Med Internet Res 2015;17:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson AJ, Puri R, Nissen SE. Statins in a Distorted Mirror of Media. Curr Atheroscler Rep 2020;22:37. [DOI] [PubMed] [Google Scholar]
- 44.Krittanawong C, Kumar A, Wang Z et al. Coronary artery disease in the young in the US population-based cohort. Am J Cardiovasc Dis 2020;10:189–194. [PMC free article] [PubMed] [Google Scholar]
- 45.Zeitouni M, Clare RM, Chiswell K et al. Risk Factor Burden and Long-Term Prognosis of Patients With Premature Coronary Artery Disease. J Am Heart Assoc 2020;9:e017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truong QA, Murphy SA, McCabe CH, Armani A, Cannon CP, Group TS. Benefit of intensive statin therapy in women: results from PROVE IT-TIMI 22. Circ Cardiovasc Qual Outcomes 2011;4:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanna MG, Wang TY, Xiang Q et al. Sex Differences in the Use of Statins in Community Practice. Circ Cardiovasc Qual Outcomes 2019;12:e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gheorghe G, Toth PP, Bungau S et al. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics (Basel) 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med 1997;12:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colantonio LD, Hubbard D, Monda KL et al. Atherosclerotic Risk and Statin Use Among Patients With Peripheral Artery Disease. J Am Coll Cardiol 2020;76:251–264. [DOI] [PubMed] [Google Scholar]
- 51.Arya S, Khakharia A, Binney ZO et al. Association of Statin Dose With Amputation and Survival in Patients With Peripheral Artery Disease. Circulation 2018;137:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.