Abstract
The present study was carried out to evaluate the effect of dietary supplemental vitamin D3 on fibroblast growth factor 23 (FGF23) signals as well as phosphorus homeostasis and metabolism in laying hens. Fourteen 40-week-old Hy-Line Brown layers were randomly assigned into 2 treatments: 1) vitamin D3 restriction group (n = 7) fed 0 IU/kg vitamin D3 diet, and 2) regular vitamin D3 group (n = 7) fed 1,600 IU/kg vitamin D3 diet. The study lasted for 21 d. Serum parameters, phosphorus and calcium excretion status, and tissue expressions of type II sodium-phosphate co-transporters (NPt2), FGF23 signals and vitamin D3 metabolic regulators were determined. Hens fed the vitamin D3 restricted diet had decreased serum phosphorus levels (by 31.3%, P = 0.028) when compared to those fed regular vitamin D3 diet. In response to the decreased serum phosphorus, the vitamin D3 restricted laying hens exhibited: 1) suppressed kidney expressions of 25-hydroxyvitamin D 1-α-hydroxylase (CYP27B1, by 52.8%, P = 0.036) and 1,25-dihydroxyvitamin D 24-hydroxylase (CYP24A1, by 99.4%, P = 0.032); 2) suppressed serum levels of FGF23 (by 14.6%, P = 0.048) and increased serum alkaline phosphatase level (by 414.1%, P = 0.012); 3) decreased calvaria mRNA expressions of fibroblast growth factor receptors (FGFR1, by 85.2%, P = 0.003, FGFR2, by 89.4%, P = 0.014, FGFR3, by 88.8%, P = 0.017, FGFR4, by 89.6%, P = 0.030); 4) decreased kidney mRNA expressions of FGFR1 (by 65.5%, P = 0.021), FGFR4 (by 66.0%, P = 0.050) and KLOTHO (by 68.8%, P = 0.038); 5) decreased kidney protein expression of type 2a sodium-phosphorus co-transporters (by 54.3%, P = 0.039); and 6) increased percent excreta calcium (by 26.9%, P = 0.002). In conclusion, the deprivation of dietary vitamin D3 decreased FGF23 signals in laying hens by reducing serum FGF23 level and suppressing calvaria and kidney mRNA expressions of FGF23 receptors.
Keywords: Dietary vitamin D3, Fibroblast growth factor 23, Fibroblast growth factor receptor, KLOTHO, Laying hen
1. Introduction
The discovery of fibroblast growth factor 23 (FGF23), a bone-derived hormone (Yamashita et al., 2000), has broadened our understandings on body phosphate homeostasis (Blau and Collins, 2015). In the last 20 years, the classic parathyroid hormone (PTH)-1,25-dihydroxyvitamin D3 (1,25(OH)2D3) axis has been expanded into the novel FGF23-PTH-1,25(OH)2D3 axis in phosphorus-calcium metabolism homeostasis. It is reported that disorders in the FGF23-PTH-1,25(OH)2D3 axis have been linked to various phosphorus-calcium metabolism-related diseases such as X-linked hypophosphatemia (Jonsson et al., 2003), Familial isolated hypoparathyroidism (Thomee et al., 2005) and Vitamin-resistant rickets type 1 (Giannakopoulos et al., 2017). Therefore, illustrating the complex cross-talk interactions among those hormones from different organs would help to develop strategies for phosphorus metabolism management in laying hens.
The effect of FGF23 on vitamin D metabolism has been well investigated. FGF23 signals could downregulate 25-hydroxyvitamin D 1-α-hydroxylase (CYP27B1) and upregulate 1,25-dihydroxyvitamin D 24-hydroxylase (CYP24A1) expressions through kidney fibroblast growth factor receptor (FGFR) (e.g. FGFR1, FGFR3 and FGFR4), subsequently promoting the catabolism of 1,25(OH)2D3 and reducing 1,25(OH)2D3 production (Gattineni et al., 2011; Han et al., 2016; Shimada et al., 2004). However, the effects of vitamin D on FGF23 physiology are not clear. In mammals, it is proposed that vitamin D metabolism may interact with FGF23 in 2 ways: 1) 1,25(OH)2D3 directly regulates FGF23 gene transcription via vitamin D receptor (VDR) (Kolek et al., 2005; Yashiro et al., 2020); 2) 1,25(OH)2D3 enhances intestinal phosphorus absorption, increases circulating phosphorus levels (Sabbagh et al., 2009) and indirectly stimulates bone FGF23 secretion (Takashi et al., 2019).
Vitamin D3 levels vary among different diets in laying hens. The National Research Council (NRC, 1994) recommends 250 IU/kg vitamin D3 for egg-laying hens. But 1,000 to 2,000 IU/kg vitamin D3 have been documented as dietary optimal addition levels based on laying performance and eggshell quality (El-Maksoud, 2010; Goodson-Williams et al., 1986a, 1986b). In order to maximize the nutritional function of vitamin D3 (improve calcium and phosphorus utilization, egg quality and bone mineralization), higher doses were used in recent studies (Barnkob et al., 2020; Wen et al., 2019). Obviously, such big differences on dietary vitamin D3 levels would affect phosphorus metabolism physiology and FGF23 secretion status in poultry (Jiang et al., 2015). Excessive FGF23 secretion has been considered as the major reason for low phosphorus utilization and high phosphorus excretion (Ren et al., 2017, 2020b). Investigating the effects of dietary vitamin D3 levels on FGF23 signals in avian species would help to broaden our understandings in phosphorus metabolism mechanisms and provide a guide for proper dietary vitamin D3 addition in poultry.
In the current study, the effects of 2 dietary vitamin D3 levels (0 and 1,600 IU/kg) on FGF23 signals were studied in laying hens. Laying performance, egg quality parameters, phosphorus excretion status and key markers of body phosphorus homeostasis were measured. The study aimed to illustrate the relationship among vitamin D3, FGF23 signals and phosphorus metabolism in avian species.
2. Materials and methods
All experimental procedures involving animals were approved by the College of Animal Science and Technology Animal Care and Use Committee at the Northwest A&F University, and performed in accordance with the guidelines.
2.1. Birds, diets and study design
A total of 14 healthy 38-week-old Hy-Line Brown laying hens were randomly selected from a chicken house for 20,000 layers in a commercial farm (Wugong, Shaanxi, China). The sample size (n = 7) of the current study was calculated using the G∗Power 3.1.9.7 software based on our previous phosphorus nutrition studies conducted at the University of Wisconsin–Madison and Northwest A&F University (Ren et al., 2019, 2020a). All of the laying hens were fed with a corn-soybean meal-based diet containing 1,600 IU/kg vitamin D3 (same as the Regular vitamin D3 diet listed in Table S1), for 2 weeks (from 38 to 39 weeks of age). Thereafter (at the age of 40 weeks), the laying hens were randomly allotted to 2 groups: 1) restricted dietary vitamin D3 (n = 7), laying hens were fed with a corn-soybean meal-based laying hen diet with no extra vitamin D3 supplementation; 2) regular vitamin D3 (n = 7), laying hens were fed with the basal diet supplemented with 1,600 IU/kg vitamin D3, which meets the Chinese Feeding Standard of Chicken (NY/T 33-2004). We recorded the cracked egg rate information on a daily basis during the whole period of the feeding trial (Fig. S1). According to the cracked egg rate, it was obvious that dietary vitamin D3 restriction effectively suppressed the eggshell quality in week 3. Therefore, the feeding trial lasted for 3 weeks (from 40 to 42 weeks of age). The composition and nutrient levels are shown in Table S1.
2.2. Animal care and experimental procedures
The birds were individually housed in laying hen cages with raised wire floors (depth × width × height, 45 cm × 35 cm × 45 cm) in the animal nutrition research laboratory at the College of Animal Science and Technology, Northwest A&F University. Feed and freshwater were supplied ad libitum. Sixteen hours of lighting (05:30 to 21:30, a combination of natural and artificial lighting was used) were provided every day. The feeding trial lasted for 21 d. Egg production, egg weight and feed intake of each laying hen were daily recorded. Laying rate, average egg weight, average feed intake and feed-to-egg ratio were calculated. On the last day of feeding trial: 1) eggs were collected for the determination of egg quality parameters. Briefly, eggshell thickness was measured using a dial pipe gauge (ETG-1061; Robotmation, Co., Ltd., Tokyo, Japan); eggshell strength was measured using a texture analyzer (EFG-0503; Robotmation, Co., Ltd., Tokyo, Japan); egg yolk color, albumen height, and Haugh units were tested using a multifunction egg quality analyzer (EMT-5200; Robotmation, Co., Ltd., Tokyo, Japan); specific gravity was measured using a flotation method in saline solutions; shell index was calculated as shell weight of whole egg; 2) 24-h total excreta from each laying hen was collected for the determination of phosphorus and calcium excretion; 3) calvaria, liver, intestinal mucosa (duodenum, jejunum and ileum) and kidney samples were collected after euthanasia, frozen in liquid nitrogen, and then transferred to a −80 °C freezer until further analysis. All experimental analysis methods were performed as previously described (Liu et al., 2018; Ren et al., 2020b, 2020c).
2.3. Biochemical assay of plasma samples
At the end of the trial, blood samples (5 mL) were collected from wing vein using vacuum tubes without anticoagulant. Serum samples were separated, aliquoted into Eppendorf tubes after 15 min centrifugation at 594g and stored at −80 °C. For serum phosphorus concentration, samples were mixed with molybdic acid to generate phosphomolybdic acid, which was then restored to molybdenum blue for colorimetric analysis based on the manual of the kit supplier (catalogue no. C006-3). For serum calcium concentration, samples were reacted with Methyl Thymol Blue using a commercial colorimetric kit (catalogue no. C004-2). Serum alkaline phosphatase (AKP) activity was analyzed using a kit (catalogue no. A059-2). These 3 kits mentioned above were purchased from Nanjing Jiancheng Bioengineering Institute. Serum concentrations of FGF23 (catalogue no. ml00321112), 1,25(OH)2D3 (catalogue no. ml00697414) and PTH (catalogue no. ml00987411) were determined by sandwich enzyme-linked immunosorbent assays using commercial kits purchased from Meilian Biological Technology Co., Ltd. (Shanghai, China) following the manufacturer's instructions. Spectrophotometric analysis was accomplished using either a Synergy HT plate reader (BioTek, Winooski, VT) or a UV-1800 spectrophotometer (Shimadzu, Japan).
2.4. Phosphorus and calcium excretion
The 24-h excreta samples from each hen were fully collected on the last day of the feeding trial. The excreta samples were oven dried, air equilibrated, weighed, ground through a 1 mm mesh screen, and mixed thoroughly before analyses. For Ca and P analysis, 1 g of sample was ashed at 550 °C for 6 h in a muffle furnace. The ash was digested with hydrochloric acid solution and diluted in a 50 mL volumetric flask with deionized water. The phosphorus content of the excreta samples was determined colorimetrically with ammonium-vanadium-molybdate using a UV-1800 spectrophotometer (Shimadzu, Japan) (Ren et al., 2017). Calcium content of the excreta samples was analyzed with a Z-2000 flame atomic absorption spectrophotometer (Hitach, Japan) (Cheng et al., 2020). Percent phosphorus and calcium concentrations in the excreta samples are presented as air dried basis, and 24-h total excretion of phosphorus and calcium were calculated accordingly.
2.5. Quantitative real-time PCR
The real-time PCR analysis was performed as previously described (Liu et al., 2018). Total RNA of the samples was extracted using AG RNAex Pro Reagent (Accurate Biotechnology, Hunan, China) according the manufacturer's specifications. The concentration and purity of the extracted RNA were determined using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, USA). Qualified RNA was subjected to cDNA synthesis using a Primer Script RT Reagent Kit (TaKaRa, Dalian, China). Then, mRNA expression levels of genes were analyzed with a SYBR Premix Ex Taq kit (TaKaRa, Dalian, China) using aniCycler iQ5 real-time PCR machine (Bio-Rad, Hercules, CA). The primers sequences used for Quantitative real-time PCR analysis are listed in Table S2. All reactions were run in triplicate. Relative mRNA expression was calculated to ACTB using 2−ΔΔCt method.
2.6. Western blot
Western blot analysis was performed as previously described (Liu et al., 2018). Total proteins were extracted and the concentrations were determined with a BCA protein assay kit (catalogue no. WB003, Hat Biotechnology, Xi'an, China). The protein components were electrophoresed in 8% SDS-PAGE, and transferred electrophoretically to PVDF blotting membranes (catalogue no. 03010040001, Roche Diagnostics, Mannheim, Germany). The membranes were blocked and incubated with the primary antibody (diluted to 1:1,000) for 1 h at room temperature and then overnight at 4 °C. After 3 times washing, the secondary antibody (HRP-conjugated, diluted to 1:1,000) was applied for 1.5 h. Then, the membranes were washed, probed, and autoradiographed with a Chemiluminescence gel imaging system (DNR, Micro Chemi, Israel) using a Super Signal West Pico Trail Kit (catalogue no. 34580; Pierce, IL). Detailed information regarding primary and secondary antibodies is provided in Table S3. The blot density (measured with Image J software 1.8.0) was normalized to ACTB.
2.7. Statistical analysis
Data were analyzed by two-sided independent student's t-test using SPSS version 23.0 (IBM Corp., Chicago, IL). The individual laying hen was considered as the statistical unit. Results are presented as means and standard error of the mean. The significance was considered at P < 0.05.
3. Results
3.1. Laying performance and egg quality
No difference (P > 0.05) was observed on the baseline body weight and egg quality parameters (shell thickness, shell index, shell strength, albumen height, yolk pigmentation, Haugh units and specific gravity) between the 2 experimental groups (Table S4).
In the 3 weeks feeding trial (40 to 42 weeks of age), dietary vitamin D3 restriction had no effects (P > 0.05) on egg production performance (Table S5; laying rate, egg weight, daily feed intake, feed-to-egg ratio) and some of the egg quality parameters (Table 1; shell thickness, shell index, albumen height and Haugh units) in Hy-Line Brown laying hens. However, decreased (P < 0.05) shell strength, yolk pigmentation, and specific gravity were observed in vitamin D3 restricted laying hens (Table 1).
Table 1.
Effects of dietary vitamin D3 restriction on egg quality in laying hens.1
| Item | Vitamin D3 restriction2 | Regular vitamin D33 | P-value |
|---|---|---|---|
| Shell thickness, mm | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.678 |
| Shell index, % of whole egg | 10.4 ± 1.0 | 11.1 ± 0.3 | 0.495 |
| Shell strength, N | 35.8 ± 4.8 | 49.1 ± 3.0∗ | 0.015 |
| Albumen height, mm | 7.90 ± 0.67 | 7.16 ± 0.44 | 0.367 |
| Yolk pigmentation | 6.07 ± 0.43 | 7.00 ± 0.13∗ | 0.040 |
| Haugh units | 89.5 ± 3.1 | 84.3 ± 2.4 | 0.242 |
| Specific gravity | 1.082 ± 0.004 | 1.092 ± 0.002∗ | 0.043 |
Data are means ± SEM (n = 7). ∗, P < 0.05.
Vitamin D3 restriction, 0 IU/kg dietary vitamin D3.
Regular vitamin D3, 1,600 IU/kg dietary vitamin D3.
3.2. Serum analysis
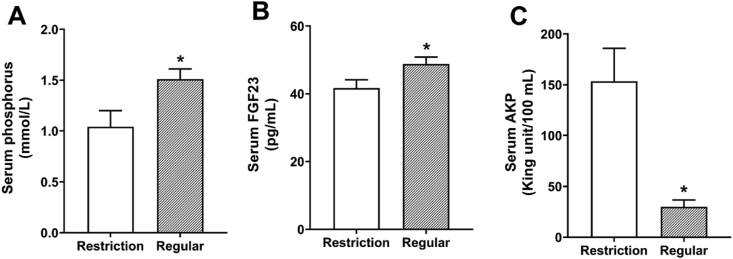
Serum levels of phosphorus and FGF23 decreased (P < 0.05) by 31.1% and 14.6% respectively, and serum levels of alkaline phosphatase increased (P < 0.05) by 414.1% in 0 IU/kg vitamin D3 group when compared with dietary 1,600 IU/kg vitamin D3 addition (Fig. 1).
Fig. 1.
Effects of dietary vitamin D3 levels on the (A) serum phosphorus, (B) FGF23, and (C) AKP of laying hens for 21 d. Data are means ± SEM (n = 7). ∗, P < 0.05. FGF23 = fibroblast growth factor 23; AKP = alkaline phosphatase. Restriction, 0 IU/kg dietary vitamin D3; Regular, 1,600 IU/kg dietary vitamin D3.
Dietary vitamin D3 restriction had no effects (P > 0.05) on serum levels of calcium, 1,25(OH)2D3 and PTH in Hy-Line Brown laying hens (Fig. S2).
3.3. mRNA expressions of FGF23, FGFR, KLOTHO and VDR
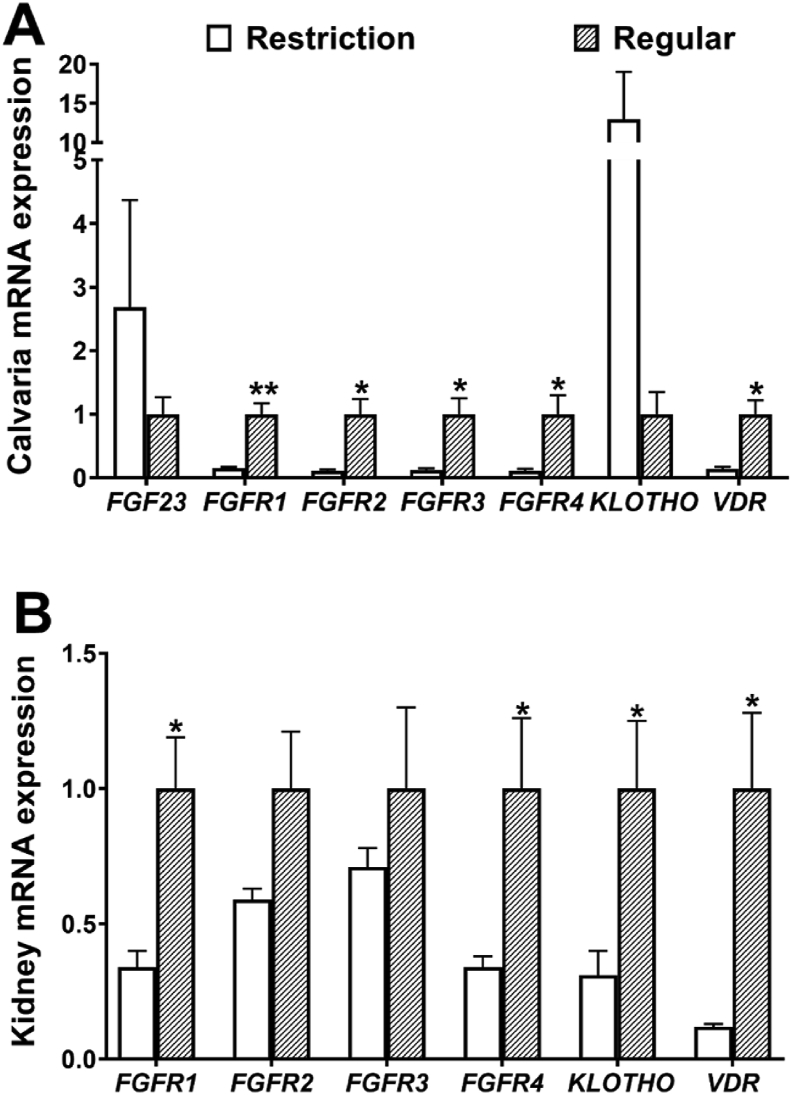
Calvaria expressions of FGFR1, FGFR2, FGFR3, FGFR4 and VDR were decreased (P < 0.05) by 85.2%, 89.4%, 88.8%, 89.6% and 86.4%, and kidney expressions of FGFR1, FGFR4, KLOTHO and VDR were reduced (P < 0.05) by 65.5%, 66.0%, 68.8% and 88.0%, respectively, when dietary vitamin D3 levels were decreased from 1,600 to 0 IU/kg (Fig. 2). Dietary vitamin D3 restriction had no effects (P > 0.05) on calvaria expressions of FGF23 and KLOTHO, as well as kidney expressions of FGFR2 and FGFR3.
Fig. 2.
Effects of dietary vitamin D3 restriction on mRNA levels of FGF23, FGFR, KLOTHO and VDR in the (A) calvaria and (B) kidney of laying hens for 21 d. Data are means ± SEM (n = 7). ∗, P < 0.05; ∗∗, P < 0.01. FGF23 = fibroblast growth factor-23; FGFR = fibroblast growth factor receptor; VDR = vitamin D receptor. Restriction, 0 IU/kg dietary vitamin D3; Regular, 1,600 IU/kg dietary vitamin D3.
Duodenum mRNA expressions of FGFR1 were decreased (P < 0.05) by 63.8% and expressions of KLOTHO were increased by 328.0%, jejunum expressions of FGFR3 and KLOTHO were decreased (P < 0.05) by 64.3% and 71.4%, ileum expressions of FGFR1, FGFR3, FGFR4 and VDR were decreased (P < 0.05) by 59.1%, 63.4%, 94.6% and 86.4%, respectively, when dietary vitamin D3 levels were decreased from 1,600 to 0 IU/kg (Fig. S3). Dietary vitamin D3 restriction had no effects (P > 0.05) on liver expressions of FGF23, FGFR1, FGFR2, FGFR3, FGFR4, VDR and KLOTHO, duodenum expressions of FGFR2, FGFR3, FGFR4 and VDR, jejunum expressions of FGFR1, FGFR2, FGFR4 and VDR, and ileum expressions of FGFR2 and KLOTHO.
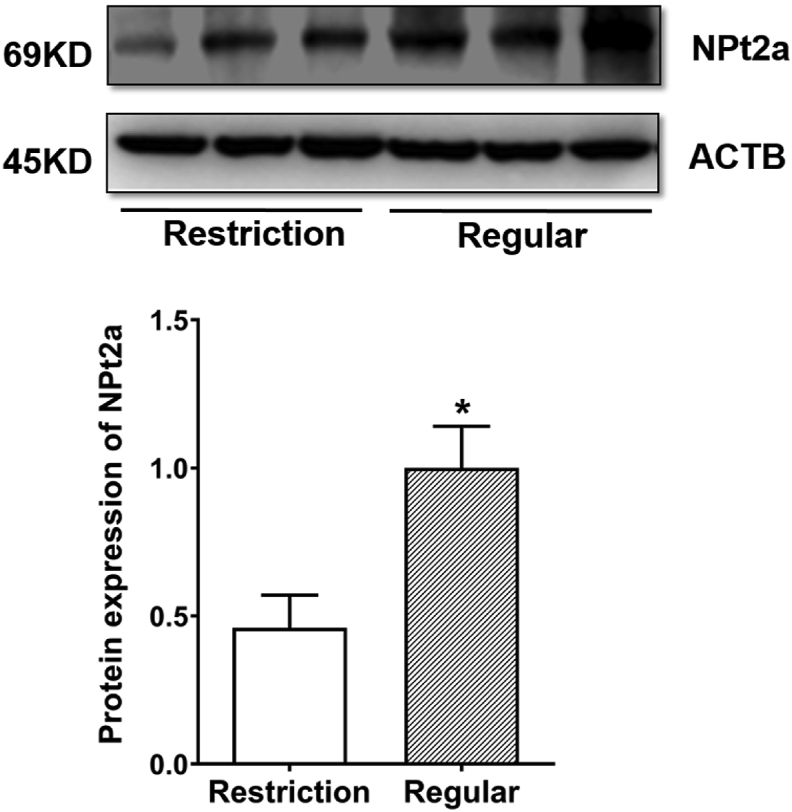
3.4. Kidney SLC34A1 mRNA expression and NPt2a protein expression
There was no difference on kidney mRNA expression of SLC34A1 (P > 0.05; Fig. S4D). However, lower (P < 0.05) kidney protein expression of NPt2a was found in the vitamin D3 restriction group (Fig. 3).
Fig. 3.
Effects of dietary vitamin D3 restriction on protein levels of NPt2a in the kidney of laying hens for 21 d. Data are means ± SEM (n = 3). ∗, P < 0.05. NPt2a = type 2a sodium-phosphate co-transporters; ACTB = actin beta. Restriction, 0 IU/kg dietary vitamin D3; Regular, 1,600 IU/kg dietary vitamin D3.
3.5. Intestine SLC34A2 mRNA expression and NPt2b protein expression
No difference (P > 0.05) was found on SLC34A2 mRNA expressions in duodenum (Fig. S4A), jejunum (Fig. S4B) and ileum (Fig. S4C) as well as NPt2b protein expressions in duodenum (Fig. S5A), jejunum (Fig. S5B) and ileum (Fig. S5C).
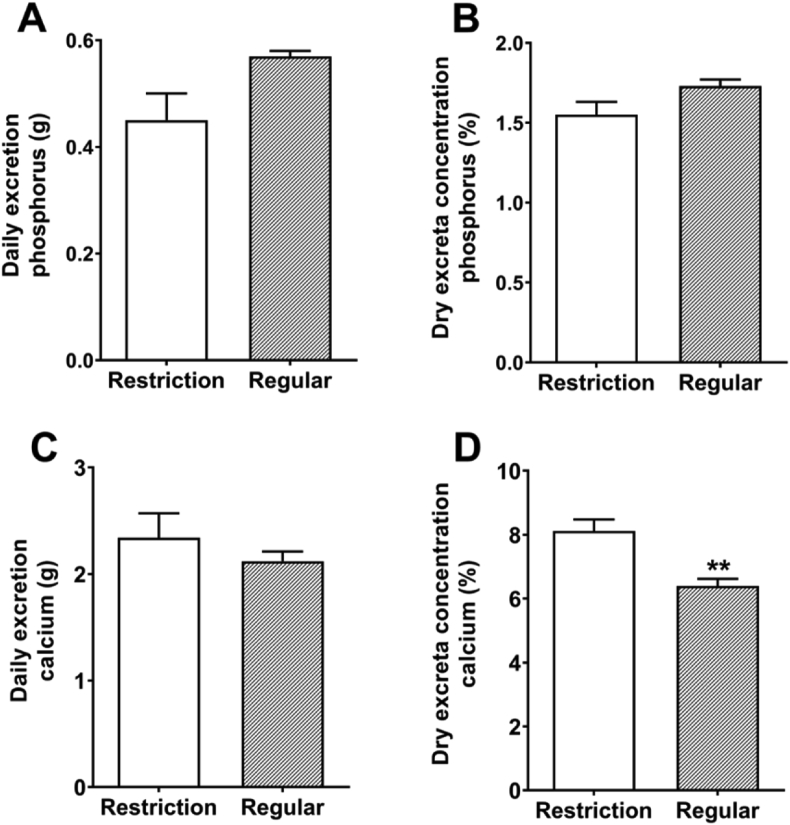
3.6. Phosphorus and calcium excretion
No differences (P > 0.05) were detected in daily phosphorus excretion and dry excreta phosphorus concentration between vitamin D3 restriction and regular vitamin D3 groups (Fig. 4). Daily calcium excretion was increased (P < 0.05) by 26.9% (increased from 6.40% to 8.12%) in regular vitamin D3 groups, while dry excreta calcium concentration was not affected (P > 0.05).
Fig. 4.
Effects of dietary vitamin D3 restriction on the excretion of phosphorus (A, daily phosphorus excretion; B, dry excreta phosphorus concentration) and calcium (C, daily calcium excretion; D, dry excreta phosphorus concentration) of laying hens. Data are means ± SEM (n = 7). ∗∗, P < 0.01. Restriction, 0 IU/kg dietary vitamin D3; Regular, 1,600 IU/kg dietary vitamin D3.
3.7. mRNA expressions of CYP2R1, CYP27B1, and CYP24A1
Dietary vitamin D3 restriction had no effect (P > 0.05) on cytochrome P450 family 2 subfamily R member 1 (CYP2R1) in liver. CYP27B1 and CYP24A1 expression were decreased (P < 0.05) by 52.8% and 99.4%, respectively, in vitamin D3 restriction group (Fig. 5).
Fig. 5.
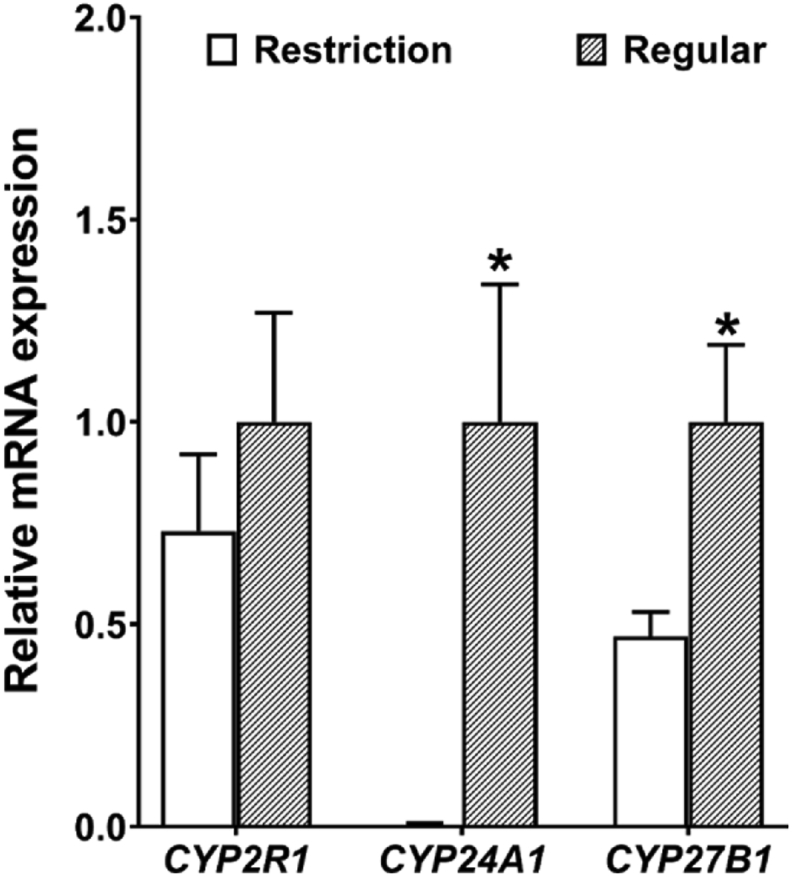
Effects of dietary vitamin D3 restriction on mRNA levels (CYP2R1, in the liver; CYP24A1 and CYP27B1 in the kidney) of laying hens for 21 d. Data are means ± SEM (n = 7). ∗, P < 0.05. CYP27B1 = 25-hydroxyvitamin D 1-α-hydroxylase; CYP24A1 = 1,25-dihydroxyvitamin D 24-hydroxylase; CYP2R1 = cytochrome P450 family 2 subfamily R member 1. Restriction, 0 IU/kg dietary vitamin D3; Regular, 1,600 IU/kg dietary vitamin D3.
4. Discussion
In this study, we found a potential regulation pathway: dietary vitamin D3 deficiency causes a decrease in serum phosphorus levels, which in turn leads to a decrease in serum FGF23 levels. These results are of great significance for revealing the phosphorus nutrition effect of dietary vitamin D3. For humans and animals, dietary vitamin D3 deficiency reduces serum phosphorus levels (ŚWiĄTkiewicz et al., 2017; Uwitonze et al., 2020). Laying hens will experience further loss of phosphorus with long time vitamin D3 deficiency, which may lead to some undesirable consequences (including bone loss and lower eggshell quality) (ŚWiĄTkiewicz et al., 2017). Our results show that the dietary vitamin D3 deficiency causes a decrease in serum phosphorus levels and an increase in serum alkaline phosphatase. Obviously, the lack of dietary vitamin D3 poses a huge challenge to the phosphorus homeostasis system of laying hens. The 3 hormones (FGF23, PTH and 1,25(OH)2D3) play important roles in maintaining the body's phosphorus homeostasis. Their levels directly reflect the trends of phosphorus absorption, metabolism and excretion in poultry (Proszkowiec-Weglarz and Angel, 2013). Circulating FGF23 levels are elevated in patients with early chronic kidney disease and are postulated to a pathophysiological role of FGF23 in the abnormal regulation of phosphorus metabolism (Hasegawa et al., 2010). Similarly, compared with normal dietary vitamin D3, dietary vitamin D3 deficiency caused a decrease in serum FGF23 levels, but had no effect on PTH and 1,25(OH)2D3 levels in this study. Presumably, serum FGF23 may be a key marker that mediates the effect of dietary vitamin D3 on phosphorus nutrition in laying hens. Previous studies have showed that FGF23 signaling plays a key role in phosphorus metabolism in poultry (Gloux et al., 2020). In laying hens, excessive FGF23 has been proven to be not conducive to the retention for phosphorus retention and improvement of eggshell quality by anti-FGF23 antibody technology (Ren et al., 2017, 2018). Therefore, clarifying the changes of FGF23 signal in various tissues will be helpful to reveal the phosphorus nutrition function of vitamin D3.
In poultry, the liver and calvaria are considered to be the main sources of FGF23 production (Wang et al., 2018). Our results showed that dietary vitamin D3 deficiency reduces the mRNA expression of FGFR (including FGFR1, FGFR2, FGFR3 and FGFR4) and VDR in the calvaria, but has no effect on the liver. Hepatic FGF23 mRNA expression is not sensitive to dietary phosphorus, nicotinamide and vitamin D3 levels (Ren et al., 2020b, 2020c), suggesting that FGF23 may be related to other physiological functions in laying hens. Calvaria FGFR1 can sense changes in extracellular phosphorus levels and regulate the production of FGF23 (Takashi et al., 2019). Similarly, serum phosphorus could change FGFR1 expression in the calvaria, which is an important factor in regulating the secretion of FGF23 (Ren et al., 2020b). It cannot be ignored that these receptors play an important role in osteogenic development, cartilage proliferation and bone loss, thereby regulating the calcium and phosphorus homeostasis of bones (Arnold et al., 2021; Starczak et al., 2018; Xie et al., 2020). Medullary bone, a highly plastic bone tissue unique to egg-laying birds, provides about 40% calcium for eggshell formation (Kerschnitzki et al., 2014). However, the regulatory mechanism of mobilization and remodeling of the medullary bone is still a mystery. Also, since the femur and tibia are more important for phosphorus metabolism and could store more phosphorus when compared to the calvaria, the mechanism of FGF23 signals in these bone tissues will need to be further investigated. By establishing a vitamin D3 deficiency model, many signaling molecules that may be related to bone mobilization have been discovered in this study. FGFR and KLOTHO are widely expressed in various tissues (Kuro, 2019). FGF23 develops its metabolic functions by binding and activating FGFR tyrosine kinases in a KLOTHO co-receptor dependent pattern (Chen et al., 2018). KLOTHO converts the classic FGFR into FGF23-specific receptors (Urakawa et al., 2006). Thus, we analyzed FGF23 signals in kidney and small intestines that control the entry and exit of the body's phosphorus. The results showed that vitamin D3 restriction reduced mRNA expression of FGFR1, FGFR4, KLOTHO and VDR in kidney, FGFR1 in the duodenum, FGFR3 and KLOTHO in the jejunum, and FGFR1, FGFR3, FGFR4 in the ileum. The kidney is the direct target organ of FGF23 (Gattineni et al., 2014). Renal phosphate transport regulated by FGF23 is mediated by FGFR1, FGFR4 and KLOTHO (Takashi and Fukumoto, 2018). However, the intestine is an unproven target organ for FGF23 (Edmonston and Wolf, 2020), where FGF23 receptor expression needs to be further studied.
NPt2b occupies 90% active absorption of phosphorus in intestine, thereby reflecting the intestinal phosphorus absorption capacity (Hernando et al., 2015). In this study, there was no significant difference in the mRNA and protein expression of NPt2b in the duodenum, jejunum and ileum between 2 groups. It is generally believed that 1,25(OH)2D3, rather than FGF23 and PTH, can directly regulate the abundance of NPt2b in the small intestine (Sabbagh et al., 2009). There is no significant difference in the serum 1,25(OH)2D3 level between the 2 groups in this study, which may be the reason why the NPt2b expression has not been changed. The phenomenon implies that the changes of FGF23 and its receptors cannot directly affect the phosphorus absorption in the small intestine. The physiological function of FGF23 signals in the small intestine needs to be further revealed. The expression of renal phosphorus transporters is strictly regulated by multiple hormones including FGF23, PTH and 1,25(OH)2D3 (Tatsumi et al., 2016). In this study, compared with the 1,600 IU/kg vitamin D3 group, the dietary vitamin D3 restriction group reduced the protein expression of NPt2a, which is in line with the previous study (Kurnik and Hruska, 1985). Tissue-specific 1,25(OH)2D3 metabolism exists in different tissues (Nguyen-Yamamoto et al., 2017). Specific 1,25(OH)2D3 metabolism in kidney, and response to dietary vitamin D3 levels, needs further study. Interestingly, dietary vitamin D3 deficiency caused a decrease in renal FGF23 signaling, while the protein expression of NPt2a did not increase. FGF23 signal might serve as a secondary factor and affect the renal expression of NPt2a in the vitamin D3 deficiency model. In this study, dietary vitamin D3 deficiency did not affect fecal phosphorus excretion and intestinal phosphorus absorption, suggesting that the kidneys had the same ability to reabsorb phosphorus between the 2 groups. The intraperitoneal injection of 1,25(OH)2D3 caused a decrease in the reabsorption of phosphorus by increasing FGF23 signals in the kidney (Hernando et al., 2020). These results implied that phosphorus excretion in the kidney is regulated by many factors. In the future, the regulatory factors of renal phosphorus excretion should be paid further attention, which will be a potential way to solve the low phosphorus utilization rate in poultry.
Our research showed that dietary vitamin D3 deficiency downregulated CYP24A1 mRNA expression in kidney, thereby maintaining the stability of 1,25(OH)2D3 levels. When dietary vitamin D3 levels were decreased from 1,600 to 0 IU/kg, mRNA expression of CYP24A1 and CYP27B1 in the kidney was reduced by 141 and 2 times, respectively, but hepatic CYP2R1 was unaffected. CYP2R1 mainly converts vitamin D3 into 25(OH)D (the storage form of vitamin D) in the liver (Christakos et al., 2016). As the active form of vitamin D, 1,25(OH)2D3 is an important implementer to develop physiological functions (Christakos et al., 2019). In order to ensure 1,25(OH)2D3 stability, the kidney, reducing the mRNA expression of CYP27B1 adaptively and CYP24A1 greatly, effectively prevents the degradation of vitamin D. Renal FGF23 signals reduce the production of 1,25(OH)2D3 by inhibiting the mRNA expression of CYP27B1 and activating CYP24A1 (Vervloet, 2019). However, in some diseases, higher CYP27B1 mRNA expression has been reported to be linked with excess FGF23 (Fujiwara et al., 2003; Yuan et al., 2004). These results indicate that CYP24A1 is a key target for regulating serum 1,25(OH)2D3 in laying hens.
Vitamin D3 is an essential vitamin in poultry diet, and a vitamin D3 short-term deficiency model is often used to evaluate the nutritional value and physiological function of vitamin D (Singh et al., 1986). This model causes calcium deficiency in laying hens, such as deterioration of eggshell quality and bone loss (Goodson-Williams et al., 1986b). In the current study, compared with the regular vitamin D3 group, the vitamin D3 restriction group had reduced eggshell quality (including eggshell strength and egg specific gravity) and increased fecal calcium excretion, which indicated that the dietary vitamin D3 deficiency model was successfully established. While the current egg number could be used to indicate the effectiveness of vitamin D3 restriction and illustrate the mechanism of vitamin D3/FGF23 signals, more eggs will need to be involved to fully reflect the effects of dietary vitamin D3 on egg quality parameters. These results indicated that dietary vitamin D3 deficiency promoted bone mobilization and thereby increased fecal calcium excretion. In order to meet the high calcium demand for eggshell formation, a special bone structure (medullary bone) has been formed in laying hens (Wang et al., 2020). The calcium storage form is composed of calcium carbonate and hydroxyapatite (Dominguez-Gasca et al., 2019). We found that bone mobilization did not increase phosphorus excretion. A large amount of calcium carbonate may be lost in medullary bone, which leads to the imbalance of bone calcium-phosphorus homeostasis, and further causes bone diseases. With the additional aggravation of dietary vitamin D3 deficiency, an overall reduction in the production performance will occur in laying hens (Goodson-Williams et al., 1986a, 1986b). However, this study showed that there was no significant difference in production performance between the 2 groups. Obviously, short-term vitamin D3 deficiency could help screen the key molecules during the process of vitamin D3 regulating the calcium and phosphorus metabolism.
5. Conclusions
In conclusion, dietary vitamin D3 deficiency decreased renal NPt2a protein expression, and subsequently decreased serum phosphorus levels and suppressed renal FGF23 signals in laying hens. The constant phosphorus excretion is a result of the sophisticated process of dietary vitamin D3 deficiency and kidney FGF23 signal suppression. FGF23 signal may be involved in the regulation of serum 1,25(OH)2D3 levels by CYP24A1 in layer kidneys. These results demonstrated the theoretical and practical significance of optimal dietary vitamin D3 supplementation, which could increase phosphorus utilization and reduce the environmental pollution of phosphorus from laying hens.
Author contributions
Jiakun Yan and Chong Pan carried out the study and wrote the manuscript. Yanli Liu helped with data analysis. Xujie Liao, Jionghao Chen, Yufei Zhu and Xinhuo Huang contributed to animal care and sample analysis. Xiaojun Yang and Zhouzheng Ren developed the research idea and designed the project. Zhouzheng Ren helped with data analysis, manuscript preparation and manuscript revision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This project was funded by the National Natural Science Foundation of China (31902175), Shaanxi Provincial Key Research and Development Program (2019NY-077), Shaanxi Feed Engineering Technology Research Center (2019HBGC-16), and the Program for Shaanxi Science & Technology from Shaanxi Provincial Science and Technology Department (2021TD-30). We acknowledge the members of the Innovative Research Team of Animal Nutrition & Healthy Feeding of Northwest A&F University for providing valuable assistance in sample collection and analysis.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.07.010.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Arnold A., Dennison E., Kovacs C.S., Mannstadt M., Rizzoli R., Brandi M.L., et al. Hormonal regulation of biomineralization. Nat Rev Endocrinol. 2021;17(5):261–275. doi: 10.1038/s41574-021-00477-2. [DOI] [PubMed] [Google Scholar]
- Barnkob L.L., Argyraki A., Jakobsen J. Naturally enhanced eggs as a source of vitamin D: a review. Trends Food Sci Technol. 2020;102:62–70. [Google Scholar]
- Blau J.E., Collins M.T. The PTH-Vitamin D-FGF23 axis. Rev Endocr Metab Disord. 2015;16:165–174. doi: 10.1007/s11154-015-9318-z. [DOI] [PubMed] [Google Scholar]
- Chen G., Liu Y., Goetz R., Fu L., Jayaraman S., Hu M.C., Moe O.W., Liang G., Li X., Mohammadi M. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Yan J.K., Sun W.Q., Chen Z.Y., Wu S.R., Ren Z.Z., Yang X.J. Effect of inorganic phosphate supplementation on egg production in Hy-Line Brown layers fed 2000 FTU/kg phytase. Animal : Int J Animal Biosci. 2020;14:2246–2252. doi: 10.1017/S1751731120001597. [DOI] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Li S.S., De La Cruz J., Bikle D.D. New developments in our understanding of vitamin D metabolism, action and treatment. Metab, Clin Exp. 2019;98:112–120. doi: 10.1016/j.metabol.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gasca N., Benavides-Reyes C., Sanchez-Rodriguez E., Rodriguez-Navarro A.B. Changes in avian cortical and medullary bone mineral composition and organization during acid-induced demineralization. Eur J Mineral. 2019;31:209–216. [Google Scholar]
- Edmonston D., Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol. 2020;16:7–19. doi: 10.1038/s41581-019-0189-5. [DOI] [PubMed] [Google Scholar]
- El-Maksoud A.A. Effect of dietary calcium and vitamin D3 levels on egg production and egg shell quality of Hy-Line Brown-egg type laying hens. Egypt Poult Sci. 2010;30:1097–1120. [Google Scholar]
- Fujiwara I., Aravindan R., Horst R.L., Drezner M.K. Abnormal regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in X-linked hypophosphatemia: a translational or post-translational defect. J Bone Miner Res : Off J Am Soc Bone and Mineral Res. 2003;18:434–442. doi: 10.1359/jbmr.2003.18.3.434. [DOI] [PubMed] [Google Scholar]
- Gattineni J., Alphonse P., Zhang Q., Mathews N., Bates C.M., Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Ren Physiol. 2014;306:F351–F358. doi: 10.1152/ajprenal.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattineni J., Twombley K., Goetz R., Mohammadi M., Baum M. Regulation of serum 1,25(OH)2 vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Ren Physiol. 2011;301:F371–F377. doi: 10.1152/ajprenal.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos A., Efthymiadou A., Chrysis D. A case of vitamin-D-dependent rickets type 1A with normal 1,25-dihydroxyvitamin D caused by 2 novel mutations of the CYP27B1 gene. Horm Res Paediat. 2017;87:58–63. doi: 10.1159/000446774. [DOI] [PubMed] [Google Scholar]
- Gloux A., Le Roy N., Meme N., Piketty M.L., Prie D., Benzoni G., Gautron J., Nys Y., Narcy A., Duclos M.J. Increased expression of fibroblast growth factor 23 is the signature of a deteriorated Ca/P balance in ageing laying hens. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson-Williams R., Roland D.A., Sr., McGuire J.A. Effects of feeding graded levels of vitamin D3 on egg shell pimpling in aged hens. Poultry Sci. 1986;65:1556–1560. doi: 10.3382/ps.0651556. [DOI] [PubMed] [Google Scholar]
- Goodson-Williams R., Roland D.A., Sr., McGuire J.A. Eggshell pimpling in young hens as influenced by dietary vitamin D3. Poultry Sci. 1986;66:1980–1986. doi: 10.3382/ps.0651556. [DOI] [PubMed] [Google Scholar]
- Han X., Yang J., Li L., Huang J., King G., Quarles L.D. Conditional deletion of Fgfr1 in the proximal and distal tubule identifies distinct roles in phosphate and calcium transport. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Nagano N., Urakawa I., Yamazaki Y., Iijima K., Fujita T., Yamashita T., Fukumoto S., Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- Hernando N., Myakala K., Simona F., Knopfel T., Thomas L., Murer H., Wagner C.A., Biber J. Intestinal depletion of NaPi-IIb/slc34a2 in mice: renal and hormonal adaptation. J Bone Miner Res : Off J Am Soc Bone and Mineral Res. 2015;30:1925–1937. doi: 10.1002/jbmr.2523. [DOI] [PubMed] [Google Scholar]
- Hernando N., Pastor-Arroyo E.M., Marks J., Schnitzbauer U., Knopfel T., Burki M., et al. 1,25(OH)2 vitamin D3 stimulates active phosphate transport but not paracellular phosphate absorption in mouse intestine. J Physiol. 2020;599(4):1131–1150. doi: 10.1113/JP280345. [DOI] [PubMed] [Google Scholar]
- Jiang S., Jiang Z., Yang K., Chen F., Zheng C., Wang L. Dietary vitamin D3 requirement of Chinese yellow-feathered broilers. Poultry Sci. 2015;94:2210–2220. doi: 10.3382/ps/pev163. [DOI] [PubMed] [Google Scholar]
- Jonsson K.B., Zahradnik R., Larsson T., White K.E., Sugimoto T., Imanishi Y., Yamamoto T., Hampson G., Koshiyama H., Ljunggren O., Oba K., Yang I.M., Miyauchi A., Econs M.J., Lavigne J., Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- Kerschnitzki M., Zander T., Zaslansky P., Fratzl P., Shahar R., Wagermaier W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone. 2014;69:109–117. doi: 10.1016/j.bone.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., Collins J.F., Haussler M.R., Ghishan F.K. 1 alpha,25-Dihydroxyvitamin D-3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol-Gastr L. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- Kurnik B.R., Hruska K.A. Mechanism of stimulation of renal phosphate transport by 1,25-dihydroxycholecalciferol. Biochim Biophys Acta. 1985;817:42–50. doi: 10.1016/0005-2736(85)90066-5. [DOI] [PubMed] [Google Scholar]
- Kuro O.M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Shen J., Yang X., Sun Q., Yang X. Folic acid reduced triglycerides deposition in primary chicken hepatocytes. J Agric Food Chem. 2018;66:13162–13172. doi: 10.1021/acs.jafc.8b05193. [DOI] [PubMed] [Google Scholar]
- Nguyen-Yamamoto L., Karaplis A.C., St-Arnaud R., Goltzman D. Fibroblast growth factor 23 regulation by systemic and local osteoblast-synthesized 1,25-dihydroxyvitamin D. J Am Soc Nephrol : JASN (J Am Soc Nephrol) 2017;28:586–597. doi: 10.1681/ASN.2016010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient requirements of poultry. 9th ed. National Academies Press; Washington (DC): 1994. [Google Scholar]
- Proszkowiec-Weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility1. J Appl Poultry Res. 2013;22:609–627. [Google Scholar]
- Ren Z., Ebrahimi M., Butz D.E., Sand J.M., Zhang K., Cook M.E. Antibody to fibroblast growth factor 23-peptide reduces excreta phosphorus of laying hens. Poultry Sci. 2017;96:127–134. doi: 10.3382/ps/pew189. [DOI] [PubMed] [Google Scholar]
- Ren Z., Piepenburg A.J., Yang X., Cook M.E. Effect of anti-fibroblast growth factor 23 antibody on phosphate and calcium metabolism in adenine gavaged laying hens. Poultry Sci. 2019;98(10):4896–4900. doi: 10.3382/ps/pez239. [DOI] [PubMed] [Google Scholar]
- Ren Z., Sun W., Cheng X., Liu Y., Han D., Yan J., Pan C., Duan Y., Yang X. The adaptability of Hy-Line Brown laying hens to low-phosphorus diets supplemented with phytase. Poultry Sci. 2020;99:3525–3531. doi: 10.1016/j.psj.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Yan J., Hu Q., Liu X., Pan C., Liu Y., Zhang X., Yang X., Yang X. Phosphorus restriction changes the expression of fibroblast growth factor 23 and its receptors in laying hens. Front Physiol. 2020;11:85. doi: 10.3389/fphys.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z.Z., Piepenburg A.J., Butz D.E., Claus J.R., Cook M.E. Vaccine to fibroblast growth factor 23 peptides increases eggshell strength. Poultry Sci. 2018;97:882–889. doi: 10.3382/ps/pex373. [DOI] [PubMed] [Google Scholar]
- Ren Z.Z., Yan J.K., Pan C., Liu Y.L., Wen H.Y., Yang X., Huang X.H., Lei X.G., Yang X.J. Supplemental nicotinamide dose-dependently regulates body phosphorus excretion via altering type II sodium-phosphate Co-transporter expressions in laying hens. J Nutr. 2020;150:2070–2076. doi: 10.1093/jn/nxaa148. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y., O'Brien S.P., Song W., Boulanger J.H., Stockmann A., Arbeeny C., Schiavi S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol : JASN (J Am Soc Nephrol) 2009;20:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Joyner C.J., Peddie M.J., Taylor T.G. Changes in the concentrations of parathyroid hormone and ionic calcium in the plasma of laying hens during the egg cycle in relation to dietary deficiencies of calcium and vitamin D. Gen Comp Endocrinol. 1986;61:20–28. doi: 10.1016/0016-6480(86)90245-5. [DOI] [PubMed] [Google Scholar]
- Starczak Y., Reinke D.C., Barratt K.R., Ryan J.W., Russell P.K., Clarke M.V., St-Arnaud R., Morris H.A., Davey R.A., Atkins G.J., Anderson P.H. Absence of vitamin D receptor in mature osteoclasts results in altered osteoclastic activity and bone loss. J Steroid Biochem Mol Biol. 2018;177:77–82. doi: 10.1016/j.jsbmb.2017.10.022. [DOI] [PubMed] [Google Scholar]
- ŚWiĄTkiewicz S., Arczewska-WŁOsek A., Bederska-Lojewska D., JÓZefiak D. Efficacy of dietary vitamin D and its metabolites in poultry - review and implications of the recent studies. World Poultry Sci J. 2017;73:57–68. [Google Scholar]
- Takashi Y., Fukumoto S. FGF23 beyond phosphotropic hormone. Trends Endocrinol Metabol: TEM (Trends Endocrinol Metab) 2018;29:755–767. doi: 10.1016/j.tem.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Takashi Y., Kosako H., Sawatsubashi S., Kinoshita Y., Ito N., Tsoumpra M.K., Nangaku M., Abe M., Matsuhisa M., Kato S., Matsumoto T., Fukumoto S. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci U S A. 2019;116:11418–11427. doi: 10.1073/pnas.1815166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi S., Miyagawa A., Kaneko I., Shiozaki Y., Segawa H., Miyamoto K. Regulation of renal phosphate handling: inter-organ communication in health and disease. J Bone Miner Metabol. 2016;34:1–10. doi: 10.1007/s00774-015-0705-z. [DOI] [PubMed] [Google Scholar]
- Thomee C., Schubert S.W., Parma J., Le P.Q., Hashemolhosseini S., Wegner M., Abramowicz M.J. GCMB mutation in familial isolated hypoparathyroidism with residual secretion of parathyroid hormone. J Clin Endocrinol Metabol. 2005;90:2487–2492. doi: 10.1210/jc.2004-2450. [DOI] [PubMed] [Google Scholar]
- Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Uwitonze A.M., Rahman S., Ojeh N., Grant W.B., Kaur H., Haq A., Razzaque M.S. Oral manifestations of magnesium and vitamin D inadequacy. J Steroid Biochem Mol Biol. 2020;200:105636. doi: 10.1016/j.jsbmb.2020.105636. [DOI] [PubMed] [Google Scholar]
- Vervloet M. Renal and extrarenal effects of fibroblast growth factor 23. Nature reviews. Nephrology. 2019;15:109–120. doi: 10.1038/s41581-018-0087-2. [DOI] [PubMed] [Google Scholar]
- Wang M., O'Connor J.K., Bailleul A.M., Li Z.H. Evolution and distribution of medullary bone: evidence from a new Early Cretaceous enantiornithine bird. Natl Sci Rev. 2020;7:1068–1078. doi: 10.1093/nsr/nwz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.M., Zhao J.P., Wang X.J., Jiao H.C., Wu J.M., Lin H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poultry Sci. 2018;97:2258–2266. doi: 10.3382/ps/pey092. [DOI] [PubMed] [Google Scholar]
- Wen J., Livingston K.A., Persia M.E. Effect of high concentrations of dietary vitamin D3 on pullet and laying hen performance, skeleton health, eggshell quality, and yolk vitamin D3 content when fed to W36 laying hens from day of hatch until 68 wk of age. Poultry Sci. 2019;98:6713–6720. doi: 10.3382/ps/pez386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Su N., Yang J., Tan Q., Huang S., Jin M., Ni Z., Zhang B., Zhang D., Luo F., Chen H., Sun X., Feng J.Q., Qi H., Chen L. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Yoshioka M., Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- Yashiro M., Ohya M., Mima T., Nakashima Y., Kawakami K., Yamamoto S., Kobayashi S., Yano T., Tanaka Y., Sonou T., Tatsuta K., Negi S., Shigematsu T. Active vitamin D and vitamin D analogs stimulate fibroblast growth factor 23 production in osteocyte-like cells via the vitamin D receptor. J Pharmaceut Biomed Anal. 2020;182:113139. doi: 10.1016/j.jpba.2020.113139. [DOI] [PubMed] [Google Scholar]
- Yuan B., Xing Y., Horst R.L., Drezner M.K. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in the hyp-mouse. Endocrinology. 2004;145:3804–3812. doi: 10.1210/en.2004-0192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.