Abstract
This experiment evaluated the impacts of essential oils (EO) and protease (PRO), independently or in combination, on growth performance, antioxidation, inflammation and intestinal function of weaned pigs. One hundred and sixty weaned pigs (21 d of age, BW of 6.74 ± 0.20 kg) were randomly divided into 4 treatments with 8 replicate pens of 5 pigs per pen. Dietary treatments included the following: 1) control diet (CON), 2) CON with 300 mg/kg essential oils (EO), 3) CON with 500 mg/kg protease (PRO), 4) CON with 300 mg/kg essential oil and 500 mg/kg protease (EO + PRO). On d 8, one pig from each pen was selected for sampling. The remaining pigs were fed for an additional week and growth performance was monitored during this period. Dietary treatments had no marked effects (P > 0.05) on the growth performance of pigs. However, pigs receiving EO diet had higher (P < 0.05) serum glutathione peroxidase (GSH-Px) activity, and tended to decrease (P = 0.063) serum concentration of tumor necrosis factor-α (TNF-α). In addition, pigs receiving EO diet had higher (P < 0.05) abundances of phylum Actinobacteria, and genera Bifidobacterium, and lower (P < 0.05) phylum Bacteroidetes and genera Alloprevotella in colonic digesta. Pigs receiving PRO diet decreased (P < 0.05) the serum concentration of malondialdehyde (MDA) and diamine oxidase activity, increased (P < 0.05) the villus height and the ratio of villus height to crypt depth in duodenum, increased sucrase activity in jejunal mucosa, and also increased the abundance of phylum Actinobacteria in colonic digesta. Furthermore, the synergistic effects of EO and PRO was observed (P < 0.05) for pigs with decreasing serum TNF-α concentration and increasing serum GSH-Px activity. Collectively, the results indicated that dietary supplementation of EO and PRO had no significant effects on growth performance of weaned pigs. EO diet appeared to improve antioxidant activity and intestinal microbiota, while PRO diet improved intestinal morphology and digestive enzyme activity, and there was a synergistic effect of EO and PRO on reducing inflammatory parameters in weaned pigs.
Keywords: Essential oil, Immune response, Intestinal health, Oxidative stress, Protease, Weaned pig
1. Introduction
Piglets are exposed to nutritional, environmental and social stresses during the immediate post-weaning period (Kim et al., 2012). This results in low feed consumption, changes in intestinal microbiota and mucosal atrophy, inducing inflammation and oxidative stress; which are often accompanied by suboptimal growth performance and higher morbidity and mortality, causing large economic losses to the livestock industry (Boudry et al., 2004; Zhu et al., 2012).
Weaning stress and intake of solid diets often leads to poor protein digestion and absorption (Zuo et al., 2015); accordingly, excessive protein residues in the large intestine disturb the intestinal microbial ecosystem, inducing diarrhea and growth retardation (Wang et al., 2018). The inclusion of exogenous feed enzymes in animal diets is considered to be an effective strategy to improve feed efficiency and reduce feed costs in animal production (Adeola and Cowieson, 2011). Protease (PRO) has the capacity to hydrolyze intact proteins that are resistant to digestion and has been widely used in pig and poultry diets (Zuo et al., 2015). Recent studies have shown that PRO was able to improve growth performance, nutrient absorption efficiency, intestinal development and health status of weaned pigs (Zuo et al., 2015; Park et al., 2020). In addition, essential oils (EO) are volatile aromatic compounds and are known to have antimicrobial, antiviral and antioxidant properties (Spisni et al., 2020). Several studies have reported that EO, such as carvacrol and thymol, could improve nutrient absorption, antioxidant capacity, intestinal ecosystem and growth performance of pigs (Li et al., 2012; Zeng et al., 2015).
To our knowledge, there is limited data about the effects of dietary supplementation with EO and PRO on growth performance, antioxidation, inflammation and intestinal function of weaned pigs. In this study, we hypothesise that the combination of EO and PRO would have interactive effects on growth performance, based on their independent bioactive properties.
2. Materials and methods
The experiment was approved by the Sichuan Agricultural University Institutional Animal Care and Use Committee (Approval number: CD-SYXK-2017-015).
2.1. Essential oils and protease products
Essential oil (Next enhance 150 premix plus, Novus International, Inc.) is a coated product containing 2.5% thymol, 2.5% carvacrol and 95% of an inert carrier. Protease (Cibenza DP100, Novus International, Inc.) is an alkaline serine endopeptidase derived from Bacillus licheniformis with an activity of 600,000 units/g. The manufacturer's recommended inclusion rate is 300 units/g of feed.
2.2. Animals and diets
One hundred and sixty weaned pigs (Duroc × [Landrace × Yorkshire]; BW of 6.74 ± 0.20 kg; 21 d of age) were randomly divided into 4 dietary treatments with 8 replicate pens and 5 pigs per pen. Dietary treatments included the following: 1) control diet (CON), 2) CON with 300 mg/kg essential oils (EO), 3) CON with 500 mg/kg protease (PRO), 4) CON with 300 mg/kg EO and 500 mg/kg PRO (EO + PRO). As shown in Table 1, the CON diet was formulated according to the NRC (2012) recommendations. All pigs were housed in a temperature-controlled room with a slatted plastic floor. Each pen (1.5 m × 2.1 m) was equipped with a sided feeder and a nipple watering device. Pigs had free access to diets and drinking water during the 14-d experimental period.
Table 1.
Composition and nutrient content of the basal diet (%, as-fed basis).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 50.60 |
| De-hulled soybean meal (46% CP) | 13.00 |
| Fermented soybean meal | 10.00 |
| Whey permeate (2% CP) | 10.00 |
| Fish meal (62.5% CP) | 4.00 |
| Milk powder (16% CP) | 2.00 |
| Soybean oil | 2.50 |
| Sucrose | 2.00 |
| Glucose | 2.00 |
| L-Lys HCl (98%) | 0.59 |
| DL-Met (98.5%) | 0.26 |
| L-Thr (98%) | 0.22 |
| L-Trp (98%) | 0.05 |
| Choline chloride (50%) | 0.16 |
| Limestone | 0.92 |
| Dicalcium phosphate | 0.75 |
| Salt | 0.40 |
| Zinc oxide (75%) | 0.20 |
| Mineral premix1 | 0.30 |
| Vitamin premix2 | 0.05 |
| Total | 100.00 |
| Nutrient composition3 | |
| DE, Mcal/kg | 3.57 |
| CP | 18.96 |
| Ca | 0.80 |
| Available P | 0.42 |
| Standardized ideal digestible AA | |
| Lys | 1.43 |
| Met | 0.54 |
| Met + Cys | 0.78 |
| Thr | 0.83 |
| Trp | 0.22 |
Mineral premix provided per kilogram of diet: Fe, 100 mg; Cu, 150 mg; Mn, 20 mg; Zn, 100 mg; I, 0.3 mg; Se, 0.3 mg.
Vitamin premix provided per kilogram of diet: vitamin A, 9,000 IU; vitamin D3, 3,000 IU; vitamin E, 20 IU; vitamin K3, 3.0 mg; vitamin B1, 1.5 mg; vitamin B2, 4.0 mg; vitamin B6, 3.0 mg; vitamin B12, 0.2 mg; niacin, 30.0 mg; pantothenic acid, 15.0 mg; folic acid, 0.75 mg; biotin, 0.1 mg.
Nutrient levels were calculated.
2.3. Growth performance
Individual pig BW and pen feed intake were determined every week for the calculation of average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F).
2.4. Sampling
In the morning of d 8, one pig with BW close to the average of each pen was chosen for bleeding via the precaval vein. Blood samples were collected into nonheparinized vacuum tubes, followed by 3,000 × g at 4 °C for 15 min, and the serum samples were frozen at −20 °C for subsequent analyses. Afterwards, these pigs were anaesthetized with sodium pentobarbital (50 mg/kg BW) and euthanized. Then, the duodenum and jejunum were removed and immediately rinsed with ice-cold phosphate buffered saline. Approximately 2-cm duodenal segment was collected and fixed in 4% formaldehyde for histological analyses. The mucosa of the jejunum was collected using a scalpel blade, rapidly frozen in liquid nitrogen and stored at −80 °C for the examination of disaccharidase activities. Additionally, digesta from the middle part of colon was aseptically collected into sterile tubes, and frozen at −80 °C for analyzing gut microbial composition.
2.5. Small intestinal morphology
The duodenal samples were removed from fixative solution, then dehydrated, and embedded in paraffin wax. Each duodenal sample was sectioned at 5-μm thickness and stained with hematoxylin and eosin. Representative photomicrographs were taken with a Leica DMI3000B microscopy (Leica, Solms, Germany). A minimum of 10 well-oriented complete crypt–villus units from each segment were measured using NIS-Elements BR software 2.20 (Nikon, Tokyo, Japan).
2.6. Disaccharidase activities
Approximately 0.4 g of frozen jejunal mucosa samples were homogenized in ice-cold saline solution (1:9, wt/vol) and then centrifuged at 3,000 × g for 10 min at 4 °C. The supernatant was used to determine the maltase and sucrase activities using spectrophotometric kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.7. Serum parameters assay
The activity of serum diamine oxidase and the concentrations of serum cytokines (interleukin 1β [IL-1β] and tumor necrosis factor-α [TNF-α]) were determined using commercial ELISA kits (Jiancheng Bioengineering Institute, Nanjing, China). Moreover, serum antioxidant-related parameters including malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) were determined using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) as described previously (Su et al., 2018).
2.8. Gut microbiome
Microbial bacterial DNA was isolated from the colon contents using a commercial kit (Qiagen, Hilden, Germany). Before sequencing, the DNA quality was checked by electrophoresis analysis. DNA concentrations were determined using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the primers F515 and R806 according to the description of previous study (Peng et al., 2019). Pyrosequencing of 16S rDNA was performed on the Illumina HiSeq PE250 platform (Novogene, Beijing, China). The taxonomy of each 16S rRNA gene sequence was analyzed according to our previous report (Peng et al., 2019). Raw sequence data were quality-filtered and demultiplexed using the Quantitative Insights into Microbial Ecology (QIIME) software (version 1.7.0). The chimeric sequences were detected and removed using the UCHIME Algorithm. Then the remaining high-quality clean tags were clustered into operation taxonomic units (OTU) using Uparse v7.0.1001 at 97% sequence similarity. Diversity within communities (Alpha diversity) calculations and taxonomic community assessments were performed by QIIME 1.7.0. The 16S rRNA gene sequence data were deposited in the National Centre for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number PRJNA779534.
2.9. Statistical analysis
Data obtained were analyzed by ANOVA using a 2 × 2 factorial design with the general linear model (GLM) procedure (SPSS 20.0; IBM-SPSS Inc., Chicago, IL, USA). The effects of EO, PRO and their interactions were included in the statistical model. The following model was used: Yijk = μ + Ai + Bj + (AB)ij + eijk (i = 1, 2; j = 1, 2; k = 1, 2, …, nij), where Yijk is the dependent variable, μ is the overall mean, Ai is the effect of EO, Bj is the effect of PRO, (AB)ij is the interaction effect between EO and PRO, and eijk is the residual error. For analysis of intestinal microbiota, data of relative abundance at phylum and genus levels were log-transformed before statistical analysis. The pen was considered as the experimental unit for growth performance, whereas all selected pigs from each pen were considered as the experimental unit for the other indices. When the interaction was significant, Duncan's multiple-comparison test was applied to examine the statistical differences among different treatments. Correlations between the differential bacterial genera and inflammatory cytokine were assessed by Spearman correlation analyses. Results are expressed as means ± SEM. P < 0.05 were considered statistically significant, while 0.05 < P < 0.10 was considered as a tendency.
3. Results
3.1. Growth performance and diarrhea index
As shown in Table 2, dietary treatments had no significant effects on the BW, ADG, ADFI, and G:F throughout the study.
Table 2.
Effects of essential oils and protease on growth performance of weaned pigs1.
| Item | CON | EO | PRO | EO + PRO |
P-value |
||
|---|---|---|---|---|---|---|---|
| EO | PRO | EO × PRO | |||||
| BW, kg | |||||||
| Initial | 6.74 ± 0.11 | 6.73 ± 0.10 | 6.74 ± 0.17 | 6.74 ± 0.25 | 0.994 | 0.988 | 0.953 |
| Wk 1 | 7.19 ± 0.10 | 7.22 ± 0.14 | 7.20 ± 0.15 | 7.24 ± 0.27 | 0.845 | 0.933 | 0.983 |
| Wk 2 | 8.82 ± 0.09 | 8.93 ± 0.20 | 8.94 ± 0.23 | 8.89 ± 0.31 | 0.872 | 0.859 | 0.730 |
| ADG, g/d | |||||||
| Wk 1 | 63.8 ± 10.7 | 69.9 ± 13.3 | 74.8 ± 6.7 | 70.8 ± 4.5 | 0.825 | 0.603 | 0.603 |
| Wk 2 | 233 ± 11 | 244 ± 15 | 247 ± 13 | 236 ± 8 | 0.966 | 0.705 | 0.476 |
| ADFI, g/d | |||||||
| Wk 1 | 108 ± 5 | 110 ± 6 | 115 ± 4 | 105 ± 8 | 0.632 | 0.774 | 0.391 |
| Wk 2 | 365 ± 16 | 389 ± 20 | 389 ± 22 | 348 ± 19 | 0.655 | 0.749 | 0.104 |
| G:F | |||||||
| Wk 1 | 0.57 ± 0.07 | 0.62 ± 0.09 | 0.63 ± 0.07 | 0.72 ± 0.12 | 0.462 | 0.403 | 0.829 |
| Wk 2 | 0.65 ± 0.03 | 0.63 ± 0.03 | 0.64 ± 0.04 | 0.69 ± 0.05 | 0.760 | 0.418 | 0.399 |
CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO; BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; G:F = gain to feed ratio; Wk = week.
Values are means ± SEM (n = 8).
3.2. Antioxidation-related parameters
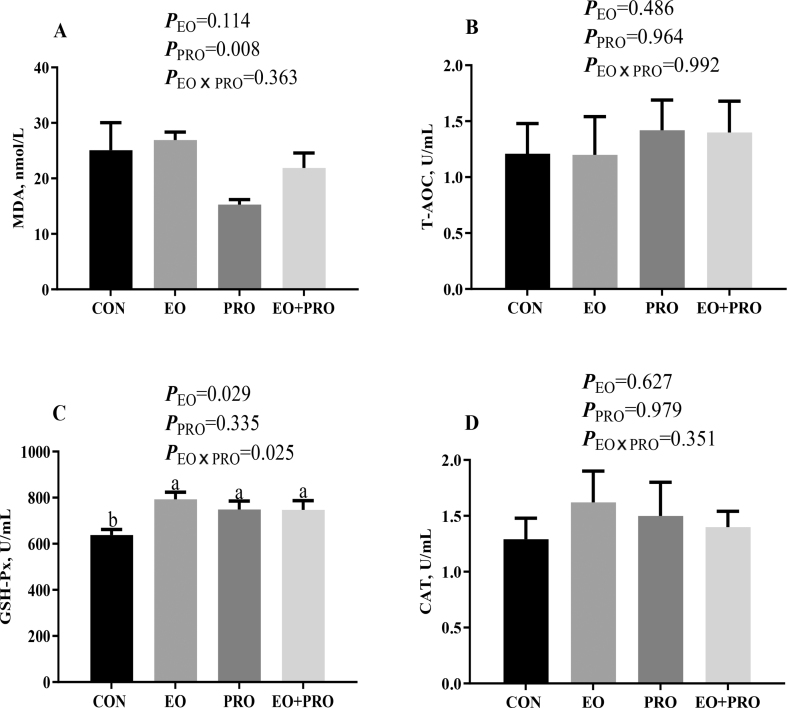
As shown in Fig. 1A, dietary supplementation of PRO decreased (P < 0.05) the serum MDA content. In addition, dietary supplementation of EO increased (P < 0.05) serum GSH-Px activity (Fig. 1C). There was an interaction between EO and PRO on the serum GSH-Px activity (P < 0.05), which was the lowest within the CON group. However, the activities of T-AOC and CAT were not markedly affected by dietary treatments (Fig. 1B, D).
Fig. 1.
Effects of essential oils and protease on redox status of weaned pigs. (A) MDA content; (B) T-AOC; (C) GSH-Px activity; (D) CAT activity. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO; MDA = malondialdehyde; T-AOC = total antioxidant capacity; GSH-Px = glutathione peroxidase; CAT = catalase. Values are means and standard errors represented by vertical bars (n = 8). a,b Means without a common letter differ (P < 0.05).
3.3. Serum cytokine concentrations
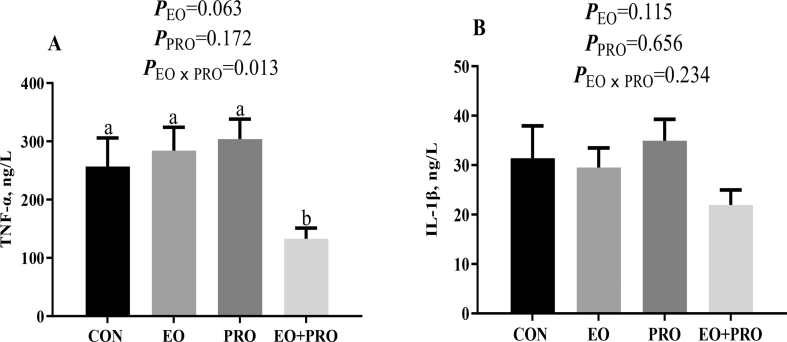
Dietary supplementation of EO had a tendency to decrease (P < 0.10) the serum concentration of TNF-α (Fig. 2A). In addition, there was an interaction between EO and PRO on the serum concentration of TNF-α (P < 0.05), which was found to be the lowest in the EO + PRO group. The serum concentration of IL-1β was not markedly affected by dietary treatments (Fig. 2B).
Fig. 2.
Effects of essential oils and protease on the serum cytokine concentrations of weaned pigs. (A) TNF-α concentration; (B) IL-1β concentration. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO; IL-1β = interleukin 1β; TNF-α = tumor necrosis factor-α. Values are means and standard errors represented by vertical bars (n = 8). a,b Means without a common letter differ (P < 0.05).
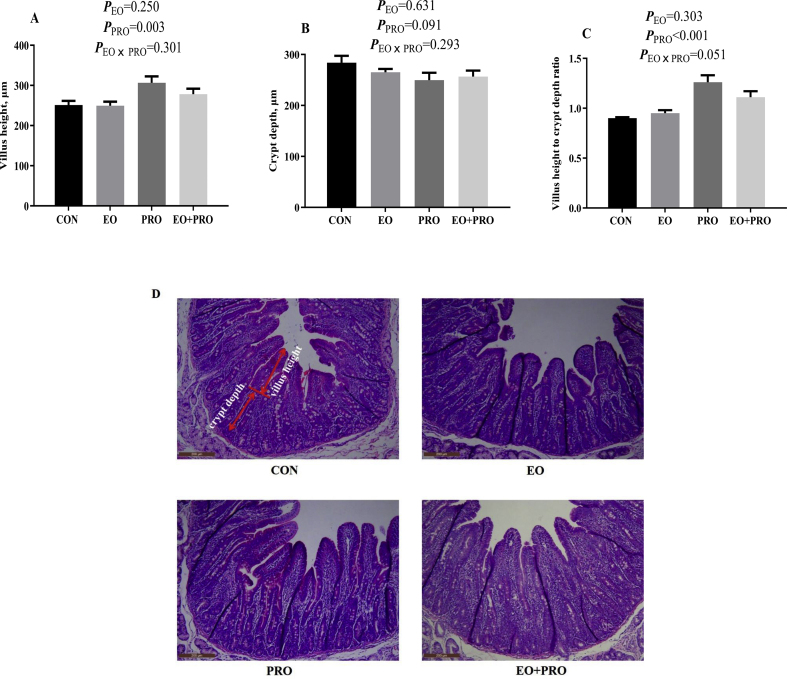
3.4. Duodenal morphology
Dietary supplementation of PRO increased (P < 0.05) the villus height in the duodenum (Fig. 3A, D). Dietary treatments had no significant effects on the crypt depth in the duodenum (Fig. 3B). Moreover, dietary supplementation of PRO increased (P < 0.05) the ratio of villus height to crypt depth (Fig. 3C). There was a tendency for an interaction between EO and PRO on the ratio of villus height to crypt depth (P < 0.10).
Fig. 3.
Effects of essential oils and protease on the duodenal morphology of weaned pigs. (A) Villus height; (B) crypt depth; (C) villus height to crypt depth ratio; (D) section image of duodenal tissue. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO. The red arrows indicate the villus height and crypt depth in the image. Values are means and standard errors represented by vertical bars (n = 6).
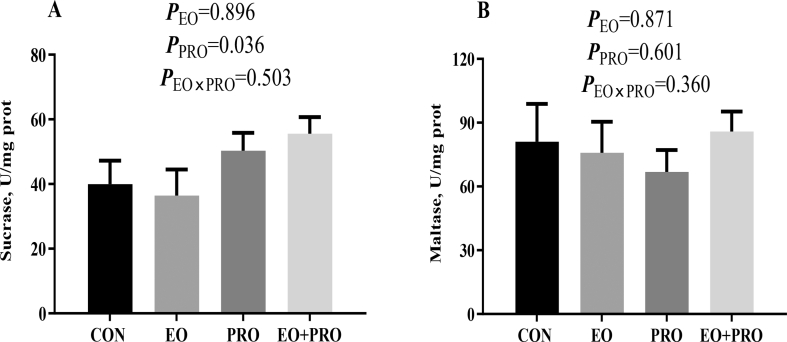
3.5. Disaccharidase activities in the jejunal mucosa
As shown in Fig. 4A, dietary supplementation of PRO increased (P < 0.05) the sucrase activity in jejunum mucosa. However, dietary treatments had no significant effects on maltase activity (Fig. 4B).
Fig. 4.
Effects of essential oils and protease on the disaccharidase activities in the jejunal mucosa of weaned pigs. (A) Sucrase activity; (B) maltase activity. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO. Values are means and standard errors represented by vertical bars (n = 8).
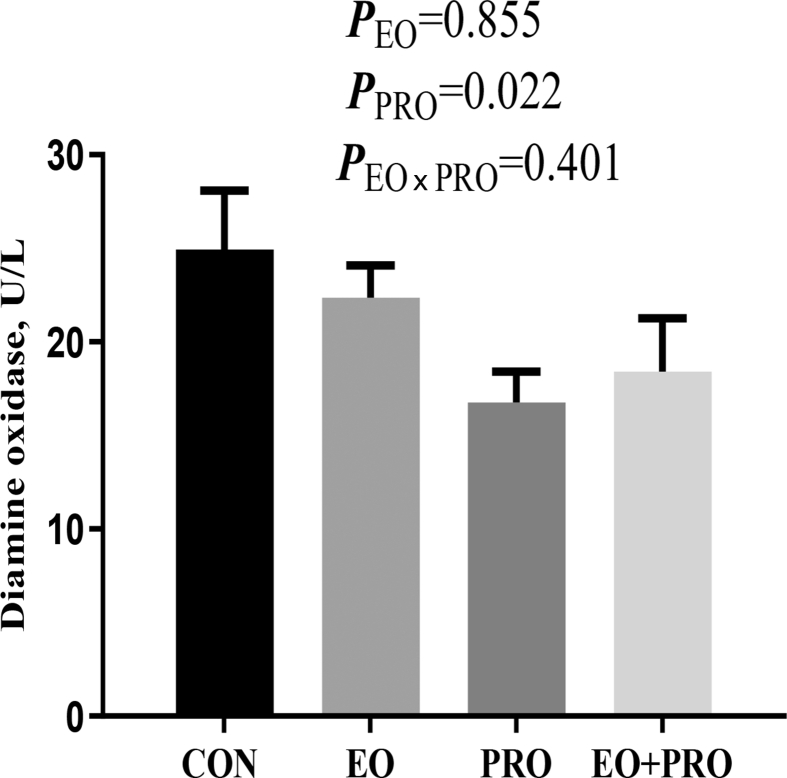
3.6. Serum diamine oxidase activity
As shown in Fig. 5, dietary supplementation of PRO decreased (P < 0.05) the serum diamine oxidase activity. However, the serum diamine oxidase activity was not significantly affected by EO or the interaction between EO and PRO.
Fig. 5.
Effects of essential oils and protease on the serum diamine oxidase activity of weaned pigs. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease; EO × PRO = interaction between EO and PRO. Values are means and standard errors represented by vertical bars (n = 8).
3.7. Colonic microbiome
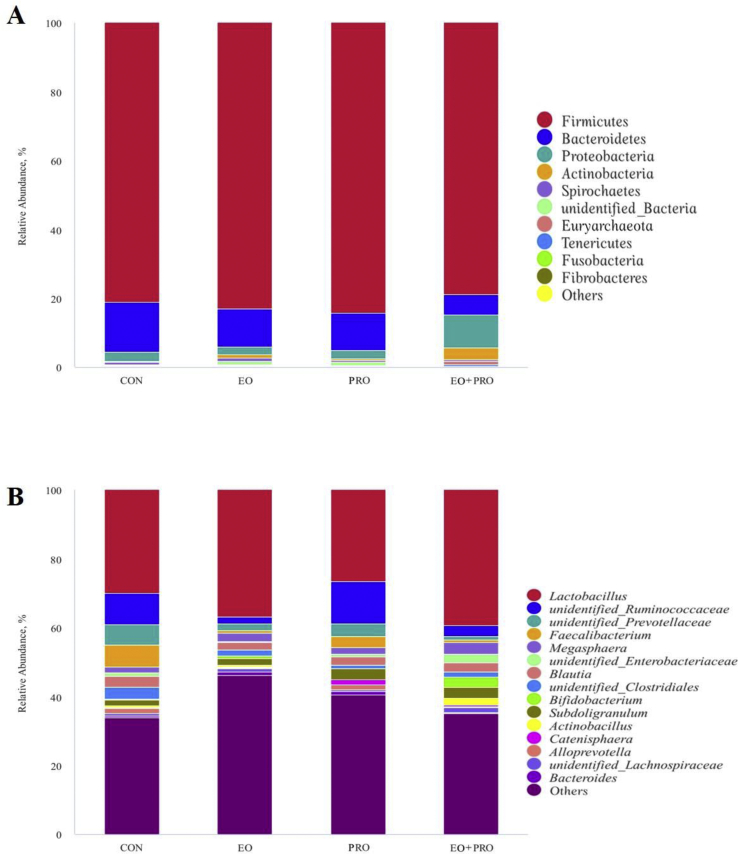
A total of 1,587,648 sequence reads were obtained from 20 digesta samples. Based on a 97% similarity level, 12,197 OTU were obtained from colonic digesta samples, with an average of 610 ± 21 OTU per sample. The alpha diversity of colonic microbiota was shown in Appendix Table 1. The observed species, Shannon index, Chao 1 index, Simpson index and Abundance Coverage Estimator (ACE) were not significantly affected by EO, PRO and their combination.
Regarding the microbial composition at phylum level, we identified 6 phyla, namely; Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes and Euryarchaeota, that had a relative abundance greater than 1.0% in at least one experimental group (Fig. 6A). Of these 6 phyla, Firmicutes (78.96% to 84.27%) and Bacteroidetes (5.73% to 14.59%) are the 2 most dominant phyla in the bacterial community of colonic digesta. Dietary supplementation of EO increased the relative abundance of Actinobacteria (P < 0.05), tended to increase the relative abundance of Euryarchaeota (P < 0.10), but decreased the relative abundance of Bacteroidetes (P < 0.05) (Appendix Table 2). In addition, dietary supplementation of PRO also increased the relative abundance of Actinobacteria (P < 0.05). The top 15 most abundant genera in the different groups were shown in Fig. 6B. Dietary supplementation of EO decreased the relative abundances of Alloprevotella and Faecalibacterium (P < 0.05), tended to decrease the relative abundances of unidentified_Ruminococcaceae and unidentified_Prevotellaceae (P < 0.10), and increased the relative abundance of Bifidobacterium (P < 0.05). Furthermore, there was a tendency of interaction between EO and PRO on the relative abundance of unidentified_Clostridiales (P < 0.10) (Appendix Table 3).
Fig. 6.
Effects of essential oils and protease on the composition of colonic microbiota in weaned pigs (n = 5) at the phylum (A) and genus (B) level. Each bar represents the relative abundance of each treatment. CON = pigs fed basal diet; EO = pigs fed basal diet supplemented with 300 mg/kg essential oils; PRO = pigs fed basis diet supplemented with 500 mg/kg protease; EO + PRO = pigs fed basal diet with 300 mg/kg essential oil and 500 mg/kg protease.
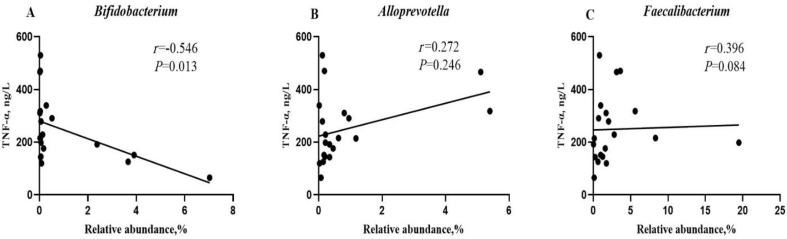
3.8. Correlation analysis between differential bacterial genera and inflammatory cytokine
The correlations between the critical differential bacterial genera and serum inflammatory cytokine (TNF-α) were analyzed (Fig. 7). Bifidobacterium was negatively correlated with the concentration of TNF-α (r = −0.546, P < 0.05).
Fig. 7.
Spearman correlation analysis between differential bacterial genera and inflammatory cytokine of the weaned pigs. TNF-α = tumor necrosis factor-α.
4. Discussion
Weaning is considered one of the most important events in the early developmental stage of pig's life. Previous studies have shown that weaning induces oxidative stress by increasing the contents of MDA and free radicals; such as hydroxyl radical and superoxide anions, and impairs the antioxidant defense system in piglets (Xu et al., 2014). A dynamic balance between the generation and the capacity to scavenge the reactive oxygen species is closely related to cell activity and animal health (Luo et al., 2020). It is difficult to maintain the balance of redox status during weaning period, which may be the crucial factor inducing the post-weaning growth check, higher morbidity and mortality (Degroote et al., 2019). In this study, dietary supplementation of PRO reduced serum MDA content, which is generally considered as a biomarker for lipid peroxidation and oxidative stress (Ito et al., 2019). The inclusion of PRO may reduce the oxidative stress by decreasing the lumen level of allergens and improving the digestibility of low digestible protein sources, which can directly interact with intestinal mucosa to induce peroxidation (Adeola and Cowieson, 2011). Additionally, dietary supplementation of EO increased the serum GSH-Px activity, and there was an interaction between EO and PRO. GSH-Px is an important component of the enzymatic antioxidant defense systems (Zhang et al., 2020). This is in accordance with Zhang et al., who reported that EO increased the serum levels of GSH-Px (Zhang et al., 2015). The positive effect of EO could probably be attributed to the presence of the phenolic compounds thymol and carvacrol, because both thymol and carvacrol have been reported to have antioxidant activity (Ündeğer et al., 2009).
It has been widely reported that TNF-α and IL-1β are typical pro-inflammatory cytokines released from various types of immune cells, and the systemic TNF-α concentration is considered to be an indicator for weaning stress (Chen et al., 2018). A previous report showed that the concentrations of pro-inflammatory cytokines were up-regulated during the immediate post-weaning period and induced intestinal damage (Novais et al., 2021). In the current study, dietary EO supplementation had a tendency to decrease the serum TNF-α concentration in weaned pigs, which is in line with the result reported by a previous study (Ahmed et al., 2013). In vitro studies also showed that thymol possessed anti-inflammatory activities and lowered the production of pro-inflammatory cytokines TNF-α and IL-6 in LPS-induced mouse mammary epithelial cells (Liang et al., 2014). In addition, a significant interactive effect was observed in pigs supplemented with EO and PRO in reducing the serum concentration of TNF-α, suggesting the synergistic interaction of EO and PRO. Protease supplementation could improve the digestibility of protein, and less protein residues accumulated in large intestine, accordingly decreased the potentially toxic metabolites in the digesta such as ammonia, amines and sulfides, which are considered detrimental for the host's health (Tactacan et al., 2016). Complimentary to protease's mode of action, the inclusion of EO in diet could manipulate gut microbiota profile with decreasing pathogenic bacteria and increasing beneficial bacteria, thereby improving the immune status of weaned piglets (Li et al., 2012).
An integrated intestinal villus-crypt morphological structure is directly related to the nutrient digestion and absorption and to the intestinal mucosa barrier (Cera et al., 1988). The intestinal morphological changes, such as villus atrophy, villous shedding, and crypt hyperplasia, could lead to pathogenic bacteria invasion influencing the nutrient digestion and absorption, as well as stunted growth (Cera et al., 1988). Our study showed that pigs receiving the PRO diet had greater villous height and villus height: crypt depth, indicating the better nutrient digestibility. Similarly, the previous study also showed that dietary supplementation of PRO would increase villous height and the ratio of villus height to crypt depth in duodenum (Zuo et al., 2015). In addition, previous studies have shown that EO can improve intestinal villus height (Zou et al., 2016; Wei et al., 2017). However, in our study, we found that pigs receiving the EO diet had no positive effects on the villous height and villus height: crypt depth. The discrepancies between studies may be due to variation in the types and doses of main components of the EO, and the length of time EO was supplied.
The activities of brush border enzymes play crucial roles in the digestion of nutrients. Previous studies reported that EO and PRO might enhance digestive function by increasing the activity of digestive enzymes and enhancing nutrient absorption for better growth performance (Zeng et al., 2015; Zuo et al., 2015). Likewise, in the present study, we observed that the activity of sucrase in the jejunum mucosal was significantly improved by PRO supplementation, which indicated that PRO improved small intestinal digestive function of weaned pigs.
As a highly active endocellular enzyme in intestinal epithelial cells, diamine oxidase releases into the blood when the intestinal mucosal epithelial cells are damaged (Lin et al., 2020). Thus, the diamine oxidase activity in the blood can serve as an indicator to determine the integrity of the intestinal mucosal (Shi et al., 2020). In the present study, the serum diamine oxidase activity was markedly decreased by PRO supplementation. This may be attributed to the fact that soybean meal contains allergenic proteins (glycinin and β-conglycinin) that can cause allergic reaction to disrupt the small intestinal structure (Chen et al., 2011). The supplementation of PRO may degrade the allergenic proteins in the pig stomach and then attenuate the allergic reaction to improve the integrity of the intestinal epithelium, but this requires further investigation.
Gut microbial communities play an important role in the health status of the host, including nutrient absorption and metabolism, defense against pathogen invasion, and mucosal barrier function (Azad et al., 2020). The fermentation of protein in the hind gut is considered to be a potential risk for the proliferation of pathogenic bacteria, and it stimulates intestinal microbiota dysbiosis (Wen et al., 2018). A previous study indicated that EO exerts antibacterial activity on various pathogens (Zhai et al., 2018). Previous study also demonstrated that the supplementation of PRO can reduce the amount of undigested proteins into hind gut, and increase the number of lactic acid bacteria to reduce the pH of the gastrointestinal tract to inhibit the colonization of pathogenic bacteria (Cowieson et al., 2017). In the present study, at the phylum level, EO or PRO supplementation significantly increased the relative abundance of Actinobacteria, and EO tended to increase the abundance of Euryarchaeota. It is worth noting that the abundances of Actinobacteria and Euryarchaeota were higher in the healthy host relative to that of the unhealthy host with coeliac disease (Bibbò et al., 2020) or irritable bowel syndrome (Mei et al., 2021). In this study, moreover, this increase can be explained by the higher abundance of Bifidobacterium spp., which belongs to the Actinobacteria phylum. Bifidobacterium has been widely reported to decrease inflammation by suppressing the growth of potentially pathogens via the production of health-promoting metabolites (Arboleya et al., 2016). The negative correlation between Bifidobacterium and TNF-α was also found in the present study. Additionally, we observed that EO significantly decreased the abundance of Bacteroidetes, which was in accordance with the results of Li et al. (2018). Bacteroidetes are involved in inflammatory pathology when the intestinal microbiota is in an unbalanced state (Lukiw, 2016; Zhai et al., 2020). EO supplementation also decreased the abundance of genus Alloprevotella, which was considered as a harmful microorganism (Zhou et al., 2019). Kang et al. (2019) reported that dietary fiber-konjac flour could remarkably improve inflammatory state and metabolism in high-fat fed mice, and was related to the decreased abundance of Alloprevotella. Interestingly, a potentially beneficial microbe, Faecalibacterium, was decreased by EO. Faecalibacterium is one of the most abundant commensal bacteria found to be decreased in patients with inflammatory bowel diseases (Zhao et al., 2021). Because of its potentially anti-inflammatory properties in promoting gut health, the flourishing of this microorganism in the non-supplemented group may be explained by improved the ability of the host to protect against pathogens to compensate for weaning stress induced inflammatory response, and the underlying mechanism is still not clear. Furthermore, there was a tendency of interaction to decrease the relative abundance of genera unidentified_Clostridiales, which was taken as opportunistic pathogens in intestinal inflammation (Leber et al., 2018).
Positive effects on oxidative status, immune function, intestinal morphology and intestinal microbiota in weaned pigs were expected to result in better growth performance. However, in this study, dietary supplementation of EO and PRO did not improve the growth performance of weaned pigs. Similar to present study, no positive effects were found on growth performance of pigs by addition of EO or PRO (Muhl and Liebert, 2007; Oshea et al., 2014). Nevertheless, increased growth performance has been observed in other studies (Zuo et al., 2015; Su et al., 2018). The differing results for the effects of EO and PRO on growth performance may be influenced by their type and dose, different dietary compositions, the experimental duration, environmental and pathogenic challenges (Jiang et al., 2015; Zeng et al., 2015).
5. Conclusions
The present study demonstrated that dietary supplementation of EO and PRO had no significant effects on growth performance of weaned pigs. The major effects of EO were to improve antioxidant ability and intestinal microbiota. The major effects of PRO were to improve duodenal morphology and jejunal sucrase activity. Moreover, the combined supplementation of EO and PRO had a synergistic effect on decreasing inflammatory parameters.
Author contributions
Xie Peng: conceptualization, investigation, writing-original draft. Qiang Zhou: data curation, investigation. Cheng Wu, Liang Hu and Ying He: methodology. Yan Lin, Xuemei Jiang, Zhengfeng Fang and Jian Li: resources, software, supervision. Shengyu Xu, De Wu, Yong Zhuo and Bin Feng: validation, visualization, Jian Zhao and Quan Tan: Funding acquisition. Chris Van Ginneken: Review & Editing. Lianqiang Che: conceptualization, validation, supervision, Funding acquisition, Writing - review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by Novus International Trading (Shanghai) Co., Ltd; International (regional) cooperation and exchange program of NSFC (3191101579).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.12.003.
Contributor Information
De Wu, Email: wude@sicau.edu.cn.
Lianqiang Che, Email: Che.lianqiang@sicau.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Adeola O., Cowieson A.J. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Hossain M., Kim G., Hwang J., Ji H., Yang C. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. AJAS (Asian-Australas J Anim Sci) 2013;26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleya S., Watkins C., Stanton C., Ross R.P. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.A.K., Gao J., Ma J., Li T., Tan B., Huang X., et al. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim Nutr. 2020;6:379–388. doi: 10.1016/j.aninu.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbò S., Abbondio M., Sau R., Tanca A., Pira G., Errigo A., et al. Fecal microbiota signatures in celiac disease patients with poly-autoimmunity. Front Cell Infect Microbiol. 2020;10:349. doi: 10.3389/fcimb.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry G., Péron V., Le Huërou-Luron I., Lallès J.P., Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Cera K., Mahan D., Cross R., Reinhart G., Whitmoyer R. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci. 1988;66:574–584. doi: 10.2527/jas1988.662574x. [DOI] [PubMed] [Google Scholar]
- Chen F., Hao Y., Piao X., Ma X., Wu G., Qiao S., et al. Soybean-derived β-conglycinin affects proteome expression in pig intestinal cells in vivo and in vitro. J Anim Sci. 2011;89:743–753. doi: 10.2527/jas.2010-3146. [DOI] [PubMed] [Google Scholar]
- Chen J., Xie H., Chen D., Yu B., Mao X., Zheng P., et al. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned Pigs. ACS Omega. 2018;3:2211–2219. doi: 10.1021/acsomega.7b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson A., Zaefarian F., Knap I., Ravindran V. Interactive effects of dietary protein concentration, a mono-component exogenous protease and ascorbic acid on broiler performance, nutritional status and gut health. Anim Prod Sci. 2017;57:1058–1068. [Google Scholar]
- Degroote J., Van Noten N., Wang W., De Smet S., Michiels J. Effects of n-acetyl-cysteine supplementation through drinking water on the glutathione redox status during the weaning transition of piglets. Antioxidants. 2019;8:24. doi: 10.3390/antiox8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F., Sono Y., Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8:72. doi: 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Agazzi A., Awati A., Vitari F., Bento H., Ferrari A., et al. Influence of a blend of essential oils and an enzyme combination on growth performance, microbial counts, ileum microscopic anatomy and the expression of inflammatory mediators in weaned piglets following an Escherichia coli infection. Anim Feed Sci Technol. 2015;209:219–229. [Google Scholar]
- Kang Y., Li Y., Du Y., Guo L., Chen M., Huang X., et al. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int J Obes. 2019;43:1631–1643. doi: 10.1038/s41366-018-0187-x. [DOI] [PubMed] [Google Scholar]
- Kim J.C., Hansen C.F., Mullan B.P., Pluske J.R. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2012;173:3–16. [Google Scholar]
- Leber A., Hontecillas R., Tubau-Juni N., Zoccoli-Rodriguez V., Abedi V., Bassaganya-Riera J., et al. nlrX1 Modulates immunometabolic Mechanisms controlling the host–gut Microbiota interactions during inflammatory Bowel Disease. Front Immunol. 2018;9:363. doi: 10.3389/fimmu.2018.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J Anim Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu X., Ma X., Geng S., Jiang X., Huang Q., et al. Intestinal microbiome-metabolome responses to essential oils in piglets. Front Microbiol. 2018;9:1988. doi: 10.3389/fmicb.2018.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Li F., Fu Y., Cao Y., Song X., Wang T., et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37:214–222. doi: 10.1007/s10753-013-9732-x. [DOI] [PubMed] [Google Scholar]
- Lin F., Li X., Wen J., Wang C., Peng Y., Feng J., et al. Effects of coated sodium butyrate on performance, diarrhea, intestinal microflora and barrier function of pigs during the first 2-week post-weaning. Anim Feed Sci Technol. 2020;263:114464. [Google Scholar]
- Lukiw W.J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's disease. Front Microbiol. 2016;7:1544. doi: 10.3389/fmicb.2016.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Zhang J.B., Liu W., Yao X.R., Guo H., Jin Z.L., et al. Leonurine improves in vitro porcine embryo development competence by reducing reactive oxygen species production and protecting mitochondrial function. Theriogenology. 2020;156:116–123. doi: 10.1016/j.theriogenology.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Mei L., Zhou J., Su Y., Mao K., Wu J., Zhu C., et al. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2021;21:105. doi: 10.1186/s12876-021-01693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl A., Liebert F. Growth and parameters of microflora in intestinal and faecal samples of piglets due to application of a phytogenic feed additive. J Anim Physiol Anim Nutr. 2007;91:411–418. doi: 10.1111/j.1439-0396.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- Novais A.K., Deschêne K., Martel-Kennes Y., Roy C., Laforest J.P., Lessard M., et al. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . National Academies Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Oshea C.J., Alpine P.O.M., Solan P.J., Curran T.P., Varley P.F., Walsh A.M., et al. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower-finisher pigs. Anim Feed Sci Technol. 2014;189:88–97. [Google Scholar]
- Park S., Lee J.W., Cowieson A.J., Pappenberger G., Woyengo T.A. Soybean meal allergenic protein degradation and gut health of piglets fed protease-supplemented diets. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Wang R., Hu L., Zhou Q., Liu Y., Yang M., et al. Enterococcus faecium NCIMB 10415 administration improves the intestinal health and immunity in neonatal piglets infected by enterotoxigenic Escherichia coli K88. J Anim Sci Biotechnol. 2019;10:72. doi: 10.1186/s40104-019-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Xun W., Peng W., Hu H., Cao T., Hou G. Effect of the single and combined use of curcumin and piperine on growth performance, intestinal barrier function, and antioxidant capacity of weaned wuzhishan piglets. Front Vet Sci. 2020;7:418. doi: 10.3389/fvets.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spisni E., Petrocelli G., Imbesi V., Spigarelli R., Azzinnari D., Donati Sarti M., et al. Antioxidant, anti-Inflammatory, and microbial-modulating activities of essential oils: implications in colonic pathophysiology. Int J Mol Sci. 2020;21:4152. doi: 10.3390/ijms21114152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G., Zhou X., Wang Y., Chen D., Chen G., Li Y., et al. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018;17:1–10. doi: 10.1186/s12944-018-0788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tactacan G.B., Cho S.-Y., Cho J.H., Kim I.H. Performance responses, nutrient digestibility, blood characteristics, and measures of gastrointestinal health in weanling pigs fed protease enzyme. Asian-Australas J Anim Sci. 2016;29:998. doi: 10.5713/ajas.15.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ündeğer Ü., Başaran A., Degen G., Başaran N. Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chem Toxicol. 2009;47:2037–2043. doi: 10.1016/j.fct.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou J., Wang G., Cai S., Zeng X., Qiao S. Advances in low-protein diets for swine. J Anim Sci Biotechnol. 2018;9:1–14. doi: 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Xue H., Zhou Z., Peng J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal. 2017;11:193–201. doi: 10.1017/S1751731116001397. [DOI] [PubMed] [Google Scholar]
- Wen X., Wang L., Zheng C., Yang X., Ma X., Wu Y., et al. Fecal scores and microbial metabolites in weaned piglets fed different protein sources and levels. Anim Nutr. 2018;4:31–36. doi: 10.1016/j.aninu.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., et al. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H., Liu H., Wang S., Wu J., Kluenter A.-M. Potential of essential oils for poultry and pigs. Anim Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S.S., Ruan D., Zhu Y.W., Li M.C., Ye H., Wang W.C., et al. Protective effect of curcumin on ochratoxin a-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poultr Sci. 2020;99:1124–1134. doi: 10.1016/j.psj.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wu Z., Heng J., Song H., Tian M., Chen F., et al. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Anim Nutr. 2020;6:160–167. doi: 10.1016/j.aninu.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhou Y., Zou Y., Hu X., Zheng L., Wei H., et al. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest Sci. 2015;180:143–149. [Google Scholar]
- Zhao H., Xu H., Chen S., He J., Zhou Y., Nie Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol. 2021;36:320–328. doi: 10.1111/jgh.15222. [DOI] [PubMed] [Google Scholar]
- Zhou L., Xiao X., Zhang Q., Zheng J., Li M., Wang X., et al. Gut microbiota might be a crucial factor in deciphering the metabolic benefits of perinatal genistein consumption in dams and adult female offspring. Food Funct. 2019;10:4505–4521. doi: 10.1039/c9fo01046g. [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhao K.L., Chen X., Xu J. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Peng J., Wei H. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. BioMed Res Int. 2016;2016:5436738. doi: 10.1155/2016/5436738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Ling B., Long L., Li T., Lahaye L., Yang C., et al. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Anim Nutr. 2015;1:276–282. doi: 10.1016/j.aninu.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.