Abstract
The objective of this study was to determine the effect of dietary supplementation of β-hydroxybutyric acid (BHBA) on performance, nutrient digestibility, organ development, and serum composition in early-weaned goat kids. Sixty-four goat kids at 30 d of age were assigned to 4 treatments in a completely randomized design: 1) control (basal diet); 2) low (basal diet with 3 g/d per animal BHBA); 3) medium (basal diet with 6 g/d per animal BHBA; and 4) high (basal diet with 9 g/d per animal BHBA). Subsequently, 48 (6 kids per treatment) goat kids were randomly selected and slaughtered at 60 and 90 d of age. Compared with the control group, BHBA at low and high doses increased body weight (P < 0.05), average daily gain (P < 0.01), and average daily starter intake (P < 0.01). The BHBA improved organ development, especially at the lowest dose (P < 0.01). The digestibility of dry matter and crude protein increased with age (P < 0.05). However, BHBA did not affect nutrient digestibility. Compared with the control group, serum ceruloplasmin increased (P < 0.05) with high BHBA level at 90 d of age. However, the serum creatinine (P < 0.05) increased over time but was not affected by BHBA. The serum total antioxidant capacity and superoxide dismutase decreased with the high dose of BHBA at 90 d of age (P < 0.01). In contrast, the serum glutathione peroxidase and malondialdehyde increased with the high doses of BHBA (P < 0.01). Overall, low doses of BHBA were positive for growth performance, organ development, and health status against weaning stress. Whereas high doses of BHBA in the long term could negatively affect antioxidant status.
Keywords: Beta-hydroxybutyric acid, Goat kid, Acute-phase protein response, Weaning stress, Antioxidant capacity
1. Introduction
Early weaning of goat kids is a common practice in intensive feeding systems, as it reduces rearing costs (Liao et al., 2019). In contrast to natural weaning, early weaning is stressful due to the sudden change from liquid to solid diets (Magistrelli et al., 2013). Weaning stress could negatively affect feed intake, growth performance, antioxidant capacity, and acute-phase proteins (Haley et al., 2005; Magistrelli et al., 2013; Chen et al., 2020). The change from milk to solid feed at weaning could also affect the rumen development of goats, which is the most crucial physiological challenge from birth to 2 months old (Jiao et al., 2015). Factors affecting rumen development at this stage include weaning time, type of solid feed, and feeding time (Eckert et al., 2015; Kim et al., 2016). In this regard, early weaning of lambs with alfalfa feeding increased creep feed intake compared with suckling lambs (Álvarez-Rodríguez et al., 2010). Whereas weaning of calves at 8 weeks of age was less stressful than weaning at 6 weeks due to the high solid feed intake before and after weaning (Eckert et al., 2015). The currently accepted theory is that solid feed intake before weaning promotes the development of rumen morphology (Kim et al., 2016), including the forestomach weight, and papillae concentration (Khan et al., 2007), and then the growth performance (Khan et al., 2007; Wang et al., 2016). Furthermore, the early feeding of a solid diet contributes to the early establishment of rumen microflora followed by a high concentration of volatile fatty acids (VFA) in the rumen, which could stimulate the early development and maturation of rumen epithelium tissue (Drackley, 2008; Cui et al., 2020). Indeed, Lv et al. (2019) found that the increase in ruminal VFA in lambs fed with starter feed was positively related to nutrient intake.
The transition from neonatal to functional rumen is accompanied by the conversion from endogenous glucose metabolism into fatty acid metabolism (Baldwin and Jesse, 1992; Girard et al., 1992). The development of rumen metabolic function depends on the ability of rumen epithelial cells to absorb VFA and produce ketones (Lane et al., 2002; Khan et al., 2011b). The ketogenic effect is a critical physiological transition for developing rumen metabolic functions (Chai et al., 2017; Zhang et al., 2017). Ketogenesis occurring in rumen epithelial cells refers to the conversion of acetyl-CoA from butyrate into β-hydroxybutyric acid (BHBA) and acetoacetic acid (Lane et al., 2002), which is the primary pathway for VFA metabolism (Penner et al., 2011). In fact, Sun et al. (2018) found that starter feeds increased butyrate concentrations and tended to increase serum BHBA. Therefore, BHBA as a biomarker is considered to play a vital role in the development of rumen epithelium metabolic functions. However, the mechanism of BHBA in rumen development during weaning transition phase remains unclear.
In summary, the substantial development of rumen is an essential factor for early-weaned goats to digest solid feed, synthesize and utilize fermentation products (VFA) (Eckert et al., 2015). The BHBA is supposed to promote early rumen metabolic functions. However, to our knowledge, the effect of dietary BHBA supplementation has not been investigated. We hypothesized that BHBA supplementation could trigger rumen anatomical development and metabolic function, and mitigate the adverse effects of weaning stress. Therefore, the objective of this study was to investigate the effect of supplementing BHBA on growth performance, organ development, and serum composition of goat kids; and to determine the appropriate dietary inclusion level of BHBA.
2. Materials and methods
This study was conducted between June 10, 2020, and August 10, 2020, at Haimen goat farm, Jiangsu, China (latitude, 31°53′ N; longitude 121°09′ E). The experimental work was performed following the guidelines approved by the Animal Ethics Committee of the Chinese Academy of Agricultural Sciences (Protocol number: AEC-CAAS-20200605; Approval date: June 3, 2020).
2.1. Animals and treatments
Sixty-four goat kids (half males and half females) of 30 d old (5.14 ± 1.08 kg of live weight) were separated from their dams and used in this study. The kids were assigned to 4 treatments in a completely randomized design (8 replicates per treatment). Dietary treatments consisted of the basal diet supplemented with 0 (CON), 3 (low dose, LD), 6 (medium dose, MD), or 9 (high dose, HD) g/d per animal BHBA. The BHBA was based on BHBA Na with a purity of 99% (Wuhan Huajiu Pharmaceutical Technology Co., Ltd., China). The experiment lasted a total of 2 months.
2.2. Feeding and management
The kids were housed in 32 covered pens (one replicate 2 kids for each pen) with a slatted floor (2 m × 2 m) and equipped with feeders and water buckets. The initial body weight was recorded at 30 d old, then the body weight was recorded monthly (at 60 and 90 d of age). The average daily gain was calculated based on body weight. The early-weaned kids were offered milk replacer at 2% of body weight and solid commercial starter feed ad libitum. After 60 d of age, milk replacer was removed, and solid feed became the only feed source. The experimental diet was provided twice daily at 08:00 and 14:00, and the offered amount was adjusted to reach 10% refusal. Orts were collected from each pen and weighed daily throughout the experiment to determine the average daily feed intake. The diets were formulated to meet the nutrient requirements of goat kids (NRC, 2007) with an average daily weight gain of 200 g. The ingredients and the chemical composition of the diets are shown in Table 1.
Table 1.
The ingredients and chemical composition of basal diets (%, DM basis).
| Item | Milk replacer | Starter diet |
|---|---|---|
| Ingredients | ||
| Corn | 50.0 | |
| Soybean meal | 25.0 | |
| Bran | 10.0 | |
| Premix1 | 2.50 | |
| Salt | 0.50 | |
| Sodium bicarbonate | 1.00 | |
| Calcium bicarbonate | 4.00 | |
| Corn husk | 15.0 | |
| Chemical composition | ||
| DM | 95.5 | 91.2 |
| ME2, MJ/kg | 19.36 | 13.38 |
| OM | 89.0 | 84.2 |
| EE | 16.0 | 3.73 |
| CP | 25.5 | 19.4 |
| Ash | 6.54 | 7.03 |
| NDF | – | 33.2 |
| ADF | – | 15.9 |
| Ca | 1.02 | 0.95 |
| P | 0.66 | 0.70 |
DM = dry matter; ME = metabolizable energy; OM = organic matter; EE = ether extract; CP = crude protein; NDF = neutral detergent fiber; ADF = acid detergent fiber.
The premix provides per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,000 IU; vitamin E, 30 IU; Cu, 12 mg; Fe, 64 mg; Mn, 56 mg; Zn, 60 mg; I, 1.2 mg; Se, 0.4 mg; Co, 0.4 mg; Ca, 3.2 g; P, 1.2 g; NaCl, 6.4 g.
ME was calculated by the equations from NRC (2001).
2.3. Digestibility trial
The digestibility trial was conducted 4 days before the end of the first and second months of the experiment. Feces from each kid were collected for 4 consecutive days using a plastic screen and weighed before morning feeding. The feed and feces samples collected during the experiment were oven-dried at 65 °C for 48 h and ground to pass through a 0.9-mm screen before determining the chemical composition. Samples were analyzed in duplicate for dry matter (DM, method No. 934.01), crude protein (CP, method No. 954.01), ash (method No. 942.05), and ether extract (EE, method No. 920.39) according to AOAC (2003). The organic matter (OM) was calculated by subtracting the ash content from the DM of samples. Neutral detergent fiber (NDF), and acid detergent fiber (ADF) were determined based on the method described by Van Soest et al. (1991). Acid-insoluble ash (AIA) was used to estimate the digestibility of a certain nutrient according to the description of Van Keulen and Young (1977) using the following formula:
where A is the content of a given nutrient in the diet (%), A1 is the content of the same nutrient in the feces (%), B is the content of AIA in the diet (%), and B1 is the content of AIA in the feces (%).
2.4. Slaughter trial
Goat kids were slaughtered at the abattoir from the Haimen goat farm. At 60 and 90 d of age, 6 goat kids per treatment were randomly selected and sacrificed to determine the rumen, kidney, and liver weights, as well as the pH of the ruminal fluid. The pH of ruminal fluid (50 mL per animal) was determined using an automatic pH detector (Testo Instruments International Trade Co., Ltd., China).
2.5. Blood samples collection and analysis
The jugular blood samples were obtained at the slaughter (60 and 90 d of age). Blood samples were collected into a 10 mL serum tube and centrifuged (1,006 × g, 10 min, 4 °C). Then, the aliquots of serum were frozen immediately at −20 °C until later analysis. The serum analysis was performed at Beijing Jinhai Keyu Biotechnology Development Co., Ltd. (Beijing, China). The serum samples were analyzed for uric acid (μmol/L), creatinine (mmol/L), serum total antioxidant capacity (TAOC, U/mL), glutathione peroxidase (GSH, U/mL), superoxide dismutase (SOD, U/mL), and malondialdehyde (MDA, nmol/mL). Biochemical analysis was determined using the standard commercial kits and a Kehua ZY KHB-1280 automatic biochemical analyzer (Shanghai Kehua Biological Engineering Co., Ltd., Shanghai, China) according to the manufacturer's instructions. Serum haptoglobin (ng/mL) and ceruloplasmin (U/L) were measured using ELISA kits (Beijing Jinhai Keyu Biotechnology Development Co., Ltd.), following the manufacturer’s instructions.
2.6. Statistical analyses
Body weight, average daily gain (ADG), and starter feed intake during 30, 60, and 90 d of age were achieved by an ANOVA with repeated measures within a completely randomized design using the MIXED procedure of SAS (SAS Enterprise Guide 5.1, SAS Institute Inc., Cary, NC) according to the following model:
| Yijk = μ + Ti + δij + Ak + (T × A)ik + eijk , |
where μ is the overall mean; Ti is the effect of treatment i (0, 3, 6 or 9 g/d per animal BHBA); Ak is the effect of age k (30, 60 or 90 d); (T × A)ik is the effect of interaction between treatment i with age k; δij is the random error related to the variance between animals within treatment; eijk is the random error related to the variance between measurements within animals.
Data are presented as least squares means using the PDIFF with no ADJUST option being specified. Significant differences were declared at P < 0.05.
In addition, nutrient digestibility, organ development, rumen pH, and serum parameters were analyzed using a two-way ANOVA design according to the following model:
| Yijk = μ + Ti + Aj +(TA)ij + eijk , |
where μ is the overall mean; Ti is the effect of treatment i; Aj is the effect of age j; (TA)ij is the interaction between treatment i and age j; eijk is the random error. Significant differences were declared at P < 0.05. Data were presented as means and pooled standard error.
3. Results
3.1. Growth performance
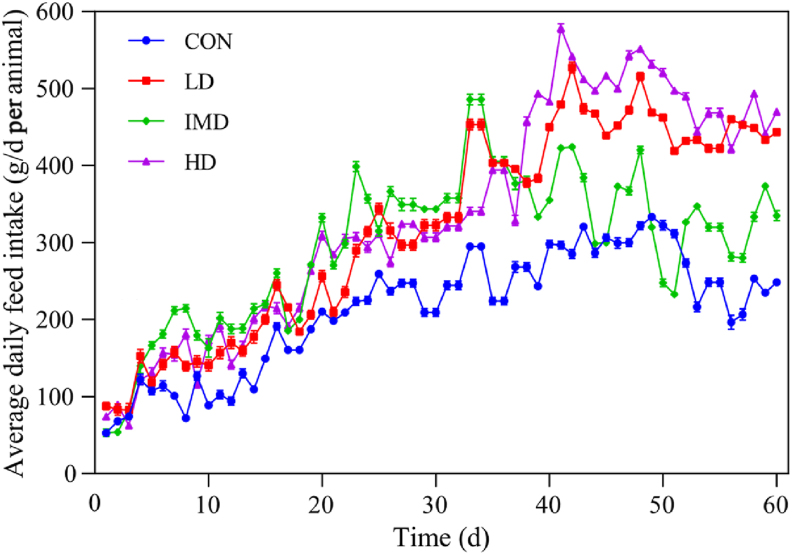
The effect of dietary supplementation of BHBA on growth performance of early-weaned goat kids is shown in Table 2 and Fig. 1. The age, BHBA, and their interaction affected (P < 0.01) the body weight and dry matter intake (DMI) of starter in goat kids. The age and the interaction between age and BHBA had no effect (P > 0.05) on the ADG of goat kids, but the BHBA had an effect (P < 0.01). Specifically, the body weight of goat kids was similar (P > 0.05) among different BHBA treatments at 30 and 60 d old, but it was higher (P < 0.01) in LD and HD groups compared with CON and MD groups at 90 d old. Similar treatment effects (P < 0.01) were observed for DMI of starter feed. The ADG had no change (P > 0.05) at different ages, but it was higher (P < 0.01) in LD and HD groups compared with the CON group across different ages.
Table 2.
Effect of dietary supplementation of BHBA on growth performance of early-weaned goat kids.
| Item | Treatment | BW, kg | DMI, g/d | ADG, g/d |
|---|---|---|---|---|
| Age | ||||
| 30 d | CON | 5.1f | ||
| LD | 5.1f | |||
| MD | 5.1ef | |||
| HD | 5.2ef | |||
| 60 d | CON | 6.2de | 182.8e | 35.2 |
| LD | 7.2d | 229.4de | 66.8 | |
| MD | 7.2d | 246.7de | 66.4 | |
| HD | 7.0d | 222.3de | 61.8 | |
| 90 d | CON | 7.7cd | 294.4cd | 35.2 |
| LD | 10.6ab | 443.6a | 100.1 | |
| MD | 9.1bc | 353.5bc | 62.5 | |
| HD | 11.1a | 436.0ab | 109.2 | |
| SEM | 0.45 | 26.50 | 13.80 | |
| Age | 30 d | 5.1 | ||
| 60 d | 6.9 | 220.3 | 57.5 | |
| 90 d | 9.6 | 381.9 | 76.7 | |
| SEM | 0.24 | 13.30 | 7.00 | |
| Treatment | CON | 6.3 | 238.6 | 35.2b |
| LD | 7.6 | 336.5 | 83.5a | |
| MD | 7.1 | 300.1 | 64.4ab | |
| HD | 7.8 | 329.2 | 85.5a | |
| SEM | 0.36 | 23.40 | 10.60 | |
| P-value | ||||
| Age | <0.01 | 0.06 | ||
| Treatment | 0.01 | <0.01 | ||
| Age × Treatment | <0.01 | 0.18 | ||
BHBA = β-hydroxybutyric acid; BW = body weight; DMI = average dry matter intake of the starter diet; ADG = average daily gain; CON = control; LD = low dose (3 g/d per animal BHBA); MD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA); SEM = the standard error of the means.
a,b,c,d,e,f Means with different superscripts within the same column differ significantly (P < 0.05).
Fig. 1.
Effect of dietary supplementation of β-hydroxybutyric acid (BHBA) on the average daily feed intake of the early-weaned goat kids. CON = control; LD = low dose (3 g/d per animal BHBA); IMD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA).
3.2. Nutrient digestibility
The age, BHBA, and their interaction had no effect (P > 0.05) on the digestibility of organic matter, ash, NDF and ADF (Table 3). The BHBA and the interaction between age and BHBA had no effect (P > 0.05) on the digestibility of DM and CP, but the age had an effect (P < 0.05). Specifically, the digestibility of DM and CP increased over time (P < 0.05).
Table 3.
Effect of dietary supplementation of BHBA on the nutrient digestibility of the early-weaned goat kids (%).
| Item | Treatment | DM | OM | CP | Ash | NDF | ADF |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 60 d | CON | 70.8 | 72.9 | 67.4 | 42.6 | 64.9 | 54.3 |
| LD | 71.0 | 73.0 | 68.6 | 40.7 | 61.6 | 53.2 | |
| MD | 69.7 | 71.9 | 66.7 | 40.6 | 62.0 | 52.6 | |
| HD | 70.7 | 72.8 | 68.1 | 43.1 | 63.5 | 55.7 | |
| 90 d | CON | 72.4 | 74.7 | 70.5 | 42.1 | 63.3 | 54.3 |
| LD | 71.6 | 73.3 | 71.4 | 49.6 | 60.3 | 47.6 | |
| MD | 73.4 | 75.8 | 68.6 | 42.0 | 69.0 | 59.6 | |
| HD | 75.1 | 77.1 | 74.0 | 47.0 | 63.4 | 53.5 | |
| SEM | 1.73 | 1.81 | 2.26 | 2.83 | 3.57 | 4.08 | |
| Age | 60 d | 70.5b | 72.6 | 67.7b | 41.8 | 63.0 | 54.0 |
| 90 d | 73.1a | 75.2 | 71.1a | 45.1 | 64.0 | 53.7 | |
| SEM | 1.07 | 0.91 | 1.13 | 1.42 | 1.79 | 2.04 | |
| Treatment | CON | 71.6 | 73.8 | 68.9 | 42.3 | 64.1 | 54.3 |
| LD | 70.3 | 73.2 | 70.0 | 45.2 | 61.0 | 50.4 | |
| MD | 71.5 | 73.8 | 67.7 | 41.3 | 65.5 | 56.0 | |
| HD | 72.9 | 75.0 | 71.0 | 45.1 | 63.5 | 54.6 | |
| SEM | 1.22 | 1.28 | 1.60 | 2.01 | 2.53 | 2.88 | |
| P-value | |||||||
| Age | 0.04 | 0.06 | 0.04 | 0.10 | 0.69 | 0.94 | |
| Treatment | 0.79 | 0.80 | 0.51 | 0.44 | 0.65 | 0.56 | |
| Age × Treatment | 0.67 | 0.65 | 0.84 | 0.39 | 0.59 | 0.48 | |
BHBA = β-hydroxybutyric acid; DM = dry matter; OM = organic matter; CP = crude protein; NDF = neutral detergent fiber; ADF = acid detergent fiber; CON = control; LD = low dose (3 g/d per animal BHBA); MD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA); SEM = the standard error of the means.
a,b Means with different superscripts within the same column differ significantly (P < 0.05).
3.3. Organ development
The age and BHBA had an effect (P < 0.01) on the weight of rumen, liver, and kidney, but the interaction of age and BHBA did not affect (P > 0.05) them (Table 4). The age and BHBA had no effect (P > 0.05) on rumen pH, but their interaction affected (P < 0.05) it. Specifically, organ weight showed an increase (P < 0.01) over time. Furthermore, the LD group increased (P < 0.01) the weight of rumen, liver, and kidney compared with the CON group. The MD group showed higher (P < 0.01) liver weight than the CON group. No significant differences were observed (P > 0.05) between the HD and the CON group. In addition, the LM group had lower (P < 0.05) pH than the other three groups at 90 d old.
Table 4.
Effect of dietary supplementation of BHBA on organ development and rumen pH of the early-weaned goat kids.
| Item | Treatment | Rumen weight, g | Liver weight, g | Kidney weight, g | Rumen pH1 |
|---|---|---|---|---|---|
| Age | |||||
| 60 d | CON | 77.5 | 118.3 | 32.5 | 5.88ab |
| LD | 114.2 | 185.0 | 42.5 | 5.90ab | |
| MD | 110.8 | 184.2 | 39.2 | 5.69ab | |
| HD | 90.0 | 160.0 | 33.3 | 5.93ab | |
| 90 d | CON | 102.5 | 210.8 | 44.2 | 5.98a |
| LD | 228.3 | 340.0 | 58.3 | 5.48b | |
| MD | 176.7 | 282.5 | 48.3 | 6.08a | |
| HD | 183.5 | 278.3 | 49.2 | 6.01a | |
| SEM | 20.80 | 22.80 | 3.37 | 5.000 × 10−7 | |
| Age | 60 d | 98.1b | 161.9b | 36.9b | 5.84 |
| 90 d | 172.7a | 277.9a | 50.0a | 5.81 | |
| SEM | 10.40 | 11.40 | 1.69 | 2.500 × 10−7 | |
| Treatment | CON | 90.0b | 164.6b | 38.3b | 5.93 |
| LD | 171.2a | 262.5a | 50.4a | 5.64 | |
| MD | 143.7ab | 233.3a | 43.7ab | 5.85 | |
| HD | 136.7ab | 219.2ab | 41.2b | 5.97 | |
| SEM | 14.70 | 16.10 | 2.39 | 3.500 × 10−7 | |
| P-value | |||||
| Age | <0.01 | <0.01 | <0.01 | 0.77 | |
| Treatment | <0.01 | <0.01 | <0.01 | 0.08 | |
| Age × Treatment | 0.18 | 0.52 | 0.70 | 0.02 | |
BHBA = β-hydroxybutyric acid; CON = control; LD = low dose (3 g/d per animal BHBA); MD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA); SEM = the standard error of the means.
a,b Means with different superscripts within the same column differ significantly (P < 0.05).
SEM for rumen pH was in the H ion form.
3.4. Acute-phase protein response
The age, BHBA, and their interaction had an effect (P < 0.05) on the serum concentrations of ceruloplasmin (Table 5). The age and BHBA had no effect (P > 0.05) on the serum concentrations of haptoglobin, but their interaction affected (P < 0.05) it. Specifically, compared with the CON group, the HD group increased ceruloplasmin concentration (P < 0.05) at 90 d old. The serum concentrations of haptoglobin in the HD group was lower (P < 0.01) at 60 d but was higher (P < 0.01) at 90 d compared with the CON group.
Table 5.
Effect of dietary supplementation of BHBA on serum acute-phase proteins of the early-weaned goat kids.
| Item | Treatment | Ceruloplasmin, U/L | Haptoglobin, ng/mL |
|---|---|---|---|
| Age | |||
| 60 d | CON | 61.8abc | 98.7ab |
| LD | 64.2a | 97.8ab | |
| MD | 62.1abc | 86.1bc | |
| HD | 63.1ab | 80.9c | |
| 90 d | CON | 58.8bc | 86.4bc |
| LD | 58.0c | 91.9abc | |
| MD | 59.5abc | 92.3abc | |
| HD | 64.0a | 100.4a | |
| SEM | 1.05 | 3.00 | |
| Age | 60 d | 62.8 | 90.9 |
| 90 d | 60.1 | 92.8 | |
| SEM | 0.53 | 1.50 | |
| Treatment | CON | 60.3 | 92.6 |
| LD | 61.1 | 94.9 | |
| MD | 60.8 | 89.2 | |
| HD | 63.5 | 90.6 | |
| SEM | 0.74 | 2.10 | |
| P-value | |||
| Age | <0.01 | 0.38 | |
| Treatment | 0.02 | 0.28 | |
| Age × Treatment | 0.02 | <0.01 | |
BHBA = β-hydroxybutyric acid. CON = control; LD = low dose (3 g/d per animal BHBA); MD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA). SEM = the standard error of the means.
a,b,c Means with different superscripts within the same column differ significantly (P < 0.05).
3.5. Serum antioxidant status and kidney function
The age, BHBA, and their interaction had no effect (P > 0.05) on the serum concentrations of uric acid (Table 6). The BHBA and the interaction between BHBA and age had no effect (P > 0.05) on the serum concentrations of creatinine, but the age affected (P < 0.05) it. The age, BHBA, and their interaction had an effect (P < 0.01) on the serum concentrations of TACO, GSH, SOD and MDA. Specifically, the serum creatinine was higher (P < 0.05) at 90 d than 60 d of age. The serum concentrations of TAOC and SOD were decreased (P < 0.01) whereas the GSH and MDA were increased (P < 0.01) in the HD group compared with the other groups at 90 d. However, at 60 d of age, the differences of antioxidant capacity between groups were not significant (P > 0.05).
Table 6.
Effect of dietary supplementation with BHBA acid and age on serum antioxidant capacity and kidney function of early-weaned goat kids.
| Item | Treatment | UA, μmol/L | CRE, mmol/L | TAOC, U/mL | GSH, U/mL | SOD, U/mL | MDA, nmol/mL |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 60 d | CON | 10.1 | 16.0 | 11.0bc | 7.72ab | 123.2bc | 4.78ab |
| LD | 14.1 | 21.7 | 11.2bc | 7.61bc | 127.8b | 4.55bc | |
| MD | 11.6 | 17.9 | 11.2bc | 7.69ab | 124.8bc | 4.74ab | |
| HD | 13.4 | 17.7 | 11.2bc | 7.80ab | 123.8bc | 4.77ab | |
| 90 d | CON | 14.9 | 19.4 | 12.3a | 6.94c | 144.1a | 3.88c |
| LD | 11.6 | 20.1 | 12.1a | 7.06bc | 143.0a | 3.92c | |
| MD | 8.27 | 26.7 | 11.7ab | 7.32bc | 131.6ab | 4.35bc | |
| HD | 11.0 | 24.2 | 10.8c | 8.31a | 111.7c | 5.24a | |
| Pooled SEM | 2.98 | 2.53 | 0.18 | 0.150 | 3.01 | 0.150 | |
| Age | 60 d | 12.3 | 18.3b | 11.2 | 7.68 | 124.9 | 4.71 |
| 90 d | 11.4 | 22.6a | 11.7 | 7.41 | 132.6 | 4.34 | |
| Pooled SEM | 1.51 | 1.27 | 0.09 | 0.070 | 1.53 | 0.080 | |
| Treatment | CON | 12.5 | 17.7 | 11.7 | 7.33 | 133.7 | 4.33 |
| LD | 12.8 | 20.9 | 11.6 | 7.34 | 135.4 | 4.23 | |
| MD | 9.94 | 22.3 | 11.5 | 7.50 | 128.2 | 4.54 | |
| HD | 12.3 | 20.9 | 11.0 | 8.00 | 117.8 | 5.01 | |
| SEM | 2.13 | 1.79 | 0.13 | 0.110 | 2.16 | 0.110 | |
| P-value | |||||||
| Age | 0.69 | 0.02 | <0.01 | 0.01 | <0.01 | <0.01 | |
| Treatment | 0.77 | 0.31 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Age × Treatment | 0.50 | 0.22 | <0.01 | <0.01 | <0.01 | <0.01 | |
BHBA = β-hydroxybutyric acid; UA = uric acid; CRE = creatinine; TAOC = total antioxidant capacity; GSH = glutathione peroxidase; SOD = superoxide dismutase; MDA = malondialdehyde; CON = control; LD = low dose (3 g/d per animal BHBA); MD = medium dose (6 g/d per animal BHBA); HD = high dose (9 g/d per animal BHBA); SEM = the standard error of the means.
a,b,c Means with different superscripts within the same column differ significantly (P < 0.05).
4. Discussion
4.1. Growth performance
Offspring spend less time eating after weaning, which reduces feed intake and weight gains (Haley et al., 2005). In this study, the interaction effects of BHBA supplementation and age on the body weight, average daily gain, and starter feed intake indicated that both the age and dietary BHBA supplementation are vital in the growth and development of goats. The positive effects of BHBA supplementation agree with previous studies on the butyrate supplementation (Kotunia et al., 2004; Mazzoni et al., 2008), which was reported to stimulate growth performance and feed intake in lambs (Cavini et al., 2015; Chai et al., 2017), calves (Guilloteau et al., 2009), post-weaned heifers (Stahl et al., 2020), pre-weaned pigs (Mazzoni et al., 2008), and growing-finishing pigs (Sun et al., 2020).
4.2. Nutrient digestibility
In this study, the digestibility of DM and CP increased over time, which could be attributed to the rumen development and the improved solid feed intake. A high feed intake of highly nutritious diets improves the intake and digestibility of nutrients due to the increased nutrient availability for rumen fermentation and microbial growth (Sauerwein et al., 2005). However, nutrient digestibility in this study was not affected by dietary BHBA. Similar to our results, sodium butyrate supplementation did not affect the nutrient digestibility in post-weaned heifers (Rice et al., 2019; Stahl et al., 2020). The lack of BHBA effect on digestibility could be due to the weaning of goats and the introduction of the solid diet, which is helpful for rumen development (Kim et al., 2016). Further research is needed to investigate the role of BHBA in young ruminants.
4.3. Organ development
The rumen development was improved over time, as evidenced by the increase of rumen weight, which was more evident after BHBA supplementation. Several studies indicated that rumen development can be promoted by higher solid feed intake during the post-weaning period (Khan et al., 2011a; Beiranvand et al., 2014), which provided readily fermentable carbohydrates and enhanced production of VFA, especially butyrate (Steele et al., 2009). As a member of VFA, butyrate promotes rumen development by increasing VFA absorption, energy mobilization, and the tight junction improvement (Baldwin et al., 2012; Steele et al., 2013). BHBA is an indicator of VFA utilization (Quigley et al., 1991) and a marker of rumen development (Quigley et al., 1991; Meale et al., 2015). The improvement of rumen and other organs development in this study indicated the potential and beneficial effects of BHBA as a growth promoter for ruminants. The ruminal fluid pH decreased (P < 0.05) in the low BHBA group at 90 d of age. The drop of pH could be attributed to rapid fermentation resulting from increased feed intake, digestibility and readily available carbohydrates (Khan et al., 2008; Laarman et al., 2012).
4.4. Acute-phase protein response
In ruminants, haptoglobin and ceruloplasmin are vital acute-phase proteins that can be increased due to infection and inflammatory conditions (Gånheim et al., 2003). Studies have shown that haptoglobin can be increased from negligible circulating concentrations in healthy animals to 100-fold in animals with infections (Earley and Crowe, 2002). The acute-phase protein can be used to evaluate weaning stress (Kim et al., 2011; Magistrelli et al., 2013). In calves, Hulbert et al. (2011) found that serum haptoglobin was high at 24 - 45 d of age and subsequently decreased at 45 - 66 d of age, suggesting that calves were less stressed at that age (Arthington et al., 2003). In this study, serum concentrations of ceruloplasmin decreased with age, whereas serum haptoglobin was not affected. One explanation for this decline may be the adaptive response against weaning over time.
The serum haptoglobin decreased with increase of dietary BHBA at 60 d, indicating the beneficial effect of BHBA to overcome the stress of weaning. However, at 90 d of age, haptoglobin and ceruloplasmin were higher in the high BHBA dose group than in the CON group. Interestingly, high concentrations of haptoglobin were observed in the circulation and milk of dairy cows with circulating concentrations of BHBA higher than 1.6 mM in the last week before calving (Hiss et al., 2009). These results indicated that in the long term, a high dose of BHBA is not recommended.
4.5. Serum antioxidant status and kidney function
Creatinine is a by-product of endogenous muscle metabolism, and its serum concentration is lower in immature animals and will increase in muscular animals (Sargent et al., 2021). In this study, serum creatinine increased from 60 d to 90 d of age, however, serum uric acid and creatinine were not affected by dietary BHBA supplements, suggesting the normal liver and renal functions of goat kids.
The complex system of antioxidant enzymes is necessary to protect the organism from harmful prooxidants. TAOC indicates the body's oxidation resistance potential (Cao et al., 2014). In comparison, MDA is one of the final products of polyunsaturated fatty acid peroxidation in cells and is considered an oxidative stress marker (Gaweł et al., 2004). In this study, serum TAOC and SOD concentrations were lower, whereas serum MDA concentration was higher at 60 d than at 90 d, indicating that weaning caused oxidative stress and free radical generation. In studies of piglets and children, it was also found that early weaning increased MDA concentration and decreased antioxidant enzyme activity (Zhu et al., 2012; Chen et al., 2020). Furthermore, the genes related to the antioxidant enzymes and digestive enzymes were downregulated after weaning (Zhu et al., 2012). The GSH activity was decreased at 3 d and recovered later due to the adaptation of animals over time (Yin et al., 2014). However, in the present study, GSH decreased (P < 0.01) at 90 d of age, which could be considered a long-term impact of early weaning stress.
At 90 d of age, the high dose of BHBA decreased the serum TAOC and SOD concentrations, and increased serum MDA concentration. However, no significant differences were observed between groups at 60 d of age. These findings suggested that long-term high-dose BHBA may increase oxidative stress due to the presence of high ketone concentration. Similarly, previous studies on cows and buffalos also confirmed that serum BHBA is associated with oxidative stress (Li et al., 2016) and serum MDA and BHBA were positively correlated (Bernabucci et al., 2005; Youssef et al., 2010).
5. Conclusions
Overall, dietary BHBA supplementation improved the growth performance and organ development of early weaned goat kids over time, especially at low doses. However, high dose BHBA increased acute-phase protein response and oxidative stress at 90 d old. The current study findings indicate that high dose BHBA is mainly effective in the early growth stage. In the long run, a low dose seems to be safer and more efficient than a high dose, as it will not increase oxidative stress.
Author contributions
Mahmoud M. Abdelsattar: Methodology, Software, Writing – original draft. Einar Vargas-Bello-Pérez: Validation, Writing – review & editing. Ymin Zhuang: Methodology. Yuze Fu: Methodology. Naifeng Zhang: Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (31872385) and the National Key R&D Program Projects (2018YFD0501902). The authors thank the goat farm for their cooperation in animal handling. We also appreciate the assistance in sampling collection from Animal Husbandry Institute of Jiangsu Academy of Agricultural Sciences.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Álvarez-Rodríguez J., Sanz A., Ripoll-Bosch R., Joy M. Do alfalfa grazing and lactation length affect the digestive tract fill of light lambs? Small Rumin Res. 2010;94:109–116. [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Arlington, Virginia: 2003. Official methods of analysis of the association of official's analytical chemists. [Google Scholar]
- Arthington J.D., Eichert S.D., Kunkle W.E., Martin F.G. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci. 2003;81:1120–1125. doi: 10.2527/2003.8151120x. [DOI] [PubMed] [Google Scholar]
- Baldwin R.L., Wu S., Li W., Li C., Bequette B.J., Li R.W. Quantification of transcriptome responses of the rumen epithelium to butyrate infusion using RNA-seq technology. Gene Regul Syst Biol. 2012;6:67–80. doi: 10.4137/GRSB.S9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R.L., Jesse B.W. Developmental changes in glucose and butyrate metabolism by isolated sheep ruminal cells. J Nutr. 1992;122:1149–1153. doi: 10.1093/jn/122.5.1149. [DOI] [PubMed] [Google Scholar]
- Beiranvand H., Ghorbani G.R., Khorvash M., Nabipour A., Dehghan-Banadaky M., Homayouni A., et al. Interactions of alfalfa hay and sodium propionate on dairy calf performance and rumen development. J Dairy Sci. 2014;97:2270–2280. doi: 10.3168/jds.2012-6332. [DOI] [PubMed] [Google Scholar]
- Bernabucci U., Ronchi B., Lacetera N., Nardone A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J Dairy Sci. 2005;88:2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2. [DOI] [PubMed] [Google Scholar]
- Cao J., Guo F., Zhang L., Dong B., Gong L. Effects of dietary Selenomethionine supplementation on growth performance, antioxidant status, plasma selenium concentration, and immune function in weaning pigs. J Anim Sci Biotechnol. 2014;5:46. doi: 10.1186/2049-1891-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavini S., Iraira S., Siurana A., Foskolos A., Ferret A., Calsamiglia S. Effect of sodium butyrate administered in the concentrate on rumen development and productive performance of lambs in intensive production system during the suckling and the fattening periods. Small Rumin Res. 2015;123:212–217. [Google Scholar]
- Chai J.M., Diao Q.Y., Wang S.Q., Wang H.C., Zhang N.F. Effect of weaning time on growth performance and rumen development of Hu lambs. Indian J Anim Res. 2017;51:423–430. [Google Scholar]
- Chen K., Liu Y., Cheng Y., Yan Q., Zhou C., He Z., et al. Supplementation of lactobacillus plantarum or macleaya cordata extract alleviates oxidative damage induced by weaning in the lower gut of young goats. Animals. 2020;10:548. doi: 10.3390/ani10040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Wu S., Li J., Yang Q.E., Chai S., Wang L., et al. Effect of alfalfa hay and starter feeding intervention on gastrointestinal microbial community, growth and immune performance of yak calves. Front Microbiol. 2020;11:994. doi: 10.3389/fmicb.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drackley J.K. Calf nutrition from birth to breeding. Vet Clin North Am Food Anim Pract. 2008;24:55–86. doi: 10.1016/j.cvfa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Earley B., Crowe M.A. Effects of ketoprofen alone or in combination with local anesthesia during the castration of bull calves on plasma cortisol, immunological, and inflammatory responses. J Anim Sci. 2002;80:1044–1052. doi: 10.2527/2002.8041044x. [DOI] [PubMed] [Google Scholar]
- Eckert E., Brown H.E., Leslie K.E., DeVries T.J., Steele M.A. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J Dairy Sci. 2015;98:6315–6326. doi: 10.3168/jds.2014-9062. [DOI] [PubMed] [Google Scholar]
- Gånheim C., Hulten C., Carlsson U., Kindahl H., Niskanen R., Waller K.P., Series B. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J Vet Med Ser B. 2003;50:183–190. doi: 10.1046/j.1439-0450.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- Gaweł S., Wardas M., Niedworok E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–458. [PubMed] [Google Scholar]
- Girard J., Ferre P., Pegorier J.P., Duee P.H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev. 1992;72:507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Zabielski R., David J.C., Blum J.W., Morisset J.A., Biernat M., et al. Sodium-butyrate as a growth promoter in milk replacer formula for young calves. J Dairy Sci. 2009;92:1038–1049. doi: 10.3168/jds.2008-1213. [DOI] [PubMed] [Google Scholar]
- Haley D.B., Bailey D.W., Stookey J.M. The effects of weaning beef calves in two stages on their behavior and growth rate. J Anim Sci (Sofia) 2005;83:2205–2214. doi: 10.2527/2005.8392205x. [DOI] [PubMed] [Google Scholar]
- Hiss S., Weinkauf C., Hachenberg S., Sauerwein H. Relationship between metabolic status and the milk concentrations of haptoglobin and lactoferrin in dairy cows during early lactation. J Dairy Sci. 2009;92:4439–4443. doi: 10.3168/jds.2008-1632. [DOI] [PubMed] [Google Scholar]
- Hulbert L.E., Cobb C.J., Carroll J.A., Ballou M.A. The effects of early weaning on innate immune responses of Holstein calves. J Dairy Sci. 2011;94:2545–2556. doi: 10.3168/jds.2010-3983. [DOI] [PubMed] [Google Scholar]
- Jiao J., Li X., Beauchemin K.A., Tan Z., Tang S., Zhou C. Rumen development process in goats as affected by supplemental feeding v. grazing: age-related anatomic development, functional achievement and microbial colonisation. Br J Nutr. 2015;113:888–900. doi: 10.1017/S0007114514004413. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Lee H.J., Lee W.S., Kim H.S., Ki K.S., Hur T.Y., et al. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J Dairy Sci. 2007;90:3376–3387. doi: 10.3168/jds.2007-0104. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Lee H.J., Lee W.S., Kim H.S., Kim S.B., Park S.B., et al. Starch source evaluation in calf starter: II. Ruminal parameters, rumen development, nutrient digestibilities, and nitrogen utilization in Holstein calves. J Dairy Sci. 2008;91:1140–1149. doi: 10.3168/jds.2007-0337. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Weary D.M., von Keyserlingk M.A. Hay intake improves performance and rumen development of calves fed higher quantities of milk. J Dairy Sci. 2011;94:3547–3553. doi: 10.3168/jds.2010-3871. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Weary D.M., von Keyserlingk M.A. Invited review: effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J Dairy Sci. 2011;94:1071–1081. doi: 10.3168/jds.2010-3733. [DOI] [PubMed] [Google Scholar]
- Kim M.H., Yang J.Y., Upadhaya S.D., Lee H.J., Yun C.H., Ha J.K. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J Vet Sci. 2011;12:151–157. doi: 10.4142/jvs.2011.12.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.H., Toji N., Kizaki K., Kushibiki S., Ichijo T., Sato S. Effects of dietary forage and calf starter on ruminal pH and transcriptomic adaptation of the rumen epithelium in Holstein calves during the weaning transition. Physiol Genom. 2016;48:803–809. doi: 10.1152/physiolgenomics.00086.2016. [DOI] [PubMed] [Google Scholar]
- Kotunia A., Wolinski J., Laubitz D., Jurkowska M., Rome V., Guilloteau P., et al. Effect of sodium butyrate on the small intestine development in neonatal piglets fed by artificial sow. J Physiol Pharmacol. 2004;55(Suppl 2):59–68. [PubMed] [Google Scholar]
- Laarman A.H., Sugino T., Oba M. Effects of starch content of calf starter on growth and rumen pH in Holstein calves during the weaning transition. J Dairy Sci. 2012;95:4478–4487. doi: 10.3168/jds.2011-4822. [DOI] [PubMed] [Google Scholar]
- Lane M.A., Baldwin R.L., Jesse B.W. Developmental changes in ketogenic enzyme gene expression during sheep rumen development. J Anim Sci. 2002;80:1538–1544. doi: 10.2527/2002.8061538x. [DOI] [PubMed] [Google Scholar]
- Li Y., Ding H.Y., Wang X.C., Feng S.B., Li X.B., Wang Z., et al. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J Anim Physiol Anim Nutr. 2016;100 doi: 10.1111/jpn.12454. [DOI] [PubMed] [Google Scholar]
- Liao R., Lv Y., Zhu L., Lin Y. Altered expression of miRNAs and mRNAs reveals the potential regulatory role of miRNAs in the developmental process of early weaned goats. PLoS One. 2019;14:1–16. doi: 10.1371/journal.pone.0220907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X., Chai J., Diao Q., Huang W., Zhuang Y., Zhang N. The signature microbiota drive rumen function shifts in goat kids introduced to solid diet regimes. Microorganisms. 2019;7:516. doi: 10.3390/microorganisms7110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistrelli D., Aufy A.A., Pinotti L., Rosi F. Analysis of weaning-induced stress in Saanen goat kids. J Anim Physiol Anim Nutr. 2013;97:732–739. doi: 10.1111/j.1439-0396.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Le Gall M., De Filippi S., Minieri L., Trevisi P., Wolinski J., et al. Supplemental sodium butyrate stimulates different gastric cells in weaned pigs. J Nutr. 2008;138:1426–1431. doi: 10.1093/jn/138.8.1426. [DOI] [PubMed] [Google Scholar]
- Meale S.J., Leal L.N., Martin-Tereso J., Steele M.A. Delayed weaning of Holstein bull calves fed an elevated plane of nutrition impacts feed intake, growth and potential markers of gastrointestinal development. Anim Feed Sci Technol. 2015;209:268–273. [Google Scholar]
- NRC . National Academy Press; Washington, DC: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- NRC . National Academy Press; Washington, DC: 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. [Google Scholar]
- Penner G.B., Steele M.A., Aschenbach J.R., McBride B.W. Ruminant Nutrition Symposium: molecular adaptation of ruminal epithelia to highly fermentable diets. J Anim Sci. 2011;89:1108–1119. doi: 10.2527/jas.2010-3378. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., Smith Z.P., Heitmann R.N. Changes in plasma volatile fatty acids in response to weaning and feed intake in young calves. J Dairy Sci. 1991;74:258–263. doi: 10.3168/jds.S0022-0302(91)78168-X. [DOI] [PubMed] [Google Scholar]
- Rice E., Aragona K., Moreland S., Erickson P. Supplementation of sodium butyrate to postweaned heifer diets: effects on growth performance, nutrient digestibility, and health. J Dairy Sci. 2019;102:3121–3130. doi: 10.3168/jds.2018-15525. [DOI] [PubMed] [Google Scholar]
- Sargent H., Elliott J., Jepson R. The new age of renal biomarkers: does SDMA solve all of our problems? J Small Anim Pract. 2021;62:71–81. doi: 10.1111/jsap.13236. [DOI] [PubMed] [Google Scholar]
- Sauerwein H., Schmitz S., Hiss S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005;10:295–302. doi: 10.1179/135100005X83725. [DOI] [PubMed] [Google Scholar]
- Stahl T., Hatungimana E., Klanderman K., Moreland S., Erickson P. Sodium butyrate and monensin supplementation to postweaning heifer diets: effects on growth performance, nutrient digestibility, and health. J Dairy Sci. 2020;103:10207–10218. doi: 10.3168/jds.2020-18584. [DOI] [PubMed] [Google Scholar]
- Steele M.A., AlZahal O., Hook S.E., Croom J., McBride B.W. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: a case report. Acta Vet Scand. 2009;51:39. doi: 10.1186/1751-0147-51-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M.A., Dionissopoulos L., McBride B.W., AlZahal O., Greenwood S.L., Laarman A.H. Butyrate and subacute ruminal acidosis affect abundance of membrane proteins involved with proton and short chain fatty acid transport in the rumen epithelium of dairy cows. Am J Anim Vet Sci. 2013;8:220–229. [Google Scholar]
- Sun D.M., Mao S.Y., Zhu W.Y., Liu J.H. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal. 2018;12:2274–2283. doi: 10.1017/S1751731118000290. [DOI] [PubMed] [Google Scholar]
- Sun W., Sun J., Li M., Xu Q., Zhang X., Tang Z., et al. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J Appl Microbiol. 2020;128:1613–1623. doi: 10.1111/jam.14612. [DOI] [PubMed] [Google Scholar]
- Van Keulen J., Young B.A. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J Anim Sci. 1977;44:282–287. [Google Scholar]
- Van Soest P.V., Robertson J., Lewis B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang W., Li C., Li F., Wang X., Zhang X., Liu T., et al. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci Rep. 2016;6:32479. doi: 10.1038/srep32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wu M.M., Xiao H., Ren W.K., Duan J.L., Yang G., et al. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 2014;92:612–619. doi: 10.2527/jas.2013-6986. [DOI] [PubMed] [Google Scholar]
- Youssef M.A., El-Khodery S.A., El-deeb W.M., Abou El-Amaiem W.E. Ketosis in buffalo (Bubalus bubalis): clinical findings and the associated oxidative stress level. Trop Anim Health Prod. 2010;42:1771–1777. doi: 10.1007/s11250-010-9636-9. [DOI] [PubMed] [Google Scholar]
- Zhang N.F., Tu Y., Diao Q.Y. Construction of young ruminant health cultivation system and its scientific issues [J] Chin Sci Bull. 2017;62:2999–3007. [Google Scholar]
- Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]