Abstract
Rhodomyrtus tomentosa (Ait.) Hassk (RTH) is a plant distributed mainly in Southeast Asia that has long been used as a food or a folk remedy for various ailments. Anthocyanins extracted from RTH have received the attention of researchers as well as manufacturers in exploiting the application of this plant for commercial purposes. Several extraction methods have been performed, however, concerns about new extraction methods, extraction conditions as well as stability of anthocyanins during storage are issues that need to be evaluated. In this study, the process of extracting anthocyanins by microwave method and the stability of anthocyanins in RTH extraction were investigated. For the extraction method, the influence of solvent concentration (30–70%), application ratio (10:1–1:1 mL/g), microwave power (100–500W), and time (1–9 min) have been evaluated. A first-order kinetic model was also used to evaluate the variation of anthocyanin content during storage at different conditions, including 5 °C (refrigerator), 30 °C (room temperature), and 45 °C (incubator). The study results showed that the anthocyanin content had the greatest value of 137.54 mg/L when extracted with 50% ethanol solvent with the material/solvent ratio of 1:3 (g/ml) microwave power is 200W for 5 min. The first-order decomposition kinetics model showed that the t1/2 values of the RTH extract were 69.64, 28.66, and 19.8, respectively, for the storage temperatures of 5 °C, 30 °C, and 45 °C, respectively. During storage, anthocyanin content tends to decrease more rapidly under high-temperature conditions. In addition, a close correlation between anthocyanin content and antioxidant capacity was recorded at a high significance level R2 > 0.98.
Keywords: Microwave-assisted extraction, Anthocyanins, Kinetic degradation, Antioxidant capacity
Microwave-assisted extraction; Anthocyanins; Kinetic degradation; Antioxidant capacity.
1. Introduction
Plants in nature have long been thought to be a valuable source of nutrients for daily meals, as well as a potential treatment for human diseases. Natural medications have become popular and effective in primary health care in recent years. Plant extracts containing physiologically active chemicals in infectious and chronic conditions enabled the search for new medications with therapeutic potential due to plants' diversity of intrinsic chemical components [1]. In general, the long history of plant remedies demonstrates man's contact with nature.
Because of their health and pharmaceutical industry benefits, anthocyanin chemicals are currently getting much public attention. Anthocyanins are natural color pigments that include purple, blue, and red in addition to being the main sub-class in polyphenols/flavonoids [2]. These pigments can be found in flowers, fruits, and vegetables. Anthocyanins are a pigment that plants use to absorb light during photosynthetic processes and to attract pollinators [3]. The sugar component of anthocyanin is made up of glucoside linkages, galactose, arabinose, rhamnose, xylose, and fructose, whereas the non-sugar component (anthocyanidin) is made up of galactose, arabinose, rhamnose, xylose, and fructose [4]. The most common anthocyanidin is cyanidin, which is followed by delphinidine, peonidine, pelargonidine, petunidine, and Malvidin. For example, delphinidine can generate bright blue, whereas cyanidine and pelargonidine can produce red and purple. Anthocyanins have been identified in over 500 different kinds, with a variety of biological actions such as anti-inflammatory, cancer support, blood pressure management, and slowing aging being discovered along the way. Furthermore, studies have demonstrated that certain natural dye extracts containing anthocyanins are advantageous in sensitive solar cells with dyes and coatings [5, 6, 7, 8, 9, 10].
Rhodomyrtus tomentosa (RTH), often known as Rose Myrtle, is a Rhodomyrtus (Ait.) Hassk (RTH) species grow in tropical and subtropical regions. Pink blossoms and violet berries, which are bell-shaped and immediately edible, can be found on RTH. The entire plant stem, nut, and fruit have been reported to be used as raw materials in the pharmaceutical industry [11]. According to the previous study, RTH contains many polyphenols/flavonoids, triterpenes, and steroids [12]. Studies have shown that Rose Myrtle is a healthful meal that may be used to make traditional jams, wine, and beverages. Antioxidants like as ascorbic acid and phenolic compounds are abundant in this fruit [13]. Anthocyanins, on the other hand, decay quickly and generate colorless or unattractive brown-colored chemicals, which are undesired in products such as fruit juices because customers see the color shift as an indication of poor quality. Temperature, solvent composition, extraction duration, pH, and solid-to-liquid ratio are all variables that influence the extraction of the bioactive molecule, according to a prior study [14]. As a result, many studies on thermal degradation of anthocyanins have been conducted; however, little is known about the effect of temperature and storage time on the quality of biological compounds and the antioxidant capacity of anthocyanin extract from Rose Myrtle.
Various extraction methods, such as enzyme-assisted extraction, soxhlet extraction, and others, are available to extract bioactive components. In that situation, microwave extraction of anthocyanins has been studied in the past with high efficiency, high efficiency, and good quality [15]. Extraction procedures using common solvents (such as acetone, methanol, and ethanol) have been described many times before, particularly for the extraction of anthocyanin compounds. On the other hand, the advantages of the simple nature of the process outweigh the disadvantages, including having to run the process for a long time and using a large amount of solvent. These are factors to consider when selecting a process for this particular program. According to Pham TN et al. (2019), the time required to maintain the extraction process at 67 °C for the optimal anthocyanin content from purple sweet potato was 39 min [16]. This procedure was found to be effective in extracting anthocyanins; however, it is clear that keeping the anthocyanin mixture at a high temperature for an extended period of time is a fatal flaw, which is closely related to anthocyanin compounds' fundamental property of being temperature sensitive. Enzyme-assisted extraction is a popular choice in the extraction of value-added compounds in general, and anthocyanins in particular, to improve extraction efficiency. The benefit of this method is that it is safe and non-toxic; however, there is a disadvantage in that it is affected by pH and time, which reduces the efficiency of the entire process [17]. In addition, the ultrasonic-assisted extraction (UAE) method has recently been used to extract anthocyanins. When compared to the efficiency of microwave-assisted extraction (MAE), the UAE method requires more time. MAE is a promising engineering model for speeding up bioactive compound extraction from natural sources. This method is known for its high separation efficiency and quick extraction [18]. These properties are due to the breakdown of cell structure caused by microwave irradiation's penetrating volume heating. The diffusion capabilities of target components can be enhanced by increasing the temperature of the solvent during microwave heating [19].
With the aim of finding a method to extract anthocyanins quickly and economically. In this study, we selected to extract anthocyanins from Rhodomyrtus tomentosa using the microwave technique. The microwave using radiation is projected to minimize extraction time while still obtaining high-value anthocyanin content. A kinetic reaction model was also used to survey the combined effect of storage temperature and storage time on anthocyanin stability in RTH extract. From there, the quality changes that occur during storage can be predicted. Furthermore, the antioxidant activity was assessed using the DPPH and ABTS free radical scavenging methods to determine their relationship with the total anthocyanin content of the extract. The study is expected to provide additional data that will aid in the understanding of this plant species, potentially increasing its value in the not-too-distant future.
2. Material and methods
2.1. Sample and chemical preparation
In January 2021, fresh RTH was purchased from a local market in Phu Quoc, Vietnam. Separating the samples was done by hand, and they were then frozen for future study.
The reagents were as follows: DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2- Azinobis-3-ethylbenzothiazoline-6- sulfonic acid) (Sigma-Aldrich, USA). Sodium acetate, acid hydrochloric, potassium persulfate, ascorbic acid, and ethanol (Merck, Germany). All of the solutions were made with distilled water.
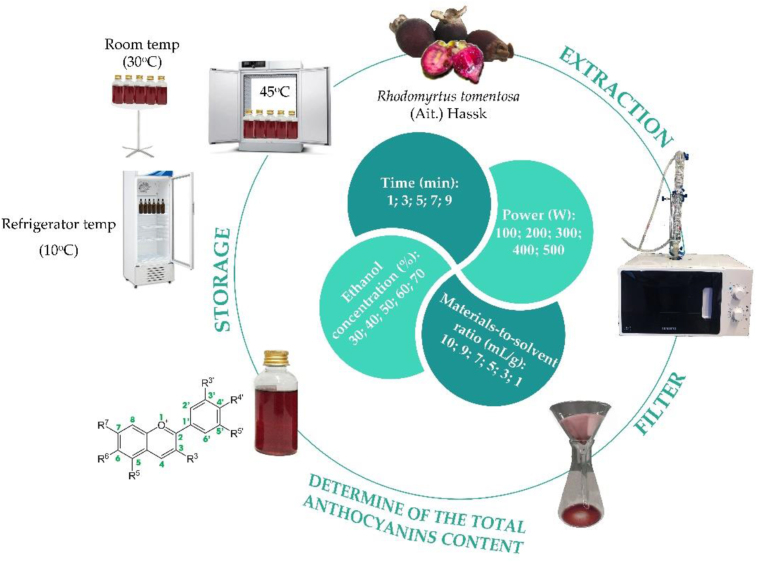
RTH was crushed before being placed in a microwave-assisted extraction system (microwave equipment code MW71E – SAMSUNG, Vietnam). Depending on the materials-to-solvent ratio, microwave radiation, and irradiation period, they were mixed with varying amounts of solvent. In a high-speed centrifuge model LACE16, the sample was centrifuged at 5000 rpm for 15 min (from COLO lab expert). The extracts were obtained to calculate the total anthocyanin content (using UV-Vis- Aligent Cary 60 Spectrophotometer – USA). Figure 1 depicts the complete experimental procedure.
Figure 1.
Routine investigation of RTH anthocyanin extract.
2.2. Anthocyanin content measurement
TAC was determined using a pH-differential technique with two buffer systems [20]: KCl, pH 1.0 (0.025 M) and CH3COONa, pH 4.5. (0.4 M). In brief, 1 mL of cleared juice was combined with 4 mL of each buffer before being measured at 510 and 700 nm against distilled water as a blank. A was used to compute absorbance. TAC was determined for each sample using Eq. (1):
| (1) |
where:
A = (Aλvis max - A700)1.0 – (Aλvismax - A700)4.5;
TAC (mg/L): the anthocyanin content in total;
Aλvis max: absorbances at λvismax;
A700: 700 nm respectively;
MW (449,2 g/mol): molecular weight of cyanidin-3-glucoside;
DF (ϵ = 26900): dilution factor; ϵ: molar absorptivity of cyanindin-3-glucoside;
l: thickness of the cuvett;
103: conversion factor between grams and milligrams.
2.3. Antiradical activity: DPPH˙ assay
The extract samples' antiradical activity was determined using the 2,2-diphenyl-1-picrylhydrazyl hydrate radical (DPPH) [21]. In a nutshell, 0.5 ml of RTH extract was mixed with 1.5 ml of DPPH (OD517nm = 1,1 ± 0,02) solution and incubated at room temperature for 30 min in the dark. The absorbance of the mixture was measured at 517 nm. The sample's ability to scavenge the DPPH radical was determined by:
| (2) |
In there:
ODcontrol: Optical absorbance of the control sample.
ODsample: Optical absorbance of the test specimen.
The results are recorded based on the IC50 value, the concentration at which the sample can reduce 50% of DPPH free radicals.
2.4. Antiradical activity: ABTS assay
This assay was carried out according to the instructions provided by Nenadis (2004) with adjustment [22]. To make the radical cation, combine 10 ml ABTS 7.4 mM stock solution with 2.6 mM potassium persulfate (1/1, v/v) and leave for 24 h, or until the reaction was complete and the absorbance was stable. For measurement, the ABTS + solution was diluted with ethanol to an absorbance of 1,1 ± 0,02 at 734 nm. After 45 s of mixing, 1.5 ml of ABTS + solution and 0.5 ml of extract were used for the photometric assay. After 30 min, the measurement was taken at 734 nm. Positive control was used, which was ascorbic acid. The antioxidative effect was determined by measuring the decrease in absorbance at various concentrations.
| (3) |
In there:
ODcontrol: Optical absorbance of the control sample.
ODsample: Optical absorbance of the test specimen.
The results are recorded based on the IC50 value, the concentration at which the sample can reduce 50% of ABTS free radicals.
2.5. Determination of kinematic parameters
A first-order depletion kinetic model was used in this study to represent the change in anthocyanin content over time. According to Jin-yu Chen, 2020 [23], the kinetic rate constant kd of thermal degradation was determined by regression of experimental data from initial concentrations with increasing time and is described as Eq. (4):
| (4) |
Where: CA denotes the total anthocyanin content after t days at a specific temperature (mg/L); CA0 is the total anthocyanin content at baseline (mg/L), t is the time (min), and kd is the kinetic rate constant (1/min).
The half-life (t1/2) of the degradation is shown in Eq. (5), according to Jin-yu Chen, 2020:
| (5) |
Where: kd is the kinematic rate constant (1/min).
2.6. Statistical analysis
All of the tests were carried out three times. Statgraphics Centurion XV (version 15.1.02) and Excel 365 were used to conduct the statistical analysis (Microsoft, Redmond, WA, USA).
3. Result
3.1. The effect of different solvent concentrations
The solvent concentration, temperature, material to solvent ratio, and extraction duration all have an impact on the anthocyanin yield. Figure 3 depicts their equivalent effects on anthocyanin yield. The solvent concentration is the first factor in determining the anthocyanin yield of extraction. Le et al. used a solvent concentration range of 25 %–100 % to evaluate fresh Carissa carandas and reported that the best anthocyanin content was obtained at 50 % [24]. Ethanol is also cost-effective and ecologically beneficial. As a result, this study looked at the effect of different ethanol concentrations (30, 40, 50, 60, and 70%) on anthocyanin extraction yield, as well as other extraction parameters like microwave irradiation power 300 W, material/solvent ratio (g/mL) (1:1), and irradiation time 5 min.
Figure 3.
Changes in anthocyanin content under microwave energy levels.
Figure 2 shows that the anthocyanin extraction yield increases as the ethanol concentration increases from 30% to 50% and decreases as the ethanol concentration exceeds 50%. The results revealed that the higher the ethanol concentration, the lower the anthocyanin concentration.
Figure 2.
The variation in anthocyanin content is caused by different material/solvent ratios.
However, the obtained content decreases when the ethanol concentration exceeds the limit at which anthocyanin molecules can be dissolved. When the ethanol concentration reached 60 percent and 70 percent, the anthocyanin content decreased by 1.1 (124.26 ± 1.05 mg/L) and 1.3 (103.81 ± 1.05 mg/L) times, respectively, compared to the landmark optimum at 50 percent ethanol (136.70 ± 1.15 mg/L). This is similar to the previous study by Nguyen et al. (2021), which found that using ethanol at a concentration of 50% increased the ability to extract anthocyanin more efficiently than using distilled water or 50% methanol [14]. Unwanted impurities such as mucus and resin may form at higher alcohol concentrations, lowering the pigment's quality. Furthermore, the ANOVA study results (p = 0.003 (<0.05)) revealed that the solvent component is 95% dependent on anthocyanin content. As a result, an ethanol concentration of 50% was chosen in the following trials.
3.2. Microwave power's influence
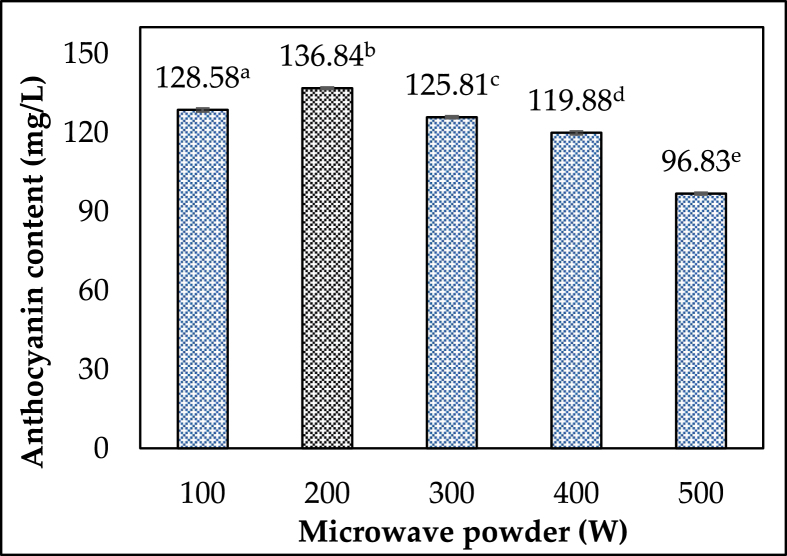
In the microwave extraction process, microwave power is critical. Choosing the appropriate microwave power for anthocyanin extraction will aid in increasing efficiency and shortening the extraction process. Figure 3 depicts the effect of various microwave power levels (100–500 W) combined with 50% ethanol, a material/solvent ratio of 1:1 mL/g, and a 5-minute irradiation time. Microwave powers ranging from 100 W to 200 W, with a peak at 200 W (p 0.05), enhanced the anthocyanin output by about 6%. The intensity of the incident microwave power, converted to heat energy in the dielectric material to raise its temperature, controls the amount of energy provided to the sample. It affects interactions and equilibrium rates and analyte partitioning between sample and solvent [25].
On the other hand, the increased microwave power can hasten the movement of the solvent, rupture the cell, and disperse the extract into the solvent. Whereas increasing the temperature increased the solubility of anthocyanin, which could reduce the viscosity of the extract and cause it to degrade [26]. Wei Liu et al. (2019) investigated the change in anthocyanin content extracted at microwave energy levels ranging from 80 to 400W and discovered that the best anthocyanin extraction efficiency was at 240W. The use of high microwave power for extraction aids in the rapid reduction of process time. However, anthocyanin is a temperature-sensitive compound by nature, so when used at higher levels, its properties may have been altered or partially degraded, resulting in low yield. As a result, a power level of 200W was used for further research.
3.3. The material-to-solvent ratio's effect
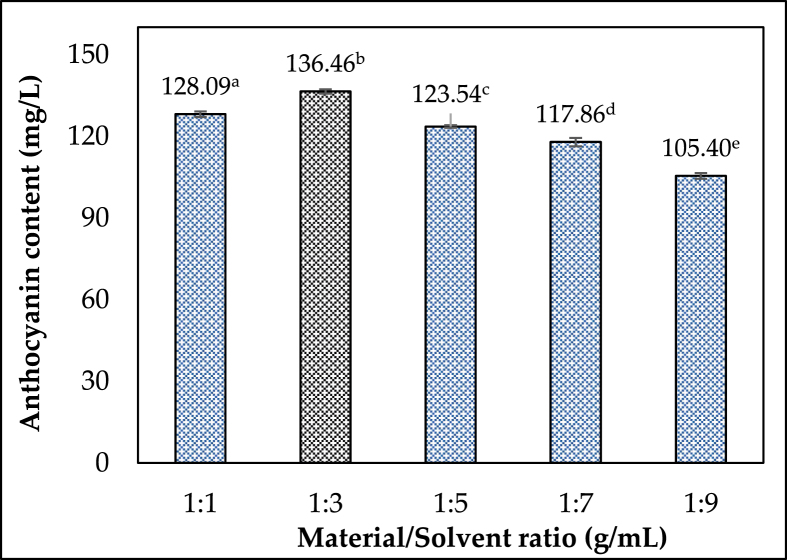
Because it aids in solute dissolution and mass transfer, the material/solvent ratio (M/S ratio) influences extraction efficiency. The influence of the M/S ratio on extraction effectiveness was investigated in the range of 1:1–1:9 g/mL, while the other variables remained constant: ethanol concentration of 50%, irradiation period of 5 min, and irradiation power of 200 W. TAC increased significantly as the M/S ratio increased, peaking at 1:3 g/mL (136.46 ± 0.78 mg/L) in Figure 4. It fell as the M/S ratio increased from 1:5 to 1:9 g/mL, reaching its lowest point at the end (108.70 ± 0.53 mg/L). Overall, this result is consistent with the previous report of Nguyen et al. (2021), which surveyed the solvent material ratios ranging from 1:2 to 1:16 (g/ml). The study of this group of authors shows that increasing the ratio of raw materials: and solvents increase anthocyanin content, but this increase reaches saturation when reaching a particular milestone and then begins an early decline (1.8 g/ml) [14]. The current study yielded similar results. This could be because the larger the solvent mass used, the longer it took to raise the mixture's temperature, resulting in more prolonged exposure to light of the anthocyanin compounds extraction and decomposition. The degradation of anthocyanin content was also observed in the report of Le et al. (2019) when the materials: the solvent ratio was increased to 1:4 and 1:5 (g/ml) [24]. The relationship between the material: solvent ratio and microwave energy and the extracted anthocyanin content was found to be statistically significant. When the material: solvent ratio and microwave energy are both increased at the same time, the anthocyanin content reaches its maximum at 2.5 g/mL and 200W [25]. Furthermore, the M/S ratio varies in comparison to other findings because it is dependent on the type and processing technique of the materials used in the study. As a result, we selected a 1:3 g/mL ratio to investigate other factors further.
Figure 4.
Changes in anthocyanin content under raw material/solvent ratios.
3.4. The impact of extraction time
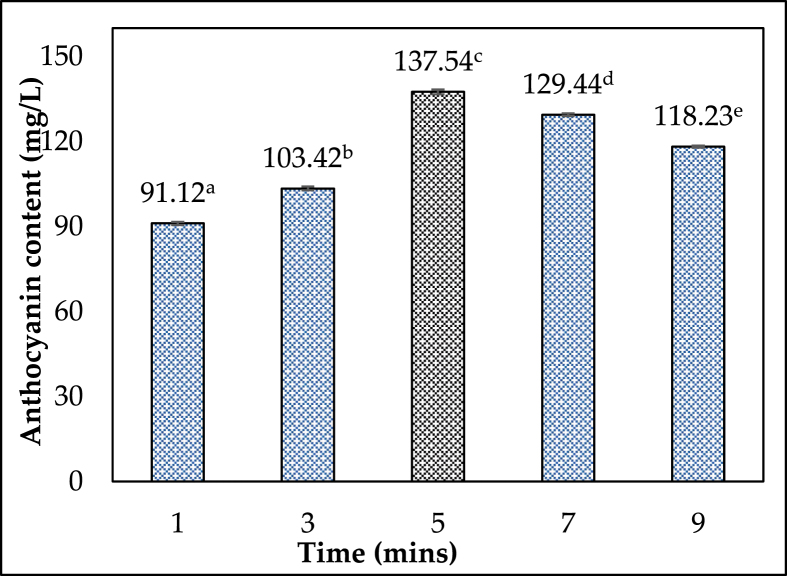
One of the most significant influences on total anthocyanin content extraction is microwave extraction time. The extraction time has a big impact on the contact between solvents and solids. Under the following conditions: ethanol concentration of 50%, M/S ratio of 1:3 g/mL, and irradiation power of 200 W, the influence of varied extraction periods (1, 3, 5, 7, and 9 min) on the extraction was examined. As the extraction period was prolonged from 1 to 5 min, the TAC value increased from 91.12 ± 0.56 to 137.54 ± 0.88 (mg/L), as shown in Figure 5. When compared to 5 min, anthocyanin content dropped 1.16 times (118.23 ± 0.37 mg/L) at 9 min. As the extraction time grew longer, the extraction efficiency began to deteriorate. The correlation of extraction time and microwave energy to the anthocyanin content obtained was studied by Wen et al. (2015), who found that increasing the microwave energy level (from 250 to 550W) increased the anthocyanin extraction efficiency. However, it was discovered that increasing the microwave irradiation time of the extraction process (from 3 min onwards) resulted in a significant reduction in anthocyanin content [26]. An optimization study on anthocyanin extraction from RTH was previously conducted by our research group, which discovered a significant correlation between microwave power and extraction time. The anthocyanin content obtained tended to increase as the microwave power and extraction time were increased simultaneously [27]. This could explain why the mass transfer equilibrium between the inside and outside material reaches the maximum threshold after a certain amount of time and why prolonging the extraction time could result in unfavorable responses. Extremely poor performance. Furthermore, the anthocyanin's nature is temperature-sensitive, causing the anthocyanin content to degrade as long as it is exposed to heat [28]. The extraction of anthocyanins from Rhodomyrtus tomentosa (Ait.) Hassk was found to be best, with a reaction time of 5 min.
Figure 5.
Change of anthocyanin content over time.
3.5. Kinetic study of pigment under different storage conditions
The thermal degradation of anthocyanins in the RTH extract under different storage conditions at 5 °C (Refrigerator), 30 °C (lab temperature), and 45 °C (incubator) are shown in Figure 6. Anthocyanin thermal degradation in RTH was demonstrated to be a first-order equation with a good regression coefficient by the linear association between the logarithm of anthocyanin concentration and time. Furthermore, the rate of anthocyanin degradation is accelerated when the temperature is changed to correspond to different storage conditions. The isothermal kinetic parameters are calculated using the first-order reaction kinetic model and are presented in Table 1 with high confidence values exceeding 95%. The results show that the decomposition rate constant (k) has a higher value at high storage conditions. At 45 °C condition, the decomposition rate constant (k = 0.03490 is 3.5 times higher than that of 5 °C (k = 0.0099), and at the same time, the t1/2 value is nearly 2.5 times lower at 30 °C (19.8 days) compared with storage at 5 °C (69.6 days). This is consistent with previous research on RTH on the trend of decreasing t1/2 value when the temperature is increased from 60 °C to 80 °C [29]. The report noted that the t1/2 value of Hibiscus sabdariffa extract was best at an average of 342 days when stored at low temperature compared to t1/2 at 180 days when stored at 40 °C [30]. In addition, when evaluating the kinetic model of the variation of anthocyanin content in Raspberry juice at 37 °C, the obtained results showed that the t1/2 of this compound did not exceed the mark of 10, with the reliability level being very high (R2 > 0.99) [31]. It shows that, in general, anthocyanins in plant extracts tend to be quickly degraded when stored at higher temperatures than when preserved at low temperatures. This is consistent with the inherent heat-sensitive properties of the theoretical anthocyanin compounds. Furthermore, it can be seen that anthocyanins of RTH have moderate temperature sensitivity when compared to Hibiscus sabdariffa and Raspberry juice. The difference may be due to the different anthocyanin structures present in the raw materials, and besides, the storage time, as well as the anthocyanin content, is also influenced by other phenolic components - factors related to the antioxidant capacity of the mixture.
Figure 6.
Thermal degradation kinetics of total anthocyanins in RTH. ( ): 5 °C; (
): 5 °C; ( ): 30 °C; (
): 30 °C; ( ): 45 °C.
): 45 °C.
Table 1.
Temperature effects on the k and t1/2 values of anthocyanin degradation in various storage conditions.
| Type of treatment | R2adj | k | t1/2 |
|---|---|---|---|
| Refrigerated (5 °C) | 0.9561 | 0.0099 | 69.6421 |
| Room (30 °C) | 0.9660 | 0.0241 | 28.6604 |
| Ambient (45 °C) | 0.9720 | 0.0349 | 19.8140 |
3.6. Antioxidant activity
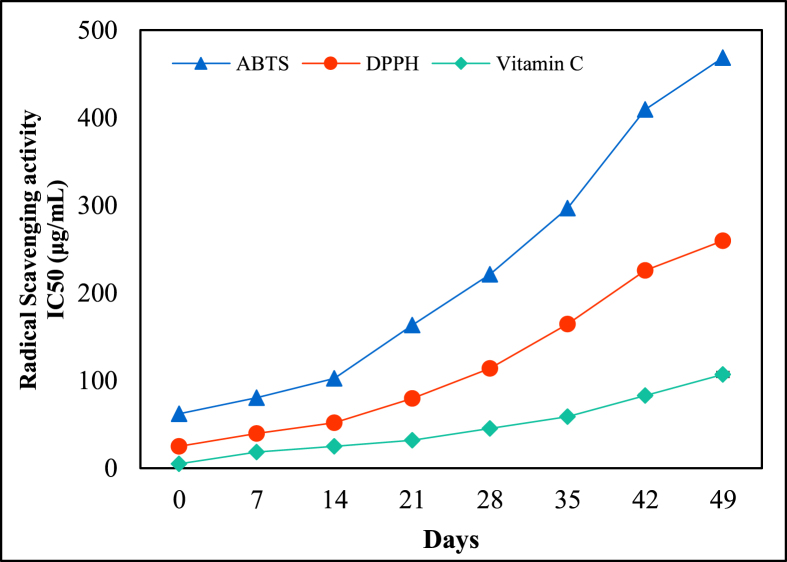
The change of antioxidant capacity of anthocyanin extracts from myrtle fruit during storage was evaluated by two antioxidant methods. The sample's ability to scavenge the DPPH radical was determined by Eq. (2), and the antioxidative effect was determined by measuring the decrease in absorbance at various concentrations, as shown in Eq. (3). The antioxidant capacity test in this study is expressed through the IC50 value. This value is expressed in the sense that the smaller this value corresponds to the better oxidation resistance. Besides, to compare the antioxidant capacity of a compound, its IC50 value will be compared with the IC50 value of a control - commonly used vitamin C.
As shown in Figure 7, the IC50 value on the first day of the DPPH method was 19.96 ± 1.7 μg/ml, and that of the ABTS method was 36.96 ± 1.7 μg/ml. When comparing this result with the previous report of Nguyen TQ et al. (2021), it was found that the antioxidant effect of RTH was collected in July (IC50 is 0.0173 ± 0.001 μg/ml and 0.0238 ± 0.0002 μg/ml, respectively. ml corresponding to DPPH and ABTS methods) was better than in January (results of the present study) [14]. Besides, Chun Cui et al. (2013) evaluated the antioxidant activity of RTH extract (harvested in August) by different methods and reported that it has a strong antioxidant capacity. Powerful through the removal of more than 90% of DPPH free radicals at a concentration of 20 μg/ml (IC50 = 6.27 ± 0.25 μg/ml) and for ABTS free radicals IC50 = 90.3 ± 1.52 μg/ml [32]. The difference in free radical scavenging activity may be due to the effect of the time of raw material harvest. In the country in the tropical climate, the period from June to August every year is when the raw materials are harvested to fruit trees reach perfect maturity – RTH is one of them. As a result, biologically active substances will be synthesized and formed in the whole fruit at this stage than at other times. The study by Mansureh et al. (2019) on the antioxidant capacity of Dracocephalum kotschyi Boiss at different harvest stages also noted that there was a significant influence on the time of harvest, the organs of the plants harvested to the total antioxidant capacity, and total phenolic and flavonoid content [33].
Figure 7.
The radical scavenging activity of anthocyanin extract from RTH.
RTH extract was followed up after two months. The results were recorded, showing the similarity of the trend of decreasing antioxidant capacity in both methods. Among them, the ABTS test showed the most significant decrease in antioxidant activity compared with the DPPH method. It is easy to see that from the 21st day onwards, the IC50 value of the ABTS method decreased more than two times compared to the first day (increased from 36.96 ± 1.74 μg/ml to 83.79 ± 3.9 μg/ml). In the DPPH trial, the 50% free radical scavenging capacity began to decline statistically significantly from day 14 (26.91 ± 3.1 μg/ml) to a peak of 7.6 times on day 49 (152.67 ± 2.3). μg/ml) from the first day. The DPPH and ABTS methods are based on an antioxidant's ability to scavenge free radicals when it has hydrogen atoms in its structure. The effectiveness of an antioxidant also depends on the reaction times of different free radicals. In addition, differences in reaction mechanisms and limitations of individual trials may account for differences in results for the same antioxidant. In parallel, some studies show that the loss of antioxidant capacity during storage is linearly correlated with the loss of anthocyanin content in antioxidants. Chen et al. (2020) observed that the decrease in total kaempferol glycoside and anthocyanin content was responsible for the decrease in antioxidant capacity during the storage of red raspberry extract [34]. In addition, Sui et al. (2016) suggested that newly formed compounds, polymerization, and partial oxidation are responsible for the decline in antioxidant activity of anthocyanins [35].
3.7. Correlations
The correlation between antioxidant activity - IC50 (μg/ml) in DPPH, ABTS, TPC (mg/g), TFC (mg/g) and anthocyanin (mg/L) had been determined and showed in Table 2.
Table 2.
Relationship between anthocyanin, polyphenol, flavonoid content, and antioxidant activity of RTH.
| Anthocyanin | DPPH | ABTS | |
|---|---|---|---|
| Anthocyanin | 1 | ||
| DPPH | -0.983969459 | 1 | |
| ABTS | -0.981542195 | 0.993233429 | 1 |
Correlation analyses were performed to determine the relationship between anthocyanin content and antioxidant activity, and color characteristics of selected plant extracts. Phenolic-rich plant materials are increasingly used in the food industry because they slow down oxidative lipid breakdown and improve foods' quality and nutritional value. The hydroxyl groups with the function of scavenging free radicals play an essential role in the value creation of phenolic compounds. Plant phenolic compounds are divided into several categories. The flavonoid is an ingredient known for effectively removing most of the oxidizing molecules, including single oxygen and various free radicals associated with various human diseases. Flavonoid derivatives have been shown to have a wide range of antibacterial, antiviral, anti-inflammatory, antitumor, and antiallergic properties [36]. In addition, it is also the main component that determines the antioxidant capacity of plant extracts, helping to protect nutritional values against environmental agents. Anthocyanin compounds are part of a chain of phenolics, so it inherits the excellent biological activities of the chain and is most clearly observed through their free radical scavenging capacity. In fact, Rhodomyrtus tomentosa (Ait.) Hassk, commonly used as a food, is a rich source of high-value phenolic compounds.
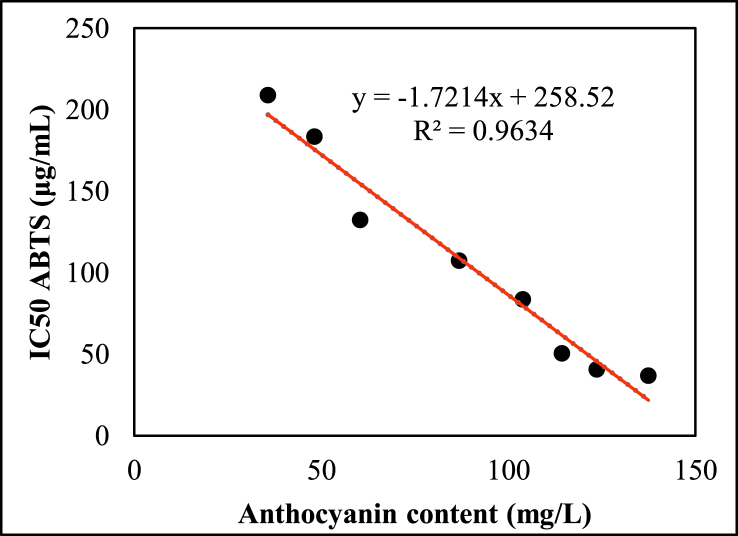
The results are shown in Table 1, and Figures 8 and 9 show a strong negative correlation between anthocyanins and the ability to remove free radicals DPPH (R2 = 0.983), and ABTS (R2 = 0.981). The results showed a stronger correlation than at the R2 = 0.842 value observed in Zea mays L. extract between the DPPH and anthocyanin test [37]. It was found that the higher the anthocyanin content, the lower the EC50 value for both DPPH and ABTS free radical scavenging methods – this indicates strong antioxidant capacity. This is also consistent with Castaneda-Ovando et al. (2009) statement that anthocyanin molecules have many advantages in donating free electrons or hydrogen atoms to reactive free radicals [38]. More specifically, they readily counteract lipid peroxidation by donating the H atom to the peroxyl radical during autooxidation, thus acting as chain-breaking antioxidants. The results of this study show that the extract from Rhodomyrtus tomentosa (Ait.) Hassk contains a large number of phytochemicals that are able to provide hydrogen for a free radical to eliminate potential damage.
Figure 8.
Relationship between total anthocyanin content (TAC) and Radical scavenging activity IC50 (DPPH) of RTH.
Figure 9.
Relationship between total anthocyanin content (TAC) and Radical scavenging activity IC50 (ABTS) of RTH.
4. Conclusion
This study showed the optimal parameters for anthocyanin extraction from Rhodomyrtus tomentosa (Ait.) Hassk by the microwave-assisted method. According to the results of this study, the use of microwaves in the extract helps to obtain anthocyanins efficiently and quickly. The results of the kinetic model-based study also showed that the preservation of anthocyanin extracts was best at the temperature of 5 °C. The findings demonstrated that anthocyanin degradation followed first-order reaction kinetics with a confidence factor greater than 0.95. Besides, a close correlation between anthocyanin content and the antioxidant capacity of this plant extract was also shown in this study. The results of this study will further enrich the anthocyanin extraction methods from Rhodomyrtus tomentosa (Ait.) Hassk serves as the basis for controlling anthocyanin variation, thereby developing methods to preserve these extracts more effectively in practice.
Declarations
Author contribution statement
Tri Nhut Pham: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xuan Tien Le: Performed the experiments; Analyzed and interpreted the data.
Van Thinh Pham: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Hoang Thien Le: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Nguyen Tat Thanh University, Ho Chi Minh city, Vietnam.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Toan T.Q., Phong T.D., Tien D.D., Linh N.M., Anh NT Mai. 2021. Optimization of Microwave-Assisted Extraction of Phlorotannin from Sargassum Swartzii (Turn.) C. Ag. With Ethanol/Water - Natural Product Communications. [Google Scholar]
- 2.Pham T.N., Lam T.D., Nguyen M.T., Le X.T., Vo N.D.V. Effect of various factors on extraction efficiency of total anthocyanins from Butterfly pea (Clitoria ternatea L. Flowers) in Southern Vietnam. IOP Conf. Ser.: Mater. Sci. Eng. 2019;544:012013. [Google Scholar]
- 3.Brito A., Areche C., Sepulveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules. 2014;19(8):10936–10955. doi: 10.3390/molecules190810936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pojer E., Mattivi F., Johnson D., Stockley C.S. The case for anthocyanin consumption to promote human health: a review. Compr. Rev. Food Sci. Food Saf. 2013;12(5):483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- 5.Blando F., Calabriso N., Berland H., Maiorano G., Gerardi C., Carluccio M.A., Andersen O.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018;19(1):169. doi: 10.3390/ijms19010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F., Liu Y., Liu J., Wang E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC Adv. 2019;9(19):10842–10853. doi: 10.1039/c9ra01772k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendrame Stefano, Klimis-Zacas Dorothy. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: a review. Nutrients. 2019;11(6):1431. doi: 10.3390/nu11061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Jie, et al. Anthocyanins from black chokeberry (Aroniamelanocarpa Elliot) delayed aging-related degenerative changes of brain. J. Agric. Food Chem. 2017;65(29):5973–5984. doi: 10.1021/acs.jafc.7b02136. [DOI] [PubMed] [Google Scholar]
- 9.Aduloju S.J., Alaba K., Shitta M.B., Modern J. Effect of extracting solvents on the stability and performances of dye-sensitized solar cell prepared using extract from Lawsonia Inermis. Fundamental J. Modern Physics. 2011;1(2):261–268. [Google Scholar]
- 10.Gómez-Ortíz, N.M., Vázquez-Maldonado I.A., Pérez-Espadas A.R. Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol. Energy Mater Sol. 2010;94(1):40–44. [Google Scholar]
- 11.Lai T.N., André C., Rogez H., Mignolet E., Nguyen T.B., Larondelle Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa) Food Chem. 2015;168:410–416. doi: 10.1016/j.foodchem.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 12.Abd Hamid H., Sileshi Zeyohannes Roziasyahira M.S., Yusoff M.M. Rhodomyrtus tomentosa: a phytochemical and pharmacological review. Asian J. Pharmaceut. Clin. Res. 2016;10(1):10. [Google Scholar]
- 13.Sang Vo Thanh, et al. Investigation of the biological activities of Phu Quoc Sim fruits Rhodomyrtus tomentosa (aiton) hassk. EurAsia J. BioSci. 2019;13(1):49–55. [Google Scholar]
- 14.Nguyen T.Q., Tran H.D.P., Nguyen D.K., Nguyen H.N.M., Lu H.K., Ngo D.H., Vo T.S. Optimization of anthocyanin extraction from Rhodomyrtus tomentosa (ait.) hassk. Fruits and their antioxidant potentials. Trop. J. Nat. Prod. Res. 2021;5(7):1179–1184. [Google Scholar]
- 15.Elez Garofulić I., Dragović-Uzelac V., Režek Jambrak A., Jukić M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca) J. Food Eng. 2013;117(4):437–442. [Google Scholar]
- 16.Pham T.N. IOP Conference Series: Materials Science and Engineering. Vol. 479. IOP Publishing; 2019. Response surface modeling and optimizing conditions for anthocyanins extraction from purple sweet potato (Ipomoea batatas (L.) Lam) grown in Lam Dong province, Vietnam. No. 1. [Google Scholar]
- 17.Zhao X., Li J. Optimization of enzyme hydrolysis of purple sweet potato by cellulase or pectinase for anthocyanin extraction. Food Sci. Technol. 2015;40(4):277–281. [Google Scholar]
- 18.Liu W., Yang C., Zhou C., Wen Z., Dong X. An improved microwave-assisted extraction of anthocyanins from purple sweet potato in favor of subsequent comprehensive utilization of pomace. Food Bioprod. Process. 2019 [Google Scholar]
- 19.Zheng X., Xu X., Liu C., Sun Y., Lin Z., Liu H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Separ. Purif. Technol. 2013;104:17–25. [Google Scholar]
- 20.Kırca A., Cemeroğlu B. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chem. 2003;81(4):583–587. [Google Scholar]
- 21.Sharma O.P., Bhat T.K. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. [Google Scholar]
- 22.Nenadis N., Wang L.-F., Tsimidou M., Zhang H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS •+ assay. J. Agric. Food Chem. 2004;52:4669–4674. doi: 10.1021/jf0400056. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Du J., Li M., Li C. LWT; 2020. Degradation Kinetics and Pathways of Red Raspberry Anthocyanins in Model and Juice Systems and Their Correlation with Color and Antioxidant Changes during Storage; p. 109448. [Google Scholar]
- 24.Le Xuan Tien, et al. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa carandas L. fruits. Processes. 2019;7(7):468. [Google Scholar]
- 25.Pham T.N., Linh H.T.K., Ngo A.Q., Anh H.L.T., Lam T.D., Toan T.Q. Optimization of microwave-assisted extraction process by response surface methodology of natural anthocyanins from Rhodomyrtus tomentosa (ait.) hassk. Solid State Phenom. 2019;298:94–99. [Google Scholar]
- 26.a Wen Y., Chen H., Zhou X., Deng Q., Zhao Y., Zhao C., Gong X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015;5(25):19686–19695. [Google Scholar]; b Routray W., Orsat V. Microwave-assisted extraction of flavonoids: a review. Food Bioprocess Technol. 2011;5(2):409–424. [Google Scholar]
- 27.Yang Zhendong, Zhai Weiwei. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.) Innovative Food Science and Emerging Technologies. 2010;11(1):169–176. [Google Scholar]
- 28.Yang L., Cao Y.-L., Jiang J.-G., Lin Q.-S., Chen J., Zhu L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantiumL. var.amaraEngl. J. Separ. Sci. 2010 doi: 10.1002/jssc.200900776. [DOI] [PubMed] [Google Scholar]
- 29.Liu G., Sun Y., Guo H. 2013. Thermal Degradation of Anthocyanins and its Impact on in Vitro Antioxidant Capacity of Downy Rose-myrtle Juice. [Google Scholar]
- 30.Pragalyaashree M.M., Tiroutchelvame D., Sashikumar S. Degradation kinetics of anthocyanin extracted from roselle calyces (Hibiscus sabdariffa) J. Appl. Pharmaceut. Sci. 2018;8:57–63. [Google Scholar]
- 31.Chen J., Du J., Li M., Li C. LWT; 2020. Degradation Kinetics and Pathways of Red Raspberry Anthocyanins in Model and Juice Systems and Their Correlation with Color and Antioxidant Changes during Storage; p. 109448. [Google Scholar]
- 32.Cui C., Zhang S., You L., Ren J., Luo W., Chen W., Zhao M. Antioxidant capacity of anthocyanins from Rhodomyrtus tomentosa (Ait.) and identification of the major anthocyanins. Food Chem. 2013;139(1-4):1–8. doi: 10.1016/j.foodchem.2013.01.107. [DOI] [PubMed] [Google Scholar]
- 33.Ghavam M. Study of antioxidant activity and some herbal compounds of Dracocephalum kotschyi Boiss. in different ages of growth. Biotechnol. Rep. 2019 doi: 10.1016/j.btre.2019.e00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Du J., Li M., Li C. LWT; 2020. Degradation Kinetics and Pathways of Red Raspberry Anthocyanins in Model and Juice Systems and Their Correlation with Color and Antioxidant Changes during Storage; p. 109448. [Google Scholar]
- 35.Sui X., Bary S., Zhou W. Changes in the color, chemical stability and antioxidant capacity of thermally treated anthocyanin aqueous solution over storage. Food Chem. 2016;192:516–524. doi: 10.1016/j.foodchem.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Saeed N., Khan M.R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Compl. Alternative Med. 2012;12(1) doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z., Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.) Innovat. Food Sci. Emerg. Technol. 2010;11(1):169–176. [Google Scholar]
- 38.Castaneda-Ovando A., Pacheco-Hernandez M.L., Paez- Hernandez M.E., Rodriguez J.A., Galan-Vidal C.A. Chemical studies of anthocyanins: a review. Food Chem. 2009;113:859–871. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.