Abstract
Diabetes mellitus patients are prone to cutaneous and subcutaneous fungal infections due to pathogenic fungi, including dermatophytes, Mucorales, Candida, Aspergillus, and Fusarium species. Here, we report a case of A. flavus mycetoma confirmed by isolation and molecular identification. The case was a 38-year-old male farmer with a seven-year history of type 2 diabetes mellitus, living in Khuzestan, southwest of Iran. The patient presented with a right foot swelling associated with a nodule and multiple discharging sinuses following trauma sustained on the foot while working barefoot on the rice farm, a year ago. The nodule appeared at the site of the trauma two months after the injury. The initial diagnosis was based on direct microscopic examination of lesions scraping using 20% potassium hydroxide and radiology. Molecular analysis confirmed the isolates to be A. flavus. In vitro susceptibility of the isolate to voriconazole, posaconazole, caspofungin, itraconazole, and amphotericin B was determined. Treatment with voriconazole (200 mg twice daily) stopped the purulent discharge, reduced the swelling, and improved the clinical condition within two months. The study emphasizes the importance of wearing footwear to prevent skin trauma as the main risk factor of patient involvement.
Keywords: Aspergillus flavus, Mycetoma, Diabetes mellitus, Molecular identification
Introduction
Chronic subcutaneous infections such as mycetoma caused by Aspergillus species are considered to be rare [1]. Results of a systematic review and meta-analysis on the global burden of human mycetoma showed that among 2704 fungal isolates from patients with mycetoma, only 5 (0.0005%) were Aspergillus species [2]. Diabetes mellitus is a common chronic endocrine metabolic disorder associated with an increased risk of fungal infections [[3], [53]]. Patients with diabetes have an immune system with a lower ability to respond to and deal with diseases of any type (fungal/bacterial/viral). This means they are more prone to illnesses than the general population [11]. Diabetic patients are susceptible to cutaneous and subcutaneous fungal infections due to pathogenic fungi, including dermatophytes, Mucorales, Candida, Aspergillus, and Fusarium species [3], [4], [5], [6]. In a study conducted by Kachi Udeani et al., of 120 diabetic patients analyzed, 63 (52.5%) had fungal infections [10].
Aspergillus flavus is a ubiquitous mold, found everywhere in the environment including air, water, soil, and plant materials. This fungus is one of the most frequently isolated Aspergillus species from human infections, especially in Asia [[7], [8], [54]]. Apart from keratitis, otitis, pulmonary and systemic infections in immunocompromised patients, A. flavus is commonly isolated from cutaneous lesions and onychomycosis in immunocompetent patients [[9], [55], [56], [57]]. Here, we report a case of subcutaneous mycetoma infection caused by A. flavus in a diabetic patient from Iran.
Case
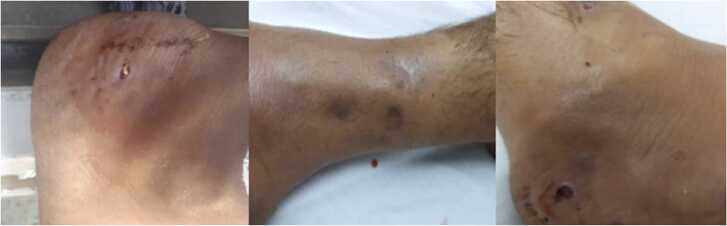
A 38-year-old male farmer from Khuzestan, southwest of Iran, with a seven-year history of type 2 diabetes mellitus was admitted at Imam Khomeini Hospital Complex, Tehran, Iran, in June 2020. The patient presented with a right foot swelling associated with a nodule and multiple discharging sinuses following trauma sustained on the foot while working on the rice farm, a year ago. The lesion started as a nodule that appeared at the site of the trauma two months after the injury. This was followed by a gradually progressing foot swelling, and after 6 months, secretory sinuses opened to the dorsal and lateral surfaces of the foot discharging purulent fluid (Fig. 1). The patient lost the ability of walking and his movement was performed only by a wheelchair. The patient stated a history of a penetrated wound due to barefoot walking on the rice farm. Also, he revealed a history of poor glycemic control despite using insulin injections, in addition to various regimens of oral hypoglycemic agents. Blood chemistry was obtained and the following results were found: fasting blood sugar (FBS): 160 mg/dL (normal range < 100 mg/dL), post-prandial blood sugar (PPBS): 230 mg/dL (normal range 110–140 mg/dL); and HbA1C: 8.3% (normal <6%). It should be noted that in previous medical reports of the patient high values for FBS, PPBS, and HbA1c in comparison with the normal ranges were found which were related to 6 months ago.
Fig. 1.
Swollen and discharging lesions on the right foot of the patient at first visit.
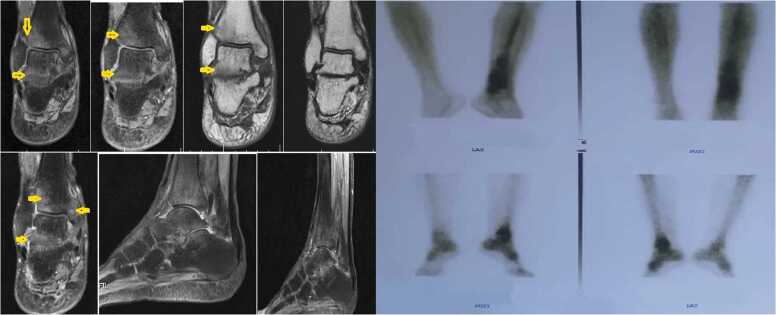
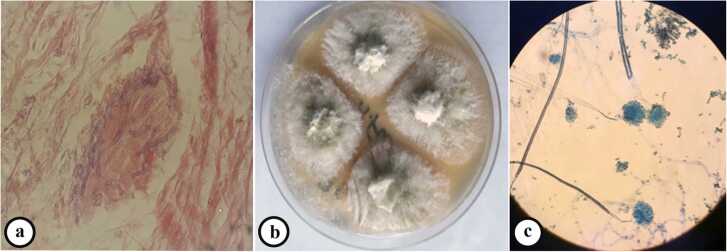
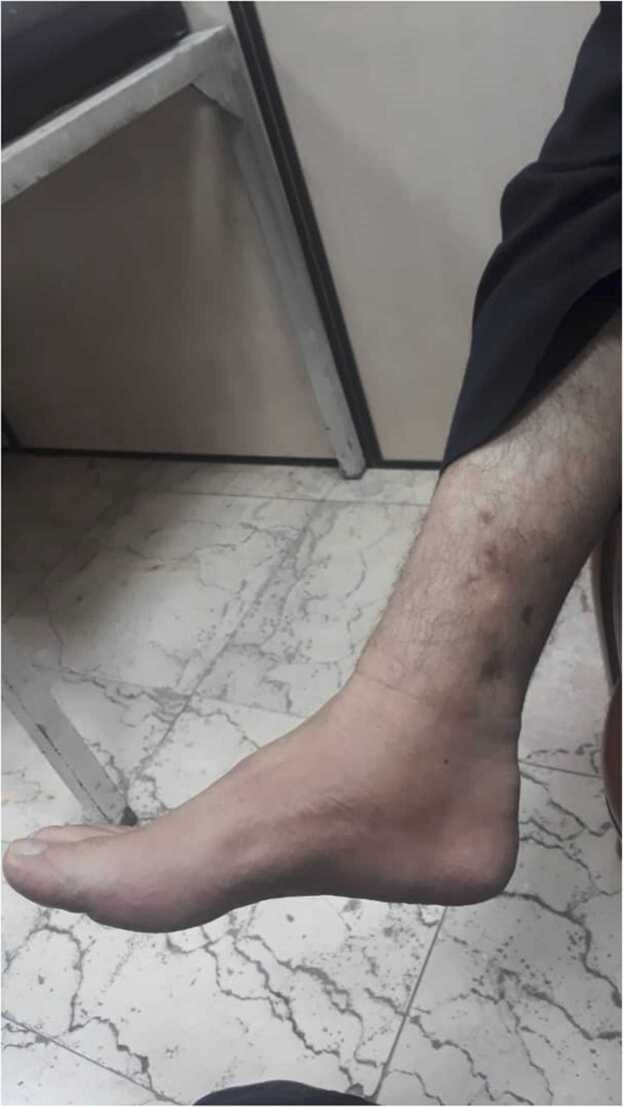
In the primary examination, the bone tumor was considered as one of the diagnostic possibilities, and with this diagnosis, other steps were taken for diagnosis and treatment. Also, even amputation of the patient's leg was considered as a treatment method for this reason. A technetium-99 m bone scan was suggestive of active infection of the left distal tibial head (osteomyelitis) as evidence by an increased flow (in angiographic phase) and mild hyperactivity (in delayed phase) at the left distal head of tibia bone (Fig. 2). With a suspicion of mycetoma, despite several examinations, grain was not observed in the purulent discharge. Thus, the lesions on the foot were scraped and a biopsy was taken from sinuses for further evaluation using potassium hydroxide and hematoxylin and eosin (H&E) stain, respectively. Microscopic examination of tissue sections revealed hyaline septate hyphae upon direct microscopy (Fig. 3a). Portions of the specimen were inoculated onto Sabouraud dextrose agar with and without chloramphenicol (Merck, Germany) at several locations on culture plates and incubated at 27 °C and 37 °C for two weeks. After five days of incubation, white, mold- colonies were seen at inoculation sites, which slowly became yellowish and finally light green (Fig. 3b); the fungus was identified as a member of the genus Aspergillus owing to macroscopic and microscopic features such as hyaline, septate hyphae with a few spherical conidia released from phialide cells (Fig. 3c). Since the initial treatment was based on bone tumor, after the evidence mentioned above and diagnosis of mycetoma, the treatment was started with itraconazole 200 mg daily. Further, molecular identification was performed via PCR-sequencing analysis to confirm the morphological identification. Briefly, the genomic DNA was isolated from fresh colonies using a method described previously [12]. Then, PCR-Sequencing was carried out bilaterally to amplify the beta-tubulin gene using the primers Bt2α: (5′-GGT AAC CAA ATC GGT GCT GCT TTC-3′) and Bt2b: (5′-ACC CTC AGT GTA GTG ACC CTT GGC-3′) [13]. Analysis of the obtained sequences demonstrated high homology (99.81%) between the investigated amplicon and the reference strain of A. flavus presented in GenBank with accession number MG991316. The obtained sequence was deposited in Gene Bank with accession number MW147358. The treatment with itraconazole was continued for one month and unfortunately, no improvement was observed. Thereafter, in vitro susceptibility of the isolate to voriconazole, posaconazole, caspofungin, itraconazole, and amphotericin B was determined based on clinical and laboratory standards institute (CLSI) M38-A2 protocol [14]. The minimum inhibitory concentrations (MIC) of antifungal agents were 0.063, 0.125, 0.5, 0.125, and 1 mg/L for voriconazole, posaconazole, caspofungin, itraconazole, and amphotericin B, respectively. Based on these results, the treatment changed to voriconazole (200 mg twice daily) and, after two months, the purulent discharge stopped, the swelling remarkably subsided, and clinical as well as radiographic signs have significantly improved. Before the treatment with voriconazole, the patient had lost the ability to walk but after two months he can walk with the help of a cane, and the leg pain was severely reduced. Treatment was continued for two months (in total four months) and in one year follow up, the lesions were healed and the patient was in a healthy status (Fig. 4).
Fig. 2.
Lateral sagittal contrast-enhanced T1 FS and axial TIRM T2 FS images of the ankle shows the abscess's disappearance around the calcaneus and the ankle.
Fig. 3.
Microscopic examination of biopsy sample stained by hematoxylin and eosin (H&E) demonstrating septate hyphae (a), macroscopic appearance of the isolate on sabouraud dextrose agar compatible with Aspergillus spp. (b), and microscopic features of the isolate (400×) compatible with A. flavus (c).
Fig. 4.
Improved lesions of the patient in one year follow.
Discussion
Mycetoma is a subcutaneous chronic infection caused by fungi (mycetoma) or bacteria (actinomycetoma). Several fungal species are the causative agents of eumycetoma. Madurella mycetomatis, Madurella grisae, and Scedosporium apiospermum are the most common causative agents. Furthermore, other fungal strains including Neotestudina rosatii, Scedosporium spp., Curvularia lunata, Falciformispora senegalensis, Biatriospora mackinnonii (P. mackinnonii), Pseudochaetosphaeronema larense and Aspergillus spp. are uncommon agents of eumycetoma [15].
In the past decade, many species of fungi such as Aspergillus, Candida, Fusarium, Cryptococcus, etc. have appeared as a considerable cause of morbidity and mortality in human and animal infections worldwide [[16], [17], [18], [58], [59]]. Aspergillus species are opportunistic microorganisms, globally distributed in air, soil, and plants, and the majority of cases reported occurred in patients with predisposing conditions such as hematologic malignancies, steroid use, human immunodeficiency virus, diabetes mellitus, etc. [[19], [20], [21], [60]]. In patients with immunodeficiency, a more severe disease that may eventually destroy tissues including bones may occur [[22], [23], [24], [61]]. The case of A. flavus mycetoma infection reported in this paper had type 2 diabetes mellitus as the main underlying illness. Hyperglycemia in diabetes is thought to cause dysfunction of the immune response, which fails to control the spread of invading pathogens in diabetic subjects [11].
To the best of our knowledge, to date, three cases of A. flavus eumycetoma have been reported in diabetic patients [25], [26], [27]. Skin involvement can be categorized as primary, following direct inoculation of Aspergillus at sites of skin injury or secondary, from the hematogenous spread [7]. In our presented case, the patient sustained a traumatic injury on his right foot while working barefoot on the rice farm. Studies reported that mycetoma typically presents in people who walk barefoot in dry, dusty conditions as was our case. Minor trauma causes the pathogens to enter the skin from the soil [1], [2], [3].
Nearly 50% of mycetoma are eumycetoma [29] and of over 30 species of Aspergillus linked to human infections, four were reported to cause eumycetoma, namely A. nidulans, A. flavus, A. fumigatus, and A. terreus [25], [26], [30]. The most prevalent Aspergillus species causing mycetoma is A. nidulans, while A. flavus is the second most common species associated with mycetoma cases [30]. Reportedly, cutaneous, mucocutaneous, and subcutaneous tissue infections are more often associated with A. flavus than other species of Aspergillus [31], [32], [33].
The clinical manifestation of cutaneous aspergillosis by A. flavus is described by the presence of macules, plaques, papules, nodules, ulcerations, pustules, or subcutaneous abscesses. In our case, nodule, swelling, and secretory sinuses were the main presentation during hospitalization [7], [34]. The progression of the infection is gradual but may affect deep structures such as muscles, joints, tendons, and bones [2]. Computed tomography (CT), X-rays, ultrasound, and magnetic resonance imaging (MRI) scans can only help to detect the extent of disease [29]. The bone scan in the presented case showed increased blood flow and mild hyperactivity at the left distal head of the tibia bone, suggestive of osteomyelitis.
Some occupational groups in rural areas such as agricultural workers, gardeners, poultry farmers, etc. [35], [36], [37], who work barefoot on land seem to be at a higher risk of ingress of causative agent into the subcutaneous tissue through direct implantation; this occurs commonly in young men aged between 20 and 40 years [38], [39]. In the present case, a 38-year-old man who was also a farmer presented with secretory sinus and swelling on his right foot. As clinical presentations are not always pathognomonic, detection of species by laboratory examination is essential [9]. Conidia of saprophytic fungal species represent one of the most common airborne laboratory contaminants [40]. There for, directly detection of the fungus in clinical specimens remains the way to corroborate the Aspergillus infection in such cases [41]. In this case, samples were inoculated on multiple points on several culture media, and the same colonies of Aspergillus species were isolated from the inoculation points. Besides, the direct microscopic examination confirmed the presence of fungi in the sample. However, because of the disintegration of the growth and sporulation pattern of the isolated strain, the morphological identification could not be specifically definite to the species level thus, further molecular evaluation became paramount for precise identification of the fungus [1]. DNA sequencing data unambiguously showed that the strain was A. flavus. Table 1 summarized all cases of mycetoma due to A. flavus reported across the world.
Table 1.
All cases of mycetoma due to A. flavus reported across the world.
| Reference | Case No | Country | Sex/Age (year) | Infection site | Occupation | Underlying condition | Diagnosis method | Treatment | |
|---|---|---|---|---|---|---|---|---|---|
| Mahgoub et al. [25]/1973 | 1 | Sudan | F/50 | Ankle | NA | None | Culture, histopathological examination, immunodiffusion tests | Multiple incisions to relieve pressure + antibiotic | |
| Padhi et al. [28]/2009 | 1 | India | 36/F | Anterior abdomen | Housewife | Diabetes Mellitus, end-stage renal disease | Culture, histopathological examination | Ketoconazole + Itraconazole + Voriconazole | |
| Witzig et al. [27]/1996 | 1 | USA | 36/F | Back | Laundry | Diabetes Mellitus, nephrotic syndrome | KOH wet mount examination, Culture, histopathological examination | Decompressive laminectomy + Itraconazole | |
| Hashemi et al. [51]/2001 | 1 | Iran | 25/F | Ankle | NA | NA | KOH wet mount examination, Culture, histopathological examination | NA | |
| Ahmed et al. [1]/2015 | 1 | Sudan | 55/M | Foot | NA | Diabetes Mellitus | PCR-sequencing, immunohistochemical analysis, MALDI-TOF MS | Operation+ ketoconazole +itraconazole + voriconazole | |
| Baishya et al. [52]/2017 | 1 | 9 months/M | Lower limb | – | NA | KOH wet mount examination, Culture, histopathological examination | Itraconazole | ||
| Present case/2021 | 1 | Iran | 38/Male | Foot | Farmer | Diabetes Mellitus | KOH wet mount examination, Culture, PCR-sequencing | Voriconazole | |
Abbreviations: M: male; F: Female; NA: not available; KOH: potassium hydroxide.
Although clinical manifestations of mycetoma caused by different etiological agents remain similar, therapy may be entirely different [42]. One core characteristic of mycetoma is poor response to treatment [43]. Also, the undesirable effects of antifungal drugs and multidrug-resistant microorganisms could be other contributing factors to treatment failure; susceptibility tests on isolated species may be of value in choosing suitable treatment [9], [47]. The newer drugs of azole antifungal agents show a broader spectrum of activity and are at lower levels of toxicity [29]. Recently, Lionakis et al. reported that 11% of the A. flavus isolates in their study were resistant to itraconazole [45]. Voriconazole, posaconazole, and caspofungin have been approved for the treatment of aspergillosis [7]. Voriconazole has good in vitro activity against a range of Aspergillus species, including A. flavus with better clinical outcomes [46], [47], [48]. Based on the results of in vitro studies, A. flavus is more susceptible to echinocandins than A. fumigatus [49], [50] thus, a species-specific difference in susceptibility to echinocandins may exist. Given that, our reported mycetoma case showed a remarkable response to treatment by voriconazole therapy in a way, the purulent discharge stopped and the swelling reduced.
In conclusion, our report highlights that A. flavus is an important mycotic pathogen that should be considered as a potential pathogenic agent in mycetoma. Although conventional mycologic techniques are powerful diagnostic tools, molecular studies substantiate identification and can lead to a better favorable clinical outcome along with appropriate treatment, especially in immunocompromised individuals such as diabetic patients.
Funding
No fund was received for this research.
Ethical approval
This study was approved by the ethics committee of Tehran University of Medical Sciences, Tehran, Iran.
Consent
Informed consent was obtained from the patient for publication of his data.
CRediT authorship contribution statement
Study design: HKS, MS, SA, ZR, MKH. Data collection: HKS, BA, MGS, MG, SM, FS, LF. Writing: BA, MG, SM. Critical review: MS, SA.
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Mohammadreza Salehi, Email: mr-salehi@sina.tums.ac.ir.
Saham Ansari, Email: saham.ansari@sbmu.ac.ir.
References
- 1.Ahmed S.A., Abbas M.A., Jouvion G., et al. Seventeen years of subcutaneous infection by Aspergillus flavus; eumycetoma confirmed by immunohistochemistry. Mycoses. 2015;58(12):728–734. doi: 10.1111/myc.12422. [DOI] [PubMed] [Google Scholar]
- 2.van de Sande W.W. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(11) doi: 10.1371/journal.pntd.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra B.K., Singh A.K., Gupta D., et al. A clinicomicrobiological study on incidence of mycotic infections in diabetic foot ulcers. Int J Sci Study. 2017;4(12):50–54. [Google Scholar]
- 4.Raiesi O., Siavash M., Mohammadi F., et al. Frequency of cutaneous fungal infections and azole resistance of the isolates in patients with diabetes mellitus. Adv Biomed Res. 2017;6:71–77. doi: 10.4103/2277-9175.191003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta P., Premkumar A., Chakrabarti A., et al. Fusarium falciforme infection of foot in a patient with type 2 diabetes mellitus: a case report and review of the literature. Mycopathologia. 2013;176(3–4):225–232. doi: 10.1007/s11046-013-9646-z. [DOI] [PubMed] [Google Scholar]
- 6.Dahiya D L., et al. Eumycetoma of the foot due to Fusarium solani in a person with diabetes mellitus: report of a case and review of literature. Mycopathologia. 2021;186(2):277–288. doi: 10.1007/s11046-020-00524-y. [DOI] [PubMed] [Google Scholar]
- 7.Hedayati M., Pasqualotto A., Warn P., et al. Aspergillus flavus: human pathogen, allergen, and mycotoxin producer. Microbiology. 2007;153(6):1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 8.Taghizadeh-Armaki M., Hedayati M.T., Ansari S., et al. Genetic diversity and in vitro antifungal susceptibility of 200 clinical and environmental Aspergillus flavus isolates. Antimicrob Agents Chemother. 2017;61(5) doi: 10.1128/AAC.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal M., Dave P., Manna A.K. Emerging role of Aspergillus flavus in human and animal disorders. J Mycopathol Res. 2014;52(2):211–216. [Google Scholar]
- 10.Udeani TK, Asogwa VN, Ezenwaka U. Assessment of systemic fungal infections among diabetic patients in Enugu, Nigeria. J Infect Dis Epidemiol., 4(2); 2018. p. 051. 〈DOI: 10.23937/2474-3658/1510051〉.
- 11.Rafat Z., Hashemi S.J., Ashrafi K., et al. Fungal isolates of the respiratory tract in symptomatic patients hospitalized in pulmonary units: a mycological and molecular epidemiologic study. J Multidiscip Health. 2020;13:661–667. doi: 10.2147/JMDH.S252371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makimura K., Tamura Y., Mochizuki T., et al. Phylogenetic classification and species identification of dermatophyte strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1999;37(4) doi: 10.1128/JCM.37.4.920-924.1999. 920-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne P. Allured Publishing Corporation; Carol Stream, IL, USA: 2008. Clinical and Laboratory Standards Institute (CLSI): reference method for broth dilution antifungal susceptibility testing of filamentous fungi. [Google Scholar]
- 15.Emery D., Denning D.W. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020 24;14(9) doi: 10.1371/journal.pntd.0008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal M. Cryptococcus gattii: An emerging global mycotic pathogen of humans and animals. J Mycopathol Res. 2014;52:1–7. doi: 10.1016/j.micinf.2011.05.009. [DOI] [Google Scholar]
- 17.Pal M., Sisay T., Asfaw Y. Growing significance of fungi in human and animal health. J Nat Hist. 2011;7(2):82–86. doi: 10.1101/cshperspect.a019273. [DOI] [Google Scholar]
- 18.Nucci M., Marr K.A. Emerging fungal diseases. Clin Infect Dis. 2005;41(4):521–526. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 19.Bennett J.W. An overview of the genus Aspergillus. Aspergillus Mol Biol Genom. 2010:1–17. [Google Scholar]
- 20.Tsai W.C., Lee C.H., Wu W.M., et al. Cutaneous manifestations of subcutaneous and systemic fungal infections in tropical regions: a retrospective study from a referral center in southern Taiwan. Int J Dermatol. 2017;56(6):623–629. doi: 10.1111/ijd.13497. [DOI] [PubMed] [Google Scholar]
- 21.Epstein M.D., Segalman K.A., Mulholland J.H., et al. Successful treatment of primary cutaneous Aspergillus flavus infection of the hand with oral itraconazole. J Hand Surg. 1996;21(6):1106–1108. doi: 10.1016/S0363-5023(96)80327-3. [DOI] [PubMed] [Google Scholar]
- 22.Nenoff P., Van De Sande W., Fahal A., et al. Eumycetoma and actinomycetoma–an update on causative agents, epidemiology, pathogenesis, diagnostics, and therapy. J Eur Acad Dermatol Venereol. 2015;29(10):1873–1883. doi: 10.1111/jdv.13008. [DOI] [PubMed] [Google Scholar]
- 23.Lazarides M., Venkatswami S., Sankarasubramanian A., et al. The Madura foot: looking deep. Int J Low Extrem Wounds. 2012;11(1):31–42. doi: 10.1177/1534734612438549. [DOI] [PubMed] [Google Scholar]
- 24.Zein H.A., Fahal A.H., Mahgoub E.S., et al. Predictors of cure, amputation, and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2012;106(11):639–644. doi: 10.1016/j.trstmh.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Mahgoub E.S. Can Aspergillus flavus cause maduromycetoma? Bull Soc Pathol Exot. 1973;66(3):390–395. [PubMed] [Google Scholar]
- 26.Bassiri-Jahromi S. Mycetoma in Iran: causative agents and geographic distribution. Indian J Dermatol. 2014;59(5):529. doi: 10.4103/0019-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witzig R., Greer D., Hyslop N., Jr Aspergillus flavus mycetoma and epidural abscess successfully treated with itraconazole. J Med Vet Mycol. 1996;34(2):133–137. doi: 10.1080/02681219680000201. [DOI] [PubMed] [Google Scholar]
- 28.Padhi S., Uppin S.G., Uppin M.S., et al. Mycetoma in South India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidiatum and Aspergillus flavus. Int J Dermatol. 2010;49(11):1289–1296. doi: 10.1111/j.1365-4632.2010.04610.x. [DOI] [PubMed] [Google Scholar]
- 29.Reis C.M.S. Reis-Filho EGdM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. Bras Dermatol. 2018;93(1):8–18. doi: 10.1590/abd1806-4841.20187075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma R., Vasudevan B., Sahni A.K., et al. First reported case of Aspergillus nidulans eumycetoma in a sporotrichoid distribution. Int J Dermatol. 2015;54(1):74–77. doi: 10.1111/ijd.12571. [DOI] [PubMed] [Google Scholar]
- 31.Myoken Y., Sugata T., Fujita Y., et al. Molecular epidemiology of invasive stomatitis due to Aspergillus flavus in patients with acute leukemia. J Oral Pathol Med. 2003;32(4):215–218. doi: 10.1034/j.1600-0714.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Heinemann S., Symoens F., Gordts B., et al. Environmental investigations and molecular typing of Aspergillus flavus during an outbreak of postoperative infections. J Hosp Infect. 2004;57(2):149–155. doi: 10.1016/j.jhin.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Pasqualotto A., Denning D. Post-operative aspergillosis. Clin Microbiol Infect. 2006;12(11):1060–1076. doi: 10.1111/j.1469-0691.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 34.Pal M. Public health concern due to emerging and re-emerging zoonoses. Int J Livest Res. 2013;3(1):56–62. doi: 10.5455/ijlr. [DOI] [Google Scholar]
- 35.Lichon V., Khachemoune A. Mycetoma. Am J Clin Dermatol. 2006;7(5):315–321. doi: 10.2165/00128071-200607050-00005. [DOI] [PubMed] [Google Scholar]
- 36.Mattioni S., Develoux M., Brun S., et al. Management of mycetomas in France. Med Mal Infect. 2013;43(7):286–294. doi: 10.1016/j.medmal.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Tomczyk S., Deribe K., Brooker S.J., et al. Association between footwear use and neglected tropical diseases: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(11) doi: 10.1371/journal.pntd.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zijlstra E.E., Van De Sande W.W., Welsh O., et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16(1):100–112. doi: 10.1016/S1473-3099(15)00359-X. [DOI] [PubMed] [Google Scholar]
- 39.Bonifaz A., Tirado-Sánchez A., Calderón L., et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8(8) doi: 10.1371/journal.pntd.0003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Hoog GS, Guarro J, Gené J, et al. Atlas of clinical fungi: Centraalbureau voor Schimmelcultures (CBS); 2000.
- 41.Hanazawa R., Murayama S.Y., Yamaguchi H. In-situ detection of Aspergillus fumigatus. J Med Microbiol. 2000;49(3):285–290. doi: 10.1099/0022-1317-49-3-285. [DOI] [PubMed] [Google Scholar]
- 42.Mhmoud N.A., Ahmed S.A., Fahal A.H., et al. Pleurostomophora ochracea, a novel agent of human eumycetoma with yellow grains. J Clin Microbiol. 2012;50(9):2987–2994. doi: 10.1128/JCM.01470-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro L.M., Piquero-Casals J. Clinical and mycologic findings and therapeutic outcome of 27 mycetoma patients from São Paulo, Brazil. Int J Dermatol. 2008;47(2):160–163. doi: 10.1111/j.1365-4632.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- 45.Lionakis M.S., Lewis R.E., Torres H.A., et al. Increased frequency of non-fumigatus Aspergillus species in amphotericin B–or triazole–pre-exposed cancer patients with positive cultures for aspergilli. Diagn Microbiol Infect Dis. 2005;52(1):15–20. doi: 10.1016/j.diagmicrobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Pfaller M., Messer S., Hollis R., et al. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob Agents Chemother. 2002;46(4):1032–1037. doi: 10.1128/AAC.46.4.1032-1037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diekema D., Messer S., Hollis R., et al. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J Clin Microbiol. 2003;41(8):3623–3626. doi: 10.1128/JCM.41.8.3623-3626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lass-Flörl C., Nagl M., Speth C., et al. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob Agents Chemother. 2001;45(1):124–128. doi: 10.1128/AAC.45.1.124-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oakley K.L., Moore C.B., Denning D.W. In vitro activity of the echinocandin antifungal agent LY303, 366 in comparison with itraconazole and amphotericin B against Aspergillus Spp. Antimicrob Agents Chemother. 1998;42(10):2726–2730. doi: 10.1128/aac.42.10.2726. PMCID: PMC105927, PMID: 9756785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinel-Ingroff A. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991) J Clin Microbiol. 2003;41(1):403–409. doi: 10.1128/JCM.41.1.403-409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashmi S.J., Geramishar M. The case report of the first Mycetoma Caused by Aspergillus flavus in Iran. J Inflamm Dis. 2001;5(3):64–67. [Google Scholar]
- 52.Baishya L., Kalita M., Sharma A., et al. White grain eumycetoma due to Aspergillus flavus in infancy: a rare case report from Assam. J Pure Appl Microbiol. 2017;11(2):969–973. doi: 10.22207/JPAM.11.2.38. [DOI] [Google Scholar]
- 53.Kamali Sarvestani Hasti, et al. Daie Ghazvini R, Hashemi SJ Molecular Characterization of Fungal Colonization on the ProvoxTM Tracheoesophageal Voice Prosthesis in Post Laryngectomy Patients. Iranian Journal of Public Health. 2022;51(1):151. doi: 10.18502/ijph.v51i1.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamali Sarwestani Zahra, et al. Evaluation of fungal air contamination in selected wards of two tertiary hospitals in Tehran, Iran. Tehran University Medical Journal TUMS Publications. 2017;75(4):299–306. [Google Scholar]
- 55.Kamali Sarwestani Zahra, et al. Species identification and in vitro antifungal susceptibility testing of Aspergillus section Nigri strains isolated from otomycosis patients. Journal de Mycologie Medicale. 2018;28(2):279–284. doi: 10.1016/j.mycmed.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Kamali Sarwestani Hasti, et al. Investigation of etiologic agents and clinical presentations of otomycosis at a tertiary referral center in Tehran, Iran. Iranian Journal of Public Health. 2019;48(2):331. [PMC free article] [PubMed] [Google Scholar]
- 57.Ardi Pegah, et al. Study on invasive aspergillosis using galactomannan enzyme immunoassay and determining antifungal drug susceptibility among hospitalized patients with hematologic malignancies or candidates for organ transplantation. Microbial pathogenesis. 2020;147:104382. doi: 10.1016/j.micpath.2020.104382. [DOI] [PubMed] [Google Scholar]
- 58.Halvaee Samaneh. A Mycological and Molecular Epidemiologic Study on Onychomycosis and Determination In Vitro Susceptibilities of Isolated Fungal Strains to Conventional and New Antifungals. Frontiers in Cellular and Infection Microbiology. 2021;11:693522. doi: 10.3389/fcimb.2021.693522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamali Sarvestani Hasti. Fatal invasive aspergillosis in a child with chronic granulomatous disease. Journal of Wound Care. 2022;31(5):427–431. doi: 10.12968/jowc.2022.31.5.427. [DOI] [PubMed] [Google Scholar]
- 60.Kamali Sarvestani Hasti. Black aspergilli as causes of otomycosis in the era of molecular diagnostics, a mini-review. Journal of Medical Mycology. 2022;32(2):101240. doi: 10.1016/j.mycmed.2021.101240. [DOI] [PubMed] [Google Scholar]
- 61.Rafat Zahra, et al. Fungal and bacterial co-infections of the respiratory tract among patients with COVID-19 hospitalized in intensive care units. Gene Reports. 2022;27:101588. doi: 10.1016/j.genrep.2022.101588. [DOI] [PMC free article] [PubMed] [Google Scholar]