Abstract
Background
Clinicians have limited time during patient encounters which can result in patients' concerns not being addressed. This study's objective was to test whether an electronic patient-reported outcome quality of life tool (PROQOL) in which patients identify their primary concern during clinic visits improves cancer patient quality of life (QOL).
Patients and methods
This single center non-blinded prospective clinical trial randomized patients (2:1) to PROQOL versus usual care (UC). Two patient cohorts were enrolled: those with hematologic malignancies (multiple myeloma [MM] or light chain amyloidosis [AL]) and solid tumors (head and neck [H/N] or gynecologic [GYN] malignancies). Primary endpoint was patient-reported QOL at 12 months measured by a single-item Linear Analog Self-Assessment. Value to patients and impact on clinician workflow was measured using a “was it worth it” survey. The study was powered to detect a 0.5 standard deviation difference between groups.
Results
Overall 383 patients were enrolled, 171 with MM, 62 AL, 113 GYN, and 37 H/N between July 2016 and April 2018, with 12-month follow-up. There were 171 (44.6%) male patients and median age was 62 years (range 31–87). The most often selected concern was physical health (30.9%), and second was cancer diagnosis and treatment (29.1%). Mean QOL was 7.12 for PROQOL and 6.98 for UC (0–10 scale) at 12 months, with no between-group difference overall (p = 0.56) or within hematologic or solid tumor cohorts, respectively. Among patients, 74% thought the PROQOL tool was worthwhile, 86% would choose PROQOL again, and 81% would recommend it to others. Among clinicians, 95% responded that PROQOL was worthwhile and did not think that PROQOL negatively impacted their workflow.
Conclusions
Although we did not demonstrate a QOL difference between PROQOL and UC groups; the PROQOL tool held considerable value in identifying patients' main concerns over time and was worthwhile for patients and clinicians.
Keywords: Quality of life, Patient-reported outcomes, Health care delivery
Abbreviations: H/N, Head and Neck malignancy; MM, Multiple myeloma; AL, Light chain amyloidosis; GYN, Gynecologic malignancies
1. Introduction
Patient-reported outcome (PRO) measures are defined by the Food and Drug administration (FDA) as “a measurement based on a report that comes directly from the patient about the status of a patient's health condition, without amendment or interpretation by a clinician or anyone else” [1]. The FDA has advocated for inclusion of PROs in clinical research and as an endpoint in clinical trials [[2], [3], [4]]. PROs can elucidate physical or functional impairment as well as emotional and psychological and social effects related to any disease or treatment administered [1,5,6]. It is well established that patients' concerns regarding functional impairments, psychosocial distress, and financial constraints are often underappreciated by clinicians and may go unaddressed [6,[7], [8]]. However, the time constraints of a clinical practice make it difficult for busy clinicians to elicit more nuanced and subjective complexities of the patient experience. PROs are a relevant tool to overcome the limitations of real-world clinical practice [9,10].
PROs hold considerable clinical importance in oncology [[11], [12], [13], [14], [15], [16]]. First, PROs serve as sensitive indicators of patients' global functioning, capturing information that may be missed by standard clinical assessment [17,18]. Second, by effectively bridging communication between clinician and patient, and focusing discussion on patients' concerns and experience, PROs can improve physicians' symptom assessment, an improvement that has translated to improved overall quality of care for patients [19,20]. Third, the prognostic significance of PROs has been supported in robust systematic reviews [17,18]. Overall, the considerable value of PROs is seen in the integration of patients’ perspectives into the clinical opinion, creating a more holistic description of the patient experience, better understanding health-related outcomes and improving patient satisfaction [[5], [6], [7],11,21].
Despite advances in systemic and locoregional therapies and improved survival, treatment-related complications and toxicities still profoundly impact cancer patients’ quality of life [6,9]. Gynecologic and head and neck (H/N) cancer patients undergo multimodality treatments that may cause sexual and urinary dysfunction, facial disfigurement, loss of oropharyngeal function, and anxiety and depression [6,10,22]. Similarly, patients with dysproteinemias like multiple myeloma (MM) and light chain amyloidosis (AL) undergo high dose chemotherapy followed by autologous stem cell transplant and often followed by indefinite chemotherapy maintenance, with associated treatment related side effects [23].
This begs the question how the patient's perspective can be elicited efficiently at every routine cancer visit? We developed an electronic patient-reported outcome quality of life (PROQOL) tool that was adapted for use in hematology and oncology [24]. The PROQOL tool asks patients to identify their biggest concern among categories, then select sub-concerns within a category, and finally complete single-item linear analogue self-assessment (LASA) scales for QOL of various domains. The LASA scales have been validated as a pragmatic screener for QOL needs with high rate of completion among patients, while still recognizing the role of more detailed standardized patient-reported QOL tools such as EORTC QLQ-C30 and FACT-G, for in depth exploration of the QOL domains [11]. The PROQOL tool systematically incorporates PROs into practice and acts as a case management system by generating a report of resources to address patient concerns identified [24]. The adaptation process was focussed on the descriptive portion of the PROQOL tool; i.e., adapting the “concern” categories and resources to aid in addressing those concerns (which were original developed for diabetes patients) to reflect those most relevant to cancer patients. The adaptation process involved an iterative process of literature review and clinician and patient focus groups or feedback. The scoring, mode of administration, number of items, and psychometric properties of LASA scales were unaltered.
This study was a prospective randomized trial conducted in two patient cohorts (hematologic and oncologic), whereby PROQOL was administered and compared to usual care with assessment of impact on clinical workflow. The objective of the study was to determine if the PROQOL tool improved patient QOL over usual care (UC) at the end of 12-month follow up. This study explored the distribution of categories selected and clinical and demographic factors associated with primary categories selected by patients.
2. Methods
2.1. Participants
The study was approved by the Mayo Clinic Institutional Review Board and is in line with the Declaration of Helsinki.
Patients with multiple myeloma (MM), light chain amyloidosis (AL), head and neck cancer (H/N) and gynecologic malignancies were eligible for inclusion in the study. Adults (≥18 years), at any disease stage, whose continued cancer care was received at Mayo Clinic in Rochester, MN, and able to use an iPad were eligible for inclusion. As PROQOL was available only in English, patients were required to be able to complete assessments in English to participate. The solid tumor and hematologic practices were distinct patient practices with separate workflows and were therefore enrolled as a hematologic cohort and solid tumor cohort. Patients were screened for eligibility the week before and approached prior to a clinical visit for consideration. Written informed consent was obtained from all patients. Patients once enrolled were followed for 12 months. Clinicians provided oral consent for involvement in the study.
2.2. Design
This was a single center non-blinded prospective clinical trial, with 2:1 randomization to PROQOL or to usual care. Patients were randomized using simple randomization stratified by cohort (hematologic versus solid tumor), and allocation was concealed using numbered envelopes which were opened in sequence as patients were registered.
2.3. Intervention
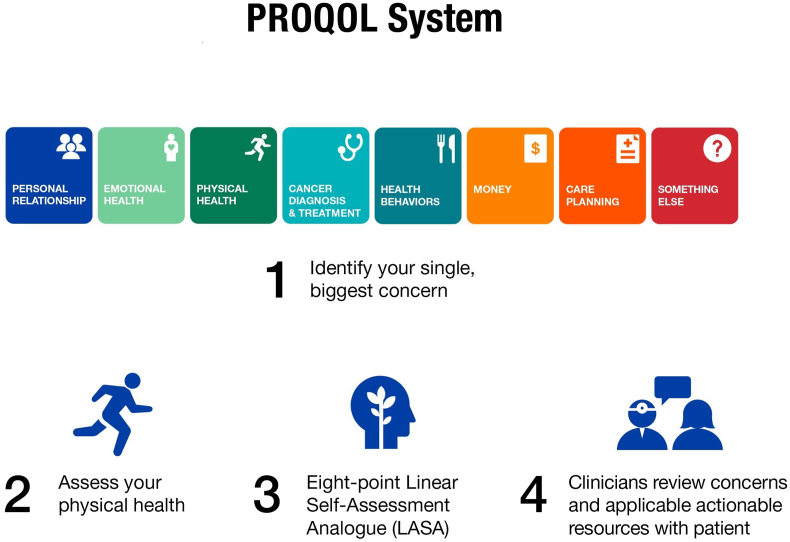
PROQOL was offered to the patients prior to every visit (maximum once per week) and each time patients selected from 8 categories that included: Personal relationship, Emotional health, Physical health, Cancer diagnosis and treatment, Health behaviors, Money, Care planning, or Something else. They were asked “which of the following if any, represents your single biggest concern right now …” Once a category was selected, patients were then presented with options to better specify their concern. Then the PROQOL system generated a printed list of actionable resources based on the selected concern which was provided to the patient and their clinician at that visit. Clinicians were able to review the generated report and the applicable actionable resources together with the patient (Fig. 1). Upon activation of the study, patients were able to select a single concern. Partway through the study based on interim monitoring of patient selection of a primary concern, the protocol was amended to allow for selection of a second distinct concern from that time onwards. The PROQOL report was scanned into the medical record.
Fig. 1.
Schema of PROQOL tool.
2.4. Usual care
Usual care (UC) was defined as patients volunteering information about symptoms or concerns during routine visits, and clinicians addressing it as they saw fit. Symptom discussions and documentation in the medical record occurred per usual practice.
2.5. Outcome measurement
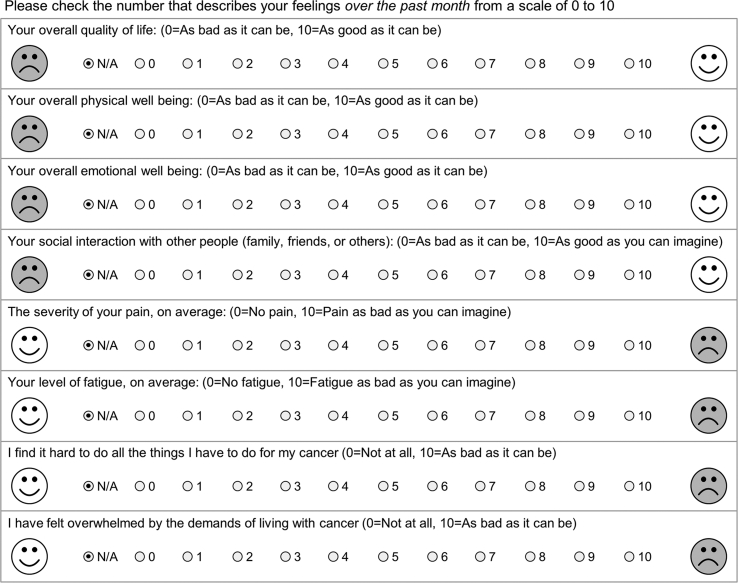
The primary endpoint was patient-reported quality of life at 12 months as measured by the single-item LASA overall QOL item. The LASA questionnaire uses a 0–10 scale to assess the overall QOL, as well as single items to evaluate various other domains: physical well-being, emotional well-being, social interactions, pain severity, fatigue, and cancer-related impacts to life (Appendix A). Secondary endpoints included distribution of categories selected and clinical and demographic factors associated with primary categories selected by patients. Providers and patients who were randomized to the PROQOL tool completed “Was it worth it” (WIWI) items (Appendix B) to gauge the patients' and providers’ opinion on the utility and ease of the PROQOL tool [25,26].
2.6. Statistical analysis
The target sample size in each cohort was 150 patients (100 randomized to PROQOL, 50 randomized to usual care) with data available for analysis at the 12-month time point. A higher rate of drop out was expected in the hematologic malignancy cohort, so the study planned to enroll 280 patients in the hematologic malignancy cohort and 167 patients in the oncologic cohort. A sample of 150 evaluable patients randomized in a 2:1 fashion provided 80% power for a two-sample t-test to detect a difference of 0.5 times the standard deviation (or 5 points on a 0–100 scale with an assumed standard deviation [SD] of 10 points) in average overall QOL between the two groups at the 12-month time point. Continuous variables were compared between groups using Wilcoxon rank sum tests, two-sample t-tests, or analysis of variance. Binary variables were compared between groups using chi-squared tests. Analysis of covariance was used as a sensitivity analysis in the comparison between groups of 12-month scores to adjust for baseline patient scores. General linear mixed models with group, continuous time, and group-by-time interaction were used to compare patient scores over time between groups. These models had random intercept and time variables to account for multiple observations within patient. Last value was carried forward for patients who did not complete a 12-month assessment; sensitivity analysis imputed the last value carried forward for only patients whose last visit was at least 9 months after the baseline visit.
3. Results
3.1. Patient characteristics
In the hematologic cohort, there were 233 patients enrolled (171 MM, 62 AL) between July 2016–April 2018; 139 (60.1%) were male, median age 62 years (range 31–87). The median distance travelled was 82 miles (range 2–1437 miles) for clinic visits. The median follow-up in months was 15 months, and the median time from diagnosis to registration was 38 months. Eleven percent of patients (n = 25) died during the study period.
In the solid tumor cohort, 150 patients enrolled (113 GYN, 37 H/N pts) between September 2016 and August 2017; 78.7% were female, median age 63 years (range 32–84). Patients were followed for a median of 9.3 months (range 0–27.5 months). Overall, patients travelled a median of 98 miles (range 3–3123) to attend clinic visits. Median time from diagnosis to enrollment in the study was 13 months. Twenty-three percent (n = 34) of patients died during the study period. Patient characteristics are summarized in Table 1a, Table 1ba and 1b.
Table 1a.
Hematologic patient baseline characteristics.
| PROQOL (N = 153) | UC (N = 80) | Total (N = 233) | p value | |
|---|---|---|---|---|
| Age, years [median, range] | 66 (31–87) | 64 (42–85) | 65 (31–87) | 0.78 |
| Gender | 0.98 | |||
| Female | 61 (39.9%) | 32 (40.0%) | 93 (39.9%) | |
| Male | 92 (60.1%) | 48 (60.0%) | 140 (60.1%) | |
| Race | 0.49 | |||
| Black | 2 (1.3%) | 2 (2.6%) | 4 (1.8%) | |
| White | 148 (98.7%) | 75 (97.4%) | 223 (98.2%) | |
| Missing | 3 | 3 | 6 | |
| Distance travelled, miles [median, range] | 80 (2–1281) | 88.5 (2–1437) | 82 (2–1437) | 0.45 |
| Diagnosis | 0.69 | |||
| Amyloidosis | 42 (27.5% | 20 (25.0%) | 62 (26.6%) | |
| Multiple myeloma | 111 (72.5%) | 60 (75.0%) | 171 (73.4%) | |
| Number of treatments [median, range] | 3 (0–23) | 3.5 (0–18) | 3 (0–23) | |
PROQOL = patient-reported outcome quality of life tool; UC = usual care.
Table 1b.
Solid tumor patient baseline characteristics.
| PROQOL (N = 100) | UC (N = 50) | Total (N = 150) | p value | |
|---|---|---|---|---|
| Age, years [median, range] | 63.5 (32–84) | 63 (39–81) | 63 (32–84) | 0.66 |
| Gender | 0.32 | |||
| Female | 81 (81.0%) | 37 (74.0%) | 118 (78.7%) | |
| Male | 19 (19.0%) | 13 (26.0%) | 32 (21.3%) | |
| Race | 0.48 | |||
| Native American | 1 (1.0%) | 0 (0.0%) | 1 (0.7%) | |
| White | 99 (99.0%) | 50 (100.0%) | 149 (99.3%) | |
| Distance travelled, miles [median, range] | 93 (3–1262) | 107 (3–3123) | 97.5 (3–3123) | 0.58 |
| Diagnosis | 0.28 | |||
| Head & Neck | 22 (22.0%) | 15 (30.0%) | 37 (24.7%) | |
| Gynecologic | 78 (78.0%) | 35 (70.0%) | 113 (75.3%) | |
| Disease status | 0.13 | |||
| Newly diagnosed | 49 (49.0%) | 18 (36.0%) | 67 (44.7%) | |
| Relapsed | 51 (51.0%) | 32 (64.0%) | 83 (55.3%) | |
PROQOL = patient-reported outcome quality of life tool; UC = usual care.
3.2. Results from PROQOL system
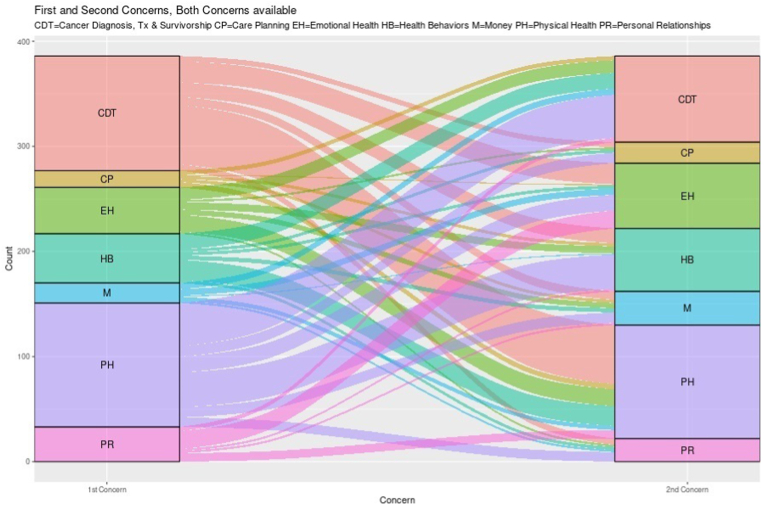
The total number of patient visits throughout the study was 1115. The single most often selected primary concern within the combined cohorts was physical health (30.9%), which was a category related to physical symptoms of disease and side effects of treatment. The second most often selected primary concern was cancer diagnosis and treatment (29.1%). In 380 visits, patients were given the option to select a second main concern and again physical health (27.8%) and cancer diagnosis and treatment (21.4%) remained most popular. The first and second selection is displayed in Fig. 2A.The election of these categories was consistent between both cohorts. Patients took an average of 2.9 min to complete PROQOL at each visit.
Fig. 2A.
First two selected concerns.
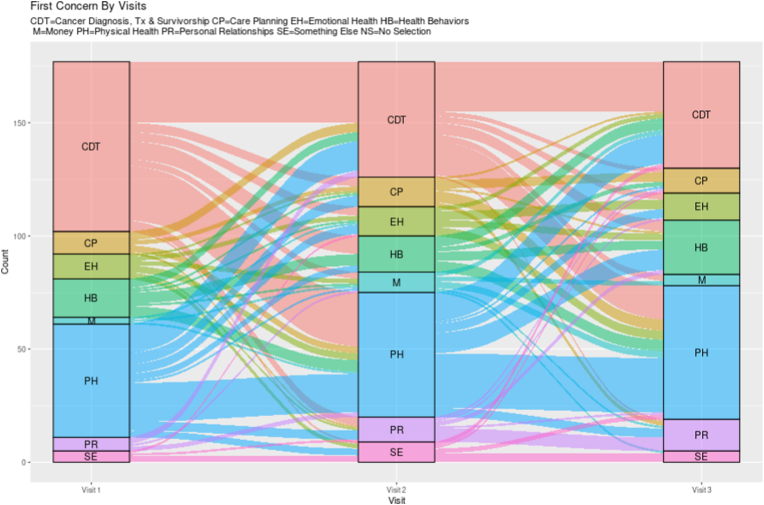
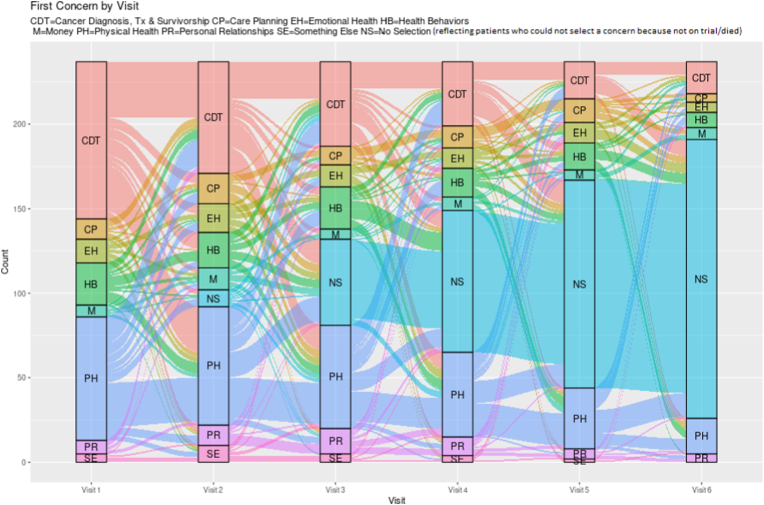
Over time the more visits patients had, the more varied their concern selections became. For instance, the mean number of unique concerns selected per patient (excluding second selections as this was an option for only a subset of encounters) for patients with 1–3 vs 4–5 vs 6 or more visits increased from 1.9 (SD 0.6) to 2.7 (SD 0.8) to 3.9 (SD 1.3) unique concerns (p < 0.001). As evident by Fig. 2B, Fig. 2CB and C which displays the first selection across 3 visits (among patients with 3 or more visits) and 6 visits (among anyone with at least 1 visit), patient selection of categories is dynamic over time for most patients. The most selected sub-concerns within physical health in descending order were related to fatigue (155/345 [47.8%]), neuropathy (150/345 [43.4%]), and difficulty sleeping (103/345 [29.9%]); note that subjects could check more than one sub-concern. Within the cancer diagnosis category, the most selected sub-concerns in descending order of frequency were related to treatment plan (196/324 [60.5%]), prognosis (161/324 [49.7%]) and chemotherapy (92/324 [28.4%]). The frequency of all concerns is shown in Appendix C.
Fig. 2B.
First Concerns over Three Visits without any Non-selections.
Fig. 2C.
First concerns over six visits including non-selection.
The hematologic cohort was significantly more likely to select the category Money (9.9% vs. 5.2%, p = 0.006) and Physical health (45.3% vs. 38.0%, p = 0.01) than the oncologic cohort. The solid tumor cohort selected health behaviors (topics related to exercise, nutrition, tobacco, & substance use) more often than hematologic cohort (23.5% vs. 13.2%, p < 0.001). Women were significantly more likely to select healthy behaviors (21.3% vs. 12.1% p < 0.001) when compared to men. Whereas men were more likely to select physical health (48.0% vs. 38.2%, p = 0.001).
When data was analyzed by age <65 years or ≥65 years, there was a strong association with concern for money in patients younger than 65 years compared to their older counterparts (12% vs 5%, p = 0.002). In addition, there was stronger interest in care planning in the patients 65 years and older (9.1% vs 4.4%, p = 0.003).
There was no statistically significant difference in mean quality of life between the PROQOL (N = 228, mean = 7.13, SD = 2.06) and UC (N = 110, mean = 6.98, SD = 2.36; p = 0.56, mean difference = 0.15, pooled SD = 2.16) patients at the end of the 12-month period for the combined cohort or the hematologic and oncologic cohorts individually, with all results remaining non-significant when adjusting for baseline patient scores in sensitivity analysis. Analysis of QOL between hematologic and solid tumor demonstrated no significance difference, nor between MM vs. AL or H/N vs. GYN. There was no statistically significant difference over time using mixed models between the PROQOL and UC groups in all sub-domains of the LASA which included physical, social, emotional well-being, fatigue, pain, difficulty in performing tasks, or their sense of being overwhelmed by cancer diagnosis.
3.3. Was it worth it
Upon completion of the 12-month follow-up period, patients were surveyed as to their thoughts on the PROQOL tool using WIWI. Seventy-four percent of patients thought the PROQOL tool was worthwhile, 86% would choose PROQOL again and 81% would recommend to others.
There were 33 clinicians surveyed (29 physicians and 4 nurse practitioners/physician's assistants), with 8 in oncology and 25 in hematology. Among the 33 surveyed clinicians with the WIWI questionnaire, 95% responded that they were satisfied with the instrument and did not think that it negatively impacted their workflow. Ninety-four percent of all the clinicians thought that they saw an improvement in their patients' well-being by receiving the additional resources provided by the PROQOL tool, and 81% would recommend and refer others to the PROQOL tool.
4. Discussion
We describe the results of a randomized trial testing clinical use of the PROQOL tool, an electronic point of care system, among the patients in hematology (MM and AL) and oncology (H/N and gynecologic malignancies). Although the primary endpoint of improving mean QOL by our PROQOL interventions was not met, the PROQOL tool demonstrated significant value in efficiently eliciting patients’ main concern and integrating the patient perspective into clinical consideration. The median time from diagnosis to enrollment was 13 months in the solid tumor cohort and 38 months in the hematologic cohort, indicating that though these patients were not new to their diagnosis, yet they continued to struggle with their cancer diagnosis and physical symptoms from disease and/or treatment. This was made evident given that their main concern and second main concern were both related to cancer diagnosis, treatment, and physical health (potential physical toxicities from treatment or disease) and suggests an unmet communication need. However, with more opportunities to interact with the system (e.g., at least 4 encounters) there was increasing diversity of selected categories over time. The mean quality of life in both arms was 7.12 and 6.98 out of 10, but the PROQOL tool did not improve overall quality of life over the course of the 12-month study period. Despite that, the PROQOL tool was considered worthwhile for the majority of both patients and clinicians, who stated that they would refer the PROQOL system to others and would opt to participate again.
The PROQOL tool demonstrated that concerns related to cancer diagnosis, treatments and prognosis and physical health (toxicities from treatment/disease) were most important for patients. Interim monitoring of patient selection of primary concern in the early part of this study demonstrated disproportionate selection of cancer diagnosis, treatment, and physical health as the predominantly selected concern compared to the other categories. With the thought that patients were potentially biased to focus on their diagnosis while at their clinic visits, the option for a second main concern was added to the selection process. Even with the option for a second main concern, cancer diagnosis, treatment, and prognosis and physical health remained the first and second most selected concerns highlighting the need for continued discussion about the treatment plans and addressing of physical symptoms. These patients had the diagnosis for at least a year and were not simply new to diagnosis. A potential gap in patient-clinician communication and counselling is suggested here, as reassurance and dissemination of this information is within the realm of the clinicians' purview. We therefore highlight here that patients’ concerns regarding their disease course and available treatments, need to be reiterated more frequently than at diagnosis and relapse This can be particularly challenging if prognosis is guarded, as has been shown in other studies about communication [27,[28], [29]].
Therefore, interventions should be aimed at improving the communications regarding patients' changing prognosis as reinforced by the American Society of Clinical Oncology's consensus statement on patient physician communication [30]. This phenomenon may be compounded by the limited time that clinicians have with their patients to discuss competing salient needs, with only 1–3 min left for patients to describe their issues, and most time spent on documentation and clerical burden thus indicating that a change in workflow in practice may be necessary [31,32]. PROQOL or a similar patient-reported tool may be the essential bridge to prioritize their needs and efficiently help address these concerns in the visit even if there is no change in their overall QOL. To this point, many studies corroborate the immense potential of information technology in empowering patients in supporting a patient-centered model of care [[33], [34], [35], [36]].
In analyzing correlations in the selection of concerns, patients younger than 65 years selected the concern for money twice as frequently as the older patients and this was strongly statistically significant. This speaks to the anticipated impact of the diagnosis and treatment course on patients who are typically actively working, with more financial responsibilities for dependents, and need for insurance. This has been shown in several studies and represents a population particularly vulnerable to financial toxicity [[37], [38], [39], [40]]. Hematology patients also selected money more frequently than solid tumor patients and is consistent with the well-established financial toxicity incurred by the specialized targeted therapies utilized in plasma cell treatments for an indefinite period [41,42]. Older patients (≥65 years) were significantly more likely to select care planning; this too reflects published literature emphasizing older patients’ unmet needs to discuss end of life concerns and issues [43,44]. Differences were also observed between other groups of subjects as well, e.g., men and women.
Patients expressed that the PROQOL tool was helpful putting forth their major concerns as demonstrated by the “Was it worth it” survey [25,26]. Seventy percent thought it was useful, with comments such as “It made me think about my medical issues” and that the linked resources helpful in addressing those specific areas. Eighty-one percent of patients indicated that they would use the PROQOL tool again. Moreover, PRQOL was self-administered and completed with a median time of 2.9 min, approximately half the time compared to other standardized quality of life assessments [45,46]. In an age when patients are inundated with questionnaires leading to survey fatigue, these results emphasize the value of the PROQOL tool in efficiently eliciting patient concerns without contributing to survey fatigue [47]. Physicians, as well, are overwhelmed by the demands imposed by the electronic health record [48]. However, physicians in this study reported minimal impedance of work flow with the PROQOL tool, supporting that integration of PROs can be successfully integrated in a high volume academic practice without a significant increase in resources or demands on the clinical team [49].
Although less prominent in our study, other psychosocial elements such as health behaviors (tobacco, alcohol, exercise, & nutrition), emotional well-being (anxiety, depression, adjustment issues), and money were still important to patients, and became more prominent with more clinical encounters. These categories are areas that the primary hematologist/oncologist may not be skilled or have the resources, but may be better addressed with a multidisciplinary approach, or even possibly remote monitoring which has shown success with maintaining physical function and symptom management in other studies [[50], [51], [52], [53]]. The recently published randomized trial by Cheville et al. is a superb example in highlighting the improved patient clinical outcomes and quality of life by engaging a robust collaborative approach to tele-rehabilitation [50].
Our study had some limitations. We were unable to assess patients’ main concerns between clinic visits, which may have put their cancer and toxicities to the forefront of their concerns. The summary of main concerns and the actionable list of resources generated by the PROQOL tool were not recorded in the electronic health record but rather printed out and scanned, thereby limiting the accessibility of the resources which were predominantly electronic links to websites, cancer societies and support groups. This limited the optimal integration of PROs into clinical practice. There was also potential contamination bias as physicians were not blinded or randomized. If a clinician had previously reviewed resources generated through the PROQOL tool with a patient, these same resources could have been unwittingly recommended to patients in the UC arm. The restriction to H/N and gynecologic malignancies, MM and AL may limit some generalizability, but the similarity in findings across these very distinct malignancies suggests that our results may generalize across broad patient populations. Given no difference in QOL, alternative unmeasured outcomes may have demonstrated other value such as knowledge increase, patient satisfaction, patient empowerment, and improvement in patient physician communication. Next steps for the PROQOL system will focus on optimization of the tool and will retain the unique component of the tool relative to other available instruments. Optimization may include integration of the PROQOL tool with the electronic medical record, coupling the selection of primary and secondary concerns with other PRO tools that are shown effective for improving patient QOL (e.g., Basch et al. [58]), and other potential automations to customize the self-care advice presented to patients.
The PROQOL tool held considerable value in aiding patients in identifying patients’ main concerns over time and was a worthwhile experience for both patients and clinicians.
Funding
This work was supported by the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic (see Table 1a, Table 1b).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Ann Engebretson, Colleen Irlbeck, Sarah Aug, Sara Ellingson, and all the clinicians and patients in hematology and oncology who were involved in this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2022.100964.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Physician and Patient Was It Worth It Questionnaires.
Fig. s1.
Linear Analog Self-Assessment (LASA) Questionnaire.
References
- 1.Group F-NBW . Silver Spring (MD); 2016. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource.https://www.ncbi.nlm.nih.gov/books/NBK326791/ [PubMed] [Google Scholar]
- 2.Arriba L.N., Fader A.N., Frasure H.E., Von Gruenigen V.E. A review of issues surrounding quality of life among women with ovarian cancer. Gynecol Oncol. 2010;119(2):390–396. doi: 10.1016/j.ygyno.2010.05.014. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 3.Mercieca-Bebber R., King M.T., Calvert M.J., Stockler M.R., Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 2018 Nov 1;9:353–367. doi: 10.2147/PROM.S156279. https://www.ncbi.nlm.nih.gov/pubmed/30464666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Administration F. and D. Guidance for Industry: patient-reported outcome measures: use in medical product development to support labelling claims Food and Drug Administration. 2009. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf cited 2019 Aug 31]
- 5.Deshpande P.R., Rajan S., Sudeepthi B.L., Abdul Nazir C.P. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011 Oct;2(4) doi: 10.4103/2229-3485.86879. http://www.ncbi.nlm.nih.gov/pubmed/22145124 [cited 2019 Apr 7] 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peach M.S., Trifiletti D.M., Vachani C., Arnold-Korzeniowski K., Bach C., Hampshire M., et al. Patient-reported outcomes in head and neck cancer: prospective multi-institutional patient-reported toxicity. Patient Relat. Outcome Meas. 2018;9:245–252. doi: 10.2147/PROM.S153919. https://www.ncbi.nlm.nih.gov/pubmed/30100773%0Ahttps://www.ncbi.nlm.nih.gov/pmc/PMC6067627/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss H.A., Havrilesky L.J. the use of patient-reported outcome tools in Gynecologic Oncology research, clinical practice, and value-based care. Gynecol. Oncol. 2018 Jan;148(1) doi: 10.1016/j.ygyno.2017.11.011. http://www.ncbi.nlm.nih.gov/pubmed/29174565 [cited 2019 Apr 7] 12–8. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey S., Blough D., Kirchhoff A., Kreizenbeck K., Fedorenko C., Snell K., et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff. 2013 Jun;32(6):1143–1152. doi: 10.1377/hlthaff.2012.1263. https://www.ncbi.nlm.nih.gov/pubmed/23676531 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meraner V., Gamper E.-M., Grahmann A., Giesinger J.M., Wiesbauer P., Sztankay M., et al. Monitoring physical and psychosocial symptom trajectories in ovarian cancer patients receiving chemotherapy. BMC Cancer. 2012 Dec 28;12(1):77. doi: 10.1186/1471-2407-12-77. http://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-12-77 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Mantia I., Rossitto F., Andaloro C. Quality of life in head and neck cancer: patients' and family caregivers' perceptions. Egypt J Ear, Nose. Throat Allied Sci. 2017 Nov 1;18(3) https://www.sciencedirect.com/science/article/pii/S2090074017300439 [cited 2019 Apr 7] 247–50. [Google Scholar]
- 11.Sloan J., Spencer M., Chauhan C., Tunis S., Lipscomb J., Basch E., et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J. Clin. Oncol. 2012 Dec;30(34):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 12.Moss H.A., Havrilesky L.J. The use of patient-reported outcome tools in Gynecologic Oncology research, clinical practice, and value-based care. Gynecol. Oncol. 2018 Jan;148(1):12–18. doi: 10.1016/j.ygyno.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande P.R., Rajan S., Sudeepthi B.L., Abdul Nazir C.P. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011 Oct;2(4):137–144. doi: 10.4103/2229-3485.86879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krantz E., Wide U., Trimpou P., Bryman I., Landin-Wilhelmsen K. Comparison between different instruments for measuring health-related quality of life in a population sample, the WHO MONICA Project, Gothenburg, Sweden: an observational, cross-sectional study. BMJ Open. 2019 Apr 1;9(4) doi: 10.1136/bmjopen-2018-024454. http://bmjopen.bmj.com/content/9/4/e024454.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers S.N. Improving quality-of-life questionnaires in head and neck cancer. Expert Rev Qual Life Cancer Care. 2017;1(1):61–71. doi: 10.1080/23809000.2016.1142357. [DOI] [Google Scholar]
- 16.F and DA. Value and use of patient-reported outcomes (PROs) in assessing effects of medical devices CDRH strategic priorities 2016-2017 [Internet]. Available from: https://www.fda.gov/media/109626/download.
- 17.Smith J.S., Chang E.F., Lamborn K.R., Chang S.M., Prados M.D., Cha S., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008 Mar 10;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. http://ascopubs.org/doi/10.1200/JCO.2007.13.9337 Available from: [DOI] [PubMed] [Google Scholar]
- 18.Mierzynska J., Piccinin C., Pe M., Martinelli F., Gotay C., Coens C., et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol. 2019 Dec;20(12):e685–e698. doi: 10.1016/S1470-2045(19)30656-4. https://linkinghub.elsevier.com/retrieve/pii/S1470204519306564 Available from: [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Ou L., Hollis S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013 Dec 11;13(1):211. doi: 10.1186/1472-6963-13-211. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-13-211 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotronoulas G., Kearney N., Maguire R., Harrow A., Di Domenico D., Croy S., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014 May 10;32(14):1480–1501. doi: 10.1200/JCO.2013.53.5948. http://ascopubs.org/doi/10.1200/JCO.2013.53.5948 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Brandt J., Scotte F., Jordan K. Patient-reported outcomes (PROs) as a routine measure for cancer inpatients: the final missing piece of the puzzle? Ann Oncol Off J Eur Soc Med Oncol. 2019 Feb;30(2):167–169. doi: 10.1093/annonc/mdy524. [DOI] [PubMed] [Google Scholar]
- 22.Ilhan T.T., Kebapcilar A.K., Ilhan T.I., Cakir T.C., Yilmaz S.A., Celik C., et al. The effect of gynecologic cancer on patient’s depression and anxiety: prospective study. Gynecol. Oncol. 2015 Apr 1;137:194. https://linkinghub.elsevier.com/retrieve/pii/S0090825815004928 [Google Scholar]
- 23.Lipe B., Vukas R., Mikhael J. The role of maintenance therapy in multiple myeloma. Blood Cancer J. 2016 Oct 21;6(10) doi: 10.1038/bcj.2016.89. http://www.nature.com/articles/bcj201689 e485–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahma Warsame M.D., Joleen Hubbard M.D., Angela Dispenzieri M.D., Carrie Thompson M.D., Katrina Croghan M.S., Juliana Perez Botero M.D., Tan Angelina, Fruth Briant, Deirdre Pachman M.D., Lafky Veronica, Jeff Sloan P. A novel case management system to address patient-identified concerns in hematology and oncology clinical pathways. J Clin Pathways. 2016;2(8):35–41. [Google Scholar]
- 25.Sloan J.A., Mahoney M.R., Sargent D.J., Hubbard J.M., Liu H., Basch E.M., et al. Was it worth it (WIWI)? Patient satisfaction with clinical trial participation: results from north central cancer treatment group (NCCTG) phase III trial N0147. J Clin Oncol. 2011 May 20;29(15_suppl):6122. doi: 10.1200/jco.2011.29.15_suppl.6122. Available from: [DOI] [Google Scholar]
- 26.Fain R., Chen Y., Rana S.R., Dang J., Haly A., Franklin A., et al. Was it worth it (WIWI)? An OHSU Knight Cancer Institute retrospective analysis of patient satisfaction following radiotherapy. J Clin Oncol. 2016 Jan 20;34(3_suppl):225. doi: 10.1200/jco.2016.34.3_suppl.225. Available from: [DOI] [Google Scholar]
- 27.Raskin W., Harle I., Hopman W.M., Booth C.M. Prognosis, treatment benefit and goals of care: what do oncologists discuss with patients who have incurable cancer? Clin. Oncol. 2016 Mar;28(3):209–214. doi: 10.1016/j.clon.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Scotté F., Bossi P., Carola E., Cudennec T., Dielenseger P., Gomes F., et al. Addressing the quality of life needs of older patients with cancer: a SIOG consensus paper and practical guide. Ann. Oncol. 2018 Jul;29(8):1718–1726. doi: 10.1093/annonc/mdy228. [DOI] [PubMed] [Google Scholar]
- 29.Chou W.-Y.S., Hamel L.M., Thai C.L., Debono D., Chapman R.A., Albrecht T.L., et al. Discussing prognosis and treatment goals with patients with advanced cancer: a qualitative analysis of oncologists' language. Heal Expect an Int J public Particip Heal care Heal policy. 2017 Oct;20(5):1073–1080. doi: 10.1111/hex.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilligan T., Coyle N., Frankel R.M., Berry D.L., Bohlke K., Epstein R.M., et al. Patient-clinician communication: American society of clinical oncology consensus guideline. J Clin Oncol [Internet] 2017 Nov 1;35(31):3618–3632. doi: 10.1200/JCO.2017.75.2311. http://ascopubs.org/doi/10.1200/JCO.2017.75.2311 Available from: [DOI] [PubMed] [Google Scholar]
- 31.Collier R. Electronic health records contributing to physician burnout. Can Med Assoc J. 2017 Nov 13;(45):189. doi: 10.1503/cmaj.109-5522. http://www.cmaj.ca/lookup/doi/10.1503/cmaj.109-5522 E1405–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baile W.F., Palmer J.L., Bruera E., Parker P.A. Assessment of palliative care cancer patients' most important concerns. Support Care Cancer. 2011 Apr 17;19(4):475–481. doi: 10.1007/s00520-010-0839-4. http://link.springer.com/10.1007/s00520-010-0839-4 Available from: [DOI] [PubMed] [Google Scholar]
- 33.Clauser S.B., Wagner E.H., Aiello Bowles E.J., Tuzzio L., Greene S.M. Improving modern cancer care through information technology. Am. J. Prev. Med. 2011 May;40(5):198–207. doi: 10.1016/j.amepre.2011.01.014. https://linkinghub.elsevier.com/retrieve/pii/S074937971100095X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisch M.J., Chung A.E., Accordino M.K. Using technology to improve cancer care: social media, wearables, and electronic health records. Am Soc Clin Oncol Educ B. 2016;36 doi: 10.1200/EDBK_156682. http://meetinglibrary.asco.org/content/156682-176 200–8. [DOI] [PubMed] [Google Scholar]
- 35.Mooney K., Berry D.L., Whisenant M., Sjoberg D. Improving cancer care through the patient experience: how to use patient-reported outcomes in clinical practice. Am Soc Clin Oncol Educ B. 2017;37:695–704. doi: 10.1200/EDBK_175418. [DOI] [PubMed] [Google Scholar]
- 36.Trautmann F., Hentschel L., Hornemann B., Rentsch A., Baumann M., Ehninger G., et al. Electronic real-time assessment of patient-reported outcomes in routine care-first findings and experiences from the implementation in a comprehensive cancer center. Support. Care Cancer. 2016 Feb 18;24(7):3047–3056. doi: 10.1007/s00520-016-3127-0. http://link.springer.com/10.1007/s00520-016-3127-0 [Internet] [DOI] [PubMed] [Google Scholar]
- 37.Yabroff K.R., Bradley C., Shih Y.-C.T. Understanding financial hardship among cancer survivors in the United States: strategies for prevention and mitigation. J Clin Oncol Off J Am Soc Clin Oncol. 2020 Feb;38(4):292–301. doi: 10.1200/JCO.19.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaddas H.K., Pannier S.T., Mann K., Waters A.R., Salmon S., Tsukamoto T., et al. Age-related differences in financial toxicity and unmet resource needs among adolescent and young adult cancer patients. J. Adolesc. Young Adult Oncol. 2020 Feb;9(1):105–110. doi: 10.1089/jayao.2019.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaul S., Avila J.C., Mutambudzi M., Russell H., Kirchhoff A.C., Schwartz C.L. Mental distress and health care use among survivors of adolescent and young adult cancer: a cross-sectional analysis of the National Health Interview. Survey. Cancer. 2017 Mar;123(5):869–878. doi: 10.1002/cncr.30417. [DOI] [PubMed] [Google Scholar]
- 40.Kirchhoff A.C., Lyles C.R., Fluchel M., Wright J., Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012 Dec;118(23):5964–5972. doi: 10.1002/cncr.27537. [DOI] [PubMed] [Google Scholar]
- 41.Roy A., Kish J.K., Bloudek L., Siegel D.S., Jagannath S., Globe D., et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: a model framework. Am Heal drug benefits. 2015 Jun;8(4):204–215. https://pubmed.ncbi.nlm.nih.gov/26157542 [Internet] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajkumar S.V. Value and cost of myeloma therapy. Am Soc Clin Oncol Educ B. 2018 May 23;(38):662–666. doi: 10.1200/EDBK_200867. [Internet] [DOI] [PubMed] [Google Scholar]
- 43.Sharp T., Moran E., Kuhn I., Barclay S. Do the elderly have a voice? Advance care planning discussions with frail and older individuals: a systematic literature review and narrative synthesis. Br J Gen Pract [Internet] 2013 Oct;63(615) doi: 10.3399/bjgp13X673667. http://bjgp.org/lookup/doi/10.3399/bjgp13X673667 e657–68. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahluwalia S.C., Levin J.R., Lorenz K.A., Gordon H.S. Missed opportunities for advance care planning communication during outpatient clinic visits. J Gen Intern Med. 2012 Apr 25;27(4):445–451. doi: 10.1007/s11606-011-1917-0. http://link.springer.com/10.1007/s11606-011-1917-0 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burckhardt C.S., Anderson K.L. The Quality of Life Scale (QOLS): reliability, validity, and utilization. Health Qual. Life Outcome. 2003 Oct 23;1:60. doi: 10.1186/1477-7525-1-60. https://www.ncbi.nlm.nih.gov/pubmed/14613562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chopra I., Kamal K.M. A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual. Life Outcome. 2012;10(1):14. doi: 10.1186/1477-7525-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolstad S., Adler J., Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011 Dec;14(8):1101–1108. doi: 10.1016/j.jval.2011.06.003. https://linkinghub.elsevier.com/retrieve/pii/S1098301511015245 [DOI] [PubMed] [Google Scholar]
- 48.Kroth P.J., Morioka-Douglas N., Veres S., Pollock K., Babbott S., Poplau S., et al. The electronic elephant in the room: physicians and the electronic health record. JAMIA Open. 2018 Jul 1;1(1):49–56. doi: 10.1093/jamiaopen/ooy016. https://academic.oup.com/jamiaopen/article/1/1/49/5035929 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basch E., Barbera L., Kerrigan C.L., Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet. 2018 May;38:122–134. doi: 10.1200/EDBK_200383. [DOI] [PubMed] [Google Scholar]
- 50.Cheville A.L., Moynihan T., Herrin J., Loprinzi C., Kroenke K. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer. JAMA Oncol. 2019 May 1;5(5):644. doi: 10.1001/jamaoncol.2019.0011. http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2019.0011 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soukup T., Lamb B.W., Arora S., Darzi A., Sevdalis N., Green J.S. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J. Multidiscip. Healthc. 2018;11:49–61. doi: 10.2147/JMDH.S117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basch E., Deal A.M., Kris M.G., Scher H.I., Hudis C.A., Sabbatini P., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2016 Feb;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basch E., Schrag D., Henson S., Jansen J., Ginos B., Stover A.M., Carr P., Spears P.A., Jonsson M., Deal A.M., Bennett A.V., Thanarajasingam G., Rogak L.J., Reeve B.B., Snyder C., Bruner D., Cella D., Kottschade L.A., Perlmutter J., Geoghegan C., Samuel-Ryals C.A., Given B., Mazza G.L., Miller R., Strasser J.F., Zylla D.M., Weiss A., Blinder V.S., Dueck A.C. Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer: a randomized clinical trial. JAMA. 2022 Jun 28;327(24):2413–2422. doi: 10.1001/jama.2022.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physician and Patient Was It Worth It Questionnaires.