Abstract
Adenosquamous carcinoma of the pancreas (ASCP) is a very rare and highly aggressive variant of pancreatic ductal adenocarcinoma, accounting for 0.5–4% of all pancreatic cancer cases in the USA. Current data indicate that epigenetic changes and MYC overexpression lead to squamous transdifferentiation of pancreatic tumor cells and development of ASCP. Minnelide™, an oral anti-super-enhancer drug that inhibits MYC expression in preclinical models of ASCP, has demonstrated safety in a phase I study. We describe the design for a phase II, open-label, single-arm trial of Minnelide in patients with advanced refractory ASCP.
Keywords: : adenosquamous carcinoma of the pancreas, anti-super-enhancer, Minnelide™, MYC, single-arm phase II trial
Plain language summary
Adenosquamous carcinoma of the pancreas (ASCP) is a rare and highly aggressive variant of pancreatic cancer, with limited treatment options. Changes in activation of DNA elements called super-enhancers drive the growth of ASCP. Minnelide™ is an oral drug that blocks the super-enhancer network and is safe to give to patients with advanced cancer. This trial is designed to determine whether Minnelide can shrink tumors in patients with ASCP who have already received at least one previous treatment for their cancer.
Clinical Trial Registration: NCT04896073 (ClinicalTrials.gov)
Tweetable abstract
Adenosquamous carcinoma of the pancreas (ASCP) is a highly aggressive variant of pancreatic cancer that overexpresses MYC. Minnelide is an oral anti-super-enhancer drug that reduces MYC expression. This phase II trial tests the efficacy of Minnelide in patients with advanced refractory ASCP.
Adenosquamous carcinoma of the pancreas (ASCP) is a rare, highly aggressive variant of pancreatic ductal adenocarcinoma (PDA). It is histologically defined as a tumor containing components with both glandular and squamous differentiation. The squamous component must constitute at least 30% of the tumor for an ASCP designation [1]. The squamous component can be identified through histopathological examination for squamous markers p63, CK5 and CK6. Prevalence of ASCP varies significantly in the literature, with most researchers reporting that it makes up 0.5–4% of pancreatic cancer cases [2]. Diagnosis of ASCP by cytological or core biopsy is difficult, leading some to explore radiomic signatures that can complement pathology-based diagnosis [3,4]. The prognosis for patients with ASCP is worse than for those with conventional PDA; overall survival (OS) is estimated to be 5–8 months shorter [5–7]. Patients are typically treated with chemotherapy and chemoradiation regimens similar to those for PDA [2,5,8]. Due to the rarity of ASCP, there have been no prospective clinical trials published and there are no existing treatment guidelines for this disease. In patients with resectable disease, retrospective case series suggest that patients with larger tumors or perineural invasion may benefit from adjuvant chemotherapy and that platinum-containing regimens may be particularly beneficial [5,9]. Despite this, a recent multicenter retrospective study reported no clinical responses among 16 patients receiving varying combinations of gemcitabine, 5-fluorouracil, irinotecan and platinum chemotherapy agents and a median OS of 7.5 months in the population, emphasizing the limited effectiveness of current treatments [10]. Given the poor prognosis of ASCP and the need for more effective treatments, patients with ASCP would benefit from clinical trial enrollment.
Genomic profiling studies have been carried out on ASCP tumors. These have noted high rates of KRAS and p53 mutations [2,11,12], as well as copy number gains in chromosome 8q which contains the MYC locus [11], MYC pathway activation [13] and specific amplification of the MYC oncogene [12,14,15]. It is therefore believed that MYC expression drives ASCP.
Introduction to the trial
Here we describe the design and rationale for the phase II, single-arm, single-cohort study (ClinicalTrials.gov: NCT04896073), which is being conducted to evaluate the efficacy of oral Minnelide™ in patients with advanced ASCP that has been previously treated (see Infographic for summary). The trial is sponsored and funded by the National Cancer Institute. Drug supply for the study is provided by Minneamrita Therapeutics, LLC (FL, USA). This trial design was presented in virtual poster form at the AACR Virtual Special Conference: Pancreatic Cancer on 29–30 September 2021.
Background & rationale
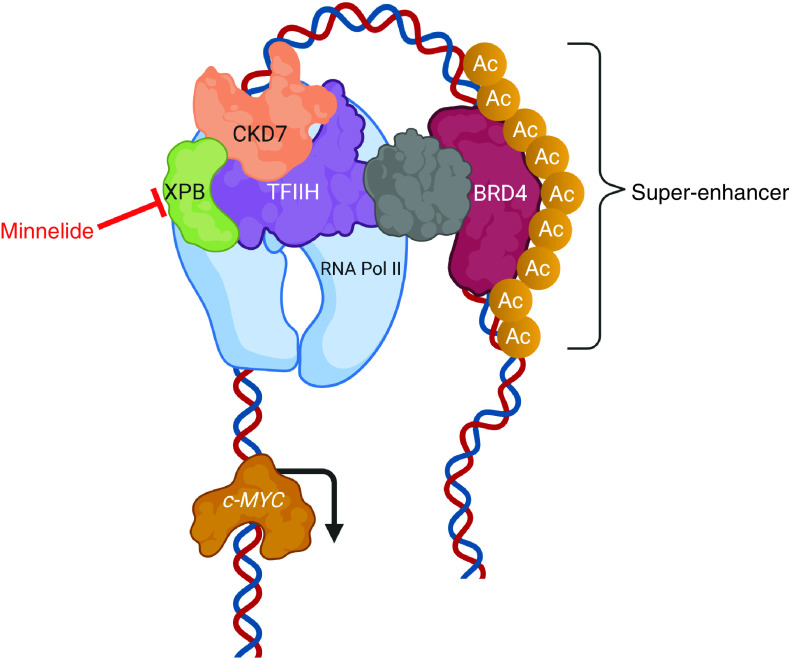
Minnelide is a water-soluble prodrug (14–0-phosponooymethyl triptolide disodium salt) of the Chinese medicinal herb triptolide. Multiple mechanisms of action for triptolide/Minnelide have been reported [16]. Ultimately, it was discovered that triptolide is a small-molecule inhibitor of the XPB subunit of the TFIIH transcription complex, a super-enhancer complex which regulates c-MYC (Figure 1). Triptolide covalently complexes with XPB, resulting in inhibition of XPB’s DNA-dependent ATPase activity [17]. This action inhibits transcription of TFIIH super-enhancer targets. For instance, inhibition of HSP70 expression by triptolide has been shown to cause HSP-70-dependent apoptosis in preclinical models of PDA, a tumor type that typically overexpresses HSP70 [18]. As MYC is also regulated by TFIIH, Minnelide/triptolide treatment causes downregulation of MYC expression and reduced expression of MYC protein in PDA cell line models. It also shrinks PDA patient-derived xenograft tumors grown in mice [19]. As MYC appears to be a key determinant in ASCP, inhibition of c-MYC with Minnelide might be advantageous for this patient population.
Figure 1. . Model for the mechanism of action of triptolide, of which Minnelide™ is a prodrug.
Inhibition of XPB as part of the transcription machinery leads to disruption of the activity of super-enhancers. This results in suppression of genes associated with that super-enhancer (in this case c-MYC).
Ac: Acetylation.
Modified with permission from [19]. Created with BioRender.com.
Oral Minnelide is undergoing ongoing clinical testing in a phase I dose escalation study. This study opened in 2017 and is still accruing (NCT03129139).
Study design
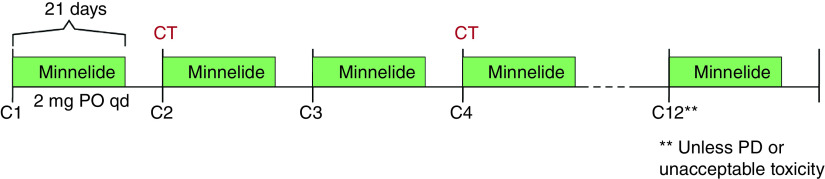
This is a phase II, single-arm, single-cohort study. Eligible participants will self-administer Minnelide at 2 mg daily by mouth on days 1–21 of each 28-day cycle. Treatment will continue for up to 12 cycles (1 year) in the absence of disease progression, investigator or participant decision to withdraw, or unacceptable toxicity (Figure 2).
Figure 2. . Study design schema.
C: Cycle; CT: Computed tomography; PO: By mouth; PD: Progressive disease; qd: Daily.
Eligibility criteria
Eligibility criteria are described in Box 1. Major eligibility criteria include presence of histologically confirmed metastatic, recurrent or locally advanced unresectable ASCP, which was previously treated with one or more systemic therapies. Eligible participants are men and women aged 18 years and older, with Eastern Co-operative Oncology Group performance status ≤2 and adequate organ and marrow function.
Box 1. Major eligibility criteria.
Inclusion criteria
Histologically confirmed ASCP, having ≥30% malignant squamous component
Presence of metastatic, recurrent or locally advanced unresectable disease
Progression or intolerance to one or more prior systemic treatment for advanced disease
Measurable disease per RECIST 1.1 criteria
Age ≥18 years
ECOG performance status 0 or 1
-
Adequate organ and marrow function
-
○
ANC ≥1500/μl, platelets ≥100,000/μl, hemoglobin ≥9.0 g/dl or ≥5.6 mmol/l
-
○
Creatinine ≤1.5 × ULN or measured creatinine clearance ≥45 ml/min (if creatinine >1.5 × ULN)
-
○
Total bilirubin ≤1.5 × ULN or direct bilirubin ≤ULN (if total bilirubin >1.5 × ULN)
-
○
AST and ALT ≤2.5 × ULN (≤5 × ULN for patients with liver metastases)
-
○
INR or PT/PTT ≤1.5 × ULN (unless on anticoagulation, then therapeutic range of PT/PTT is acceptable)
-
○
Agreement to use contraception for women of child-bearing potential and men
Exclusion criteria
Uncontrolled vomiting or medical condition preventing oral ingestion and digestion
Pregnant and/or women who are breastfeeding
Currently participating and receiving trial therapy, or had done so within 3 weeks of first planned treatment on study
Prior chemotherapy, targeted therapy or radiation therapy within 2 weeks prior to cycle 1/day 1
Requires use of ondansetron (other 5-HT3 inhibitors are not prohibited)
Major surgery within last 4 weeks, minor endoscopic procedure within last 2 weeks, or percutaneous procedure within 24 h of planned treatment start date
Additional malignancy that is progressing and requiring active treatment
Known active CNS metastases and/or carcinomatous meningitis
Active infection requiring systemic therapy
Known psychiatric or substance abuse disorders that would affect co-operation with trial requirements
Known uncontrolled or poorly controlled HIV infection.
Active or currently under treatment for HBV or HCV
Received live vaccine within 30 days of planned start of treatment
History of allergic reaction to compounds of similar chemical or biological composition to Minnelide™
ASCP: Adenosquamous carcinoma of the pancreas; ANC: Absolute neutrophil count; ECOG: Eastern Co-operative Oncology Group; HBV: Hepatitis B virus; HCV: Hepatitis C virus; INR: International normalized ratio; PT: Prothrombin time; PTT: Partial thromboplastin time; RECIST: Response Evaluation Criteria In Solid Tumors; ULN: Upper limit of normal.
Planned sample size & study period
To assess study objectives, up to 25 evaluable participants will be enrolled. The study opened for patient accrual in July 2021. It is expected that approximately one participant may initiate treatment every 1–2 months; thus, accrual to the trial may be completed in 3 years.
Outcome measures/end points
The primary end point of the study is disease control rate, defined as the sum of complete responses (CRs), partial responses (PRs) and at least 16 weeks of stable disease, as per Response Evaluation Criteria In Solid Tumors (RECIST) v.1.1. All end points are listed in Box 2.
Box 2. End points.
Primary end points
Disease control rate (complete response + partial response + stable disease for ≥16 weeks)
Secondary end points
Drug safety
Progression-free survival
Overall survival
Exploratory end points
-
Serial biopsies to assess drug effects on:
-
○
MYC expression
-
○
Accessibility of MYC and other super-enhancer loci
-
○
Tumor microenvironment, including stroma and immune cell populations
-
○
Post treatment
-
○
Changes in circulating tumor DNA
Circulating immune cell populations
Safety of Minnelide in this population will be assessed as a secondary end point. The rate of grade ≥2 adverse events (per Common Terminology Criteria for Adverse Events v.5.0) and serious adverse advents related to Minnelide will be determined.
Two alternative measures of antitumor activity will also be determined as secondary end points. Objective response rate (ORR) will be calculated as the percentage of participants with CR or PR as per RECIST 1.1 criteria, measured every two cycles (8 weeks), via radiographic assessment. Median time until disease progression, switch to a new systemic treatment or death in the treated population will also be measured with a cutoff of 1 year after last Minnelide treatment. Progression-free survival and OS will also be evaluated as secondary end points.
A number of scientific correlatives will be evaluated as exploratory end points. Optional pre- and on-treatment tumor biopsies will allow assessment of the effect of Minnelide on MYC expression and accessibility of loci for the MYC gene. Changes to fibroblast and immune cell populations in the tumor microenvironment will also be examined in participant tumor tissue. The effect of Minnelide on circulating immune cell populations will be determined. Given that KRAS mutation occurs in most ASCPs, we will also examine the feasibility of quantitating KRAS-mutated circulating tumor DNAs and whether changes in this potential serum tumor marker are associated with response to treatment or tumor progression.
Study procedures
Participant assessments will occur on days 1, 8, 15 and 22 of the first cycle, followed by days 1 and 15 of all subsequent cycles. Notably, participants only need to be at the NIH Clinical Center for assessments that include a physical exam and vital signs, which will occur on days 1 and 15 of the first cycle and then on day 1 of each subsequent cycle. If only symptom assessment is required, a telehealth visit may be conducted so that the participant need not travel to Bethesda, unless preferred by the participant or felt to be clinically indicated by the provider.
Participants will be provided with Minnelide capsules sufficient for one cycle. Participants with baseline nausea and/or vomiting will be continued on their home antiemetic regimen. If this contains ondansetron (a prohibited medication), it will be transitioned to an acceptable alternative. Participants without an antiemetic regimen at baseline will be prescribed oral granisetron in case of nausea and/or vomiting.
Adverse events (assessed using the Common Terminology Criteria for Adverse Events v.5.0) and concomitant medications will be continuously monitored throughout the study until 30 days from last dose of Minnelide or start of new anticancer treatment, whichever comes first. Participants will be given a dosing diary to self-report Minnelide administration which will be reviewed for drug adherence/accountability. Two dose reductions per participant – to 75 and 50% of the dose – will be permitted in response to either hematological or non-hematological adverse events as defined in the protocol.
A mandatory safety follow-up should be conducted approximately 30 days after the last dose of study drug or before initiation of a new anticancer treatment, whichever comes first. Participants will be contacted remotely (e.g., by phone or email) approximately every 90 days to make this assessment.
Radiographic tumor assessments via CT scan or MRI will be performed at baseline and every two cycles.
Clinical laboratory investigations will be performed on days 1, 8, 15 and 22 of cycle 1 and on days 1 and 15 of each subsequent cycle. Of note, these can be also done at a local laboratory in case the participant is not being physically evaluated at the NIH Clinical Center.
Archival tissue is required for confirmation of diagnosis. Optional tumor biopsies will be requested at baseline, mid-cycle 1 and/or at time of progression. Correlative blood samples will be collected at the NIH Clinical Center on days 1 and 15 of cycle 1, day 1 of cycle 2 and at the end-of-treatment visit/time of disease progression.
Notably, NIH does not bill health insurance companies or participants for any research or related clinical care that participants receive at the NIH Clinical Center. The National Cancer Institute will cover the costs of some expenses associated with protocol participation, which may be paid directly by the NIH or reimbursed to the participant/guardian as appropriate. The amount and form of these payments are determined by the National Cancer Institute Travel and Lodging Reimbursement Policy.
Statistics
The trial will be conducted using a Simon optimal two-stage phase II trial design.
The first stage has a null hypothesis of unacceptably low clinical benefit rate of 20% (p0 = 0.20). The probability of accepting a poor treatment (α) is set at 10%, and the probability of rejecting a good treatment (β) is set at 20%. If the null hypothesis of the first stage is true, the study treatment will be deemed ineffective.
Stage two is initiated if the null hypothesis of stage one is rejected and will test for an improved clinical benefit rate of 40% (p1 = 0.40).
The first stage will enroll a total of 12 evaluable participants. If two or fewer of these 12 participants have clinical benefit, then no further participants will be accrued. If three or more of the first 12 participants have clinical benefit, then accrual would continue until a total of 25 evaluable participants have been enrolled. If there are eight or more of 25 (32%) who experience clinical benefit, this would be sufficiently interesting to warrant further study in later trials. To account for screen failures and inevaluable participants (i.e., participants who are not evaluable for the primary end point of response), the accrual ceiling will be set at 55 participants.
Primary end point will be calculated as a fraction of evaluable participants who experience clinical benefit, reported along with a 95% two-sided CI (disease control rate = CR + PR + stable disease for ≥16 weeks).
Secondary end points of progression-free survival and OS will be determined using the Kaplan–Meier method and will be reported along with a 95% CI for the median. Objective response rate will be calculated as the percentage of participants with CR or PR as defined by RECIST 1.1 criteria.
Conclusion
This is a newly opened phase II single-center trial that is investigating the efficacy of Minnelide in patients with advanced ASCP that was previously treated. ASCP is a rare and highly aggressive subtype of PDA, associated with frequent MYC amplifications and super-enhancer network activation. Minnelide is a small-molecule anti-super-enhancer drug that inhibits MYC. Preclinical data support its use in ASCP, and safety has been shown in a phase I trial for advanced gastrointestinal tumors [20]. Testing of Minnelide in combination with nab-paclitaxel in PDA patients is ongoing (NCT03129139). If the current study demonstrates antitumor activity of single-agent Minnelide in ASCP patients, this would represent an important advance for patients with ASCP and could also lead to future studies examining combinations of Minnelide with chemotherapy or immunotherapy.
Executive summary.
Adenosquamous carcinoma of the pancreas
Adenosquamous carcinoma of the pancreas (ASCP) is a rare, highly aggressive variant of pancreatic ductal adenocarcinoma, with an overall worse prognosis.
Due to its rarity, there have been no prospective clinical trials published and there are no existing guidelines for treatment of patients with ASCP.
A few genomic profiling studies carried out on ASCP tumors demonstrate MYC copy number gains, MYC pathway activation and specific amplification of MYC, leading to a conclusion that MYC expression drives ASCP pathogenesis.
Minnelide
Minnelide™ is a prodrug of triptolide, which is a small-molecule inhibitor of the XPB subunit of the TFIIH transcription complex – a super-enhancer complex regulating c-MYC.
Oral Minnelide is undergoing clinical testing in a phase I dose-escalation study for patients with advanced solid tumors and has been demonstrated to be safe.
Trial description
This is a phase II, single-arm, single-cohort study testing efficacy of oral Minnelide in patients with advanced ASCP that had been previously treated.
Minnelide will be administered for 21 days of each 28-day cycle for up to 12 cycles unless disease progression or unacceptable toxicity occur.
The primary end point of the study is disease control rate, defined as the sum of complete responses, partial responses and at least 16 weeks of stable disease, as per Response Evaluation Criteria In Solid Tumors v.1.1.
The study will be performed at the NIH Clinical Center, with an option for televisits on certain occasions, minimizing the need for travel. The National Cancer Institute will cover the costs of some expenses associated with protocol participation, travel and lodging.
Conclusion
As MYC appears to be a key determinant in ASCP, inhibition of c-MYC with Minnelide might be advantageous for this patient population with limited treatment options.
Supplementary Material
Footnotes
Supplementary data
An infographic accompanies this paper. To view or download this infographic in your browser please click here: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-1609
Financial & competing interests disclosure
D Von Hoff received research funding from Minneamrita Therapeutics. C Alewine receives funding through the NCI Center for Cancer Research (NIH funding information: NCI CCR ZIA BC 011652). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Human data sharing plan
Human data generated in this research will be shared as coded, linked data in an NIH-funded or approved public repositories (such as clinicaltrials.gov) and in BTRIS (at the NIH Clinical Center). Identified or coded, linked data may be shared with collaborators outside NIH Clinical Center under appropriate agreements. Data will also be shared through publication and public presentation. Unlinked genomic data will be deposited in public genomic databases such as dbGaP in compliance with the NIH Genomic Data Sharing Policy.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 76(2), 182–188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borazanci E, Millis SZ, Korn R et al. Adenosquamous carcinoma of the pancreas: molecular characterization of 23 patients along with a literature review. World J. Gastrointest. Oncol. 7(9), 132–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren S, Zhao R, Cui W et al. Computed tomography-based radiomics signature for the preoperative differentiation of pancreatic adenosquamous carcinoma from pancreatic ductal adenocarcinoma. Front. Oncol. 10, 1618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, Jia Z, Chen X et al. CT and MR imaging features of pancreatic adenosquamous carcinoma and their correlation with prognosis. Abdom. Radiol. 44(8), 2822–2834 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Voong KR, Davison J, Pawlik TM et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum. Pathol. 41(1), 113–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol. 57(2), 139–146 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest. Cancer Res. 6(3), 75–79 (2013). [PMC free article] [PubMed] [Google Scholar]
- 8.Moslim MA, Lefton MD, Ross E, Mackrides N, Reddy SS. Clinical and histological basis of adenosquamous carcinoma of the pancreas: a 30-year experience. J. Surg. Res. 259, 350–356 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wild AT, Dholakia AS, Fan KY et al. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J. Gastrointest. Oncol. 6(2), 115–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunetti O, Aprile G, Marchetti P et al. Systemic chemotherapy for advanced rare pancreatic histotype tumors: a retrospective multicenter analysis. Pancreas 47(6), 759–771 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, Su Z, Xie J et al. Genomic signatures of pancreatic adenosquamous carcinoma (PASC). J. Pathol. 243(2), 155–159 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Lenkiewicz E, Malasi S, Hogenson TL et al. Genomic and epigenomic landscaping defines new therapeutic targets for adenosquamous carcinoma of the pancreas. Cancer Res. 80(20), 4324–4334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Findings of a study identifying MYC amplification as a potential therapeutic target based on genomic analysis of patient-derived tumor samples.
- 13.Bailey P, Chang DK, Nones K et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531(7592), 47–52 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Hayashi A, Fan J, Chen R et al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat. Cancer 1(1), 59–74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkiewicz AK, McMillan EA, Balaji U et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6(1), 6744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel P, Hoff DDV, Saluja AK, Velagapudi M, Borazanci E, Han H. Triptolide and its derivatives as cancer therapies. Trends Pharmacol. Sci. 40(5), 327–341 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Titov DV, Gilman B, He QL et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 7(3), 182–188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips PA, Dudeja V, McCarroll JA et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 67(19), 9407–9416 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Noel P, Hussein S, Ng S et al. Triptolide targets super-enhancer networks in pancreatic cancer cells and cancer-associated fibroblasts. Oncogenesis 9(11), 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study demonstrating that triptolide, of which Minnelide is a prodrug, disrupts the super-enhancer network, downregulates MYC and has an antitumor effect in pancreatic cancer cells and mouse models.
- 20.Greeno E, Borazanci EH, Gockerman JP, Korn RL, Saluja A, VonHoff DD. Phase I dose escalation and pharmokinetic study of a modified schedule of 14-o-phosphonooxymethyltriptolide. J. Clin. Oncol. 34(Suppl. 4), TPS472–TPS472 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.