Abstract
Objective:
Compare health-related quality of life (HRQoL) of selinexor versus placebo in patients with dedifferentiated liposarcoma.
Materials & methods:
HRQoL was assessed at baseline and day 1 of each cycle using the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire. Results were reported from baseline to day 169 (where exposure to treatment was maximized while maintaining adequate sample size).
Results:
Pain scores worsened for placebo versus selinexor across all postbaseline visits, although differences in HRQoL at some visits were not significant. Other domains did not exhibit significant differences between arms; however, scores in both arms deteriorated over time.
Conclusion:
Patients treated with selinexor reported lower rates and slower worsening of pain compared with patients who received placebo.
Keywords: : advanced liposarcoma, pain, patient-reported outcomes, progression-free survival, quality of life, selective inhibitor of nuclear export, selinexor
Lay abstract
The goal of this study was to compare the health-related quality of life (HRQoL) of patients with advanced unresectable dedifferentiated liposarcoma treated with selinexor compared with those treated with placebo. HRQoL was measured prior to treatment initiation and at the first day of each cycle of their treatment using the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire. Pain scores worsened for placebo compared with selinexor across all visits after treatment, but differences at some visits were not significant. Other domains did not exhibit significant differences between arms; however, scores in both arms worsened over time reflecting the progressive disease burden in this patient population. As pain is one of the most devastating symptoms associated with advanced and progressing cancers, the significant reduction in pain in the selinexor arm, according to patient perception, represent a relevant added value of this drug in dedifferentiated liposarcoma.
As one of the most common soft-tissue sarcomas (STS) in adults, liposarcomas represent 24 and 45% of extremity and retroperitoneal sarcomas, respectively [1]. Among liposarcomas, the well-differentiated liposarcoma (WDLS)/dedifferentiated liposarcoma (DDLPS) subgroup is the most common, with an incidence of approximately 1000 new cases per year in the USA [2]. It was originally believed that DDLPS was a higher-grade tumor progression from WDLS; however, DDLPS has more recently been understood to have evolved from a common precursor to WDLS, harboring 12q amplification [3].
Localized STS is treated with surgery; however, recurrence is common and systemic treatment options are limited [4,5]. For advanced STS, anthracyclines are considered the standard front-line therapy, with doxorubicin being the most commonly prescribed. While response rates exceeding 20% with doxorubicin alone or in combination with ifosfamide have been reported, median survival in patients with metastatic STS has not improved beyond 20.5 months [6,7]. Subsequent lines of therapy for advanced/metastatic disease include eribulin and trabectedin with median progression-free survival (PFS) of 2.0 and 2.2 months, respectively [8–10]. Thus, current treatment options for DDLPS are limited to parenteral, cytotoxic chemotherapeutic agents, and off-label CDK-4 and -6 inhibitors, presenting an unmet clinical need for more therapeutic options [11,12].
The impact of STS and its treatment on health-related quality of life (HRQoL) can be substantial. Patients receiving treatment commonly report issues with pain, role and social functioning, fatigue, insomnia, loss of appetite, anxiety and depression [13]. A study evaluating the impact of preradiation and postradiation and surgery outcomes among patients with localized extremity STS reported that the magnitude of surgery-related impairment explained 54% of the decline in HRQoL while the ability to participate in activities with friends and family explained 61% of the variation in HRQoL [14]. Another study in the metastatic setting, using the European Organization for Research and Treatment of Cancer 30-item core quality of life (QoL) questionnaire (EORTC QLQ-C30) indicated that disease progression was associated with a 30-point decline in Global Health Status [15]. Understanding patient reported outcomes (PROs) and HRQoL in this population of patients with advanced, incurable disease is critical and has become an increasingly important end point in clinical trials as they provide information on the impact of a disease and its treatment from the perspective of the patient [5,16]. Additionally, the US FDA recognizes HRQoL as a meaningful primary outcome in cancer clinical trials [17]. This study focuses on the HRQoL impact of selinexor, a selective inhibitor of the nuclear export protein exportin 1 in the treatment of advanced and metastatic DDLPS in patients who have experienced disease progression while on at least two prior lines of systemic therapy.
Exportin 1 (XPO1 and CRM1) is one of seven encoders of nuclear export receptors responsible for the transport of many proteins and RNA species; overexpression or mutation of XPO1 has been shown to function as an oncogenic driver [18]. The majority of tumor suppressor proteins are exported from the nucleus solely by XPO1; this results in functional inactivation of their anticancer regulatory functions [19–22]. Selinexor is a potent, oral, first-in-class selective inhibitor of nuclear export (SINE) compound that enhances nuclear sequestration of tumor suppressor proteins, growth regulators and messenger RNAs of oncogenic proteins by blocking XPO1 [23–25]. Currently approved in previously treated multiple myeloma and diffuse large B-cell lymphoma [23,26,27], selinexor is being investigated across a broad range of solid tumors. In a Phase I clinical study, oral selinexor demonstrated antitumor activity in patients with DDLPS, reducing target lesion size in 40% of patients, with 47% of patients experiencing stable disease for 4 months or longer. Activity in heavily pretreated leiomyosarcoma and other STS was also observed [28].
The Selinexor In Advanced Liposarcoma (SEAL) trial (ClinicalTrials.gov: NCT02606461) is a Phase II/III, multicenter, randomized, double-blinded, placebo-controlled study initiated to assess the efficacy, safety and HRQoL of patients with advanced unresectable DDLPS treated with either selinexor or placebo. Patients with advanced and metastatic DDLPS that experienced disease progression after at least two prior lines of systemic therapy were enrolled in May 2017 through September 2020 at 70 sites in North America, Europe and Israel. In the Phase III portion of the study, the focus of this manuscript, eligible patients were randomly assigned in a 2:1 ratio to treatment with selinexor or placebo. Patients with other subtypes of liposarcoma or with known CNS metastases were excluded. Patients were administered selinexor (60 mg) or matching placebo in a blinded fashion twice weekly in 6-week cycles. Patients were stratified by prior eribulin or trabectedin use, and the number of prior system therapies (2 vs ≥3). Treatment was administered until disease progression, discontinuation or unacceptable toxic effects. Following objectively confirmed progression, crossover from the placebo arm to open label selinexor was permitted [29].
The primary objective of the SEAL trial was to compare PFS based on the Independent Review Committee’s disease outcome assessments in patients randomized to the selinexor arm versus the placebo arm. The selinexor regimen conferred a 30% increase in median PFS (hazard ratio [HR]: 0.70; median 2.83 vs 2.07; p = 0.0228) [29]. This trial was one of the largest global Phase III trials in patients with relapsed DDLPS and showed a significantly improved PFS with twice-weekly oral selinexor at 60 mg. The most common side effects, primarily nausea, anorexia and fatigue, were reversible and could be mitigated with standard supportive care; cytopenia was uncommon [30]. In this study, the secondary end point of HRQoL outcomes, as measured by the EORTC QLQ-C30, were compared between patients randomized with the selinexor arm versus the placebo arm.
Materials & methods
Patient population
The patient-reported outcome population (PRP) was a subset of the per-protocol population of the Phase III portion of the trial (NCT02606461) and included those who completed the EORTC QLQ-C30 at baseline. All analyses were based on the PRP.
PRO assessments
At baseline and day 1 of each cycle, the EORTC QLQ-C30 questionnaire was assessed. The EORTC QLQ-C30 contains 30 questions and includes five functional scales (physical, role, emotional, social and cognitive functioning), three symptom scales (fatigue, nausea/vomiting and pain), a Global Health Status/QoL scale and six single-item symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). Scores range from 0 to 100 and higher scores on the functional scales indicate better functioning, while higher scores on the symptom scales indicate more severe symptoms [31]. For this study, prespecified analyses focused on physical functioning, role functioning, pain and global health/QoL.
Statistical analyses
Quality of completion of each PRO assessment measure was described at each visit by treatment arm. For PRO assessments collected at the visits, the number and percentage of patients who completed all questions were reported. The denominator of the percentage was the number of patients who remained in the study at each assessment. Due to attrition loss, results were reported from change in baseline through day 169 for selinexor versus placebo. By day 211, there was an 85% drop and less than 30 patients in the selinexor arm and a 93% drop and less than ten patients in the placebo arm that completed a PRO questionnaire. Therefore, statistical analyses were unable to be performed past day 169 due to inadequate sample size.
Mixed-effect model repeated measures
Mixed effects models for repeated measures (MMRM) were fit to the longitudinal data to estimate differences in change from baseline on the respective PRO domains in both adjusted and unadjusted models. Likelihood-based mixed-effect models are a principled approach for handling missing at random data, using all available observations and yielding unbiased estimates by assuming missing data follow the same distribution as the observed data, conditional on observed data [32]. The adjusted MMRM model included treatment, visit, baseline PRO score and a randomization stratification variable. All mixed models included a fixed-effect interaction term between visit and treatment arm and a random effect for patient. Restricted Maximum Likelihood estimation was applied as the primary method.
Responder analysis
Using literature-based meaningful change thresholds (MCTs) for the EORTC QLQ-C30 domains, at each postbaseline visit, results were reported for the overall population and by treatment arm [33–36]. Meaningful change status for each scale of interest in the direction of the PRO end point were defined as follows: if the score improved from baseline by at least one MCT, the patient was categorized at this cycle as ‘improved’; if the change from baseline was within ±1 MCT, the patient was categorized at this cycle as ‘stable’; and if the score worsened from baseline by at least one MCT, the patient was categorized at this cycle as ‘worsened’.
For the EORTC QLQ-C30, Osoba et al. estimated important differences in patients with breast and small-cell lung cancer as 5–10 points for a small effect and 10–20 points for a moderate effect [33]. Kemmler et al. recommended a threshold of 20 points for classifying individual patient change based on findings that thresholds of 5–10 points are too low when focusing on the individual patient [34]. A recent evaluation of quality of life in patients with STS used a threshold of 10 points for MCT [35]. Based on this range of recommendations, an intermediate value of 10 points was selected as the MCT for all EORTC QLQ-C30 scales for the following analyses [36]. Chi-squared tests were performed to test for differences between the treatment arms at postbaseline visits.
Time to definitive deterioration
Time to definitive deterioration (TDD) was defined as the time from randomization to the first occurrence of meaningful deterioration (worsened by at least one MCT) that was not followed by subsequent improvement during the blinded treatment. For patients without a meaningful deterioration, TDD was censored at the time of the last PRO assessment. For patients without a postbaseline PRO assessment, TDD was censored at randomization. Median TDD and its 95% CI were summarized using the Kaplan–Meier method for each treatment arm. Cox proportional hazard models compared the hazard rates between arms adjusted for the stratification variable as the covariate.
Post hoc exploratory analysis
Analyses to examine the association between baseline HRQoL scores and PFS and overall survival (OS) were performed using Kaplan–Meier techniques and Cox proportional hazards regression. The exploratory analyses were stratified by treatment arm.
Results
Quality of completion
A total of 277 patients were enrolled in the Phase III portion of the trial; 255 (92.1%) completed a baseline QLQ-C30 assessment and were included in these analyses: 168 (65.6%) patients in the selinexor arm and 88 (34.4%) patients in the placebo arm. Overall, completion rates for all items for the EORTC QLQ-C30 in the PRP were generally greater than 95% across most time points in both treatment arms (Table 1). Specifically, at baseline, day 43, day 85, day 127, day 169, day 211 and day 253, the percentage of patients who completed select domains for the EORTC-QLQ-C30 (pain, physical function, role function and Global Health/QoL) ranged from 95.0 to 100.0% in the selinexor arm and 83.3 to 100.0% in the placebo arm.
Table 1. . Quality of completion of the European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire.
| Time point | Domain | Completed all items n (%) | |||
|---|---|---|---|---|---|
| n | Selinexor (n = 168) | n | Placebo (n = 88) | ||
| Baseline | Pain | 168 | 167 (99.4%) | 88 | 88 (100.0%) |
| Physical function | 168 | 166 (98.8%) | 88 | 87 (98.9%) | |
| Role function | 168 | 167 (99.4%) | 88 | 88 (100.0%) | |
| Global Health/QoL | 168 | 168 (100.0%) | 88 | 88 (100.0%) | |
| Day 43 | Pain | 144 | 142 (98.6%) | 71 | 69 (97.2%) |
| Physical function | 144 | 142 (98.6%) | 71 | 70 (98.6%) | |
| Role function | 144 | 144 (100.0%) | 71 | 70 (98.6%) | |
| Global Health/QoL | 144 | 140 (97.25) | 71 | 71 (100.0%) | |
| Day 85 | Pain | 94 | 94 (100.0%) | 33 | 33 (100.0%) |
| Physical function | 94 | 92 (97.9%) | 33 | 32 (97.0%) | |
| Role function | 94 | 94 (100.0%) | 33 | 33 (100.0%) | |
| Global Health/QoL | 94 | 94 (100.0%) | 33 | 33 (100.0%) | |
| Day 127 | Pain | 53 | 53 (100.0%) | 17 | 17 (100.0%) |
| Physical function | 53 | 52 (98.1%) | 17 | 17 (100.0%) | |
| Role function | 53 | 53 (100.0%) | 17 | 17 (100.0%) | |
| Global Health/QoL | 53 | 53 (100.0%) | 17 | 17 (100.0%) | |
| Day 169 | Pain | 40 | 40 (100.0%) | 11 | 11 (100.0%) |
| Physical function | 40 | 39 (97.5%) | 11 | 11 (100.0%) | |
| Role function | 40 | 40 (100.0%) | 11 | 11 (100.0%) | |
| Global Health/QoL | 40 | 40 (100.0%) | 11 | 11 (100.0%) | |
| Day 211 | Pain | 26 | 26 (100.0%) | 6 | 6 (100.0%) |
| Physical function | 26 | 25 (96.2%) | 6 | 6 (100.0%) | |
| Role function | 26 | 26 (100.0%) | 6 | 6 (100.0%) | |
| Global Health/QoL | 26 | 26 (100.0%) | 6 | 5 (83.3%) | |
| Day 253 | Pain | 20 | 20 (100.0%) | 4 | 4 (100.0%) |
| Physical function | 20 | 19 (95.0%) | 4 | 4 (100.0%) | |
| Role function | 20 | 20 (100.0%) | 4 | 4 (100.0%) | |
| Global Health/QoL | 20 | 20 (100.0%) | 4 | 4 (100.0%) | |
EORTC QLQ-C30: European Organization for research and treatment of cancer quality of life cancer-30 item questionnaire; QoL: Quality of life.
Baseline patient characteristics
Age, sex, race and ethnicity was balanced between the two arms (Table 2). The median age was 65 years (range: 33–84). Overall, a higher percentage of males versus females (total: 62.1 vs 37.9%, respectively) were enrolled. Most patients were White (77.7%) and 4.7% were Hispanic or Latino. The majority of patients reported received at least one concomitant pain or steroid medication (75.4%) at any point during the study period. The proportion of patients treated with a concomitant steroid medication was higher in the selinexor arm versus placebo (43.5 vs 19.3%, respectively). Among the 43.5% in the selinexor arm that received a concomitant steroid medication, 71.2% consisted of low dose (2–4 mg) dexamethasone. Approximately 60.5% of patients had an Eastern Cooperative Oncology Group score of 1. At baseline, pain scores were significantly higher in the selinexor arm compared with the placebo arm (28.27 vs 20.27; p = 0.0264) (Table 3). Median baseline tumor burden (defined as the sum of the longest diameter among target lesions per RECIST v1.1) [37] was 156.2 mm in diameter (range: 20.7–486.7) with no significant difference between treatment arms.
Table 2. . Baseline patient characteristics (patient-reported outcome population).
| Selinexor arm (n = 168) | Placebo arm (n = 88) | Total (n = 256) | |
|---|---|---|---|
| Age (years) † | |||
| n | 168 | 88 | 256 |
| Median | 64.5 | 65.0 | 65.0 |
| Min, Max | 33, 84 | 31, 85 | 31, 85 |
| Age category n (%) † | |||
| 18–50 | 17 (10.1) | 11 (12.5) | 28 (10.9) |
| 51–64 | 67 (39.9) | 32 (36.4) | 99 (38.7) |
| 65–74 | 62 (36.9) | 36 (40.9) | 98 (38.3) |
| ≥75 | 22 (13.1) | 9 (10.2) | 31 (12.1) |
| Sex n (%) | |||
| Male | 102 (60.7) | 57 (64.8) | 159 (62.1) |
| Female | 66 (39.3) | 31 (35.2) | 97 (37.9) |
| Race n (%) | |||
| Asian | 8 (4.8) | 2 (2.3) | 10 (3.9) |
| Black or African–American | 3 (1.8) | 1 (1.1) | 4 (1.6) |
| Native Hawaiian or other Pacific islander | 2 (1.2) | 0 | 2 (0.8) |
| White | 126 (75.0) | 73 (83.0) | 199 (77.7) |
| Other | 28 (16.7) | 12 (13.6) | 40 (15.6) |
| Missing | 1 (0.6) | 0 | 1 (0.4) |
| Ethnicity n (%) | |||
| Hispanic or Latino | 6 (3.6) | 6 (6.8) | 12 (4.7) |
| Not Hispanic or Latino | 136 (81.0) | 71 (80.7) | 207 (80.9) |
| Not reported | 21 (12.5) | 8 (9.1) | 29 (11.3) |
| Unknown | 5 (3.0) | 3 (3.4) | 8 (3.1) |
| Geographic region n (%) | |||
| North America | 86 (51.2) | 51 (58.0) | 137 (53.5) |
| Europe and Israel | 82 (48.8) | 37 (42.0) | 119 (46.5) |
| Patients with at least one concomitant pain or steroid medication, n (%) | 128 (76.2%) | 65 (73.9%) | 193 (75.4%) |
| Pain | 112 (66.7%) | 61 (69.2%) | 173 (67.6%) |
| Steroid | 73 (43.5%) | 17 (19.3%) | 90 (35.2%) |
| Baseline tumor burden (mm) | |||
| n | 161 | 84 | 245 |
| Median | 153.8 | 160.8 | 155.2 |
| Min, Max | 20.7, 486.7 | 30.7, 473.6 | 20.7, 486.7 |
| ECOG performance status, n (%) ‡ | |||
| 0 | 64 (38.1%) | 37 (42.0%) | 101 (39.5%) |
| 1 | 104 (61.9%) | 51 (58.0%) | 155 (60.5%) |
Age is the age at date of randomization.
ECOG performance-status scores range from 0 to 5, with higher scores reflecting greater disability.
ECOG: Eastern Cooperative Oncology Group; Max: Maximum; min: Minimum.
Table 3. . Model-based change from baseline on European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire.† .
| Domain | Least square adjusted mean change (standard error) | Selinexor vs placebo | ||
|---|---|---|---|---|
| Selinexor (n = 168) | Placebo (n = 88) | Difference (95% CI) | p-value | |
| QLQ-C30 | ||||
| Baseline | ||||
| – n | 168 | 88 | – | – |
| – Pain | 28.27 (2.13) | 20.27 (2.94) | 8.11 (0.99 to 15.22) | 0.0264 |
| – Physical function | 75.55 (1.66) | 77.01 (2.29) | -1.46 (-7.01 to 4.09) | 0.6070 |
| – Role function | 69.96 (2.26) | 76.70 (3.11) | -6.74 (-14.27 to 0.78) | 0.0801 |
| – Global Health/QoL | 63.19 (1.70) | 66.19 (2.35) | -3.00 (-8.68 to 2.68) | 0.3017 |
| Day 43 | ||||
| – n | 144 | 71 | – | – |
| – Pain | 1.35 (2.44) | 8.05 (3.11) | -6.70 (-13.69 to 0.29) | 0.0603 |
| – Physical function | -8.19 (1.79) | -1.94 (2.30) | -6.25 (-11.40 to -1.09) | 0.0177 |
| – Role function | -15.58 (2.56) | -6.46 (3.25) | -9.12 (-16.47 to -1.77) | 0.0152 |
| – Global Health/QoL | -7.67 (1.94) | -2.14 (2.46) | -5.53 (-11.05 to -0.01) | 0.0495 |
| Day 85 | ||||
| – n | 94 | 33 | – | – |
| – Pain | 3.17 (2.70) | 9.44 (3.98) | -6.26 (-15.10 to 2.57) | 0.1641 |
| – Physical function | -11.73 (2.00) | -3.90 (2.96) | -7.83 (-14.38 to -1.29) | 0.0192 |
| – Role function | -15.17 (2.91) | -6.38 (4.37) | -8.79 (-18.53 to 0.95) | 0.0767 |
| – Global Health/QoL | -8.71 (2.14) | -6.60 (3.15) | -2.10 (-9.08 to 4.87) | 0.5534 |
| Day 127 | ||||
| – n | 53 | 17 | – | – |
| – Pain | 3.98 (3.13) | 16.16 (5.04) | -12.18 (-23.31 to -1.06) | 0.0320 |
| – Physical function | -14.73 (2.34) | -8.61 (3.77) | -6.11 (-14.42 to 2.20) | 0.1490 |
| – Role function | -20.26 (3.49) | -11.20 (5.75) | -9.06 (-21.77 to 3.64) | 0.1616 |
| – Global Health/QoL | -12.46 (2.48) | -6.54 (3.99) | -5.91 (-14.71 to 2.88) | 0.1869 |
| Day 169 | ||||
| – n | 40 | 11 | – | – |
| – Pain | 6.76 (3.436) | 20.99 (5.883) | -14.24 (-27.15 to -1.32) | 0.0308 |
| – Physical function | -11.94 (2.581) | -3.92 (4.405) | -8.02 (-17.70 to 1.66) | 0.1039 |
| – Role function | -14.63 (3.906) | -14.28 (6.821) | -0.35 (-15.35 to 14.65) | 0.9633 |
| – Global Health/QoL | -9.16 (2.726) | -8.17 (4.652) | -0.99 (-11.20 to 9.22) | 0.8489 |
A positive increase in pain indicates worsening of symptoms. A positive increase in function scores and global health/QoL indicate improvement of symptoms.
EORTC QLQ-C30: European Organization for research and treatment of cancer quality of life cancer-30 item questionnaire; QoL: Quality of life.
MMRM analysis
Overall, pain scores worsened in the placebo arm compared with the selinexor arm across all postbaseline visits, though some visits (day 43 and 85) were not statistically significant (Table 3 and Figure 1). By day 127, average pain scores significantly worsened by a mean 16.16 points in the placebo arm compared with 3.98 points in the selinexor arm, where a mean -12.18 difference was observed, in favor of the selinexor arm (95% CI: -23.31 to -1.06; p = 0.0320). By day 169, pain scores significantly worsened in the placebo arm compared with the selinexor arm, where a mean -14.24 difference was observed, in favor of the selinexor arm (95% CI: -27.15 to -1.32; p = 0.0308). At day 43, physical function (-6.25; 95% CI: -11.40 to -1.09; p = 0.0177), role function (-9.12; 95% CI: -16.47 to -1.77; p = 0.0152) and Global Health/QoL (-5.53; 95% CI: -11.05 to -0.01; p = 0.0495) significantly worsened for selinexor compared with placebo. This significant trend was observed at day 85 for physical function in favor of placebo (-7.83; 95% CI: -14.38 to -1.29; p = 0.0192). While scores gradually deteriorated across both groups, no significant differences were observed across treatment arms beginning at day 127 for physical function, day 85 for role function and day 85 for Global Health/QoL.
Figure 1. . Model-based change from baseline on European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire.
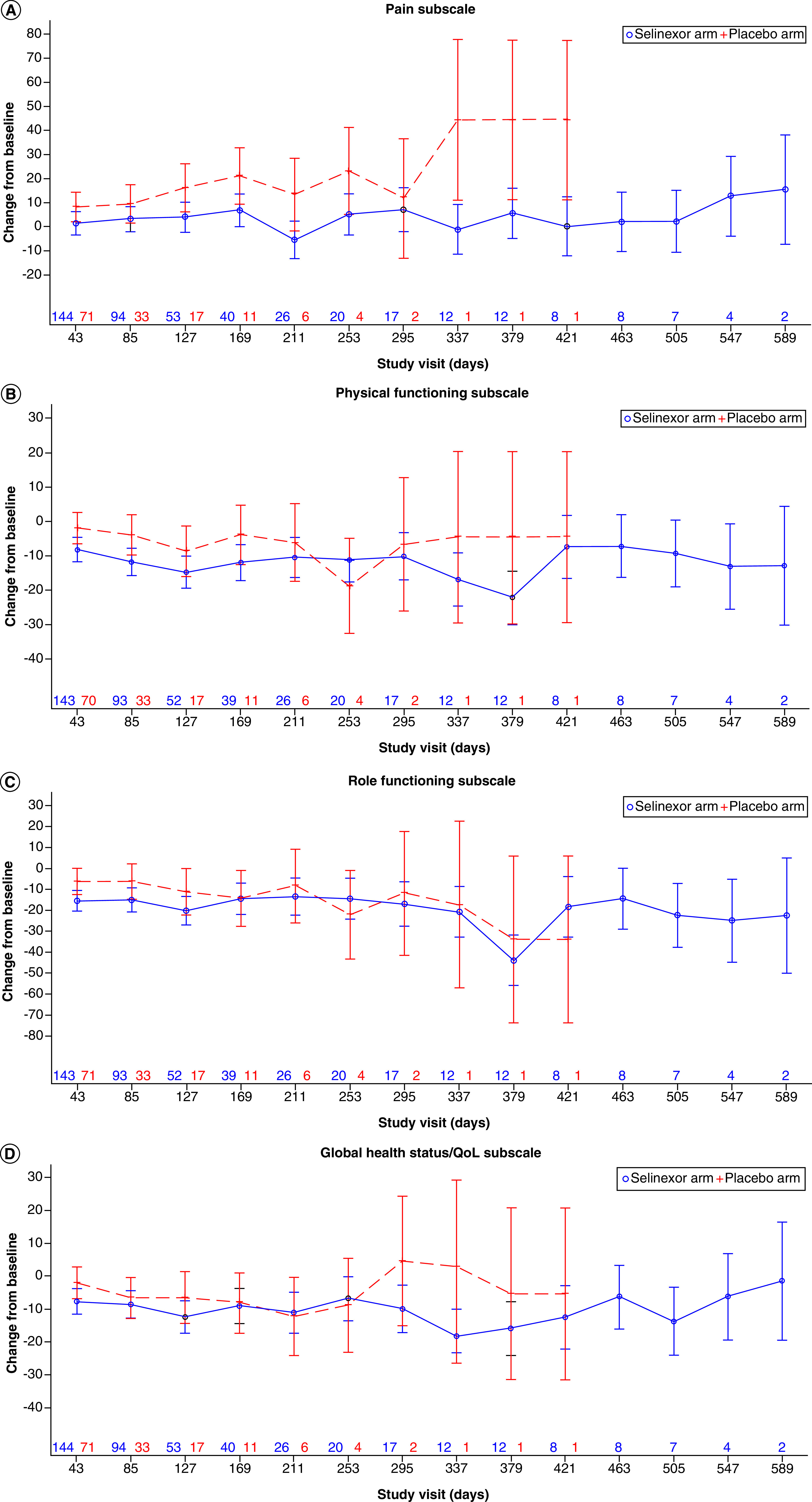
QoL: Quality of life.
Responder analysis
For the pain domain, the percentage of selinexor patients who experienced worsening of pain was lower compared with the placebo arm (27.1 vs 40.8%, respectively) and the percentage of selinexor patients who experienced an improvement in pain was higher than in the placebo arm (28.5 vs 15.5%, respectively) at day 43 (p = 0.0458) (Table 4). These trends were observed for day 85, 127 and 169, although they were not statistically significant. The percentage of selinexor patients who experienced worsening of symptoms was higher than in the placebo arm for physical function at day 43 (36.4 vs 15.7%; p = 0.0070). However, results became nonsignificant after day 85 (p = 0.0532 and 0.0587 for pain and physical functioning, respectively). Although not considered significant, the percentage of patients who did not experience a deterioration in pain (stable or improved) was higher in the selinexor arm compared with the placebo arm for pain (75.0 vs 63.6%, respectively; p = 0.4835), role function (61.6 vs 36.4%, respectively; p = 0.1508) and Global Health/QoL (67.5 vs 63.6%, respectively; p = 0.8759) but lower for physical function (69.2 vs 81.8%, respectively; p = 0.6453) at day 169.
Table 4. . Categorical change from baseline to day 169 by treatment arm for European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire scores.
| Domain | Status | Selinexor | Placebo | p-value |
|---|---|---|---|---|
| EORTC QLQ-C30 pain | ||||
| Day 43 | n | 144 | 71 | 0.0458 |
| Worsened | 39 (27.1%) | 29 (40.8%) | ||
| Stable | 64 (44.4%) | 31 (43.7%) | ||
| Improved | 41 (28.5%) | 11 (15.5%) | ||
| Day 85 | n | 94 | 33 | 0.0532 |
| Worsened | 28 (29.8%) | 16 (48.5%) | ||
| Stable | 41 (43.6%) | 14 (42.4%) | ||
| Improved | 25 (26.6%) | 3 (9.1%) | ||
| Day 127 | n | 53 | 17 | 0.2926 |
| Worsened | 17 (32.1%) | 6 (35.3%) | ||
| Stable | 20 (37.7%) | 9 (52.9%) | ||
| Improved | 16 (30.2%) | 2 (11.8%) | ||
| Day 169 | n | 40 | 11 | 0.4835 |
| Worsened | 10 (25.0%) | 4 (36.4%) | ||
| Stable | 20 (50.0%) | 6 (54.5%) | ||
| Improved | 10 (25.0%) | 1 (9.1%) | ||
| EORTC QLQ-C30 physical function | ||||
| Day 43 | n | 143 | 70 | 0.0070 |
| Worsened | 52 (36.4%) | 11 (15.7%) | ||
| Stable | 77 (53.8%) | 48 (68.6%) | ||
| Improved | 14 (9.8%) | 11 (15.7%) | ||
| Day 85 | n | 93 | 33 | 0.0587 |
| Worsened | 33 (35.5%) | 7 (21.2%) | ||
| Stable | 53 (57.0%) | 19 (57.6%) | ||
| Improved | 7 (7.5%) | 7 (21.2%) | ||
| Day 127 | n | 52 | 17 | 0.1973 |
| Worsened | 26 (50.0%) | 5 (29.4%) | ||
| Stable | 21 (40.4%) | 8 (47.1%) | ||
| Improved | 5 (9.6%) | 4 (23.5%) | ||
| Day 169 | n | 39 | 11 | 0.6453 |
| Worsened | 12 (30.8%) | 2 (18.2%) | ||
| Stable | 19 (48.7%) | 7 (63.6%) | ||
| Improved | 8 (20.5%) | 2 (18.2%) | ||
| EORTC QLQ-C30 role function | ||||
| Day 43 | n | 143 | 71 | 0.7417 |
| Worsened | 64 (44.8%) | 28 (39.4%) | ||
| Stable | 53 (37.1%) | 28 (39.4%) | ||
| Improved | 26 (18.2%) | 15 (21.1%) | ||
| Day 85 | n | 93 | 33 | 0.0352 |
| Worsened | 45 (48.4%) | 14 (42.4%) | ||
| Stable | 22 (23.7%) | 15 (45.5%) | ||
| Improved | 26 (28.0%) | 4 (12.1%) | ||
| Day 127 | n | 52 | 17 | 0.0298 |
| Worsened | 30 (57.7%) | 5(29.4%) | ||
| Stable | 15 (28.8%) | 11 (64.7%) | ||
| Improved | 7 (13.5%) | 1 (5.9%) | ||
| Day 169 | n | 39 | 11 | 0.1508 |
| Worsened | 15 (38.5%) | 7 (63.6%) | ||
| Stable | 15 (38.5%) | 4 (36.4%) | ||
| Improved | 9 (23.1%) | 0 (0.0%) | ||
| EORTC QLQ-C30 Global Health/QoL | ||||
| Day 43 | n | 140 | 71 | 0.1266 |
| Worsened | 57 (40.7%) | 21 (29.6%) | ||
| Stable | 66 (47.1%) | 35 (49.3%) | ||
| Improved | 17 (12.1%) | 15 (21.1%) | ||
| Day 85 | n | 94 | 33 | 0.9194 |
| Worsened | 39 (41.5%) | 13 (39.4%) | ||
| Stable | 39 (41.5%) | 15 (45.5%) | ||
| Improved | 16 (17.0%) | 5 (15.2%) | ||
| Day 127 | n | 53 | 17 | 0.3194 |
| Worsened | 22 (41.5%) | 6 (35.3%) | ||
| Stable | 26 (49.1%) | 7 (41.2%) | ||
| Improved | 5 (9.4%) | 4 (23.5%) | ||
| Day 169 | n | 40 | 11 | 0.8759 |
| Worsened | 13 (32.5%) | 4 (36.4%) | ||
| Stable | 21 (52.5%) | 6 (54.5%) | ||
| Improved | 6 (15.0%) | 1 (9.1%) |
MCT values: QLQ-C30 functioning and pain = 10. Improved was defined as an improvement in score of at least MCT value, stable was defined as a change within ± MCT value and worsening was defined as a worsening in score of at least MCT value.
p-value was for Wald chi-square test.
EORTC QLQ-C30: European Organization for research and treatment of cancer quality of life cancer-30 item questionnaire; MCT: Meaningful change threshold; QoL: Quality of life.
Time to definitive deterioration
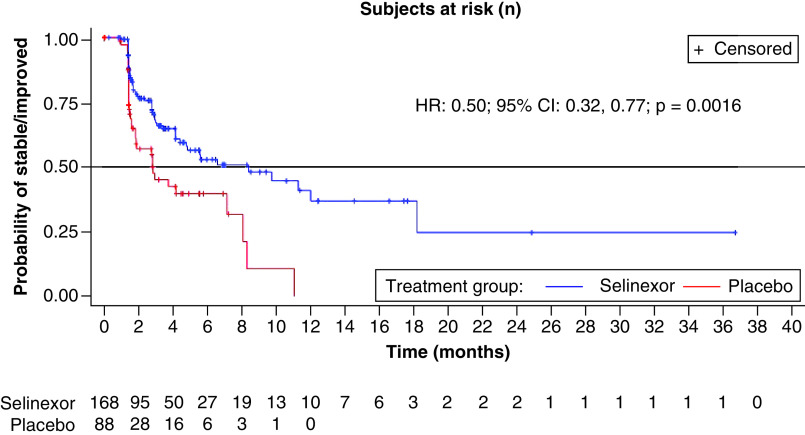
The number of patients with definitive deterioration in QLQ-C30 pain scores was greater in the placebo arm (38 patients, 43.2%) compared with the selinexor arm (58 patients, 34.5%) (Table 5). The median TDD was 5.5 months longer in the selinexor arm compared with the placebo arm (8.4 vs 2.9 months). The adjusted HR comparing time with deterioration in pain scores between selinexor and placebo was 0.50 (95% CI: 0.32–0.77; p = 0.0016) in favor of selinexor (Figure 2). There were no significant differences in the TDD for physical function, role function or Global Health/QoL scores.
Table 5. . Time to definitive deterioration on European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire.
| MCT | Selinexor (n = 168) | Placebo (n = 88) | Cox proportional hazards model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Definitive deteriorations, n (%) | Median TDD (95% CI) | n | Definitive deteriorations, n (%) | Median TDD (95% CI) | HR – selinexor/placebo (95% CI) | p-value | ||
| QLQ-C30 | |||||||||
| Pain | 10 | 168 | 58 (34.5%) | 8.4 (4.8–NE) | 88 | 38 (43.2%) | 2.9 (1.8–11.1) | 0.50 (0.32–0.77) | 0.0016 |
| Physical function | 10 | 168 | 76 (45.2%) | 4.2 (2.9–12.0) | 88 | 20 (22.7%) | 8.1 (4.2–NE) | 1.42 (0.85–2.36) | 0.1705 |
| Role function | 10 | 168 | 83 (49.4%) | 4.1 (2.8–9.7) | 88 | 35 (39.8%) | 4.1 (1.9–9.7) | 0.81 (0.53–1.23) | 0.3496 |
| Global Health/QoL | 10 | 168 | 75 (44.6%) | 4.7 (4.1–10.6) | 88 | 29 (33.0%) | 4.2 (2.8–NE) | 0.96 (0.62–1.50) | 0.8764 |
Time to definitive deterioration units = months.
EORTC QLQ-C30: European Organization for research and treatment of cancer quality of life cancer-30 item; HR: Hazard ratio; MCT: Meaningful change threshold; NE: Not estimable; QoL: Quality of life; TDD: Time to definitive deterioration.
Figure 2. . Time to definitive deterioration on European Organization for Research and Treatment of Cancer quality of life pain score.
Exploratory analysis
Patients treated with selinexor had significantly longer PFS compared with placebo across all domains despite health status (≥50 or <50 for pain and ≤50 or >50 for physical function, role function and Global Health/QoL) and no difference in tumor burden at baseline (Table 6). In the context of crossover, where many of the patients on placebo did cross over to selinexor, there was no significant differences observed for OS.
Table 6. . Association of progression-free survival and overall survival on European Organization for Research and Treatment of Cancer quality of life-30 item questionnaire scores at baseline.
| Selinexor | Placebo | Cox proportional hazards model | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients (n) | Observed events, n | Median duration, months | Patients (n) | Observed events, n | Median duration, months | HR – selinexor/placebo (95% CI) | p-value | |
| PFS | ||||||||
| Baseline pain score | ||||||||
| – ≥50 | 45 | 32 (71.1%) | 2.83 | 15 | 12 (80.0%) | 1.45 | 0.46 (0.23–0.91) | 0.0236 |
| – <50 | 123 | 90 (73.2%) | 2.89 | 73 | 57 (78.1%) | 2.07 | 0.62 (0.44–0.87) | 0.0053 |
| Baseline physical function score | ||||||||
| – ≤50 | 21 | 13 (61.9%) | 2.61 | 9 | 8 (88.9%) | 1.33 | 0.34 (0.14–0.84) | 0.0139 |
| – >50 | 146 | 108 (74.0%) | 2.86 | 79 | 61 (77.2%) | 1.87 | 0.63 (0.46–0.87) | 0.0050 |
| Baseline role function score | ||||||||
| – ≤50 | 49 | 34 (69.4%) | 2.76 | 19 | 16 (84.2%) | 1.41 | 0.45 (0.24–0.83) | 0.0092 |
| – >50 | 118 | 87 (73.7%) | 2.89 | 69 | 53 (76.8%) | 2.10 | 0.63 (0.45–0.90) | 0.0100 |
| Baseline Global Health/QoL score | ||||||||
| – ≤50 | 62 | 47 (75.8%) | 2.79 | 25 | 20 (80.0%) | 1.45 | 0.57 (0.33–0.97) | 0.0369 |
| – >50 | 106 | 31 (29.2%) | 4.11 | 63 | 49 (77.8%) | 1.87 | 0.59 (0.41–0.85) | 0.0045 |
| OS | ||||||||
| Baseline pain score | ||||||||
| – ≥50 | 45 | 30 (66.7%) | 7.59 | 15 | 9 (60.0%) | 9.17 | 1.09 (0.51–2.30) | 0.8262 |
| – <50 | 123 | 65 (52.8%) | 11.63 | 73 | 43 (58.9%) | 11.66 | 0.77 (0.52–1.14) | 0.1956 |
| Baseline physical function score | ||||||||
| – ≤50 | 21 | 13 (61.9%) | 6.05 | 9 | 6 (66.7%) | 4.47 | 0.75 (0.28–2.02) | 0.5835 |
| – >50 | 146 | 82 (56.2%) | 11.07 | 79 | 46 (58.2%) | 12.71 | 0.89 (0.62–1.28) | 0.5196 |
| Baseline role function score | ||||||||
| – ≤50 | 49 | 33 (67.3%) | 7.56 | 19 | 14 (73.7%) | 4.47 | 0.65 (0.35–1.23) | 0.1845 |
| – >50 | 118 | 62 (52.5%) | 11.63 | 69 | 38 (55.1%) | 13.86 | 0.93 (0.62–1.40) | 0.7343 |
| Baseline Global Health/QoL score | ||||||||
| – ≤50 | 62 | 44 (71.0%) | 9.10 | 25 | 19 (76.0%) | 4.70 | 0.68 (0.39–1.17) | 0.1565 |
| – >50 | 106 | 51 (48.1%) | 11.60 | 63 | 33 (52.4%) | 14.69 | 0.89 (0.57–1.38) | 0.6055 |
EORTC QLQ-C30: European Organization for research and treatment of cancer quality of life cancer-30 item; HR: Hazard ratio; PFS: Progression-free survival; QoL: Quality of life.
Discussion
Liposarcomas are rare malignancies with substantial heterogeneity and these differences are exacerbated by prior surgeries, cytotoxic chemotherapies and radiation. These factors pose profound challenges in clinical practice and confound our understanding of the patient experience. To better understand the experience of patients with advanced DDLPS, this study aimed to assess changes in HRQoL between the selinexor and placebo arms. The underlying hypothesis for this study was that first-in-class oral selinexor provides equivalent (or better) PROs in HRQoL domains when compared with placebo in the context of improving PFS and tumor shrinkage. These analyses focused on the pain, physical functioning, role function and Global Health/QoL scores from the EORTC QLQ-C30 from baseline to day 169 (the time point where exposure to treatment was maximized while maintaining adequate sample size across both arms).
Overall, pain worsened in the placebo arm compared with the selinexor arm across all postbaseline visits, despite higher average baseline pain scores in the selinexor arm. When examining mean scores over time, patients who received twice weekly selinexor reported lower rates and slower worsening of pain compared with patients treated with placebo. This is consistent with the reported reduction in tumor size in 27.1% of patients treated with selinexor versus 15.5% treated with placebo [29]. Physical function, role function and Global Health/QoL initially worsened in the selinexor arm but became similar to placebo at later time points; the initial worsening may potentially be related to initial side effects of active therapy. Together, the results show that selinexor significantly reduced pain, a relevant symptom in patients with heavily pretreated, progressive DDLPS. Selinexor also prolonged the time to marked clinical deterioration of pain. All of this is consistent with the prolonged PFS and tumor shrinkage observed in the selinexor arm compared with the placebo arm and indicate that the radiographic changes in DDLPS were accompanied by meaningful clinical benefits.
The trial permitted patients treated with placebo with Independent Review Committee-approved objective progression of their tumors to cross over to open label selinexor, and a substantial number of patients (58.8%) did cross over. In this context, the OS in the selinexor arm was noninferior to placebo (HR = 1.0039) [29].
Baseline HRQoL scores can act as a universal prognostic factor across many different types of cancer for both PFS and OS [38–40]. The prognostic value of the baseline EORTC QLQ-C30 scores support previous reported findings in that those with poorer HRQoL scores (≥50 for pain and ≤50 for physical function, role function and Global Health/QoL) at baseline had lower PFS compared with those with higher HRQoL scores. Results from our study are consistent with previous findings demonstrating the association of cancer-related pain with poor survival in advanced cancer patients [41]. However, across all baseline scores, patients treated with selinexor had significantly higher PFS compared with placebo despite health status and no difference in tumor burden at baseline, which may potentially be related to effective disease control [29].
Sarcomas can have a detrimental impact on the HRQoL of patients during diagnosis, treatment and follow-up. Compared with the general population, sarcoma patients confer poorer outcomes despite showing improvements in HRQoL during follow-up and specifically reported issues with their role and social functioning, as well as pain, loss of appetite, fatigue and anxiety and depression [13]. In the current study, the differences in the mean values for pain were greater than the published interpretation threshold of 10 points and indicate that it is possible to observe clinically meaningful differences in pain and highlight the worsening pain observed in the placebo arm. Prior studies have evaluated symptom burden associated with treatment among adult patients with STS and found that pain was the most frequent and significant symptom [42–45]. Our results are consistent with the literature in that more than half of all patients in our study utilized some form of pain medication. Although patients in the selinexor arm had baseline levels of pain that were significantly higher than those on placebo, there was no significant differences in the utilization of concomitant pain medication. In addition, while the proportion of patients with concomitant corticosteroid medication was higher in the selinexor arm compared with the placebo arm (43.5 vs 19.3%, respectively), the majority of patients in the selinexor arm that received a concomitant corticosteroid medication were treated with low dose (2–4 mg) dexamethasone (71.2%). Despite this difference, there is conflicting evidence demonstrating steroid efficacy in cancer patients with pain [46,47]. Vecht et al. compared dexamethasone doses of 10 versus 100 mg in 37 patients with spinal cord compression who then received radiotherapy and found no significant differences in respect to pain relief [46]. The WHO GDG found a moderate quality of evidence which indicates that steroids may improve pain relief and QoL; however, it is uncertain whether, in cancer patients, steroids increase risks of gastrointestinal bleeds or psychiatric adverse events [47]. These results from SEAL show that oral selinexor provides an improved patient experience by reducing the worsening of pain in patients with liposarcoma.
There is a paucity of literature assessing the impact of advanced STS on HRQoL, particularly pain, in patients [13,48]. Primary issues in this patient population are invasive surgical procedures and impairments in physical functioning [49]. Previous studies of HRQoL and health state utilities in patients with metastatic STS found declines in both outcomes, particularly among those with progressive disease. Baseline HRQoL scores and change in HRQoL scores reported in this study are consistent with other work in advanced STS [15,35]. In addition, approximately 68% of patients progressed during this study. While HRQoL was assessed at each cycle in our study, future research that evaluates HRQoL preprogression and postprogression in liposarcoma is warranted. Reichardt et al. reported HRQoL and health state utility results among patients with metastatic soft tissue and bone sarcomas (excluding gastrointestinal stromal tumors), who had achieved a favorable response to therapy, and found that those with progressive disease had an average decline of 30 points in their Global Health/QoL status on the EORTC QLQ-C30 and decline of 0.21 utility on the EuroQoL EQ-5D generic health status instrument [15]. Results from our study observed a lower but similar rate of decline in EQ-5D utility (data not presented). Shingler et al. reported that those with progressive disease saw a substantial decline in utility (-0.473) on the EuroQoL EQ-5D generic health status instrument [50]. Using the MD Anderson Symptom Inventory, Jones et al. found that among patients treated with trabectedin, at the end of treatment, pain, fatigue and feeling sad were among the most frequently high-scoring symptoms [51]. They reported declines in HRQoL over time were likely attributed to the underlying disease burden, both emphasized the significant negative impact of disease progression, because patients who developed progressive disease consistently reported lower HRQoL [15,50].
Studies assessing the PROs and HRQoL of patients on treatments often report mixed results. When assessing HRQoL in STS patients treated with pazopanib, patients reported worse diarrhea, appetite loss, nausea/vomiting and fatigue than those treated with placebo; however, there were no statistically significant differences between the two treatment arms in HRQoL [52]. Another study, comparing eribulin and dacarbazine in patients with STS, found that while HRQoL scores worsened from diagnosis to treatment, patients treated with eribulin maintained their HRQoL, while patients treated with dacarbazine reported lower global health and physical functioning scores [35]. However, it is important to note that the eribulin trial included both liposarcoma (31.9%) and leiomyosarcoma (68.1%) while SEAL focused exclusively on patients with advanced and more heavily pretreated DDLPS, one of the most aggressive forms of liposarcoma; therefore, findings may not be directly comparable. Of note, 35.1% of patients in the SEAL study had already received eribulin and 35.8% had received trabectedin. Results from our study showed a general decline across all measures in both treatment arms and support previous work highlighting the importance of the underlying disease burden and the negative impact of disease progression.
The Phase III SEAL study demonstrated enhanced clinical activity and a manageable safety profile in patients with DDLPS compared with placebo [29]. In addition to meeting its primary end point with a statistically significant improvement in PFS, selinexor efficacy was consistent across subgroups, including in patients who were older, had prior eribulin and trabectedin therapy, distant metastases, retroperitoneal location, nonresponse to prior therapy, and were heavily treated with ≥3 lines of prior systemic therapy and ≥3 prior antineoplastic surgeries. Median time to next treatment was significantly longer in patients receiving selinexor (5.75 months [95% CI: 4.83–6.93]) versus those receiving placebo (3.22 months [95% CI: 2.56–4.21]) (HR = 0.4988; p = <0.0001). Compared with other therapies in DDLPS, selinexor provides a novel, oral treatment option in this population with an unmet need and can potentially reduce patient burden [29]. The results presented here confirm that the reduction in tumor growth (as measured by objective radiographic PFS) is accompanied by clinically important reduction in pain, with minimal effects on other aspects of quality of life. As pain is one of the most devastating symptoms associated with advanced and progressing cancers, the significant reduction of pain in the selinexor arm, according to patient perception, represent a relevant added value of this drug in DDLPS. Furthermore, evidence suggests that oncology patients prefer oral treatment to intravenous therapy due to a number of factors, including convenience, perception of efficacy and past experience [53]. These factors may be enhanced during the COVID-19 pandemic [54].
This study had several limitations. Missing data are a common challenge to HRQoL assessments in clinical trials [55]. Compliance in the SEAL trial was relatively good and remained well within acceptable limits to allow the performance of analyses as intended. Although SEAL was the largest study to date in patients exclusively with heavily pretreated DDLPS, due to the relatively small sample size across both groups, results should be interpreted with caution. It is also important to note that currently, there is no disease-specific PRO questionnaire for patients with advanced STS or DDLPS. The EORTC QoL Group uses a module-based approach to questionnaire development, in which the core questionnaire (EORTC QLQ-C30) is often supplemented with disease-, symptom- or treatment-specific modules depending on the therapeutic area. No supplement module exists to date for STS, but a symptom-based module on targeted therapies is in development [56]. Although the QLQ-C30 is one of the most commonly used and validated measures within oncology clinical trials, when applied to this particular study, it is possible that disease- or symptom-based effects within the DDLPS population were lacking.
Conclusion
These findings support that HRQoL is an important secondary end point in this comparison between selinexor and placebo in patients with DDLPS. Overall, HRQoL scores tended to decline over time in both arms, partially reflecting the progressive disease burden in this patient population. Patients treated with placebo, despite initially reporting lower pain at baseline, experienced higher reported pain and a more rapid worsening of pain compared with patients treated with selinexor at day 169. With no treatment options of known clinical benefit in the heavily pretreated population with DDLPS studied in SEAL, selinexor provides an effective, novel, oral therapy option, potentially providing convenience, improved adherence and a reduced caregiver burden compared with existing parenteral therapies.
Summary points.
Liposarcoma is the most common soft-tissue sarcomas in adults, representing 24 and 45% of extremity and retroperitoneal sarcomas, respectively. The impact of soft-tissue sarcomas and its treatment on health-related quality of life (HRQoL) can be substantial.
The selinexor in advanced liposarcoma (SEAL) trial (ClinicalTrials.gov: NCT02606461) is a Phase II/III, multicenter, randomized, double-blinded, placebo-controlled study initiated to assess the efficacy, safety and HRQoL of patients with advanced and metastatic unresectable dedifferentiated liposarcoma (DDLPS) treated with either selinexor, a selective inhibitor of the nuclear export protein exportin 1, or placebo.
The primary end point in SEAL was progression-free survival, which significantly improved in the selinexor versus placebo arm (hazard ratio = 0.70; p = 0.023). HRQoL was a secondary end point assessed at baseline and day 1 of each cycle using the European Organization for Research and Treatment of Cancer QoL-30 item questionnaire.
This study focused on the HRQoL impact of selinexor in the treatment of advanced and metastatic unresectable DDLPS in patients who have experienced disease progression while on at least two prior lines of systemic therapy.
A total of 277 patients were enrolled in the Phase III portion of the trial; 255 (92.1%) completed a baseline QoL cancer-30 item questionnaire assessment and were included in these analyses as the patient-reported outcome population: 168 (65.6%) patients in the selinexor arm and 88 (34.4%) patients in the placebo arm.
Pain worsened in the placebo arm compared with the selinexor arm across all postbaseline visits, despite higher average baseline pain scores in the selinexor arm.
When examining mean scores over time, patients who received twice weekly selinexor reported lower rates and slower worsening of pain compared with patients treated with placebo.
Overall, HRQoL scores tended to decline over time in both arms, partially reflecting the progressive disease burden in this patient population.
As pain is one of the most devastating symptoms associated with advanced and progressing cancers, the significant reduction in pain in the selinexor arm, according to patient perception, represent a relevant added value of this drug in DDLPS.
With no treatment options of known clinical benefit in the heavily pretreated population with DDLPS studied in SEAL, selinexor provides an effective, novel, oral therapy option, potentially providing convenience, improved adherence and a reduced caregiver burden compared with existing parenteral therapies.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-0284
Financial & competing interests disclosure
This study was funded by Karyopharm Therapeutics, Inc. M Gounder: reports an institutional research grant from Karyopharm, personal fees from Karyopharm, Epizyme, Springworks, Daiichi, Bayer, Amgen, Tracon, Flatiron, Medscape, Physicians Education Resource, Guidepoint, GLG and UpToDate; and grants from the National Cancer Institute, National Institutes of Health (P30CA008748) – core grant (CCSG shared resources and core facility). ARA Razak: consulting/Ad board: Merck & Adaptimmune Research support: Karyopharm Therapeutics, Deciphera, Blueprint Medicines, Pfizer, Adaptimmune, Merck, Roche/Genentech, Bristol-Myers Squibb, Medimmune, Amgen, GSK, AbbVie, Iterion Therapeutics. AM Gilligan: employee of Karyopharm Therapeutics, Inc. H Leong: employee of Karyopharm Therapeutics, Inc. X Ma: employee of Karyopharm Therapeutics, Inc. N Somaiah: consultant for Deciphera, Blueprint, Bayer Research Support from Ascentage, Astra-Zeneca, Daiichi-Sankyo, Deciphera, Eli Lilly, Karyopharm and GSK. SP Chawla: consultant for Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx Corporation, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, SARC: Sarcoma Alliance for Research though Collaboration, Janssen, Advenchen Laboratories, Bayer, NKMax, InhibRx. Grants or contracts from Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx Corporation, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, SARC: Sarcoma Alliance for Research though Collaboration, Janssen, Advenchen Laboratories, Bayer, InhibRx, NKMax. G Grignani: consultant for Eli Lilly, Novartis, Glaxo, Pharmamar, EISAI, Bayer, Merck. SM Schuetze: consultant – NanoCarrier, UpToDate. Research funding to institution – Adaptimmune, Amgen, Blueprint, Glaxo-SmithKline, Karyopharm. B Vincenzi: Consultant for Pharmamar Eisai, Lilly, Abbott, Novartis, Accord AJ Wagner: consultant for Daiichi-Sankyo, Deciphera, Eli Lilly, Epizyme, NovoCarrier, Mundipharma, and Research Support to My Institution from Aadi Bioscience, Daiichi-Sankyo, Deciphera, Eli Lilly, Karyopharm and Plexxikon. RL Jones: consultant for Adaptimmune, Athenex, Bayer, Boehringer Ingelheim, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar, Springworks, Tracon, Upto Date. J Shah: employee of Karyopharm Therapeutics, Inc. S Shacham: employee of Karyopharm Therapeutics, Inc. M Kauffman: employee of Karyopharm Therapeutics, Inc. RF Riedel: ownership - Limbguard, LLC (Spouse); Institutional Clinical Research Support - AADi, AROG, Blueprint, Daiichi-Sankyo, Deciphera, Glaxo-SmithKline, Karyopharm, Ignyta, Immune Design, NanoCarrier, Oncternal, Philogen, Plexxikon, Roche, Springworks, Tracon; Consultant/Advisor - Bayer, Blueprint, Daiichi-Sankyo, Deciphera, Ignyta, NanoCarrier. S Attia: reports research funding from Desmoid Tumor Research Foundation and research funding to their institution from: AB Science, TRACON Pharma, Bayer, Novartis, Lilly, Immune Design, Karyopharm Therapeutics, Epizyme, Blueprint Medicines, Genmab, CBA Pharma, Merck, Philogen, Gradalis, Deciphera, Takeda, Incyte, Springworks, Adaptimmune, Advenchen Laboratories, Bavarian Nordic, BTG, PTC Therapeutics, GlaxoSmithKline, FORMA Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: NCT02606461. The data will not be made publicly available. For original data please contact hoyee.leong@karyopharm.com.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Crago AM, Singer S. Clinical and molecular approaches to well-differentiated and dedifferentiated liposarcoma. Curr. Opin. Oncol. 23(4), 373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock S, Hoffmann DG, Jiang Y, Chen H, Il'yasova D. Increasing incidence of liposarcoma: a population-based study of National Surveillance Databases, 2001–2016. Int. J. Environ. Res. Public Health 17(8), 2710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin-Mansour A, George S, Sioltec S et al. Genomic evolutionary patterns of leiomyosarcoma and liposarcoma. Clin. Cancer Res. 25(16), 5135–5142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston J, Bugano D, Barbo A et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci. Rep. 7(1), 1–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somaiah N, Beird HC, Barbo A et al. Targeted next generation sequencing of well-differentiated/dedifferentiated liposarcoma reveals novel gene amplifications and mutations. Oncotarget 9(28), 19891 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tap WD, Aj W, S P et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas The ANNOUNCE randomized clinical trial. JAMA 323(13), 1266–1276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judson I, Verweij J, Gelderblom H et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled Phase III trial. Lancet Oncol. 15(4), 415–423 (2014). [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (NCCN) Practice Guidelines in oncology: soft tissue sarcoma NCCN evidence blocks. (2020). https://jnccn.org/view/journals/jnccn/18/12/article-p1604.xml?ArticleBodyColorStyles=pdf-5590 ; • This reference provides the NCCN guidelines for treating soft tissue sarcoma.
- 9.Demetri GD, Von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a Phase III randomized multicenter clinical trial. J. Clin. Oncol. 34(8), 786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demetri GD, Schöffski P, Grignani G et al. Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized Phase III study of eribulin versus dacarbazine. J. Clin. Oncol. 35(30), 3433–3439 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Dickson MA, Tap WD, Keohan ML et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J. Clin. Oncol. 31(16), 2024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson MA, Schwartz GK, Keohan ML et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: a Phase II clinical trial. JAMA Oncol. 2(7), 937–940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonough J, Eliott J, Neuhaus S, Reid J, Butow P. Health-related quality of life, psychosocial functioning, and unmet health needs in patients with sarcoma: a systematic review. Psycho‐oncology 28(4), 653–664 (2019). [DOI] [PubMed] [Google Scholar]; • Provides a systematic review of health-related quality of life in patients with sarcoma.
- 14.Schreiber D, Bell RS, Wunder JS et al. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Qual. Life Res. 15(9), 1439–1446 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Reichardt P, Leahy M, Garcia Del Muro X et al. Quality of life and utility in patients with metastatic soft tissue and bone sarcoma: the sarcoma treatment and burden of illness in North America and Europe (SABINE) study. Sarcoma 2012, 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 9, 353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg. Oncol. Clin. N. Am. 27(4), 675 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azizian NG, Li Y. XPO1-dependent nuclear export as a target for cancer therapy. J. Hematol. Oncol. 13, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca R, Abouzaid S, Bonafede M et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia 31(9), 1915–1921 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camus V, Miloudi H, Taly A, Sola B, Jardin F. XPO1 in B cell hematological malignancies: from recurrent somatic mutations to targeted therapy. J. Hematol. Oncol. 10(1), 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill R, Cautain B, De Pedro N, Link W. Targeting nucleocytoplasmic transport in cancer therapy. Oncotarget 5(1), 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conforti F, Wang Y, Rodriguez JA, Alberobello AT, Zhang Y-W, Giaccone G. Molecular pathways: anticancer activity by inhibition of nucleocytoplasmic shuttling. Clin. Cancer Res. 21(20), 4508–4513 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Kalakonda N, Maerevoet M, Cavallo F et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, Phase II trial. Lancet Haematol. 7(7), e511–e522 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Ben-Barouch S, Kuruvilla J. Selinexor (KTP-330)-a selective inhibitor of nuclear export (SINE): anti-tumor activity in diffuse large B-cell lymphoma (DLBCL). Expert Opin. Investig. Drugs 29(1), 15–21 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Razak ARA, Mau-Soerensen M, Gabrail NY et al. First-in-class, first-in-human Phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J. Clin. Oncol. 34(34), 4142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chari A, Vogl DT, Gavriatopoulou M et al. Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381(8), 727–738 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Grosicki S, Simonova M, Spicka I et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, Phase III trial. Lancet 396(10262), 1563–1573 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Gounder MM, Zer A, Tap WD et al. Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J. Clin. Oncol. 34(26), 3166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Presents the early phase results for selinexor in soft-tissue sarcoma.
- 29.Gounder M, Razak Aa, Somaiah N et al. SEAL: Phase III, randomized, double blind, cross-over, study of selinexor versus placebo in advanced unresectable dedifferentiated liposarcoma (DDLS). Connective Tissue Oncology Society (2020). [Google Scholar]; •• This study presents the results of the selinexor in advanced liposarcoma trial.
- 30.Karyopharm Therapeutics, Inc. XPOVIO (selinexor), prescribing information. MA, USA: (2019). [Google Scholar]
- 31.Fayers P, Aaronson NK, Bjordal K, Sullivan M. EORTC QLQ–C30 Scoring Manual. European Organisation for research and treatment of cancer; (1995). https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf [Google Scholar]
- 32.Beunckens C, Molenberghs G, Kenward MG. Direct likelihood analysis versus simple forms of imputation for missing data in randomized clinical trials. Clinical Trials 2(5), 379–386 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 16(1), 139–144 (1998). [DOI] [PubMed] [Google Scholar]; •• Provides results for the validation of meaningful change threshholds used.
- 34.Kemmler G, Zabernigg A, Gattringer K et al. A new approach to combining clinical relevance and statistical significance for evaluation of quality of life changes in the individual patient. J. Clin. Epidemiol. 63(2), 171–179 (2010). [DOI] [PubMed] [Google Scholar]; • Provides results for the validation of meaningful change threshholds used.
- 35.Hudgens S, Forsythe A, Kontoudis I, D'Adamo D, Bird A, Gelderblom H. Evaluation of quality of life at progression in patients with soft tissue sarcoma. Sarcoma 2017, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvam AK, Fayers PM, Wisloff F. Responsiveness and minimal important score differences in quality‐of‐life questionnaires: a comparison of the EORTC QLQ‐C30 cancer‐specific questionnaire to the generic utility questionnaires EQ‐5D and 15D in patients with multiple myeloma. Eur. J. Haematol. 87(4), 330–337 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Eisenhauer E, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Cella D, Cappelleri JC, Bushmakin A et al. Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J. Oncol. Pract. 5(2), 66–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinten C, Coens C, Mauer M et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 10(9), 865–871 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Le Cesne A, Kapnang RK, Foulon S, Bonastre J. Health-related quality of life in patients with advanced soft tissue sarcoma (ASTS): results from the TSAR randomized Phase III trial of the French Sarcoma Group. Ann. Oncol. 29, viii577 (2018). [Google Scholar]
- 41.Zheng J, He J, Wang W et al. The impact of pain and opioids use on survival in cancer patients: results from a population-based cohort study and a meta-analysis. Medicine 99(9), 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan A, Lim E, Ng T, Shih V, Quek R, Cheung YT. Symptom burden and medication use in adult sarcoma patients. Support. Care Cancer 23(6), 1709–1717 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Kuo P, Yen J, Parker G et al. The prevalence of pain in patients attending sarcoma outpatient clinics.Sarcoma 2011, 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gough NJ, Smith C, Ross JR, Riley J, Judson I. Symptom burden, survival and palliative care in advanced soft tissue sarcoma. Sarcoma 2011, 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagar SP, Mytelka DS, Candrilli SD et al. Treatment patterns and survival among adult patients with advanced soft tissue sarcoma: a retrospective medical record review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecht CJ, Haaxma-Reiche H, van Putten W, de Visser M, Vries E, Twijnstra A. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology 39, 1255–1257 (1989). [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. World Health Organization (WHO) guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. (2018). https://www.who.int/publications/i/item/9789241550390 [PubMed]
- 48.Sharma S, Takyar S, Manson SC, Powell S, Penel N. Efficacy and safety of pharmacological interventions in second-or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review. BMC Cancer 13(1), 1–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thijssens KM, Hoekstra-Weebers JE, van Ginkel RJ, Hoekstra HJ. Quality of life after hyperthermic isolated limb perfusion for locally advanced extremity soft tissue sarcoma. Ann. Surg. Oncol. 13(6), 864–871 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Shingler SL, Swinburn P, Lloyd A et al. Elicitation of health state utilities in soft tissue sarcoma. Qual. Life Res. 22(7), 1697–1706 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Jones RL, Maki RG, Patel SR et al. Safety and efficacy of trabectedin when administered in the inpatient versus outpatient setting: clinical considerations for outpatient administration of trabectedin. Cancer 125, 4435–4441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coens C, van der Graaf WT, Blay JY et al. Health‐related quality‐of‐life results from PALETTE: a randomized, double‐blind, Phase III trial of pazopanib versus placebo in patients with soft tissue sarcoma whose disease has progressed during or after prior chemotherapy – a European Organization for research and treatment of cancer soft tissue and bone sarcoma group global network study (EORTC 62072). Cancer 121(17), 2933–2941 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Eek D, Krohe M, Mazar I et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer. Adherence 10, 1609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claudia Castro, Manoogian A. COVID-19 and the impact on oral oncolytics: a specialty pharmacy perspective. Pharmacy Time 2(4), (2020). https://www.pharmacytimes.com/view/covid-19-and-the-impact-on-oral-oncolytics-a-specialty-pharmacy-perspective [Google Scholar]
- 55.Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for research and treatment of cancer experience. J. Clin. Oncol. 25(32), 5082–5086 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Sodergren SC, White A, Efficace F et al. Systematic review of the side effects associated with tyrosine kinase inhibitors used in the treatment of gastrointestinal stromal tumours on behalf of the EORTC Quality of Life Group. Crit. Rev. Oncol. Hematol. 91(1), 35–46 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.