Abstract
Background
Myelodysplastic syndrome (MDS) is a type of heterogeneous myeloid clonal disorder usually diagnosed based on a combination of multiple laboratory examinations, including analysis of peripheral blood cells, bone marrow cell morphology and cytogenetics. However, there is a certain difficulty in cases with no distinct changes in hematology and marrow cell morphology.
Methods
We adopt flow cytometry to quantitatively analyze the immunophenotypic changes of marrow monocytes according to the surface antigens and their combinations at different differentiation stages, so as to study the changes of monocytes during differentiation in patients with bone marrow failure. In the meantime, the relationship between the immunophenotypic changes of marrow monocytes and IPSS-R score and prognosis of MDS patients was analyzed.
Results
Our results demonstrated disorders of maturation and differentiation of monocytes in patients with MDS and clonal cytopenias of undetermined significance as compared to those with aplastic anemia and healthy individuals. In addition, the differentiation abnormality gradually increased with the disease progression. Furthermore, CD300e expression was found to show significant associations with the clinical stage and disease progression of MDS, and the progression-free survival and AML-free survival were much longer in MDS patients highly expressing CD300e on monocytes.
Conclusions
CCUS and MDS patients have disorders of differentiation and maturation of monocytes, which tends to be more critical with MDS progression or transforms to AML. Moreover, high CD300e expression has the potential to be a favorable prognostic marker for MDS. This study provides important insights to the role of monocyte immunotyping in the diagnosis, differentiation and prognosis of MDS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-022-00856-7.
Keywords: Myelodysplastic syndrome, Monocytes, Immunophenotype, Flow cytometry
Introduction
Myelodysplastic syndrome (MDS) is a type of heterogeneous myeloid clonal disorder derived from hematopoietic stem cells. It is mainly manifested by single or multi-lineage cytopenias and morbid hematopoiesis in bone marrow, and a risk of transformation to acute myelogenous leukemia (AML) [1]. Generally, the diagnosis of MDS relies on the reduction of peripheral blood cells, increased proportion of blasts, morphological abnormalities and aberrant cytogenetics [2]. However, it is usually an exclusion diagnosis. There is no specific diagnostic marker but similar characteristics (such as cytopenias and the natural history) with some common bone marrow failure disorders, such as aplastic anemia (AA) and clonal cytopenia of undetermined significance (CCUS) [3]. In suspected cases presenting with typical aberrant chromosomal karyotype or cytopenias but absent of morphological findings regarding aberrant cell development, there might be some possible divergence between clinicians before reaching a definite diagnosis. In addition, cytogenetic abnormality somatic mutation can also occur in either MDS patients presenting with cytopenias or normal individuals, and it is also a risk factor of other clonal disorders [4]. Under this background, the diagnosis of unexplained cytopenias remains a great challenge in hematologists and physicians.
For the past few years, flow cytometry (FCM) has shown significance in diagnosis and prognosis of MDS, which has received much attention in increasing medical researchers. With its wide application and continuous development, a variety of immunophenotypic changes can be detected in bone marrow and peripheral blood cells in patients with bone marrow failure disorders. There was a study which adopted FCM and reported significant higher proportions of CD13 + , CD33 + , CD34 + and HLA-DR + granulocytes while lower proportions of CD15 + and CD10 + granulocytes in MDS patients than AA patients and normal people. In addition, CD13 + , CD33 + , CD34 + and HLA-DR + granulocytes were much more abundant during the transition from refractory anemia (RA) to refractory anemia with excess blasts (RAEB), but CD15 + granulocytes were less abundant [5]. These data suggest that the immunophenotype of bone marrow granulocytes could bring more benefit in differentiation between MDS and other bone marrow failure disorders. However, the immunophenotype of bone marrow monocytes has seldom been studied in bone marrow failure disorders.
The immunophenotypic changes are also important in prognosis of MDS. Research found that CD25 was highly expressed in MDS patients especially those at a high risk, and the increase of CD25 + hematopoietic stem cells was predictive for disease progression [6]. Chen JX et al. [7] found increased expression of CD200 on CD33 + bone marrow cells in MDS, which was associated with poor overall survival (OS) of patients and a high risk of transformation to leukemia. This indicated that CD200 could be a good diagnostic marker of MDS as well as a predictor for clinical staging and progression of this disease. Nathalie et al. [8] reported that CD141 expression on monocytes could be used as a favorable marker for early prognosis in patients with low-risk MDS, as it was associated with lower risk of MDS, better OS and leukemia-free survival. CD300e composes a single Ig-like domain and encodes a member of the CD300 glycoprotein family of cell surface proteins expressed on myeloid cells [9]. Its expression can be used to predict the maturation of monocytes and the differentiation to myeloid dendritic cells (mDC) [10, 11]. In addition, it plays a role in immunoregulation via inducing the release of cytokines and increasing the expression of immune costimulatory molecules [12]. It was reported that CD300e has diagnostic potential for fulminant type 1 diabetes [13]. In addition, its decreased expression in monocytes is prognostic for disease progression in patients with Coronavirus disease 2019 (COVID-19) [14]. There is no previous report of CD300e in bone marrow failure disorders. In this context, a comprehensive, thorough understanding on the immunophenotypic changes in MDS may facilitate further research on the immune-related pathogenesis of MDS and provide certain evidence for the diagnosis and differentiation of this disease and the development of targeted drugs.
Here, we adopted FCM to study the immunophenotypic changes of bone marrow monocytes in MDS patients to identify whether such changes are of clinical significance in diagnosis and differentiation of low-risk MDS and other bone marrow failure disorders. In the meantime, the associations with IPSS-R score and prognosis in patients were analyzed. Furthermore, this study, for the first time, revealed the role of high CD300e expression as a favorable prognostic marker for MDS, which provides important insights to the diagnosis, differentiation and prognosis of MDS based on immunophenotypic changes of monocytes.
Materials and methods
Subjects
This study totally enrolled 98 patients newly diagnosed as MDS in the Department of Hematology, Tianjin Medical University General Hospital between November 2017 and November 2019. There were 59 males and 39 females, with the mean age of 62 (20–84) years old. According to the WHO classification of MDS (2016) [15] and the Revised international prognostic scoring system (IPSS-R) [16], the participants were categorized into lower-risk group (LR-MDS) (including very low risk, low risk and intermediate risk with IPSS-R score ≤ 3.5) and higher-risk group (HR-MDS) (including intermediate risk with IPSS-R score > 3.5, high risk and very high risk). The detailed information of the two groups is displayed in Table 1. There were 21 CCUS cases, including 8 males and 13 females aged 45 (14–82) years old, and 24 AA cases, including 13 males and 11 females aged 41.5 (11–69) years old. A total of 38 healthy people were enrolled as control, including 13 males and 25 females, with the mean age of 54 (17–80) years old (Table 2). This study followed the Declaration of Helsinki and was approved by the ethics committee of Tianjin Medical University General Hospital. All participants signed the informed consent.
Table 1.
Baseline characteristics of MDS patients
| Subtype | MDS | ||||||
|---|---|---|---|---|---|---|---|
| SLD | MLD | RS-SLD | RS-MLD | MDS-5q- | EB-1 | EB-2 | |
| n = 9 | n = 9 | n = 23 | n = 7 | n = 3 | n = 21 | n = 26 | |
| Age (Year) | 62 | 53 | 62 | 68 | 68 | 65 | 55 |
| 47–82 | 20–69 | 34–84 | 40–84 | 57–83 | 30–82 | 33–82 | |
| Male/Female | 7:2 | 5:4 | 14:9 | 4:3 | 1:2 | 12:9 | 16:10 |
| Blast (%) | 0.5 | 0 | 0.5 | 0.5 | 0 | 8 | 16.5 |
| 0–4 | 0–4 | 0–4.5 | 0–4.5 | 0–1 | 5.5–9.5 | 10.5–19.5 | |
| HGB (g/L) | 108 | 77 | 74 | 87 | 79 | 72 | 76 |
| 70–149 | 57–149 | 38–138 | 61–120 | 69–126 | 42–129 | 53–145 | |
| PLT × 10^9/L | 92 | 40 | 122 | 52 | 114 | 82 | 49 |
| 11–591 | 9–189 | 3–428 | 8–503 | 51–210 | 3–295 | 6–323 | |
| ANC × 10^9/L | 1.34 | 1.58 | 2.23 | 0.85 | 1.84 | 1.16 | 0.9 |
| (0.73–6.12) | (0.21–10.70) | (0.26–8.97) | (0.56–3.84) | (0.94–2.44) | (0.11–6.50) | (0.09–6.99) | |
MDS myelodysplastic syndrome, RS ring sideroblasts, SLD single lineage dysplasia, MLD multilineage dysplasia, EB1 excess blasts type 1, EB2 excess blasts type 2, HGB hemoglobin, PLT platelet, ANC absolute neutrophil count
Table 2.
Baseline characteristics of patients with low-risk MDS, CCUS, AA and normal people
| Group | Lower risk MDS | CCUS | AA | Control |
|---|---|---|---|---|
| n = 45 | n = 21 | n = 24 | n = 38 | |
| Age (Year) | 63(26–84) | 45(14–82) | 41.5(11–69) | 54(17–80) |
| Male: Female | 25:20 | 8:13 | 13:11 | 13:25 |
| HGB (g/L) | 101(38–149) | 81(39–118) | 71(28–117) | 78(49–109) |
| PLT × 10^9/L | 112(3–591) | 39(6–186) | 29(3–98) | 324(84–642) |
| ANC × 10^9/L | 1.84 | 1.61 | 1.13 | 3.29 |
| (0.29–8.97) | (0.25–4.28) | (0.27–2.41) | (1.38–9.60) |
MDS myelodysplastic syndrome, CCUS clonal cytopenias of undetermined significance, AA aplastic anemia, HGB hemoglobin, PLT platelet, ANC absolute neutrophil count
Flow cytometry
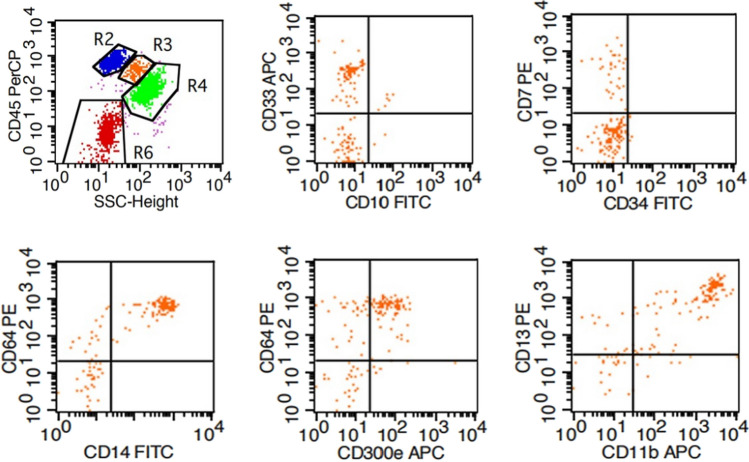
Four tubes corresponding to each group were used, numbered and added with 10 μl CD33-APC, CD10-FITC, CD45-PerCP antibodies; 10 μl CD7-PE, CD34-FITC and CD45-PerCP antibodies; 10 μl CD64-PE/CD14-FITC, CD300e-APC and CD45-PerCP antibodies; 10 μl CD13-PE, CD11b-APC and CD45-PerCP antibodies, respectively. All antibodies were provided by BD Biosciences. Fresh bone marrow was transferred to the tubes for heparin anticoagulation. In short, 50 μl bone marrow fluid was added to each tube for 30 min of incubation away from light at routine temperature, followed by addition of 2 ml red cell lysis solution (BD Biosciences) for 10 min. Subsequently, the mixture was centrifuged at 1500 r/min for 5 min. The supernatant was discarded. The remaining fraction was then centrifuged with 2 ml PBS solution at 1500 r/min for another 5 min. The supernatant was removed. The remaining fraction was washed 3 times and then suspended by 500 μl PBS solution for further FCM analysis on a flow cytometry machine (BD FACS Calibur). Each tube contained 10,000–50,000 cells. Monocytes were identified by CD45 int/bright and SSC int (Fig. 1 R3). The expression of CD14 was detected to predict the maturation of monocytes. In addition, CD64 expression can also be present in the maturation of monocytes. The proportions of CD33 + , CD10 + , CD7 + , CD34 + , CD64 + , CD14 + , CD300e + , CD13 + and CD11b + cells in total monocytes (CD45 int/bright and SSC int) were calculated. According to the changing patterns of cell surface expression of antigens during normal differentiation, antigen expression patterns in MDS were analyzed based on the combinations of CD64-CD14-, CD64-CD300e-, CD64 + CD14-, CD64 + CD300e-, CD64 + CD14 + and CD64 + CD300e + (Fig. 1), and the proportion of each combination was recorded.
Fig. 1.
Flow cytometry diagram of the expression of antigens/antigen combinations on surface of bone marrow monocytes
Fluorescence in situ hybridization (FISH)
The bone marrow specimens were permeabilized (0.075 M KCl solution), fixed (methanol: glacial acetic acid = 3:1), incubated on a glass slide containing fresh 2 × SSC (pH7.0) solution at 37 ℃ for 1 h, dehydrated with gradient concentration of ethanol and then dried at room temperature. FISH probes were used to detect −5/5q-, −7/7q-, + 8, −Y, 20q- and 17p13- karyotypes. Following the instructions, genomic DNA was denatured and hybridized with the probes. The cells were then washed, counterstained and observed microscopically.
Gene mutation detection
Gene mutation analysis was performed using next-generation sequencing (NGS) with the DNA samples extracted from the peripheral or bone marrow monocytes in patients (Qiagen, Hilden, Germany). DNA library was constructed according to the specifications. Genomic sequencing was completed on the Illumina Miseq platform (San Diego, CA, USA), with the gene sequencing panel containing 38 genes which have mutations in myeloid tumors. The variant allele frequency was set as 5%. Each mutation was analyzed in Catalogue of Somatic Mutations in Cancer (COSMIC) and Database of Single Nucleotide Polymorphisms (dbSNP) databases.
Statistical analysis
SPSS 22.0 was applied for statistical analysis. Experimental data were reported as mean ± standard deviation (± SD). Independent-sample t test was adopted to compare two sets of data with normal distribution. One-way ANOVA was performed to compare two sets of data among multiple groups. Spearman correlation analysis was conducted to study correlation. Kaplan–Meier method was used to perform survival analysis. GraphPad Prism 6 was run to generate graphs. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Monocytes surface expression of antigens and antigen combinations in bone marrow failure disorders
Expression of antigens
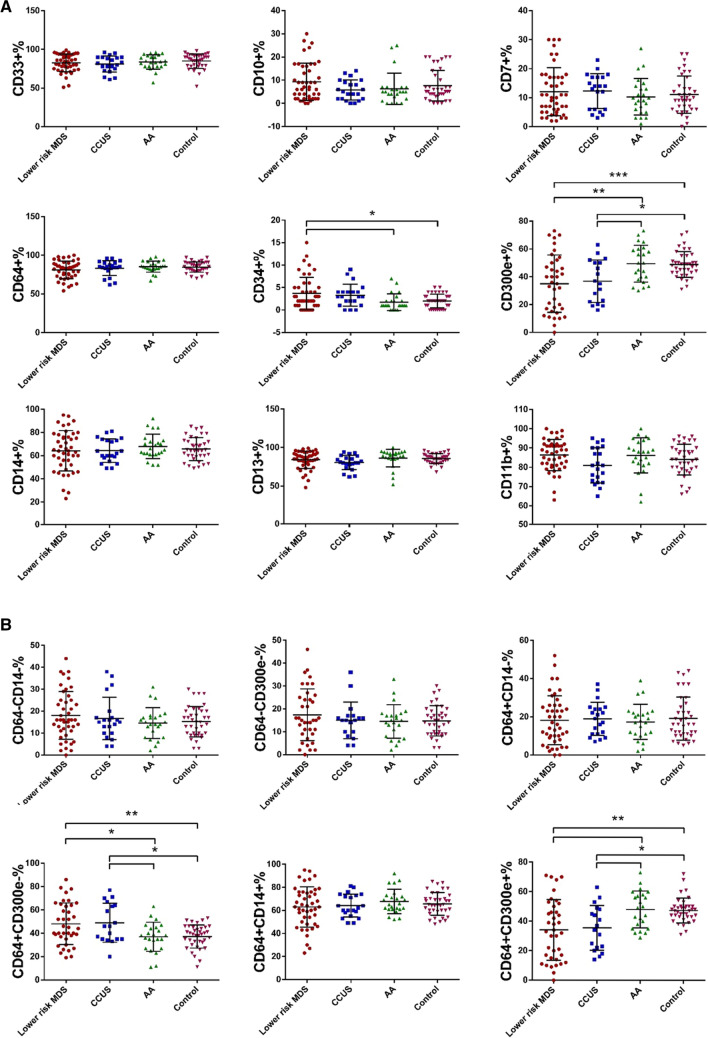
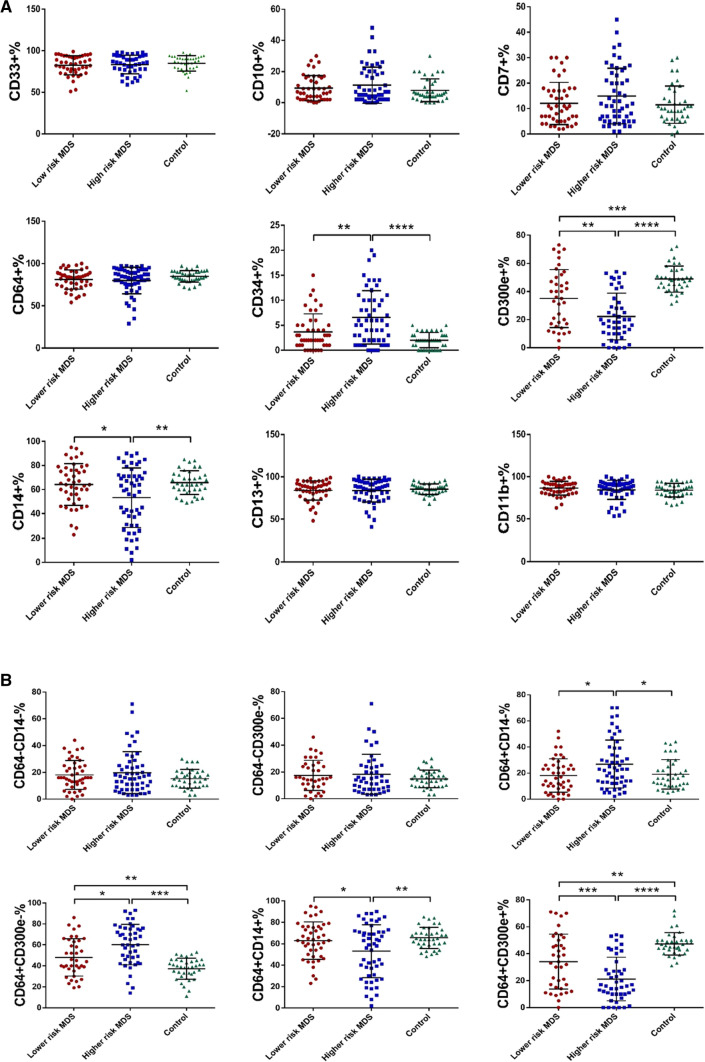
To investigate the changes in the immunophenotype on bone marrow monocytes in patients with bone marrow failure disorders, monocytes were identified by FCM with CD45 int/bright and SSC int. The proportions of CD33 + , CD10 + , CD7 + , CD34 + , CD64 + , CD14 + , CD300e + , CD13 + and CD11b + cells in total monocytes (CD45 int/bright and SSC int) were calculated. The expression of CD34 in the LR-MDS, CCUS, AA and control groups was 3.69% ± 3.60%, 3.27% ± 2.47%, 1.75% ± 1.85% and 2.03% ± 1.53%, respectively (p = 0.006). Comparatively, the CD34 expression in the LR-MDS group was significantly higher than that in the AA (p = 0.022) and control groups (p = 0.025), but no evident difference was shown in other between-group comparisons (p > 0.05). The expression of CD300e in the LR-MDS, CCUS, AA and control groups was 35.05% ± 20.59%, 36.83% ± 15.19%, 49.44% ± 13.19% and 48.84% ± 9.25%, respectively (p = 0.000). Comparatively, the CD300e expression in the LR-MDS and CCUS groups was significantly lower than those in the AA (LR-MDS, p = 0.003; CCUS, p = 0.049) and control groups (LR-MDS, p = 0.001; CCUS, p = 0.036), but no evident difference was shown in other between-group comparisons (p > 0.05). There were no evident differences in terms of CD33, CD10, CD7, CD64, CD14, CD13 and CD11b in any between-group comparisons (p > 0.05) (Fig. 2a). CD34 expression suggests that the cells are in a more primitive stage. The results suggested that monocytes from LR-MDS patients are impaired in differentiation and maturation compared to AA patients and normal controls. And we speculated that reduced CD300e expression might be associated with impaired monocyte maturation.
Fig. 2.
Expression of antigens/antigen combinations on surface of bone marrow monocyte in bone marrow failure disorders. a Expression of antigens on surface of bone marrow monocyte in lower risk MDS, CCUS, AA and Control. b Expression of antigen combinations on surface of bone marrow monocyte in lower risk MDS, CCUS, AA and Control. (*p < 0.05, **p < 0.01, ***p < 0.001)
Expression of antigen combinations
The proportion of CD64 + CD300e- cells in total monocytes of the LR-MDS, CCUS, AA and control groups was 48.08% ± 17.89%, 48.94% ± 16.64%, 36.96% ± 12.51% and 37.18% ± 9.93%, respectively (p = 0.001). Comparatively, the proportion in the LR-MDS and CCUS groups was significantly higher than those in the AA (LR-MDS, p = 0.022; CCUS, p = 0.047) and control groups (LR-MDS, p = 0.007; CCUS, p = 0.027), but no evident difference was shown in other between-group comparisons (p > 0.05). The proportion of CD64 + CD300e + cells in total monocytes of the LR-MDS, CCUS, AA and control groups was 34.11% ± 20.45%, 35.50% ± 15.10%, 47.96% ± 12.52% and 47.37% ± 8.34%, respectively (p = 0.000). Comparatively, the proportion in the LR-MDS and CCUS groups was profoundly lower than those in the AA (LR-MDS, p = 0.004; CCUS, p = 0.046) and control groups (LR-MDS, p = 0.001; CCUS, p = 0.033), but no evident difference was shown in other between-group comparisons (p > 0.05). No evident differences regarding the proportions of CD64-CD14-, CD64-CD300e-, CD64 + CD14- and CD64 + CD14 + cells in any between-group comparisons was detected (p > 0.05) (Fig. 2b). The results showed that CD300e is promising as a marker for differentiating bone marrow failure disorders.
Association between monocytes surface expression of antigens/antigen combinations and clinical characteristics of MDS
Association with single and multi-lineage cytopenias
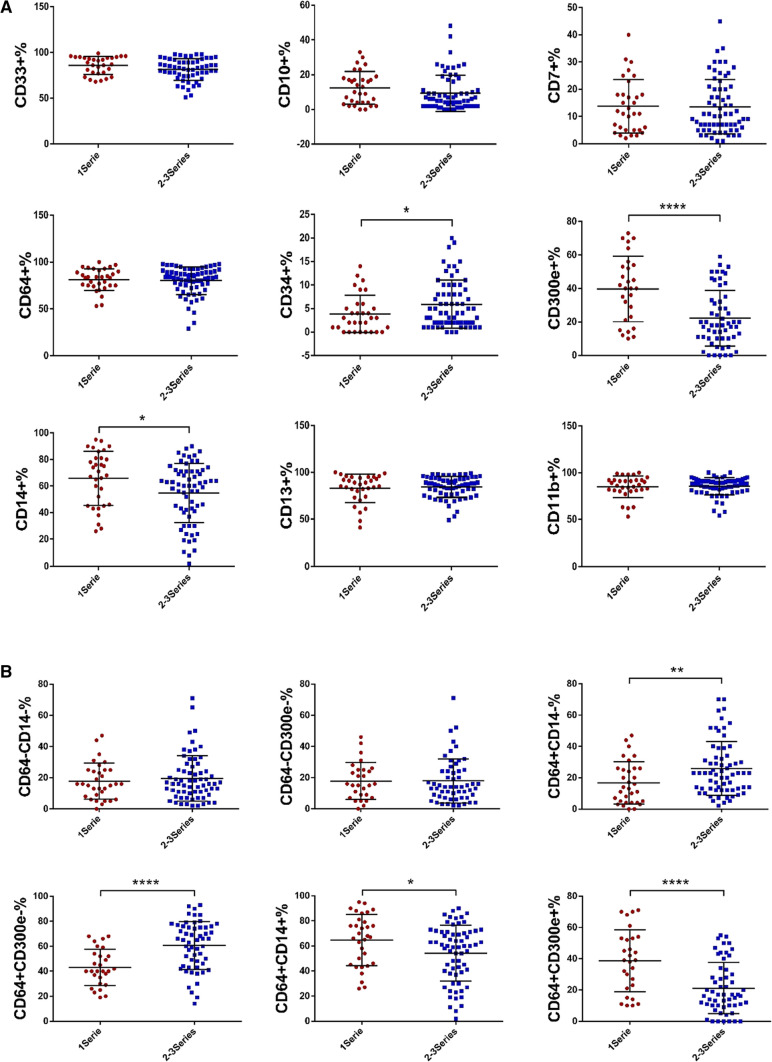
Since patients with MDS generally present with single or multi-lineage cytopenias, where multilineage cytopenias often suggest more severe disease or has a worse prognosis. Here we analyzed their association with the expression of antigens and antigen combinations on surface of bone marrow monocytes. As analyzed, the expression of CD34 was lower in patients with single lineage cytopenias than that in patients with multilineage cytopenias (3.84% ± 3.94% vs 5.92% ± 5.08%, p = 0.044), but the expression levels of CD14 and CD300e were much higher (CD14: 65.75% ± 20.48% vs 54.68% ± 22.32%, p = 0.020; CD300e: 39.71% ± 19.61% vs 22.20% ± 16.66%, p = 0.000). There were no evident differences between the two groups in terms of CD33, CD10, CD7, CD64, CD13 and CD11b (p > 0.05) (Fig. 3a). Furthermore, the proportions of CD64 + CD14- (16.69% ± 13.47% vs 25.91% ± 17.22%, p = 0.009) and CD64 + CD300e- cells (43.11% ± 14.59% vs 60.57% ± 19.21%, p = 0.000) were significantly lower in patients with single lineage cytopenias than those in patients with multi-lineage cytopenias, while the proportions of CD64 + CD14 + (64.69% ± 20.36% vs 54.18% ± 22.39%, p = 0.027) and CD64 + CD300e + cells (38.68% ± 19.72% vs 21.20% ± 16.28%, p = 0.000) were much higher. No evident differences regarding the proportions of CD64-CD14- and CD64-CD300e- cells were noted (p > 0.05) (Fig. 3b). Increased CD34 expression and lack of CD14 expression suggest that monocytes are in a more primitive state. The results suggested that the maturation of bone marrow monocytes is more impaired in MDS patients with multi-lineage cytopenias.
Fig. 3.
Expression of antigens/antigen combinations in MDS patients with single and multi-lineage cytopenias. a Expression of antigens in MDS patients with single and multi-lineage cytopenias. b Expression of antigen combinations in MDS patients with single and multi-lineage cytopenias. (*p < 0.05, **p < 0.01, ****p < 0.0001).1 Series: Decreased numbers of one of the red blood cells, white blood cells and platelets. 2–3 Series: Decreased numbers of more than one of red blood cells, white blood cells and platelets
Association with bone marrow blasts
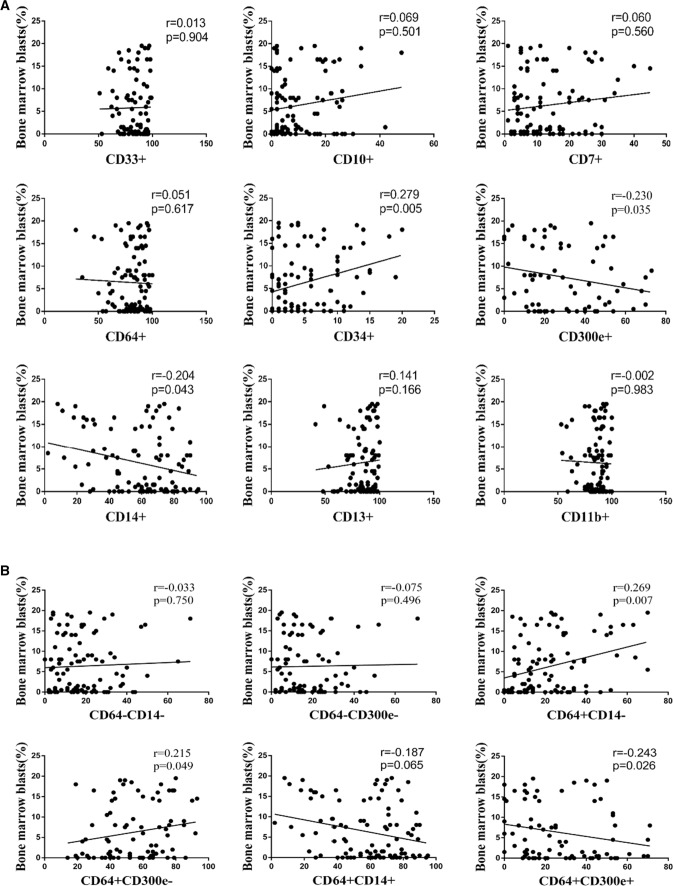
The proportion of bone marrow blasts in MDS patients as an important factor in the typing of MDS and the transformation to acute leukemia. In the above results we found abnormal expression of immune antigens (CD34, CD14) indicating monocyte maturation, and we next explored whether there was a correlation between the immune phenotype of bone marrow monocytes and blasts. We found that the proportion of bone marrow blasts was positively associated with the expression of CD34 on monocyte surface (r = 0.279, p = 0.005), but negatively associated with the expression levels of CD14 (r = −0.204, p = 0.043) and CD300e (r = −0.230, p = 0.035). The expression levels of CD33, CD10, CD7, CD64, CD13 and CD11b were independent of the proportion of blasts in bone marrow (p > 0.05) (Fig. 4a). In addition, the proportion of bone marrow blasts was positively associated with the CD64 + CD14- (r = 0.269, p = 0.007) and CD64 + CD300e- cells (r = 0.215, p = 0.049), but negatively associated with CD64 + CD300e + cells (r = −0.243, p = 0.026). The proportions of CD64-CD14-, CD64-CD300e- and CD64 + CD14 + cells were independent of the proportion of blasts in bone marrow (p > 0.05) (Fig. 4b).
Fig. 4.
Association of expression of antigens/antigen combinations with bone marrow blasts in MDS. a Association of expression of antigens with bone marrow blasts in MDS. b Association of expression of antigen combinations with bone marrow blasts in MDS
Association with the number of mutated genes
To investigate the relationship between monocyte immune phenotype and gene mutations in MDS patients, we found that no significant differences were observed in patients with < 2 mutated genes and > = 2 mutated genes, in terms of either expression levels of CD33, CD10, CD7, CD34, CD64, CD14, CD300e, CD11b, CD13 or the proportions of CD64-CD14-, CD64-CD300e-, CD64 + CD14-, CD64 + CD300e-, CD64 + CD14 + , CD64 + CD300e + cells (p > 0.05).
Association with FISH
To explore the association between monocyte immunophenotype and cytogenetics of MDS patients, we found that there were no evident differences between patients with normal and abnormal FISH in terms of the expression levels of CD33, CD10, CD7, CD34, CD64, CD14, CD300e, CD13 and CD11b (p > 0.05) (Supplementary table 1). As for the antigen combinations, the proportion of CD64 + CD14- cells was much higher in patients with normal FISH (25.28 ± 17.84% vs 18.21 ± 12.85%, p = 0.046). There were no evident differences in terms of the proportions of CD64-CD14-, CD64-CD300e-, CD64 + CD300e-, CD64 + CD14 + and CD64 + CD300e + cells (p > 0.05) (Supplementary Table 2).
Monocytes surface expression of antigens and antigen combinations in higher-risk and lower-risk patients
Expression of antigens
The degree of risk in MDS patients is very closely related to disease prognosis. Therefore, we explored the differences in the expression of bone marrow monocyte immunophenotypes in low- and high-risk MDS patients. In the LR-MDS, HR-MDS and control groups, the expression levels of CD34 were 3.69% ± 3.60%, 6.57% ± 5.34% and 2.03% ± 1.53%, respectively (p = 0.000); the expression levels of CD14 were 64.18% ± 17.38%, 53.30% ± 24.75% and 65.71% ± 9.88%, respectively (p = 0.003); and the expression levels of CD300e were 35.05% ± 20.59%, 22.24% ± 16.51% and 48.84% ± 9.25%, respectively (p = 0.000). Comparatively, CD34 expression was much higher in the HR-MDS group as compared to that in the other two groups (LR-MDS, p = 0.002; control, p = 0.000), but CD14 expression was profoundly lower in the HR-MDS group (LR-MDS, p = 0.016; control, p = 0.008). No evident difference between the LR-MDS and control groups regarding the expression of CD14 was noted (p > 0.05). The expression of CD300e in the LR-MDS (p = 0.001) and HR-MDS (p = 0.000) was remarkably lower than that in the control group, and that in the HR-MDS group was even much lower than that in the LR-MDS group (p = 0.001). No evident differences among the three groups regarding CD33, CD10, CD7, CD64, CD13 and CD11b were observed (p > 0.05) (Fig. 5a).
Fig. 5.
Expression of surface antigens/antigen combinations in patients with higher- and lower-risk MDS. a Expression of surface antigens in patients with higher- and lower-risk MDS. b Expression of surface antigen combinations in patients with higher- and lower-risk MDS. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Expression of antigen combinations
The proportions of CD64 + CD14-, CD64 + CD300e-, CD64 + CD14 + and CD64 + CD300e + cells among the LR-MDS, HR-MDS and control groups were statistically different. Specifically, the proportion of CD64 + CD14- cells upon HR-MDS was the highest among the three groups (26.93% ± 18.44% vs 18.16% ± 12.78% vs 19.08% ± 11.24%; LR-MDS, p = 0.012; control, p = 0.038), and the difference between the LR-MDS and control groups was not remarkable (p > 0.05). The proportion of CD64 + CD300e- cells in the HR-MDS group was significantly higher than that in the LR-MDS and control groups (60.26% ± 19.33% vs 48.08% ± 17.89% vs 37.18% ± 9.93%; LR-MDS, p = 0.003; control, p = 0.000), and the proportion in the LR-MDS group was also much larger as compared to the control (p = 0.013). The proportion of CD64 + CD14 + cells in the HR-MDS group was significantly lower than that in the LR-MDS and control groups (53.08% ± 24.76% vs 62.96% ± 17.56% vs 65.50% ± 9.87%; LR-MDS, p = 0.033; control, p = 0.008), and the difference between the LR-MDS and control groups was not remarkable (p > 0.05). The proportion of CD64 + CD300e + cells in the HR-MDS group was significantly lower than that in the LR-MDS and control groups (21.17% ± 16.22% vs 34.11% ± 20.45% vs 47.37% ± 8.34%; LR-MDS, p = 0.001; control, p = 0.000), and the proportion in the LR-MDS group was also much lower than that in the control group (p = 0.001). No evident differences among the three groups regarding the proportions of CD64-CD14- and CD64-CD300e- cells were observed (p > 0.05) (Fig. 5b). The results suggest that impaired monocyte maturation is more obvious in HR-MDS patients and the abnormal differentiation of monocytes in MDS tends to be more critical with disease progression.
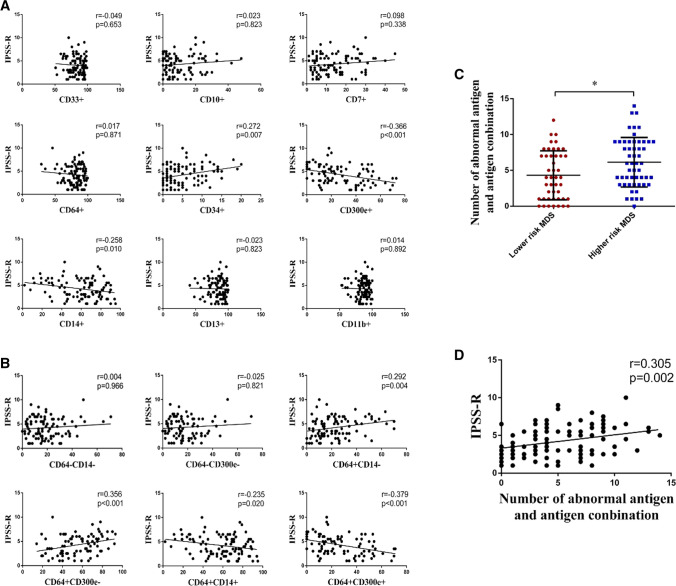
Association with IPSS-R score
The IPSS-R score was positively associated with the expression of CD34 on monocyte surface (r = 0.272, p = 0.007), but negatively correlated to the expression levels of CD14 (r = −0.258, p = 0.010) and CD300e (r = −0.366, p < 0.001). The IPSS-R score was independent of the expression levels of CD33, CD10, CD7, CD64, CD13 and CD11b (p > 0.05) (Fig. 6a). The IPSS-R score was positively correlated to the proportions of CD64 + CD14- (r = 0.292, p = 0.004) and CD64 + CD300e- cells (r = 0.356, p < 0.001) in MDS, but negatively associated with the proportions of CD64 + CD14 + (r = −0.235, p = 0.020) and CD64 + CD300e + cells (r = −0.379, p < 0.001). The IPSS-R score was independent of the proportions of CD64-CD14- and CD64-CD300e- cells (p > 0.05) (Fig. 6b). Furthermore, the relationship between the IPSS-R score and the number of abnormalities in surface antigens and antigen combinations was analyzed. With the expression of the control as reference (x̅ ± s), the number of abnormalities in monocyte surface antigens and antigen combinations in the LR-MDS group was much lower than that in the HR-MDS group (4.31 ± 3.41 vs 6.13 ± 3.46, p = 0.010) (Fig. 6C). In addition, the number was positively associated with the IPSS-R score (r = 0.305, p = 0.002) (Fig. 6d). The results suggest that monocyte immunophenotypes are more abnormal in patients with MDS at higher risk, and the expression of antigens on the surface of bone marrow monocytes changes during the MDS progression.
Fig. 6.
Association of expression of antigens/antigen combinations with IPSS-R score in MDS. a Association of expression of antigens with IPSS-R score in MDS. b Association of expression of antigen combinations with IPSS-R score in MDS. c The number of abnormalities in surface antigens and antigen combinations [With the expression of the control as reference (x̅ ± s), the number of abnormalities in monocyte surface antigens and antigen combinations in each MDS patient were counted] in the higher- and lower-risk MDS (*p < 0.05). d Association of the number of abnormalities in surface antigens and antigen combinations with IPSS-R score in MDS
CD300e expression and survival outcome in MDS patients
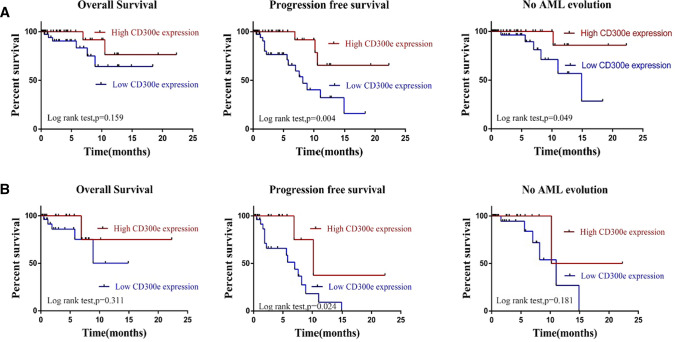
In the above results, we found that CD300e expression on the surface of monocytes showed negative correlation with impaired monocyte maturation, proportion of blasts and IPSS-R, suggesting that CD300e might be a marker of good prognosis in MDS. To study the prognostic significance of CD300e, patients at 24-month follow-up were divided into the high- and low-expression groups according to the expression of CD300e (25%). Of the 98 patients, 18 patients had disease progression (increase of blasts confirmed by cell morphology: from MDS with few blasts to MDS with excessive blasts, or from MDS-EB1 to MDS-EB2 or AML) or died. Kaplan–Meier survival analysis was performed and revealed that the Overall survival (OS) on average was not remarkably different between the high- and low-expression groups (19.2 months vs 13.9 months, p = 0.159), while the progression-free survival (PFS) (17.9 months vs 9.3 months, p = 0.004) and AML-free survival (20.6 months vs 12.9 months, p = 0.049) were significantly different (Fig. 7a). Among the 53 HR-MDS patients, there were 17 patients who had disease progression or died. Kaplan–Meier survival analysis showed that the PFS on average was significantly different between the high- and low-expression groups (13.9 months vs 6.4 months, p = 0.024), while the differences regarding OS (18.5 months vs 10.5 months, p = 0.311) and AML-free survival (16.3 months vs 10.0 months, p = 0.181) were not evident (Fig. 7b). The results indicated that high expression of CD300e might be a favorable prognostic marker for MDS.
Fig. 7.
Survival analysis for expression of CD300e in MDS patients. a Overall survival, progression-free survival and no AML evolution curves survival of MDS patients with different CD300e expression. b Overall survival, progression-free survival and no AML evolution survival curves of higher risk MDS patients with different CD300e expression
Discussion
In normal biological process, some cell surface antigens will appear or disappear in a certain order with the gradual differentiation of primitive, naive and mature monocytes. Upon the initiation of MDS, the monocytes in bone marrow will aberrantly express some antigens including CD33, CD14, CD13 and CD11b. Besides, CD64 and CD34 are also abnormally expressed but not synchronously, and there are some other markers of other spectrums, such as CD7 and CD10, expressed in dysplastic monocytes [10, 17]. FCM has shown advantages in analysis of aberrant immunophenotypes of blood cells in patients with MDS, and it is proved to have clinical value in prognostic stratification of MDS and differentiation between low-risk MDS and other cases of cytopenias [18]. In the current study, we adopted FCM to analyze whether the antigens mentioned above (CD33, CD14, CD13, CD11b, CD64, CD34, CD7 and CD10) exhibit differential expression between low-risk MDS and other bone marrow failure disorders. In the meantime, antigen combinations in different differentiation stages of monocytes, on the basis of the expression patterns during normal differentiation, were analyzed. The results demonstrated that CD34 expression in the LR-MDS group was much higher than that in the AA and control groups; the expression of CD300e and the proportion of CD64 + CD300e + cells were profoundly lower in the LR-MDS and CCUS groups as compared to the AA and control groups, while the proportion of CD64 + CD300e- cells was much higher. This demonstrated that the monocytes in MDS patients have disorders in differentiation and maturation, and abnormal blood cell development already occurs in CCUS. In addition, this also provides certain evidence for the differentiation between CCUS/MDS and AA. As for the differentiation between CCUS and low-risk MDS, gene mutation, including the mutation type and number, should be taken into account in diagnosis due to the certain overlapped mutation profile, and some genetic characteristics such as variant allele frequency should also be considered [19].
Here, we found that there was an association between the surface antigen expression by monocytes in MDS and clinical characteristics. Specifically, the expression of CD14, CD300e and the proportions of CD64 + CD14 + , CD64 + CD300e + cells were evidently increased in patients with single lineage cytopenias than patients with multilineage cytopenias (p < 0.05), while the proportions of CD64 + CD14- and CD64 + CD300e- cells were remarkably decreased (p < 0.05). Notably, the proportion of blasts was positively associated with the expression of CD34 and the proportions of CD64 + CD14-, CD64 + CD300e- cells, but negatively associated with the expression of CD14, CD300e and the proportion of CD64 + CD300e + cells. This implied that the abnormal differentiation of monocytes in MDS tends to be more critical with disease progression.
MDS is a disorder of bone marrow failure mainly characterized by ineffective hematopoiesis, refractory cytopenias with abnormal differentiation and development of myeloid cells. It has a chance to transform to AML, leading to a high risk of patient death. Research revealed that the abnormal immunophenotypic changes of bone marrow cells in MDS were tightly associated with the risk stratification of MDS, AML transformation and prognosis [20–22]. CD300e which is expressed by monocytes and mDCs can prevent the apoptosis of these two types of cells by triggering intracellular calcium mobilization and inducing the production of superoxide anion O(2)(-). In that way, proinflammatory cytokines will be released and the expression of cell surface costimulatory molecules will be increased [12]. An animal experimental showed that CD300e could interact with DNAX-activating protein 12 (DAP12) to transmit immune-activating signals [23], which suggested that CD300e might be an immune-activating receptor involved in the innate immune response of bone marrow cells. However, Matteo Pagliari et al. [24] reported that H pylori (helicobacter pylori) could affect the ability of macrophages to express and expose MHC II molecules by regulating the expression of CD300e via miR-4270, without altering phagocytosis. As a consequence, the capability of effector T cells to recognize antigens and activate the killing potential of macrophages were impaired. Similarly, another study also found that the CD300e expression could negatively regulate T cell activation by causing damages to the STAT1-dependent antigen presentation [25]. The studies demonstrate that CD300e might play a dual part in immunoregulation. In cases with MDS, the impaired immunosurveillance system and most importantly, the cellular immuno-deficiency medicated by T cells and natural killer (NK) cells, can facilitate the immune escape and tolerance of malignant clonal cells [26–28]. There has been no report devoted to the role of CD300e in MDS. Under this background, research into the expression of CD300e in MDS and its association with disease prognosis is conducive to further studies on pathogenesis of MDS and outcome evaluation.
The present study also divided patients into HR-MDS and LR-MDS groups according to the IPSS-R scores. Comparatively, the expression of CD14, CD300e and the proportions of CD64 + CD14 + and CD64 + CD300e + cells were much lower in the HR-MDS group than those in the LR-MDS group (p < 0.05), while the expression of CD34 and the proportions of CD64 + CD14- and CD64 + CD300e- cells were evidently higher (p < 0.05). In addition, it was also noted that the IPSS-R score was positively associated with the number of abnormal antigens on surface of monocytes in MDS. Collectively, the findings suggest that the expression of antigens on the surface of bone marrow monocytes in MDS changes during the disease progression. In the meantime, there are disorders of the maturation and differentiation of monocytes, which indicates the gradual aggravation of differentiation abnormality and increased risk of AML transformation. Besides, the important role of CD300e in MDS progression was also revealed. Furthermore, we also analyzed the survival outcome of the patients after 24 months of follow-up. It was found that patients highly expressing CD300e had prolonged PFS and AML-free survival than patients lowly expressing CD300e, which indicated that high expression of CD300e might be a favorable prognostic marker for MDS.
To conclude, this study focused on immunophenotypic changes of bone marrow monocytes to study their effect in MDS transformation to AML and disease prognosis. It was revealed that patients with CCUS and MDS have disorders of differentiation and maturation of monocytes as compared to patients with AA and normal people. In addition, MDS progresses or transforms to AML during that process. Moreover, CD300e expression was found to show significant associations with the clinical stage and disease progression of MDS, and high CD300e expression has the potential to be a favorable prognostic marker for MDS. This study provides important insights to the diagnosis, differentiation and prognosis of MDS based on the cell surface antigens on monocytes. Given the large variance between MDS patients regarding the natural disease course and prognosis and the multiple factors that affect MDS prognosis and risky for the AML transformation, a prospective study is required to further prove the clinical significance of CD300e as a marker for diagnosis and prognosis of MDS.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
LL, SY, and XH designed the research study, performed the analysis, and wrote the paper. ZL, XT, XR, and XG abstracted the data and assisted in the collection and analysis of the data. LL and RF critically revised the paper, performed data analysis, and ensured correct analysis of the data. All authors reviewed the manuscript.
Funding
Not applicable.
Data availability
The datasets generated for this study are available on request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The study was done following the Declaration of Helsinki, confirmed by the Ethics Committee of Tianjin Medical University General Hospital and provided written informed consent to participate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijuan Li, Shunjie Yu and Xian Hu have contributed equally to this work and share first authorship.
Contributor Information
Lijuan Li, Email: lilijuan20@qq.com.
Rong Fu, Email: furong8369@tmu.edu.cn.
References
- 1.Cazzola M. Myelodysplastic syndromes. N Engl J Med. 2020;383(14):1358–1374. doi: 10.1056/NEJMra1904794. [DOI] [PubMed] [Google Scholar]
- 2.Montalban-Bravo G, Garcia-Manero G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol. 2018;93(1):129–147. doi: 10.1002/ajh.24930. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka TN, Bejar R. MDS overlap disorders and diagnostic boundaries. Blood. 2019;133(10):1086–1095. doi: 10.1182/blood-2018-10-844670. [DOI] [PubMed] [Google Scholar]
- 4.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371–3378. doi: 10.1182/blood-2017-01-763425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang M, Li J, Zhao G, Sui X, Zhao X, Xu H. Immunophenotype of myeloid granulocytes: a pilot study for distinguishing myelodysplastic syndrome and aplastic anemia by flow cytometry. Int J Lab Hematol. 2010;32(3):275–281. doi: 10.1111/j.1751-553X.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Jiang H, Che M, et al. Abnormal CD25 expression on hematopoietic cells in myelodysplastic syndromes. Leuk Res. 2018;67:12–16. doi: 10.1016/j.leukres.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Chen JX, Mei LP, Chen BG, et al. Over-expression of CD200 predicts poor prognosis in MDS. Leuk Res. 2017;56:1–6. doi: 10.1016/j.leukres.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen-Kerkhoff N, Westers TM, Poddighe PJ, de Gruijl TD, Kordasti S, van de Loosdrecht AA. Thrombomodulin-expressing monocytes are associated with low-risk features in myelodysplastic syndromes and dampen excessive immune activation. Haematologica. 2020;105(4):961–971. doi: 10.3324/haematol.2019.219303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood. 2013;121(11):1951–1960. doi: 10.1182/blood-2012-09-435057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aanei CM, Picot T, Tavernier E, Guyotat D, Campos CL. Diagnostic utility of flow cytometry in myelodysplastic syndromes. Front Oncol. 2016;6:161. doi: 10.3389/fonc.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark GJ, Jamriska L, Rao M, Hart DN. Monocytes immunoselected via the novel monocyte specific molecule, CD300e, differentiate into active migratory dendritic cells. J Immunother. 2007;30(3):303–311. doi: 10.1097/01.cji.0000211342.65964.9e. [DOI] [PubMed] [Google Scholar]
- 12.Brckalo T, Calzetti F, Pérez-Cabezas B, Borràs FE, Cassatella MA, López-Botet M. Functional analysis of the CD300e receptor in human monocytes and myeloid dendritic cells. Eur J Immunol. 2010;40(3):722–732. doi: 10.1002/eji.200939468. [DOI] [PubMed] [Google Scholar]
- 13.Haseda F, Imagawa A, Nishikawa H, et al. Antibody to CMRF35-like molecule 2, CD300e a novel biomarker detected in patients with fulminant type 1 diabetes. PLoS ONE. 2016;11(8):e0160576. doi: 10.1371/journal.pone.0160576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenarruzabeitia O, Astarloa-Pando G, Terrén I, et al. T cell activation, highly armed cytotoxic cells and a shift in monocytes CD300 receptors expression is characteristic of patients with severe COVID-19. Front Immunol. 2021;12:655934. doi: 10.3389/fimmu.2021.655934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Loosdrecht AA, Alhan C, Béné MC, et al. Standardization of flow cytometry in myelodysplastic syndromes: report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica. 2009;94(8):1124–1134. doi: 10.3324/haematol.2009.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duetz C, Westers TM, van de Loosdrecht AA. Clinical implication of multi-parameter flow cytometry in myelodysplastic syndromes. Pathobiology. 2019;86(1):14–23. doi: 10.1159/000490727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karner K, George TI, Patel JL. Current aspects of clonal hematopoiesis: implications for clinical diagnosis. Ann Lab Med. 2019;39(6):509–514. doi: 10.3343/alm.2019.39.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chee L, Ritchie D, Ludford-Menting M, et al. Dysregulation of immune cell and cytokine signaling correlates with clinical outcomes in myelodysplastic syndrome (MDS) Eur J Haematol. 2021 doi: 10.1111/ejh.13742.10.1111/ejh.13742. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Moon A, Hoyle E, et al. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytometry B Clin Cytom. 2019;96(1):67–72. doi: 10.1002/cyto.b.21744. [DOI] [PubMed] [Google Scholar]
- 22.Majcherek M, Kiernicka-Parulska J, Mierzwa A, et al. The diagnostic and prognostic significance of flow cytometric bone marrow assessment in myelodysplastic syndromes according to the European LeukemiaNet recommendations in single-centre real-life experience. Scand J Immunol. 2021;94(2):e13028. doi: 10.1111/sji.13028. [DOI] [PubMed] [Google Scholar]
- 23.Isobe M, Izawa K, Sugiuchi M, et al. The CD300e molecule in mice is an immune-activating receptor. J Biol Chem. 2018;293(10):3793–3805. doi: 10.1074/jbc.RA117.000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliari M, Munari F, Toffoletto M, et al. Helicobacter pylori affects the antigen presentation activity of macrophages modulating the expression of the immune receptor CD300E through miR-4270. Front Immunol. 2017;8:1288. doi: 10.3389/fimmu.2017.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coletta S, Salvi V, Della Bella C, et al. The immune receptor CD300e negatively regulates T cell activation by impairing the STAT1-dependent antigen presentation. Sci Rep. 2020;10(1):16501. doi: 10.1038/s41598-020-73552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montes P, Bernal M, Campo LN, et al. Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol Immunother. 2019;68(12):2015–2027. doi: 10.1007/s00262-019-02420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarhan D, Wang J, Sunil Arvindam U, et al. Mesenchymal stromal cells shape the MDS microenvironment by inducing suppressive monocytes that dampen NK cell function. JCI Insight. 2020;5(5):e130155. doi: 10.1172/jci.insight.130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fozza C, Crobu V, Isoni MA, Dore F. The immune landscape of myelodysplastic syndromes. Crit Rev Oncol Hematol. 2016;107:90–99. doi: 10.1016/j.critrevonc.2016.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.