Abstract
We present a divergent strategy for the fluorination of phenylacetic acid derivatives that is induced by a charge-transfer complex between Selectfluor and 4-(dimethylamino)pyridine. A comprehensive investigation of the conditions revealed a critical role of the solvent on the reaction outcome. In the presence of water, decarboxylative fluorination through a single-electron oxidation is dominant. Non-aqueous conditions result in the clean formation of α-fluoro-α-arylcarboxylic acids.
Fluorination increases the lipophilicity and metabolic stability of organic molecules, resulting in improved active pharmaceutical ingredients and agrochemicals.1−4 Position emission tomography (PET) of 18F-labeled radiopharmaceuticals is important for studying biochemical pathways and physiological processes.5 Consequently, the development of efficient and selective protocols for constructing C–F bonds is desirable.6 Nucleophilic fluorination and electrophilic fluorination dominate the field, but radical fluorinations recently gained significant momentum for two reasons.6 First, the restriction to hazardous radical fluorine sources, such as XeF2 and F2, was overcome by the discovery that electrophilic N–F reagents transfer fluorine atoms to carbon-centered radicals.7 Second, the increasing interest in synthetic radical chemistry resulted in attractive methods for generating C-centered radicals using dedicated catalysts and reagents.8
The formation of benzylic C(sp3)–F bonds using 1-(chloromethyl)-4-fluoro-1,4-diazabicyclo[2.2.2]octane-1,4-diium ditetrafluoroborate (Selectfluor)9 is among the most studied C–F bond formations that proceed via a radical mechanism.6,7,10−13 These transformations are promising tools for modifying drug candidates for preventing undesired benzylic oxidation by cytochrome P450 oxidases.14 Common strategies are decarboxylative fluorinations that use photocatalysts15−18 or silver catalysts to induce single-electron-transfer (SET) oxidation19−21 and the direct fluorination of benzylic C(sp3)–H bonds using catalysts or reagents that enable hydrogen atom transfer (HAT) (Scheme 1A).22−26 It must be noted that the α-fluorination of phenylacetic acids can also be carried out using Selectfluor via a silyl ketene acetal that is formed using a strong base21,27,28 or with the aid of catalytic amounts of a strong Lewis acid and an organic base to generate an enediolate intermediate.29
Scheme 1. Benzylic C(sp3)–F Bond Formation Using Selectfluor through Radical Intermediates.
A dedicated catalyst or reagent is regularly used to generate the key benzylic radical, which ultimately undergoes F atom transfer with Selectfluor to yield the desired product. Such benzylic fluorinations may also proceed through electron transfer or proton-coupled electron transfer rather than HAT.30 Catalyst-free benzylic fluorination of aza-heterocycles was reported to proceed through the formation of a charge-transfer (CT) complex between N-heterocyclic substrates and Selectfluor. This induces a stepwise electron/proton transfer or a concerted proton-coupled electron-transfer process.31 Nitrogen–fluorine halogen bonding between Selectfluor and pyridine additives was proposed to facilitate silver-catalyzed radical fluorinations, but no product was observed in the absence of the metal catalyst.32
We envisioned that a CT complex between Selectfluor and an aromatic N-heterocyclic compound could generate the N-(chloromethyl)triethylenediamine radical dication (TEDA2+•), a potent single-electron oxidant and hydrogen atom-transfer reagent.33 This would access an operationally simple and divergent strategy for the generation of benzylic carbon-centered radicals that could ultimately engage with Selectfluor to form C–F bonds. Here we present that this mechanistic blueprint can indeed be applied to achieve the direct fluorination of benzylic C(sp3)–H bonds that likely proceeds via a HAT mechanism and the decarboxylative formation of benzylic C(sp3)–F bonds through a SET process (Scheme 1B).
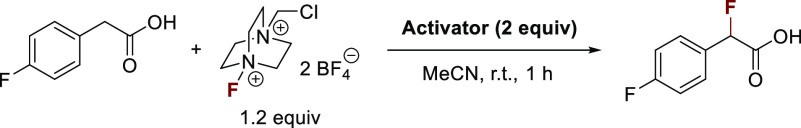
We started our investigations by studying whether a combination of Selectfluor and 4-(dimethylamino)pyridine (DMAP) triggers decarboxylative C–F bond formation or direct fluorination of benzylic C(sp3)–H bonds. We chose 2-(4-fluorophenyl)acetic acid as model substrate that serves as an ideal probe for both scenarios (Scheme 2A). To our delight, we indeed observed a mixture of both fluorination products at room temperature with good selectivity toward the decarboxylative product in an acetonitrile/water mixture. Under non-aqueous conditions, α-fluorination occurred selectively.
Scheme 2. Initial Results and Scope of the Benzylic C(sp3)–F Bond Formation Using Selectfluor and DMAP.
Yields were determined by 1H NMR using dimethyl maleate as the internal standard. Isolated yields are in parentheses.
After considerable experimentation (see the Supporting Information), we found that the decarboxylative fluorination works best in an acetone/water (1:1) mixture using 2 equiv of DMAP and an excess of Selectfluor (3 equiv) (Scheme 2B). Also, the addition of sodium fluoride (2 equiv) and an increased temperature (70 °C) were beneficial for converting a series of phenylacetic acid derivatives to the corresponding, volatile SET products (1–10) within 30 min in moderate to good NMR yields. Unreacted starting material was observed in many cases, and no major side product could be identified (see the Supporting Information).
A combination of 2 equiv of DMAP and 1.2 equiv of Selectfluor in acetonitrile produced the α-fluorination products (11–30) at room temperature in good to excellent NMR yields (Scheme 2B). Phenylacetic acid derivatives with electron-rich and electron-deficient substituents were cleanly converted to the respective α-fluoro-α-arylacetic acids. Small amounts of unreacted starting material were detected in all cases, which were difficult to separate by column chromatography and resulted in modest isolated yields in certain cases. A reaction time of 1 h was used for practical reasons, but detailed investigations revealed that the fluorination occurs in <5 min (Tables S14 and S15). Interestingly, the carboxylic acid functionality is crucial for α-fluorination. No reaction was observed using other functional groups, such as ketones, esters, amides, boronic acid esters, or boronates (see the Supporting Information).
We propose that a mixture of DMAP and Selectfluor spontaneously produces TEDA2+•, which acts as a chain carrier in a SET or HAT process (Scheme 3). The radical chain is efficient for the HAT route (1.2 equiv of Selectfluor under optimized conditions), whereas the SET pathway seems to suffer from a significant amount of undesired termination events (3 equiv of Selectfluor under optimized conditions). The switch between the SET and HAT pathway is a consequence of different pKa values of phenylacetic acids and DMAP under the applied conditions. The organic base deprotonates the carboxylic acid in an aqueous environment, enabling single-electron oxidation of the carboxylate by TEDA2+•. SET oxidation triggers decarboxylation to produce a C-centered radical that ultimately reacts with Selectfluor to yield the desired product and TEDA2+•. Phenylacetic acid derivatives have low acidity in aprotic polar solvents (pKa of phenylacetic acid in MeNO2 > 19).34 The pKa of the conjugated acid of pyridine derivatives in MeCN is lower.35 As a result, the amount of carboxylate under these conditions is negligible. This reduces the likelihood of decarboxylative SET and HAT becoming the dominant pathway.
Scheme 3. Proposed Mechanism for the Formation of Benzylic C(sp3)–F Bonds via the Activation of Selectfluor Using DMAP.
While there are no plausible alternatives to the SET mechanism, α-fluoro-α-arylacetic acid formation may occur through a more traditional “electrophilic” fluorination mechanism rather than HAT. Surprisingly, we also observed product formation using NFSI (N-fluorobenzenesulfonimide) instead of Selectfluor (Table S10), which was, to the best of our knowledge, never reported to produce a radical chain carrier. We therefore carried out a series of experiments to shed some light on the mechanism. An enediolate species was not observed upon treatment of phenylacetic acid with DMAP in MeCN-d3 (Figure S3). This is in agreement with a study that shows that the formation of such species using organic bases requires strong Lewis acids as activators.29 However, a radical clock experiment did not prove the formation of the proposed benzylic radical intermediate (Scheme S2). The addition of the radical scavenger TEMPO resulted in the consumption of the substrate, but no trace of the fluorination product was formed, indicative of a radical mechanism (Scheme S3). Competition experiments involving deuterium-labeled substrates indicate that C–H bond cleavage is the rate-determining step (Scheme S4). In addition, competitive experiments between phenylacetic acids with different substituents on the aromatic ring showed that electron-rich aromatic systems react slower (Scheme S5). These experiments suggest that the reaction does not proceed through a SET oxidation followed by deprotonation.
The simple preparation of α-fluoro-α-arylcarboxylic acids through our method is attractive compared to the most common approach for synthesizing such scaffolds that requires formation of a silyl ketene acetal using a strong base, followed by treatment with Selectfluor.21,27,28 Our α-fluorination is not sensitive to air or moisture, works with bench-stable reagents, and produces the desired products in up to quantitative yields as determined by 19F NMR (isolated yields range from 32% to 86%). This operational simplicity is promising for the synthesis of 18F-labeled radiopharmaceuticals using [18F]Selectfluor. In particular, the short reaction times are ideally suited for such applications, due to the short half-lives of 18F-labeled radionuclides (110 min).36 Monitoring the fluorination of 4-tert-butylphenylacetic acid using in situ FTIR spectroscopy showed that the reaction forms the desired product instantaneously once Selectfluor is added to a solution of DMAP and the substrate in MeCN (Figure 1A). Fast consumption of the fluorination reagent was also monitored in the absence of the substrate (Figure S6). This supports our mechanistic proposal that the fluorine source and the organic base form a reactive, labile species that leads to productive fluorination or, if no substrate is present in the reaction mixture, to degradation products. 1H NMR experiments using Teflon inserts showed the formation of a DMAP·HF adduct upon mixing DMAP and Selectfluor along with several unidentified compounds that are likely N-fluorinated DMAP derivatives.37 We carried out a series of “delayed addition” experiments to clarify if the order of reagent addition is crucial for successful fluorination (Figure 1B). When Selectfluor and DMAP were mixed in MeCN and the substrate was added after 30 min, no reaction was observed. Premixing the substrate with Selectfluor or DMAP is possible.
Figure 1.
Practical aspects of the HAT protocol. (A) Reaction monitoring using in situ FTIR spectroscopy. (B) Delayed addition experiments.
Finally, we sought to study if a strong electron-donating substituent on the pyridine base is crucial for reactivity. Exchanging DMAP with 4-aminopyridine or 4-methoxypyridine reduced the efficacy of this reaction (Table 1). Modest conversions were obtained with pyridine, emphasizing that a strong Lewis basicity is key for the generation of TEDA2+•.
Table 1. Influence of Lewis Basicity on N–F Bond Activation of Selectfluora.
Reaction conditions: 4-fluorophenylacetlc acid (0.3 mmol), Selectfluor (0.36 mmol), DMAP (0.6 mmol), MeCN (1.5 mL), rt, 4 h.
Converaion of 4-fluorophenylacetic acid determined by 1H NMR using dimethyl maleate as an internal standard.
NMR yield determined by 1H NMR using dimethyl maleate as an internal standard.
In summary, we developed a new strategy for the formation of benzylic C(sp3)–F bonds that is proposed to proceed via the formation of TEDA2+• from Selectfluor and 4-(dimethylamino)pyridine. Controlling the pKa of phenylacetic acid derivatives via the reaction media enables switching between reaction mechanisms that enables the selective formation of different products. Under aqueous conditions, a decarboxylative fluorination was observed, whereas non-aqueous conditions allow for direct fluorination of benzylic C(sp3)–H bonds. This enables a facile and clean formation of α-fluoro-α-arylacetic acids within a few minutes at room temperature.
Acknowledgments
The authors gratefully acknowledge the Max Planck Society for generous financial support. A.M. and B.P. acknowledge the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy-EXC 2008/1 (UniSysCat)-390540038 for financial support. L.A. and B.P. acknowledge the DFG for financial support (PI 1635/2-19). B.P. acknowledges financial support by a Liebig Fellowship from the German Chemical Industry Fund [Fonds der Chemischen Industrie (FCI)]. The authors thank our colleague John J. Molloy (Max-Planck-Institute of Colloids and Interfaces) for fruitful scientific discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02050.
Experimental procedures and characterization data (PDF)
Open access funded by Max Planck Society.
A version of this research was previously posted to chemRxiv.38
The authors declare no competing financial interest.
Supplementary Material
References
- Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- O’Hagan D. Fluorine in health care: Organofluorine containing blockbuster drugs. J. Fluorine Chem. 2010, 131, 1071–1081. 10.1016/j.jfluchem.2010.03.003. [DOI] [Google Scholar]
- Fujiwara T.; O’Hagan D. Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem. 2014, 167, 16–29. 10.1016/j.jfluchem.2014.06.014. [DOI] [Google Scholar]
- Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci. 2014, 5, 4545–4553. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne P. A.; Desroches J.; Hamel J.-D.; Vandamme M.; Paquin J.-F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174. 10.1021/cr500706a. [DOI] [PubMed] [Google Scholar]
- Chatalova-Sazepin C.; Hemelaere R.; Paquin J.-F.; Sammis G. M. Recent Advances in Radical Fluorination. Synthesis 2015, 47, 2554–2569. 10.1055/s-0034-1378824. [DOI] [Google Scholar]
- Crespi S.; Fagnoni M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833. 10.1021/acs.chemrev.0c00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler P. T.; Durón S. G.; Burkart M. D.; Vincent S. P.; Wong C. H. Selectfluor: mechanistic insight and applications. Angew. Chem., Int. Ed. 2005, 44, 192–212. 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- Tarantino G.; Hammond C. Catalytic C(sp3)–F bond formation: recent achievements and pertaining challenges. Green Chem. 2020, 22, 5195–5209. 10.1039/D0GC02067B. [DOI] [Google Scholar]
- Szpera R.; Moseley D. F. J.; Smith L. B.; Sterling A. J.; Gouverneur V. The Fluorination of C–H Bonds: Developments and Perspectives. Angew. Chem., Int. Ed. 2019, 58, 14824–14848. 10.1002/anie.201814457. [DOI] [PubMed] [Google Scholar]
- Lantaño B.; Postigo A. Radical fluorination reactions by thermal and photoinduced methods. Org. Biomol. Chem. 2017, 15, 9954–9973. 10.1039/C7OB02402A. [DOI] [PubMed] [Google Scholar]
- Xiao P.; Pannecoucke X.; Bouillon J.-P.; Couve-Bonnaire S. Wonderful fusion of organofluorine chemistry and decarboxylation strategy. Chem. Soc. Rev. 2021, 50, 6094–6151. 10.1039/D1CS00216C. [DOI] [PubMed] [Google Scholar]
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- Ventre S.; Petronijevic F. R.; MacMillan D. W. C. Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137, 5654–5657. 10.1021/jacs.5b02244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Meng C.; Yuan X.; Jia X.; Qian X.; Ye J. Transition-metal-free visible-light photoredox catalysis at room-temperature for decarboxylative fluorination of aliphatic carboxylic acids by organic dyes. Chem. Commun. 2015, 51, 11864–11867. 10.1039/C5CC04527D. [DOI] [PubMed] [Google Scholar]
- Tarantino G.; Hammond C. Catalytic Formation of C(sp3)–F Bonds via Heterogeneous Photocatalysis. ACS Catal. 2018, 8, 10321–10330. 10.1021/acscatal.8b02844. [DOI] [Google Scholar]
- Pieber B.; Shalom M.; Antonietti M.; Seeberger P. H.; Gilmore K. Continuous Heterogeneous Photocatalysis in Serial Micro-Batch Reactors. Angew. Chem., Int. Ed. 2018, 57, 9976–9979. 10.1002/anie.201712568. [DOI] [PubMed] [Google Scholar]
- Nguyen T. V.; Pham V. T.; Nguyen T. V. T.; Phan N. T. S.; Truong T. Decarboxylative fluorination of aliphatic carboxylic acids under heterogeneous delafossite AgFeO2 nanoparticle catalysis: The utilization of bimetallic cooperativity. J. Catal. 2018, 360, 270–276. 10.1016/j.jcat.2018.02.018. [DOI] [Google Scholar]
- Wang Z.; Guo C.-Y.; Yang C.; Chen J.-P. Ag-Catalyzed Chemoselective Decarboxylative Mono- and gem-Difluorination of Malonic Acid Derivatives. J. Am. Chem. Soc. 2019, 141, 5617–5622. 10.1021/jacs.9b00681. [DOI] [PubMed] [Google Scholar]
- Mizuta S.; Stenhagen I. S. R.; O’Duill M.; Wolstenhulme J.; Kirjavainen A. K.; Forsback S. J.; Tredwell M.; Sandford G.; Moore P. R.; Huiban M.; Luthra S. K.; Passchier J.; Solin O.; Gouverneur V. Catalytic Decarboxylative Fluorination for the Synthesis of Tri- and Difluoromethyl Arenes. Org. Lett. 2013, 15, 2648–2651. 10.1021/ol4009377. [DOI] [PubMed] [Google Scholar]
- Xia J.-B.; Zhu C.; Chen C. Visible Light-Promoted Metal-Free C–H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination. J. Am. Chem. Soc. 2013, 135, 17494–17500. 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P.; Guo S.; Wang L.; Tang P. Silver-Catalyzed Oxidative Activation of Benzylic C-H Bonds for the Synthesis of Difluoromethylated Arenes. Angew. Chem., Int. Ed. 2014, 53, 5955–5958. 10.1002/anie.201400225. [DOI] [PubMed] [Google Scholar]
- Amaoka Y.; Nagatomo M.; Inoue M. Metal-Free Fluorination of C(sp3)–H Bonds Using a Catalytic N-Oxyl Radical. Org. Lett. 2013, 15, 2160–2163. 10.1021/ol4006757. [DOI] [PubMed] [Google Scholar]
- Hua A. M.; Mai D. N.; Martinez R.; Baxter R. D. Radical C–H Fluorination Using Unprotected Amino Acids as Radical Precursors. Org. Lett. 2017, 19, 2949–2952. 10.1021/acs.orglett.7b01188. [DOI] [PubMed] [Google Scholar]
- Ma J.-j.; Yi W.-b.; Lu G.-p.; Cai C. Transition-metal-free C–H oxidative activation: persulfate-promoted selective benzylic mono- and difluorination. Org. Biomol. Chem. 2015, 13, 2890–2894. 10.1039/C4OB02418D. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Song J. Z. A novel general method for preparation of α-fluoro-α-arylcarboxylic acid. Direct fluorination of silyl ketene acetals with Selectfluor®. Tetrahedron Lett. 2006, 47, 7641–7644. 10.1016/j.tetlet.2006.08.057. [DOI] [Google Scholar]
- Wang H.; Liu C.-F.; Song Z.; Yuan M.; Ho Y. A.; Gutierrez O.; Koh M. J. Engaging α-Fluorocarboxylic Acids Directly in Decarboxylative C–C Bond Formation. ACS Catal. 2020, 10, 4451–4459. 10.1021/acscatal.0c00789. [DOI] [Google Scholar]
- Hu H.; Wang C.; Wu X.; Liu Y.; Yue G.; Su G.; Feng J. Boron-catalyzed α-C–H fluorination of aryl acetic acids. Org. Chem. Front. 2022, 9, 1315–1320. 10.1039/D1QO01814K. [DOI] [Google Scholar]
- Bloom S.; McCann M.; Lectka T. Photocatalyzed benzylic fluorination: shedding ″light″ on the involvement of electron transfer. Org. Lett. 2014, 16, 6338–6341. 10.1021/ol503094m. [DOI] [PubMed] [Google Scholar]
- Danahy K. E.; Cooper J. C.; Van Humbeck J. F. Benzylic Fluorination of Aza-Heterocycles Induced by Single-Electron Transfer to Selectfluor. Angew. Chem., Int. Ed. 2018, 57, 5134–5138. 10.1002/anie.201801280. [DOI] [PubMed] [Google Scholar]
- Hua A. M.; Bidwell S. L.; Baker S. I.; Hratchian H. P.; Baxter R. D. Experimental and Theoretical Evidence for Nitrogen–Fluorine Halogen Bonding in Silver-Initiated Radical Fluorinations. ACS Catal. 2019, 9, 3322–3326. 10.1021/acscatal.9b00623. [DOI] [Google Scholar]
- Aguilar Troyano F. J.; Merkens K.; Gómez-Suárez A. Selectfluor® Radical Dication (TEDA2+.) – A Versatile Species in Modern Synthetic Organic Chemistry. Asian J. Org. Chem. 2020, 9, 992–1007. 10.1002/ajoc.202000196. [DOI] [Google Scholar]
- Subirats X.; Porras S. P.; Rosés M.; Kenndler E. Nitromethane as solvent in capillary electrophoresis. J. Chrom A 2005, 1079, 246–253. 10.1016/j.chroma.2005.02.072. [DOI] [PubMed] [Google Scholar]
- Tshepelevitsh S.; Kütt A.; Lõkov M.; Kaljurand I.; Saame J.; Heering A.; Plieger P. G.; Vianello R.; Leito I. On the Basicity of Organic Bases in Different Media. Eur. J. Org. Chem. 2019, 2019, 6735–6748. 10.1002/ejoc.201900956. [DOI] [Google Scholar]
- Teare H.; Robins E. G.; Kirjavainen A.; Forsback S.; Sandford G.; Solin O.; Luthra S. K.; Gouverneur V. Radiosynthesis and evaluation of [18F]Selectfluor bis(triflate). Angew. Chem., Int. Ed. 2010, 49, 6821–4. 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- Chauhan M. S.; Yadav G. D.; Hussain F.; Singh S. N-Fluorobenzenaminium tetrafluoroborate generated in situ by aniline and Selectfluor as a reusable catalyst for the ring opening of epoxides with amines under microwave irradiation. Catal. Sci. Technol. 2014, 4, 3945–3952. 10.1039/C4CY00609G. [DOI] [Google Scholar]
- Madani A.; Anghileri L.; Heydenreich M.; Möller H. M.; Pieber B. Benzylic fluorination induced by N–F bond activation of Selectfluor with a solvent-dependent selectivity switch. chemRxiv 2022, 10.26434/chemrxiv-2022-mstv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.