Abstract
Background/Objective:
Pediatric neurocritical care survivorship is frequently accompanied by functional impairments. Lack of prognostic biomarkers is a barrier to early identification and management of impairment. We explored the association between blood biomarkers and functional impairment in children with acute acquired brain injury.
Methods:
Secondary analysis of a randomized control trial evaluating early versus usual care rehabilitation in the pediatric intensive care unit (PICU). Forty-four children (17 [39%] female, median age 11 [interquartile range 6-13] years) with acute acquired brain injury admitted to the PICU were studied. A single center obtained serum samples on admission days 0, 1, 3, 5, and the day closest to hospital discharge. Biomarkers relevant to brain injury (neuron specific enolase [NSE], S100b), inflammation (interleukin [IL- 6], C-reactive protein [CRP]), and regeneration (brain derived neurotrophic factor [BDNF], vascular endothelial growth factor [VEGF]) were collected. Biomarkers were analyzed using a Luminex® bioassay. Functional Status Scale (FSS) scores were abstracted from the medical record. New functional impairment was defined as a (worse) FSS score at hospital discharge compared to pre-PICU (baseline). Individual biomarker fluorescence index (FI) values for each sample collection day were correlated with new functional impairment using Spearman rank correlation coefficient (ρ). Trends in repeated measures of biomarker FI over time were explored graphically, and the association between repeated measures of biomarker FI and new functional impairment were analyzed using covariate adjusted linear mixed effect models.
Results:
Functional impairment was inversely correlated with markers of regeneration and plasticity including BDNF at day 3 (ρ= −.404, p=.015), day 5 (ρ= −.549, p=.005) and hospital discharge (ρ= −.420, p=.026), and VEGF at day 1 (ρ= −.282, p=.008) and hospital discharge (ρ= −.378, p=.047), such that lower levels of both markers at each time point were associated with greater impairment. Similarly, repeated measures of BDNF and VEGF were inversely correlated with new functional impairment (B=−.001, p=.001and B=−.001, p=.003, respectively). NSE, a biomarker of acute brain injury, showed a positive correlation between day 0 levels and new functional impairment (ρ=.320, p=.044).
Conclusions:
Blood-based biomarkers of regeneration and plasticity may hold prognostic utility for functional impairment among pediatric patients with neurocritical illness and warrant further investigation.
Keywords: Brain injury, biomarkers, pediatrics, critical care, rehabilitation
Introduction
In highly resourced pediatric intensive care units (PICUs), mortality has become an uncommon event while acquisition of new morbidities impairing function has increased [1]. Children admitted to the PICU with acute neurologic injuries have 4 to 6 times the mortality rate of the general PICU population [2]. Furthermore, neurocritical care survivors are at high risk for acquired cognitive, physical, social, and emotional health morbidities, collectively referred to as Post-Intensive Care Syndrome in Pediatrics (PICS-p)[3]. Recovery trajectories vary by neurological condition, age, and rehabilitation response[1,4–8]. In a prospective cohort study on functional outcomes post-PICU, Choong and colleagues found that acute neurologic condition was the greatest predictor of unfavorable functional outcomes 6 months post discharge after controlling for illness severity and pre-PICU functioning [5].
Healthcare utilization is high among ICU survivors with associated post hospital healthcare costs ranging from $18,847–$148,454 during the first year following ICU discharge in the United States (US) in 2011 [9]. Furthermore, children with neurologic diagnoses are 3 times as likely to be admitted to the ICU, have longer lengths of stay (LOS), and greater hospital costs compared to other hospitalized children[10]. Emerging evidence suggests early ICU-based rehabilitative therapy holds potential for improving functional recovery [11]. However, the lack of validated screening tools that facilitate early PICU-based assessment of children at higher-risk for new functional impairment is a barrier to identifying those who could benefit from rehabilitation services.
Blood-based biomarkers are potential clinical tools to elucidate risk of future functional impairment. In critically ill adults, inflammatory biomarkers including C-reactive protein (CRP), Interleukin-6 and 10 (IL-6, IL-10), and regeneration biomarker brain derived neurotrophic factor (BDNF) measured early after admission (e.g., within 24-48 hours) and at ICU discharge predicted post-discharge mortality and functional recovery [12–15]. In children with specific neurocritical conditions (e.g., traumatic brain injury [TBI] and cardiac arrest), brain injury-based biomarkers (e.g., neuron specific enolase and S100b) have been assessed for diagnostic and prognostic properties [16–22]. However, markers of regeneration and plasticity have not been tested to prognosticate outcome in acute pediatric acute acquired brain injury. A validated screening tool that includes patient characteristics and biomarkers could facilitate personalized approaches to prognostication, therapeutic interventions, and to monitor response to interventions.[17,21,23–25].
Our objective was to perform an exploratory analysis of the relationships among blood-based biomarkers of injury, inflammation, regeneration and plasticity with child functional outcome status among survivors of neurocritical illness who participated in a trial of ICU-based rehabilitation. We hypothesized that these serum biomarkers of injury, inflammation, and regeneration during the PICU stay would predict functional impairment at hospital discharge.
Methods
Design and Setting.
We utilized data from a randomized control pilot trial evaluating the safety and feasibility of an early, protocolized rehabilitation intervention in the PICU compared to usual care [7]. The University of Pittsburgh Institutional Review Board approved the parent trial at the UPMC Children’s Hospital of Pittsburgh (CHP) (NCT02209935). Children enrolled at the Ann & Robert H. Lurie Children’s Hospital of Chicago and Cincinnati Children’s Hospital Medical Center did not have blood samples collected, and thus, were not included in this biomarker study. Informed consent was obtained from each child’s parent or guardian (hereinafter referred to as “caregiver”) by the study coordinator or site investigator, and assent was obtained from the child when appropriate.
Sample.
Eligible children (N=44) were between 3 and 17 years of age and enrolled at CHP with expected PICU stay of >2 days and diagnosed with one of the following acute brain conditions: TBI, cardiac arrest, stroke, brain mass, or central nervous system (CNS) infection or inflammation. Children with non-English speaking caregivers, a do-not-resuscitate (DNR) status, expected survival of less than 24 hours, or severe neurological dysfunction at baseline as indicated by a Pediatric Cerebral Performance Category (PCPC) score 4-5 (4=severe disability and 5=persistent vegetative state) were excluded [26]. For the purposes of this study, only children with blood samples who were living at hospital discharge and <18 years old were included.
Data Collection and Measures.
We sought to explore the association between blood biomarkers and functional impairment in pediatric patients with acute acquired brain injury, data from both groups (intervention and control) were combined for all analyses. Data used for this study were collected at study enrollment and throughout hospitalization via medical record abstraction and caregiver report.
Functional Impairment.
The primary outcome measure was new functional impairment as captured by the Functional Status Scale (FSS). The FSS rates items from 1 (normal) to 5 (very severe dysfunction) over 6 domains (mental status, sensory, communication, motor function, feeding, and respiratory) for a total score of 6 (normal)-30 (very severe dysfunction) [27]. Pre-PICU and hospital discharge FSS scores were assigned by the site study team using chart review; study team members were blinded to biomarker levels. Although the FSS domains descriptions are specific, each subdomain score description remains inclusive to allow for data obtained from thorough chart review. To capture new functional impairment, a change score was created by subtracting pre-PICU FSS from FSS score at hospital discharge. This change score was included as a continuous outcome for the Spearman Rank test and for the mixed effect models. To explore sample characteristics, this score was dichotomized into groups of FSS change score of < 3 or FSS change score of ≥ 3 as described by Pollack et al .[28]
Serum Biomarkers.
Blood samples were collected, processed, and stored on PICU admission day 0, 1, 3, and 5, and closest to hospital discharge (the longest hospital stay was 42 days) either prospectively using an existing vascular access or from remnant, banked hospital laboratory specimens. Dates were purposefully selected to represent biomarkers levels across the hospital stay. Serum biomarkers were analyzed using a custom Luminex high sensitivity bead bioassay at the University of Pittsburgh Luminex Core Facility. The bioassay measured biomarkers of brain injury (neuron specific enolase [NSE], S100b), regeneration (brain derived neurotrophic factor [BDNF], vascular endothelial growth factor [VEGF]), and inflammation (interleukin [IL- 6], C-reactive protein [CRP]). Biomarker concentrations were calculated by subtracting the background fluorescence from the sample fluorescence and are reported as fluorescence index (FI) (arbitrary units). FI values were included individually by day and as a time variant (i.e., repeated measure) variable across days for between person analyses.
Child and Clinical Characteristics.
Medical condition, illness severity (Pediatric Index of Mortality [PIM] score), hospital LOS and PICU LOS, duration of mechanical ventilation, discharge disposition, and demographic data (age, gender) were collected via medical chart abstraction.
Statistical Analysis.
Categorical variables are presented as frequencies or percentages. Continuous variables are presented as medians with interquartile ranges (IQR). Fisher’s exact test was used for categorical variables and Wilcoxon rank sum test was used for continuous variables, as data were not normally distributed.
Between day comparisons.
The strength of the relationship between individual biomarker FI values by day with new functional impairment at hospital discharge were assessed using Spearman rank correlation coefficient (ρ). Biomarker FI values over time were explored graphically using error bars with 95% confidence intervals.
Between person comparisons.
The association between repeated (daily) measures of biomarker FI values within participants and new functional impairment were explored using linear mixed effect models with fixed intercepts. Assumptions were checked and this approach was appropriate for our data Before analyses, an unadjusted model was run to explore within and between participant variability on new functional impairment. The intraclass correlation coefficient for this model was 0.98, demonstrating that most of the variability was between participants. However, we proceeded with nesting our data as repeated biomarker values as level 1 and the participant as level two to ensure separate effects were measured. All predictors were then cluster mean centered (i.e., centered on participant mean scores) before running mixed effects models. Given the small sample size, we ran a mixed effect model for each biomarker. Each model included the main effects of the biomarker, time of biomarker collection (day 0, 1, 3, 5, and hospital discharge), and illness severity as measured by the PIM. Missing data were not imputed, given mixed-effect models ability to handle missing observations [29], but are reported for each biomarker in Tables 2, 4, and 5.
Table 2.
Biomarkers of brain injury by day and their correlation with new functional impairment
| N | Median (IQR) | Correlation coefficient (ρ) | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Neuron Specific Enolase | |||||
| Day 0 | 42 | 354.50 (147.50-568.75) | .32 | .044 | |
| Day 1 | 42 | 204.00 (100.00-585.50) | .10 | .552 | |
| Day 3 | 37 | 253.50 (102.00-560.25) | −0.02 | .900 | |
| Day 5 | 27 | 202.50 (84.50-568.50) | −0.13 | .539 | |
| Hospital discharge | 28 | 268.75 (161.50-837.75) | −0.31 | .111 | |
| S100b | |||||
| Day 0 | 42 | 10.25 (4.75-27.13) | .19 | .236 | |
| Day 1 | 42 | 8.00 (3.00-15.50) | .02 | .928 | |
| Day 3 | 37 | 8.00 (4.00-14.50) | −.04 | .801 | |
| Day 5 | 27 | 7.00 (5.00-18.00) | .14 | .510 | |
| Hospital discharge | 28 | 7.50 (3.00-18.50) | −.15 | .462 | |
Table 4.
Biomarkers of inflammation by day and their correlation with new functional impairment
| N | Median (IQR) | Correlation coefficient (ρ) | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| C-Reactive Protein | |||||
| Day 0 | 42 | 19,200.75 (15,478.37 – 20,222.13) | −.094 | .563 | |
| Day 1 | 42 | 19,228.75 (16,549.75-20,350.88) | .047 | .772 | |
| Day 3 | 37 | 17,644.00 (8,955.00-19,404.50) | .228 | .183 | |
| Day 5 | 27 | 14,210.00 (10,800.50-18,587.00) | .173 | .398 | |
| Hospital discharge | 28 | 9,968.00 (5,434.38-15,602.00) | .083 | .675 | |
| Interleukin-6 | |||||
| Day 0 | 42 | 243.75 (109.63-734.88) | .184 | .256 | |
| Day 1 | 42 | 89.25 (39.75-352.50) | .246 | .126 | |
| Day 3 | 37 | 55.00 (25.50-222.25) | .318 | .059 | |
| Day 5 | 27 | 66.00 (24.00-164.00) | .295 | .144 | |
| Hospital discharge | 28 | 34.00 (21.75-94.13) | .205 | .295 | |
Table 5.
Correlation between biomarkers of regeneration with new functional impairment
| N | Median (IQR) | Correlation coefficient (ρ) | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Brain-Derived Neurotrophic Factor | |||||
| Day 0 | 42 | 390.00 (180.25-954-38) | −.207 | .200 | |
| Day 1 | 42 | 324.75 (129.63-846.38) | −0.304 | .057 | |
| Day 3 | 37 | 435.50 (152.75-1,619.25) | −0.404 | .015 | |
| Day 5 | 27 | 425.00 (162.00-968.50) | −0.529 | .005 | |
| Hospital discharge | 28 | 1,022.75 (627.38-1,816.88) | −0.420 | .026 | |
| Vascular Endothelial Growth Factor | |||||
| Day 0 | 42 | 291.75 (143.63-807.25) | −.247 | .125 | |
| Day 1 | 42 | 271.75 (66.13-718.13) | −0.282 | .008 | |
| Day 3 | 37 | 419.00 (159.00-1,130.00) | −.287 | .090 | |
| Day 5 | 27 | 619.00 (287.00-1,082.50) | −.222 | .277 | |
| Hospital discharge | 28 | 747.75 (444.75-1,397.00) | −.378 | .047 | |
All analyses were conducted using Stata (StataCorp LLC, College Station, Texas) and SPSS version 26 using the GENLINMIXED procedure.
Results
As shown in Table 1, the median age for the study cohort was 11 (IQR: 6-14) years, and the majority were male (61%) and received mechanical ventilation (74%). The median PIM score was 3.38 (IQR: 1.29-16.88), and the most frequent neurocritical illness was brain mass (32%) followed by TBI (27%). There were no statistically significant differences in age, sex, condition, or illness severity score between children with FSS < 3 and FSS ≥ 3 at hospital discharge. There was no difference between parent trial group assignment (ICU-based rehabilitation n=20 [46%] and usual care n=24 [34%]) and new functional impairment. Compared to children with FSS change of < 3, those with an FSS change of ≥ 3 had a longer hospital LOS (20 [9–28] versus 11 [7–18] days, p=.027) and were more likely to be discharged to inpatient rehabilitation (77% versus 19%, p=.001). Figure 1 displays the trajectories of biomarkers by day for children with FSS change of < 3 and ≥ 3.
Table 1.
Patient and disease characteristics by difference in functional status prior to onset of acute neurocritical illness and hospital discharge.
| n (%); median (IQR) | Total sample N=44 |
Functional decrease < 3 N=31 |
Functional decrease ≥ 3 N=13 |
p-value |
|---|---|---|---|---|
| Age, years | 11 (6-14.) | 11 (6-13) | 12 (8-14) | .439 |
| Female | 17 (39) | 11 (36) | 6 (46) | .521 |
| Condition | .100 | |||
| Traumatic brain injury | 12 (27) | 5 (16) | 7 (54) | |
| Brain mass | 14 (32)) | 10 (32) | 4 (31) | |
| Cardiac arrest | 9 (21) | 8 (26) | 1 (7) | |
| Inflammation/Infection | 8 (18) | 7 (23) | 1 (7) | |
| Stroke | 1 (2) | 1 (2) | 0 (0) | |
| Pediatric Index of Mortality | 3.38 (1.29-16.88) | 3.33 (1.28-27.00) | 3.69 (1.71-14.23) | .922 |
| Hospital disposition | .001 | |||
| Home | 27 (61) | 24 (77) | 3 (23) | |
| Inpatient rehabilitation | 16 (36) | 6 (19) | 10 (77) | |
| Long term care facility | 1 (2) | 1 (3) | 0 (0) | |
| PICU length of stay, days | 6 (4-16) | 5 (4-8) | 16 (4-24) | .089 |
| Hospital length of stay, days | 12 (8-20) | 11 (7-18) | 20 (9-28) | .027 |
| PICU rehabilitation randomized group | 20 (46%) | 15 (48%) | 5 (39%) | .742 |
| Received mechanical ventilation | 31 (74%) | 20 (67%) | 11 (92%) | .097 |
| Length of mechanical ventilation, days | 4 (2-8) | 3 (2-6) | 8 (1-23) | .152 |
PICU = Pediatric intensive care unit
Figure 1.
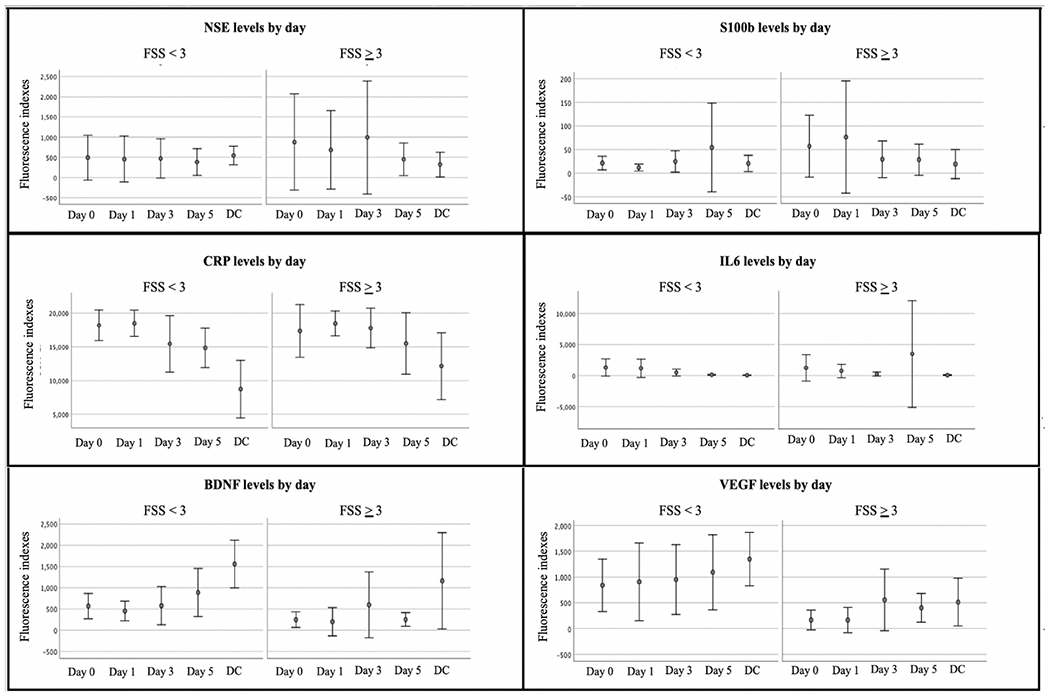
Error bar graphs of mean biomarker levels by day and by change in functional status score
Bars represented 95% confidence interval for the mean. BDNF = Brain-Derived Neurotrophic Factor; CRP = C-Reactive Protein; DC = Hospital discharge; FSS = Functional Status Scale; IL6 = interleukin-6; NSE = Neuron specific enolase; VEGF = Vascular Endothelial Growth Factor, SI00b needs definition
Serum biomarker concentrations and functional status
Brain Injury.
Increased NSE on day 0 was weakly correlated with worse functioning (ρ = .320, p=.044) (Table 2). The mixed effect model showed no association between repeated measures of NSE levels with new functional impairment (Table 3) when controlling for illness severity and time.
Table 3.
Linear mixed effects models of repeated measures of biomarkers on development of new functional impairment.
| B | 95% CI | p-value | |
|---|---|---|---|
| Neuron Specific Enolase Model | |||
| Intercept | 2.566 | (1.992, 3.140) | <.001 |
| Neuron specific enolase | 0.000 | (0.000, 0.001) | .270 |
| Time | 0.064 | (−0.015, 0.143) | .111 |
| Illness severity | 0.120 | (0.089, 0.151) | <.001 |
| S100b Model | |||
| Intercept | 2.555 | (1.979, 3.131) | <.001 |
| s100b | 0.002 | (−0.009, 0.014) | .699 |
| Time | 0.064 | (−0.016, 0.144) | .114 |
| Illness severity | 0.117 | (0.084, 0.151) | <.001 |
| C-Reactive Protein Model | |||
| Intercept | 2.552 | (1.979, 3.125) | <.001 |
| C-reactive protein | 0.000 | (0.000, 0.000) | .182 |
| Time | 0.081 | (0.000, 0.165) | .057 |
| Illness severity | 0.114 | (0.082, 0.146) | <.001 |
| Interleukin-6 Model | |||
| Intercept | 2.559 | (1.983, 3.135) | <.001 |
| Interleukin-6 | 0.000 | (0.000, 0.000) | .857 |
| Time | 0.062 | (−0.018, 0.142) | .120 |
| Illness severity | 0.120 | (0.087, 0.154) | <.001 |
| Vascular Endothelial Growth Factor | |||
| Intercept | 2.562 | (2.002, 3.122) | <.001 |
| Vascular Endothelial Growth Factor | −0.001 | (−0.002, 0.000) | .003 |
| Time | 0.081 | (0.003, 0.169) | .043 |
| Illness severity | .120 | (0.089, 0.150) | <.001 |
| Brain-Derived Neurotrophic Factor Model | |||
| Intercept | 2.550 | (1.993, 3.106) | <.001 |
| Brain-derived neurotrophic factor | −0.001 | (−0.002, −0.001) | .001 |
| Time | 0.096 | (0.017, 0.175) | .018 |
| Illness severity | 0.106 | (0.075, 0.138) | <.001 |
Inflammation.
There were no significant correlations between CRP or IL-6 with new functional impairment (Table 4). Similarly, the mixed effect models showed no association between repeated measures of CRP and IL6 levels with new functional impairment when controlling for illness severity and time (Table 3).
Regeneration and Plasticity.
BDNF levels on day 3, day 5, and hospital discharge were inversely correlated with functional impairment at hospital discharge (ρ = −.404, p =.015; ρ = −.529, p=.005; and ρ = −.420, p=.026, respectively) (Table 5). VEGF levels on day 1 and hospital discharge were inversely correlated with new functional impairment at hospital discharge (ρ = −.282, p=.008 and ρ = −.378, p=.047, respectively). In the mixed effect model, repeated measures of increased BDNF and VEGF levels (i.e., measures across days) were both inversely associated with new functional impairment (both β = −0.001, p=.003 and p=.001, respectively) when controlling for illness severity and time post-admission (Table 3).
Discussion
Most children survive neurocritical illness, however many acquire new functional morbidities, known as PICS-p, which may not be apparent until later in their hospital course or with reintegration into the community and resuming previous activities [3,30]. Early recognition and treatment of children at risk for developing new functional impairment is a critical next step in advancing care and improving outcomes for pediatric critical care survivors. However, screening tools that predict future new functional impairment are unavailable for this population. Our preliminary results suggest that serum levels of regeneration biomarkers BDNF and VEGF measured in the hospital may serve a novel role in screening for the development of new functional impairment among survivors of pediatric neurocritical illness. Given the inverse associations that we observed with functional status, low serum levels of BDNF and VEGF may inform the future development of functional impairment.
Brain injury biomarkers.
Increased blood NSE and S100b concentrations show promise to distinguish between abusive head trauma and accidental TBI and predicting outcome after cardiac arrest [17,18,22,31]. The half-lives and trajectories of NSE and S100b vary by condition studied and cellular origin, with both S100b and NSE peaking earlier in TBI compared to cardiac arrest and S100b peaking earlier than NSE in both conditions [17,32–38]. Thus, in our current study, the association between NSE levels and new functional impairment was only significant early after admission, and the lack of association with S100b and other NSE time points may be in part explained by blood sampling timing in relation to biomarker trajectory post-insult (e.g., S100b and NSE peak earlier in TBI vs. cardiac arrest patients) [22]. While there is increasing evidence that NSE and S100b have prognostication value, their use to predict functional outcome in neurocritical illness may warrant further research in a larger trial to elucidate their roles as screening tools; however, our findings suggest that BDNF and VEGF may have some promise in identifying new functional impairment, and perhaps, have potential to identify candidates that should be carefully evaluated for rehabilitation services. It may also be helpful in future studies to explore blood biomarkers specific to brain injury, such as Ubiquitin C-terminal hydrolase L1 (UCHL1) and glial fibrillary acidic protein (GFAP) measured serially during acute injury and recovery. [39] Such studies should consider adjusting for relevant covariates, such as illness severity, age, and type of injury (e.g., hypoxic brain injury or TBI) when establishing the association of these brain-based biomarkers with functional recovery.
Inflammation biomarkers.
IL-6 and CRP are biomarkers of inflammation, which commonly accompanies neurocritical illness [40,41]. We did not find an association between IL-6 or CRP and new functional impairment at any individual time point or over time, despite the growing body of evidence that persistent inflammation negatively affects outcomes following neurocritical and non-neurocritical illness [40,42,43]. Acutely increased levels of IL-6 and other cytokines both in the CSF and serum have been associated with mortality and poor outcome in adults and children with TBI, respectively [44–46]. Additionally, inflammation at discharge was associated with worse mobility among adults with critical illness, even after adjusting for the most likely confounding factors [14]. It is possible our findings may be due in part to small sample size or the specific outcome measure selected. In the previously mentioned study, mobility was assessed using the Rivermead Mobility Index, which provides slightly more detailed information on functional capacities than the FSS. It is also possible that the course and/or role of inflammation among children during recovery or after discharge from neurointensive care differs importantly from adults. The role of biomarkers of inflammation among children who survive neurocritical illness warrants further investigation to ascertain its role in identifying children with rehabilitative needs.
Regeneration and plasticity biomarkers.
BDNF is a growth factor that supports neuronal survival, plasticity, memory, and myelination during brain development and is notably lower in older age in association with hippocampal atrophy and memory dysfunction [47–49]. We found that serum BDNF levels in the latter days of hospitalization (Days 3+) were inversely associated with new functional impairment among children who survived neurocritical illness; i.e., lower levels of BDNF are associated with an increase in new functional impairment. A prior study of 14 children with acute TBI found serum BDNF levels were higher among children with a “good” versus “poor” clinical outcome (Glasgow Outcome Scale score dichotomized as 1-3 [poor], 4-5 [good]) with a small effect size (Cohen’s d = .32); however, this difference was not statistically significant, possibly due to sample size [50]. In prior research among adults, individuals with TBI had lower levels of serum BDNF compared to healthy controls; however, serum BDNF levels 6 days post TBI were positively correlated with neuropsychological testing scores evaluating memory domains and also a functional cognition scale that measures memory 6 and 12 months post injury [12]. Further, in adults who experienced an ischemic stroke, low levels of serum BDNF were significantly correlated with poor outcomes 2 and 7 years post injury [51]. These findings are consistent with the current study, suggesting a potential role for serum BDNF levels as a screening tool for new functional impairment s following brain insult [12,15,51]. Indeed, we speculate that low circulating BDNF levels may indicate a failure to initiate recovery processes in plasticity that are essential to prevent or mitigate future functional impairment in acute brain injury.
Furthermore, we found an association between repeated measures of BDNF levels captured during acute hospitalization with new functional impairment at hospital discharge in children after acute brain injury. Similarly, BDNF levels measured post-discharge in adults with TBI (12 months after injury) tended to be lower among individuals with worse depression scores [12], consistent with previous findings of the role of BDNF in emotional function [52–55]. Given the profound impact of depression on other aspects of functional outcomes, this association with BDNF may illuminate a way to help target recovery interventions and improve outcomes.
VEGF is a mitogen that plays a dual role in promoting angiogenesis and neurogenesis during brain development [56]. Interestingly, a rodent study that exposed animals to exercise preconditioning prior to TBI showed less sensorimotor and cognitive deficits following TBI, with histologic evidence of increased neuronal VEGF compared to rodents without preconditioning exercise [57]. Literature on blood VEGF concentrations and outcomes in humans after brain injury is limited. Among adults with TBI, high levels of VEGF acutely following injury (day 7) is associated with poor outcome, as measured by Glasgow Outcome Scale-Extended and Glasgow Coma Scale (GCS) [58,59], while high levels during recovery (day 21) is associated with improved neurologic status, as measured by GCS [59]. Our finding that lower VEGF levels are associated with new functional impairment suggests further investigation of the relationship between VEGF levels with functional outcomes in this population is indicated.
While our study was not powered to detect whether randomization groups of the parent study modified the relationship between biomarkers and new functional impairment, it is possible that receipt of early ICU-based rehabilitation interventions may have contributed to the biomarker levels found in our study. For example, a study of healthy adults randomized to an aerobic exercise intervention showed increased cortical connectivity on neuroimaging in the setting of increased levels of BDNF and VEGF as well as greater connectivity in the exercise group with higher pre-intervention VEGF levels compared to the control group [60]. Furthermore, in studies assessing healthy adults and adults with a variety of diagnoses (e.g., stroke, Parkinson’s disease, or generalized frailty) an exercise intervention resulted in increased BDNF levels and improved cognition [60–64]. The feasibility of implementing an exercise intervention in children acutely in the PICU suggests the possibility that it could represent an excellent approach to increase BDNF levels [7,65] and warrants further investigation. It is worth noting that rehabilitation utilization in the PICU remains relatively low, with recent point prevalence study finding that only 32% of the sample receiving a physical or occupational therapy consultation by the third day of their PICU stay[66].
In our graphical exploration of regeneration and plasticity biomarkers, individuals with great impairment (FSS score change of ≥3 versus FSS score change of < 3) presented with lower levels of BDNF and VEGF (Figure 1). These biomarkers may aid in both identifying patients who have the most potential to benefit from rehabilitation efforts and monitoring their response to rehabilitation. A framework to contextualize biomarker relationships to function has been proposed for rehabilitation populations broadly and has been evaluated for adults with TBI in particular.[67] Termed “Rehabilomics”, this research model is informed by the International Classification of Functioning, Disability and Health, and along with biomarkers, considers patient factors, clinical course, and environmental barriers when assessing multiple facets of function as a means to inform precision care and personalize recovery [67–70]. Our findings may contribute towards development and validation of a clinical tool that includes patient characteristics and biomarkers to facilitate personalized approaches to prognostication, therapeutic interventions, and monitoring responses to interventions for children with acute brain injury. For instance, a patient identified as high risk for poor cognitive outcome based on biomarker screening could be enrolled in a prescribed “early rehabilitation” regimen targeting cognitive recovery with early interventions acutely during critical illness in addition to environmental modifications, such as provision of audiobooks or preferred music to improve attention and executive function, and caregiver education on strategies to promote their child’s functioning in the home and at school [17,21,23–25,71–73].
Limitations
Our study had several limitations. First, biomarkers were collected at both objective (days 0, 1, 3, 5) and subjective (at hospital discharge) time points, which adds variability in time to final biomarker collection. Second, analyses only included functional status impairment at hospital discharge, therefore generalizability of our results to long-term functional outcomes given the potential for rehabilitation post-discharge remain to be defined. Third, this sample came from a pilot RCT on rehabilitation therapy utilization in the PICU and while there were no differences on outcome between those who were randomized to the early ICU rehabilitation group versus those in usual care group,[7] it is possible that our population may have had improved outcomes due to receiving rehabilitation during their stay. Further research is needed with adequate sample size to control for relevant covariates, e.g., age, and to stratify by endotype in order to explore diagnostic-specific relationship between serum biomarkers and functional outcome to validate our findings for generalizability. Next, biomarkers often have trajectories that are dependent on population (e.g., age) and injury (e.g., specific neurologic condition, severity) characteristics and should be validated with consideration of these factors [74].
Lastly, the outcome in our study is a relatively early and gross measure of functional status. As research on post-PICU functional recovery continues to evolve, pairing biomarkers with long-term, validated outcome measures with normative data references that align with International Classification of Functioning, Disability and Health functional domains (e.g., functioning at the level of activity performance and participation) and caregiver priorities may improve the accuracy and value of assessing functional outcomes [68,75–77].
Conclusion
Blood biomarkers of regeneration BDNF and VEGF have potential to identify children at risk for new functional impairment among pediatric neurocritical care survivors and warrant further investigation. Validation studies are needed to test whether clinical integration of biomarkers can assist in personalizing rehabilitation planning.
DETAILS PAGE.
This manuscript complies with all instructions to authors from Neurocritical Care.
All authors meet the authorship requirements and have approved the final manuscript.
This study adhered to ethical guidelines and institutional approval was obtained from all sites prior to study enrollment. IRB approved informed consent was used for all participants.
This manuscript conforms to the reporting requirements of the STROBE checklist, as this study involved secondary analyses of a randomized clinical trial but did not include trial group comparisons. Therefore, the study design is not placed in the title.
Acknowledgements
We thank Denise Prosser at the Luminex Core Facility at the University of Pittsburgh, Keri Feldman for her assistance with sample management and all of the children and families who generously participated in this study.
Funding:
This study received funding from Patient Centered Outcomes Research Institute CER-1310-08343 (E.L.F.) and the National Institutes of Health T32 HD040686 (J.M.J.) and K01 HD097030 (A.T-B.) Luminex bioassay used UPCI Cancer Biomarkers Facility: Luminex Core Laboratory, which is supported in part by award P30CA047904.
Footnotes
Conflict of Interest: The authors declare that they have no other conflicts of interest.
Trial Registration: This project is registered with clinicaltrials.gov under NCT02209935
References
- 1.Pollack MM, Holubkov R, Funai T, et al. Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med [Internet] 2014;15(9):821–7. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00130478-201411000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink EL, Kochanek PM, Tasker RC, et al. International Survey of Critically Ill Children With Acute Neurologic Insults : The PANGEA study To cite this version : HAL Id : hal-01717475 HHS Public Access. Pediatr crit care med 2017;4(18):330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in children - The PICS-p framework. Pediatr Crit Care Med 2018;19(4):298–300. [DOI] [PubMed] [Google Scholar]
- 4.Bone MF, Feinglass JM, Goodman DM. Risk Factors for Acquiring Functional and Cognitive Disabilities During Admission to a PICU*. Pediatr Crit Care Med 2014;15(7):640–8. [DOI] [PubMed] [Google Scholar]
- 5.Choong K, Fraser D, Al-Harbi S, et al. Functional recovery in critically ill children, the “WeeCover” multicenter study. Pediatr Crit Care Med 2018;19(2):145–54. [DOI] [PubMed] [Google Scholar]
- 6.Bedell GM. Functional outcomes of school-age children with acquired brain injuries at discharge from inpatient rehabilitation. Brain Inj 2008;22(4):313–24. [DOI] [PubMed] [Google Scholar]
- 7.Fink EL, Beers SR, Houtrow AJ, et al. Early protocolized versus usual care rehabilitation for pediatric neurocritical care patients: A randomized controlled trial. Pediatr Crit Care Med 2019;20(6):540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepas JJ, Leaphart CL, Pieper P, et al. The effect of delay in rehabilitation on outcome of severe traumatic brain injury. J Pediatr Surg 2009;44(2):368–72. [DOI] [PubMed] [Google Scholar]
- 9.Lone NI, Seretny M, Wild SH, Rowan KM, Murray GD, Walsh TS. Surviving intensive care: A systematic review of healthcare resource use after hospital discharge. Crit Care Med 2013;41(8):1832–43. [DOI] [PubMed] [Google Scholar]
- 10.Moreau JF, Fink EL, Hartman ME, et al. Hospitalizations of children with neurologic disorders in the United States. Pediatr Crit Care Med 2013;14(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker T, Kudchadkar SR. Early Mobility in the Pediatric Intensive Care Unit: Can We Move On? J Pediatr 2018;1–3. [DOI] [PubMed] [Google Scholar]
- 12.Failla MD, Juengst SB, Arenth PM, Wagner AK. Preliminary Associations between Brain-Derived Neurotrophic Factor, Memory Impairment, Functional Cognition, and Depressive Symptoms Following Severe TBI. Neurorehabil Neural Repair 2016;30(5):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yende S, D’Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008;177(11):1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith DM, Lewis S, Rossi AG, et al. Systemic inflammation after critical illness: Relationship with physical recovery and exploration of potential mechanisms. Thorax 2016;71(9):820–9. [DOI] [PubMed] [Google Scholar]
- 15.Korley FK, Diaz-Arrastia R, Wu AHB, et al. Circulating Brain-Derived Neurotrophic Factor Has Diagnostic and Prognostic Value in Traumatic Brain Injury. J Neurotrauma 2016;33(2):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au AK, Bell MJ, Fink EL, Aneja RK, Kochanek PM, Clark RSB. Brain-Specific Serum Biomarkers Predict Neurological Morbidity in Diagnostically Diverse Pediatric Intensive Care Unit Patients. Neurocrit Care 2017;1–9. [DOI] [PubMed] [Google Scholar]
- 17.Fink EL, Berger RP, Clark RSB, et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest. Crit Care Med 2014;42(3):664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topjian AA, Lin R, Morris MC, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med 2009;10(4):479–90. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Hwang SK. Prognostic Value of Serum Levels of S100 Calcium-Binding Protein B, Neuron-Specific Enolase, and Interleukin-6 in Pediatric Patients with Traumatic Brain Injury. World Neurosurg 2018;118:e534–42. [DOI] [PubMed] [Google Scholar]
- 20.Bembea MM, Rizkalla N, Freedy J, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med 2015;43(10):2202–11. [DOI] [PubMed] [Google Scholar]
- 21.Daoud H, Alharfi I, Alhelali I, Charyk Stewart T, Qasem H, Fraser DD. Brain injury biomarkers as outcome predictors in pediatric severe traumatic brain injury. Neurocrit Care 2014;20(3):427–35. [DOI] [PubMed] [Google Scholar]
- 22.Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: Insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci 2006;28(4–5):327–35. [DOI] [PubMed] [Google Scholar]
- 23.Spadaro S, Park M, Turrini C, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (United Kingdom) 2019;16(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA - J Am Med Assoc 2019;15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson AA, Dennis M, Simic N, et al. Brain biomarkers and pre-injury cognition are associated with long-term cognitive outcome in children with traumatic brain injury. BMC Pediatr 2017;17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121(1):68–74. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack MM, Holubkov R, Funai T, et al. Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med 2014;15(9):821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holden JE, Kelley K, Agarwal R. Analyzing change: A primer on multilevel models with applications to nephrology. Am J Nephrol 2008;28(5):792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross KA, Dorris L, Mcmillan T. A systematic review of psychological interventions to alleviate cognitive and psychosocial problems in children with acquired brain injury. Dev Med Child Neurol 2011;53(8):692–701. [DOI] [PubMed] [Google Scholar]
- 31.Berger RP, Dulani T, Adelson PD, Leventhal JM, Richichi R, Kochanek PM. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: A possible screening tool. Pediatrics 2006;117(2):325–32. [DOI] [PubMed] [Google Scholar]
- 32.Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg 2005;103(Suppl. 1):61–8. [DOI] [PubMed] [Google Scholar]
- 33.Bechtel K, Frasure S, Marshall C, Dziura J, Simpson C. Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatrics 2009;124(4). [DOI] [PubMed] [Google Scholar]
- 34.Müller K, Townend W, Biasca N, et al. S100B serum level predicts computed tomography findings after minor head injury. J Trauma - Inj Infect Crit Care 2007;62(6):1452–6. [DOI] [PubMed] [Google Scholar]
- 35.Thelin EP, Nelson DW, Bellander BM. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir (Wien) 2017;159(2):209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghanem G, Loir B, Morandini R, Al. E. on the Release and Half-Life of S100B Protein in the Peripheral. Int J Cancer 2001;94:586–90. [DOI] [PubMed] [Google Scholar]
- 37.Stojanovic Stipic S, Carev M, Bajic Z, et al. Increase of plasma S100B and neuron-specific enolase in children following adenotonsillectomy: a prospective clinical trial. Eur Arch Oto-Rhino-Laryngology 2017;274(10):3781–8. [DOI] [PubMed] [Google Scholar]
- 38.Berger RP, Bazaco MC, Wagner AK, Kochanek PM, Fabio A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev Neurosci 2011;32(5–6):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta T, Fayyaz M, Giler GE, et al. Current trends in biomarkers for traumatic brain injury. Open Access J Neurol Neurosurg 2020;12(4):86–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Bell MJ, Kochanek PM, Doughty LA, et al. Interleukin-6 and Interleukin-10 in Cerebrospinal Fluid after Severe Traumatic Brain Injury in Children. J Neurotrauma 2009; [DOI] [PubMed] [Google Scholar]
- 41.Muszynski JA, Thakkar R, Hall MW. Inflammation and innate immune function in critical illness. Curr Opin Pediatr 2016;28(3):267–73. [DOI] [PubMed] [Google Scholar]
- 42.Peberdy MA, Andersen LW, Abbate A, et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study. Resuscitation 2016;103:117–24. [DOI] [PubMed] [Google Scholar]
- 43.Buerki SE, Grandgirard D, Datta AN, et al. Inflammatory markers in pediatric stroke: An attempt to better understanding the pathophysiology. Eur J Paediatr Neurol [Internet] 2016;20(2):252–60. Available from: 10.1016/j.ejpn.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 44.Chiaretti A, Genovese O, Aloe L, et al. Interleukin 1β and interleukin 6 relationship with paediatric head trauma severity and outcome. Child’s Nerv Syst 2005;21(3):185–93. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira LCB, Regner A, Miotto KDL, et al. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj 2014;28(10):1311–6. [DOI] [PubMed] [Google Scholar]
- 46.Kumar RG, Diamond ML, Boles JA, et al. Acute CSF interleukin-6 trajectories after TBI: Associations with neuroinflammation, polytrauma, and outcome. Brain Behav Immun [Internet] 2015;45(2015):253–62. Available from: 10.1016/j.bbi.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 47.Wurzelmann M, Romeika J, Sun D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res 2017;12(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci 2013;33(11):4947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 2010;30(15):5368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiaretti A, Piastra M, Polidori G, et al. Correlation between neurotrophic factor expression and outcome of children with severe traumatic brain injury. Intensive Care Med 2003;29(8):1329–38. [DOI] [PubMed] [Google Scholar]
- 51.Stanne TM, Aberg ND, Nilsson S, et al. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke 2016;47(7):1943–5. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto K Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin Neurosci 2010;64(4):341–57. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Roberts RE. The power of positive emotions: it’s a matter of life or death--subjective well-being and longevity over 28 years in a general population. Health Psychol 2010;29(1):9–19. [DOI] [PubMed] [Google Scholar]
- 54.Seale GS, Berges I-M, Ottenbacher K, Ostir GV. Change in Positive Emotion and Recovery of Functional Status Following Stroke. Rehabil Psychol 2010;55(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarvis JM, Downer B, Baillargeon J, Khetani M, Ottenbacher KJ, Graham JE. The modifying effect of positive emotion on the relationship between cognitive impairment and disability among older Mexican Americans: a cohort study. Disabil Rehabil 2018;41(13):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogensis 2010;6(2):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor JM, Montgomery MH, Gregory EJ, Berman NEJ. Exercise preconditioning improves traumatic brain injury outcomes. Brain Res [Internet] 2015;1622:414–29. Available from: 10.1016/j.brainres.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou S, Yin DP, Wang Y, Tian Y, Wang ZG, Zhang JN. Dynamic changes in growth factor levels over a 7-day period predict the functional outcomes of traumatic brain injury. Neural Regen Res 2018;13(12):2134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M, Jia Q, Chen T, Zhao Z, Chen J, Zhang J. The role of vascular endothelial growth factor and vascular endothelial growth inhibitor in clinical outcome of traumatic brain injury. Clin Neurol Neurosurg [Internet] 2016;144(2016):7–13. Available from: 10.1016/j.clineuro.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 60.Voss MW, Erickson KI, Prakash RS, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun 2013;28:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Tamawy MS, Abd-Allah F, Ahmed SM, Darwish MH, Khalifa HA. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. NeuroRehabilitation 2014;34(1):209–13. [DOI] [PubMed] [Google Scholar]
- 62.Coelho FM, Pereira DS, Lustosa LP, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr 2012;54(3):415–20. [DOI] [PubMed] [Google Scholar]
- 63.Rahmani F, Saghazadeh A, Rahmani M, et al. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res 2019;1704(October 2018):127–36. [DOI] [PubMed] [Google Scholar]
- 64.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity – Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sport Med 2010;40(9):765–801. [DOI] [PubMed] [Google Scholar]
- 65.Choong K, Awladthani S, Khawaji A, et al. Early exercise in critically ill youth and children, a preliminary evaluation: The wEECYCLE pilot trial. Pediatr Crit Care Med 2017;18(11):e546–54. [DOI] [PubMed] [Google Scholar]
- 66.Kudchadkar SR, Nelliot A, Awojoodu R, et al. Physical Rehabilitation in Critically Ill Children: A Multicenter Point Prevalence Study in the United States. Crit Care Med 2020;48(5):634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner AK, Sowa G. Rehabilomics research: A model for translational rehabilitation and comparative effectiveness rehabilitation research. Am J Phys Med Rehabil 2014;93(10):913–6. [DOI] [PubMed] [Google Scholar]
- 68.The World Health Organization. International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. 2007;
- 69.Wagner AK. TBI translational rehabilitation research in the 21st Century: Exploring a Rehabilomics research model. Eur J Phys Rehabil Med 2010;46(4):549–55. [PubMed] [Google Scholar]
- 70.Wagner AK. TBI Rehabilomics Research: an Exemplar of a Biomarker-Based Approach to Precision Care for Populations with Disability. Curr Neurol Neurosci Rep 2017;17(11). [DOI] [PubMed] [Google Scholar]
- 71.Särkämö T, Tervaniemi M, Laitinen S, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain [Internet] 2008. [cited 2014 Jan 21];131(Pt 3):866–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18287122 [DOI] [PubMed] [Google Scholar]
- 72.Jarvis JM, Gurga A, Lim H, et al. Caregiver strategy use to promote children’s home participation after pediatric critical illness. Arch Phys Med Rehabil [Internet] 2019;100(11):2144–50. Available from: 10.1016/j.apmr.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 73.Jarvis JM, Choong K, Khetani MA. Associations of participation-focused strategies and rehabilitation service use with caregiver stress after pediatric critical illness. Arch Phys Med Rehabil [Internet] 2019;100(4):703–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003999318315399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomaz A, Jaeger M, Buendia M, et al. BDNF/TrkB Signaling as a Potential Novel Target in Pediatric Brain Tumors: Anticancer Activity of Selective TrkB Inhibition in Medulloblastoma Cells. J Mol Neurosci 2016;59(3):326–33. [DOI] [PubMed] [Google Scholar]
- 75.Fink EL, Jarvis JM, Maddux AB, et al. Development of a core outcome set for pediatric critical care outcomes research. Contemp Clin Trials [Internet] 2020;91(March):105968. Available from: 10.1016/j.cct.2020.105968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddux AB, Pinto N, Fink EL, et al. Postdischarge Outcome Domains in Pediatric Critical Care and the Instruments Used to Evaluate Them: A Scoping Review. Crit Care Med 2020;48(12):e1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarvis JM, Kaelin V, Anaby D, Teplicky R, Khetani M. Electronic participation-focused care planning support for families: A pilot study. Dev Med Child Neurol 2020;62:954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


