Abstract
Tuberculosis (TB), the world’s deadliest bacterial infection, afflicts more human males than females, with an M/F ratio of 1.7. Sex disparities in TB prevalence, pathophysiology and clinical manifestations are widely reported, yet the underlying biological mechanisms remain largely undefined. This review assesses existing epidemiologic data on sex disparity in TB, and possible underlying hormonal and genetic mechanisms that might differentially modulate innate and adaptive immune responses in males and females, leading to sex differences in disease susceptibility. We consider whether sex disparity can be extended to efficacy of vaccines and discuss novel animal models which may offer mechanistic insights. A better understanding of the biological factors underpinning sex-related immune responses in TB may enable sex-specific candidate personalized therapies for TB.
Sex inequality in World Health Organization tuberculosis data
Global epidemiological data suggest that tuberculosis (TB) case notification rates among human males are consistently higher than females and that this phenomenon has been sustained for many decades [1]. Despite a 18% reduction in case notifications between 2019 and 2020 due to the COVID-19 pandemic, the global M/F ratio of TB incidence in HIV-1-negative adults varied from 1.12 in the Eastern Mediterranean Region to >2 in the Americas and the Western Pacific Region [1]. Data indicating that males are at a higher risk for developing TB than females across geographical regions are compelling [2]; however, research on sex differences in TB susceptibility remains understudied, and few underlying immune mechanisms have been elucidated. Given the importance of sex equality combined with the World Health Organization (WHO)-endorsed End TB Strategy goal to eradicate the TB epidemic by 2035 [3], this striking sex-based difference warrants new research. This review describes our current understanding of mechanisms that may account for this sex-based difference in TB pathophysiology with a focus on immune regulation–hormonal and genetic–that may be at play.
Biological intricacy or epidemiologic error?
Demographic factors affecting sex bias in TB
Sex-bias in TB is still an intriguing conundrum. One potential explanation for the male sex-bias is that it may represent an epidemiological artifact resulting from various socioeconomic, geographical, and cultural factors. A common hypothesis has been that females do not have equal access to healthcare [4,5]. The male bias, however, also occurs in developed countries where barriers to healthcare for female TB diagnosis are reduced [6]. Regarding bias reporting, a study found that male TB prevalence was 2-fold higher than for females, and diagnosis took 1.5 times longer in males in low- and middle-income countries from 1993–2016 [2]. These findings concurred with other studies suggesting that pulmonary TB in females is more likely to be diagnosed than in males [7].
Multiple lines of evidence from humans also support a biologic basis for this sex difference. First, the 1929 Lübeck disaster showed convincing evidence for a direct biological basis for male sex bias in young children. In this tragic event, 251 neonates were accidentally inoculated with Bacillus Calmette Guérin (BCG) contaminated with virulent Mycobacterium tuberculosis (Mtb) [8]. In the aftermath investigation, death was concluded to be due to TB, which was significantly more frequent in male (50/137, 36.5%) than female children (27/114, 23.7%) with an odds-ratio of 1.67 [8]. Second, in a study of 74 castrated males and 170 age-matched human controls from the same era, castrated males were statistically significantly less likely to die from TB (6/74, 8.1%) than gonadal-males (35/170, 20.6%) [9]. Third, a recent retrospective cohort study and parallel meta-analysis of the last 10 years’ published reports on the clinical outcomes of TB, revealed that male patients had higher mortality, and higher sputum culture and smear positivity rates, during and after TB treatment than females[10]. Fourth, studies examining immune biomarkers for TB, found higher IFN-γ response to Mtb antigens CFP-10 and ESAT-6, and lower IgM titers in males, while a strong tuberculin reaction was observed in smear and culture positive female patients [11]. Also, male contacts of pulmonary TB patients who did not develop TB exhibited significantly larger positive tuberculin test reactions than the general cutoff -- an observation suggesting that sex-specific and age matched cut-offs for the defining tuberculin positivity might be warranted [11]. Of note, pulmonary TB produces a spectrum of abnormalities, some of which like cavitary disease, show equal distribution among sexes. Also, lower lung field TB, could be more prominent in women, requiring more risk stratification tools to evaluate sex differences [12,13].
Does the sex bias hold up in immunocompromised patients?
Given the substantial evidence that the male sex bias has a biologic basis, a follow-up question is whether the sex bias is evident even in immunocompromised patients. Since HIV-1 infection is one of the highest risk factors for TB [14], the male sex bias has been studied in TB-HIV-1 co-infected patients. Out of 128 Mtb/HIV-1 co-infected patients in Colombia, 79.7% were men [15]. In a similar retrospective study of 666 drug-resistant TB/HIV-1 co-infected patients in Uganda, 60.2% were men, with a median age of 37 years. Also, mortality following treatment failure was higher in men (25.7%) than in women (18.5%, p=0.04) [21]. Table 1 provides a summary of studies that have addressed the male sex bias in TB-HIV-1 co-infection.
Table 1.
Male predominance in TB/HIV-1 co-infection
| Type | Setting | Number | M/F ratio | p-val, HR or AHR* | Ref. |
|---|---|---|---|---|---|
| New TB incidence among HIV-1+ subjects | |||||
| Retrospective | South Africa | 2423 | 2.16 | <0.001 AHR | [16] |
| Prospective | South Africa | 2778 | 1.46 | <0.05 HR** | [17] |
| Retrospective | South Africa | 240 | 1.45 | NA | [18] |
| TB registries in a large region (with data on sex and HIV-1 status) | |||||
| Retrospective | Zimbabwe | 13,802 | 1.38 | p 0.017 | [19] |
| Retrospective | Ethiopia | 2252 | 1.19 | NA | [20] |
HR = hazard ratio, AHR = adjusted HR,
The AHR was 1.22 with p = 0.08.
Is there a sex bias in latent TB infection (LTBI)?
Sex disparity in the prevalence of LTBI is not as well-studied as active TB, and existing studies reveal conflicting findings. Despite the global AT M/F ratio of 1.7, many regional surveys have not found a sex bias in human LTBI [22–24]. For example, a study on high-risk individuals revealed that LTBI was diagnosed predominantly in males than in females (32.6% vs. 25.2%, p=0.010). However, in a following multivariate analysis after considering age, smoking status, and clinical factors, male sex was not found to be an independent factor for LTBI [22]. One interpretation of the available data is that although females and males may have a similar risk of developing LTBI, males with LTBI might progress to AT more readily. Stated another way, male immunologic containment responses during LTBI might fail to provide the same degree of Mtb containment as those in females, although this remains conjectural. Of note, a serological survey of 383 Canadian subjects with active TB (AT) or LTBI using multivariate analysis, found that antibody titers against the 14 kDa antigen of Mtb were significantly associated with LTBI and female sex, suggesting their potential use in identifying individuals at high risk of LTBI reactivation [25]. Thus, biological factors clearly play important roles, and research addressing the biologic bases for the male bias are likely to unmask novel insights into the pathogenesis of human AT and LTBIs. Altogether, under-diagnosis and under-reporting in females presumably may affect the epidemiology data in some settings, but these alone cannot account for the global sex-bias towards males in TB, which further raises the question as to why males are more susceptible to TB than females.
Why are males more likely to develop active TB?
Two different hypotheses- physiological versus behavioral have been proposed to explain the phenomenon of sexual inequality in infectious diseases [26]. The latter posits that the biased infection rates are primarily due to sex-specific differences in pathogen exposure. In contrast, the physiological hypothesis is that sex hormones along with the genetic effectors encoded on the sex chromosomes are the likely determinants of immunity and disease susceptibility [26]. Indeed, a survey of case notifications and age-stratified M/F prevalence pattern in Brazil, suggests that TB male sex bias is minimal during childhood and becomes prominent only after puberty –an observation indirectly implicating sex hormones as putative key mediators of the TB sex bias (Figure 1) [26] [27]. A study of TB incidence in Tuscany showed that the male proportion ranged from 51.4% in children (0–14 years) and adolescents (15–18 years), but escalated to 60.9% post puberty (≥18 years), suggesting that male TB incidence could rise after sexual maturation [28]. Importantly, the female protective effect from TB is observed both at pre- and post-menopausal phases, and although the incidence of female TB increases in post-menopausal elderly age groups compared to adults, it does so at a lower rate than males, suggesting that aging perhaps intensifies pulmonary TB incidence in males [29]. A recent study from China’s Shandong Province from 2005 to 2017 showed that compared to non-elderly, pulmonary TB incidence was higher in the elderly population (aged ≥60 years) and of the 77,192 elderly TB cases, 76.8% occurred among males, indicating an even higher M/F ratio of TB incidence among the elderly population [29]. Alhough further investigations are needed to ascertain if the observed resistance in females is modulated hormonally or has a genetic basis, sex- and age-specific methods for prevention and control of pulmonary TB should be certainly adopted.
Figure 1. Sex hormone effects that may contribute to the male bias in immune responses to tuberculosis.
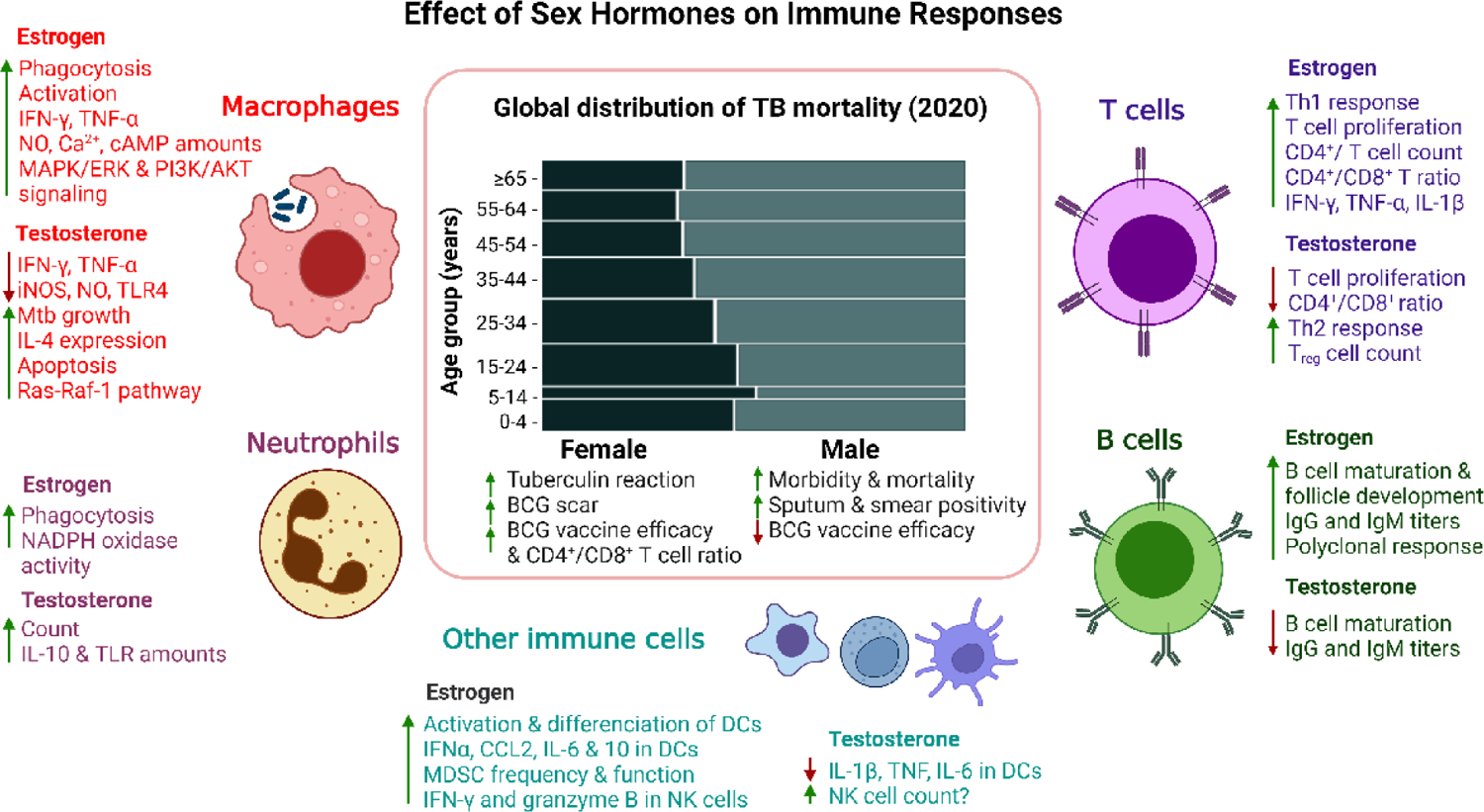
Effects of estrogen and testosterone on human or mice immune cells that are known to play a key role in anti-tuberculosis (TB) immunity [31,73]. Center panel: depiction of the global sex- and age-based distribution of TB mortality in 2020 (adapted from the 2021 WHO TB report) [1]. Schematic representation, images not scaled. Abbreviations: IFN-γ, interferon γ; TNF-α, tumor necrosis factor α; NO, nitric oxide; Ca2+, calcium ion, cAMP, Cyclic Adenosine Monophosphate; MAPK/ERK, mitogen-activated protein kinase/Extracellular signal-regulated kinase 1/2; PI3K/AKT, phosphatidylinositol 3-kinase/protein kinase B; iNOS, nitric oxide synthase; TLR-4, toll-like receptor 4, IL-4, interleukin 4; IL-10, interleukin 10; IgG, immunoglobulin G; IgM, immunoglobulin M; Treg, regulatory T cell; CD, cluster of differentiation; Th, T helper cell; IL-1β, interleukin 1β; CCL2, chemokine (C-C motif) ligand 2; IL-6, interleukin 6; DCs, dendritic cells, MDSCs, Myeloid-derived suppressor cells; NK cells, natural-killer cells.
Animal models reveal a physiologic basis for TB sex differences
Various animal models have been employed to assess physiological factors in TB sex differences. A 1947 study showed that castrated guinea pigs of both sexes exhibited increased survival upon TB infection, and no male versus female differences were observed [30]. However, two recent murine studies have now demonstrated that TB disease severity is greater in male mice than in females [31] [32]. Specifically, gonadectomized (Gdx) and sham-operated female and male BALB/c mice were assessed for Mtb susceptibility. Sham-operated male mice showed more rapid lethality and higher organ bacterial burdens than Gdx-males or sham-operated females [31]. Compared to non-castrated males, sham-operated female and Gdx-male lung tissues showed significantly higher mRNA expression of pro-inflammatory cytokines TNF-α, IFN-γ, IL-12, and IL-17, besides iNOS, suggesting a more robust immunologic response in animals lacking testes.[31]. A following study observed the same male-skewed increased lethality and higher organ burdens compared to females, in the C57BL/6 mice background upon aerosol infection with Mtb [32]. However, in contrast to the previous study [29], infected male C57BL/6 mice lung homogenates contained significantly higher pro-inflammatory cytokines such as IL1α, IL1β, IFNγ and IL-6, and wide range of chemokines such as RANTES, MIP-3α, CXCL1, etc., over time, than female mice [32]; this re-emphasizes the notion that pro-inflammatory cytokines are essential for the containment of Mtb infection, but they may become detrimental when produced in a dysregulated manner. Lymphoid aggregates rich in B cell follicles are archetypal for both mouse and human lung granulomas, and are essential for immune control during the containment of Mtb infection [33]. In this milieu, the development of smaller B cell follicles (see Glossary) in males over time, compared to Mtb infected female C57BL/6 mice, may contribute to reduced resistance and higher TB-susceptibility in males [34]. In addition, the expression of CXCL13 and CCL19, chemokines involved in the homing of lymphocytes and myeloid cells to infected areas of the lung, was significantly elevated in females compared to males, consistent with the impairment of B-cell follicle formation in males [34]. Also, BCG-vaccinated females were better protected from TB than males in mouse models (Box 1) [35]. Compared to males, similar protection from disease progression in females has also been observed for M. bovis infection in red deer and certain non-tuberculous mycobacterial (NTM) infections in mice [36–40]. Of note, in humans, the clinically important NTM pulmonary disease syndromes caused by M. avium and M. abscessus are more common in females than in males, possibly due to related age- and sex-specific risk factors (Box 2) [41–43]. These discrepancies suggest that host immune responses to Mtb could be different from those evoked by NTMs, leading to differing sex ratios. Altogether, these animal studies clearly indicate a physiologic basis for increased male susceptibility to TB, and reveal how gonads can influence the course of experimental TB in both the sexes to different degrees.
Box 1 |. Sex differences in BCG vaccine efficacy.
BCG is used to vaccinate neonates in most nations for TB prevention [145]. It protects children from disseminated TB during the first decade of life, but has little efficacy in preventing adult forms of TB [146]. The magnitude of the BCG response as measured either by scar size or the tuberculin skin test reaction shows a strong correlation with higher overall childhood survival among female vs male children [147]. Post-BCG vaccination, a sex-dependent reactivity to TLR2, 4, 7 and 8 ligands has been observed, with significantly elevated IL-10 and IL-17 responses in males than females [148]. A number of recent studies have identified sex differences in immunologic responses to BCG in adult volunteers receiving it as an experimental vaccine. Using a proteome platform to assess systemic inflammatory markers, BCG vaccination of adults enhanced cytokine responses (L-1β, IL-6, and TNF-α) to Mtb re-stimulation but reduced systemic inflammation to a significantly greater degree in males than females, as evidenced from significantly lower concentrations of circulating inflammatory proteins such as CXCL1, CXCL5, CXCL6, MCP-4, CD40, CASP-8, etc., 3 months post vaccination [149].
In a cohort of hundred healthy individuals, BCG-vaccinated females showed higher CD4+/CD8+ ratios and their peripheral blood mononuclear cells controlled Mtb growth better than males [150]. Using the TB-susceptible 129 S2 mouse model, a recent study revealed lower lung Mtb burden and hence better protection in BCG-vaccinated females mice compared to males, reiterating sex differences to BCG vaccination [35]. Further, 4 weeks post Mtb infection, the lung of vaccinated male mice showed a significantly higher number of neutrophils, inflammatory monocytes, CD11b+GR-1− monocytes/macrophages and myeloid cell to T cell ratio, than females [35]. Conversely, female mice tended to have an overall increased number of total and CD4+ T cells in lungs compared to males, which was consistent with the well-known effect of androgens on abbreviating T cell responses [35]. Also, Treg cell expansion following human BCG vaccination has been postulated to correlate with relatively poor efficacy of BCG, and human males possess more Treg cells than females [151–153]. Therefore, it is possible that Treg cells might contribute to modulating BCG efficacy in a sex-dependent manner, although this remains to be rigorously tested.
Box 2 |. Non-tuberculous mycobacterial infections: more common in human females than males.
To date, over 170 naturally occurring non-tuberculous mycobacterial (NTM) species are known, but pulmonary disease (PD) caused by Mycobacterium avium complex (MAC), M. kansasii, and M. abscessus are the most common [41]. NTMs may also cause cutaneous infections and lymphadenitis, though less frequently. Demographically, the incidence rate of NTM-PD is growing each year and many studies across different countries show that NTM-PD is more common in adult females than males by a margin of 1.1–1.6-fold [41–43]. Particularly, NTM-PDs have higher prevalence among elderly postmenopausal women than men, and in contrast to TB, underlying chronic lung disease is a key risk factor [43]. Typical underlying risk factors for NTM-PD include chronic obstructive pulmonary disease (COPD), non-cystic fibrosis bronchiectasis (NCFB), and primary ciliary dyskinesia. Certain body morphotypes (pectus excavatum or scoliosis) and slender body habitus are also risk factors particularly among adult females [43]. Among the younger population, cystic fibrosis is a strong risk factor for NTM infection, but in this population, males and females are at equal risk [154]. These observations suggest that post-menopausal sex hormone concentrations, adipocytokine signaling, low-fat, connective tissue disorders, might contribute to the female-bias in NTM-PD. Also, host immune responses to Mtb could be different from those evoked by NTM, leading to differing sex ratios. However, experimental studies in murine models have not successfully re-capitulated the clear-cut human female sex bias for NTM diseases, perhaps due to the paucity of animal models that mimic the underlying lung diseases which predispose humans.
In multiple mice variants, gonadal males were more susceptible to M. marinum than females and castrated males, while testosterone treatment increased the infection susceptibility of gonadal females [38]. Similarly, a male-sex bias was observed in mice to M. intracellulare and M. lepramurium infections [37,40]. After M. avium challenge, ovariectomized mice exhibited increased lung bacillary burden compared with sham-operated controls, and the effect was reversed upon exogenous estradiol treatment [39]. Because of the rising rates of NTM disease and the need for improved therapies, considerable attention is now being devoted to developing better animal models for NTM infections [155].
Sex hormones as modulators of anti-TB immune responses
During pulmonary TB, gene regulation and immune cell recruitment in the lung may be modulated by sex hormones (Box 3 & Figure 1) [44]. Using the human genotype-tissue expression project (GTEx) data from non-diseased, healthy tissues of 838 individuals (557 males and 281 females), 13,294 genes were identified that showed sex-based differential expression across 44 different tissues [45]. Sex-dependent expression of autosomal genes suggested regulation either via sex hormones and their receptors or stemming from sex-dependent epigenetic changes influencing transcription [45]. Accordingly, the promoters of a large number of sex-biased genes harboring hormone receptor elements (HREs) were recognized by transcription factors such as the estrogen receptor-α (ERα), glucocorticoid receptor (GR), as well as two X-linked transcription factors (TFs) - the androgen receptor (AR) and ELK1 [45]. Androgen response elements (AREs) and estrogen response elements (EREs) are present in the promoters of several innate immunity genes [46].
Box 3 |. Sex hormones and TB.
Sex steroids such as estrogens, progesterone, testosterone, dihydrotestosterone (DHT), vary extensively between sexes. Estrogen exists in 3 different isoforms: estrone (E1), estradiol (E2), and estriol (E3). Estradiol predominates in the sera of menstruating females (100–600 pg/ml), while the E1 isoform dominates in post-menopausal women when estradiol concentrations fall similar to, or lower than age-matched males (5–20 pg/ml). Female pulmonary TB patients of reproductive age show significantly reduced serum estradiol and progesterone concentrations relative to age-matched healthy females [156]. In post-menopausal females, TB patients’ mean estrogen concentrations have been found to be significantly higher than in age-matched women without TB [157]. Compared to healthy male controls, male active TB patients exhibit significantly reduced dehydroepiandrosterone (DHEA) and testosterone titers with moderately higher estradiol, cortisol, prolactin, and thyroid hormones [158]. However, the existence of serum testosterone in three different forms, viz., free, globulin-bound and albumin-bound, and its rate of conversion to estrogens, further complicates assessment of its effect on retrospective studies in TB. Although the major male androgen is testosterone, the effects of other important androgens such as dihydrotestosterone and androstenedione on immune responses are to be assessed.
These sex steroids are known to influence immune responses positively or negatively, most prominently in the reproductive years of females. They can modulate the proliferation and immune function of monocytes, macrophages, neutrophils, dendritic cells, natural killer cells, B and T cells. The human ERα estrogen receptor (ER) is strongly expressed on immune cells within the TB granuloma including DCs, monocytes, macrophages, NK cells, and T cells while ERβ appears mostly on B cells and subset of T cells [73]. Androgens mainly function by binding to their cognate androgen receptor (AR). In addition to AR, testosterone also cross-reacts with progesterone and estrogen receptor, though with a lower affinity, while, DHT binds more specifically to AR. Genes encoding many immunostimulatory proteins (e.g., IFN-γ, TLR7) have hormone response elements (HREs) in their promoters, which allow bound sex hormone receptors with nuclear localization sequences to act as transcription factors (TFs), leading to altered gene expression [46]. In human studies, estrogen has been deemed to promote immunoenhancing effect on the immune system, while testosterone and progesterone are considered immunosuppressive [159].
Effect on innate immune responses
Innate immune cells such as macrophages, neutrophils, and natural killer (NK) cells that mediate Type I immune responses play important roles in determining the outcome of disease in Mtb infection, since they influence the early inflammatory response, secretion of cytokines, and the recruitment and activation of other innate and adaptive immune cells, eventually leading to either containment of infection or progress of disease [47]. Since these cells express sex hormone receptors, steroid signaling pathways mediated by them could induce differing functional responses between males and females during early host interactions with Mtb (Figure 1) [48]. Lung macrophages are the first cells to encounter Mtb during infection, and they mount multiple defense strategies to contain the infection, including phagocytosis, induction of anti-microbial effectors such as nitric oxide (NO), production of cytokines, and induction of autophagy [47]. Compared to males, macrophages from adult female C57BL/6 mice show greater phagocytosis of zymosan particles and NADPH oxidase–mediated killing of Group B streptococci in vitro [49]. Human and mouse macrophages express ERα, the intracellular estrogen receptor; however, phagocytosis of macrophages is impaired in bone marrow derived macrophages from C57BL/6 mice that carry a myeloid-specific ERα deletion [50]. This study showed that ERα was important for maintaining other macrophage functions including oxidative metabolism, and responses to LPS and pro-inflammatory fatty acids [50]. Neutrophils play dual and opposing roles during Mtb infection: during early infection, neutrophils phagocytose Mtb and also generate NADPH oxidase-mediated reactive oxygen species (ROS), and peptides such as cathelicidin LL-37 and lipocalin 2 -- all of which are anti-microbial, as shown in studies examining Mtb growth in human peripheral blood cells [51–54]. Studies using zebrafish show that neutrophils exert a protective effect through oxidative killing of the mycobacteria phagocytosed from dead granuloma macrophages [55]. Human neutrophils express ERα and ERβ [56]. Phagocytosis of heat killed Staphylococcus aureus by neutrophils derived from the peripheral blood of healthy human females of reproductive age has been founf to be higher than by cells from age matched males [57]. Moreover, neutrophils from granulomas of Mtb infected macaques express pro- (IFNγ, TNFα) and anti-inflammatory cytokines (IL-4, and IL-10) suggesting that they modulate immune homeostasis in the granuloma microenvironment [58]. During later stages of Mtb infection, excess neutrophilic infiltration leads to increased severity of inflammation, and is associated with pathology in active human TB [59]. Although not evaluated in TB, neutrophil recruitment in prostate inflammation induced by bacterial infection is higher in testosterone-treated rats, associated with infiltration of inefficient “N2-like” neutrophil, as well as expression of IL-10, prolonging inflammation and tissue damage [60]. Type I invariant natural killer T cells (iNKT) cells also are important innate immune cells that contribute to early anti-mycobacterial responses via the production of IFNγ and GM-CSF [61,62]. In vitro and in vivo challenge with specific iNKT cell ligand aGalCer induces higher IFNγ responses in female vs male C57BL/6 mice, and in vitro, via exposure of iNKT cells to estradiol, but not in ovariectomized females [63]. Inhibition of IFNγ secretion by testosterone has also been observed in a mouse model of amoebic liver abscess, a parasitic infection with a strong male bias, in which disease control is influenced by NKT cells [64]. The differential roles these cells might play in Mtb infection in males and females is yet to be elucidated. However, since early events dictate clinical outcome in TB, sex hormones might play an important role in modulating initial responses mediated by innate immune cells following Mtb infection.
The MAPK/ERK pathway is essential for the secretion of TNFα, IL-8, and monocyte chemotactic protein-1 (MCP-1), as revealed by analysis of ERK1/2 and p38 kinases and quantitation of cytokines in culture supernatants following Mtb infection of human peripheral blood monocytes in the presence of specific inhibitors of p38 and MAPK-1 [65,66]. Mtb infection of primary human macrophages also suppresses the PI3K/AKT/mTORC1 pathway that negatively regulates MMP-1 -- a primary mediator of tissue destruction and disease spread, as shown by gene expression and MMP-1 secretion [67]. To impede phagosome-lysosome fusion and promote intracellular survival, Mtb suppresses calcium signaling in human macrophages [68]. In this milieu, estrogen-signaling via ERs localized on the inner cell membrane is associated with increased intracellular NO (anti-mycobacterial) and Ca2+ concentrations, activation of cAMP synthesis, and stimulation of the MAPK/ERK and Pl3K/AKT signaling cascades [69]. Hence, estrogen signaling through intracellular ERs may serve a protective role against TB. In contrast to the impact of estrogen stimulating signaling cascades to elevate cytokine production, testosterone induces apoptosis (thought to be host-protective in TB) in mouse bone marrow derived macrophages (BMDM) via the Fas/FasL-death-inducing signaling pathway and by activating caspase-3, caspase-8, and poly(ADP-ribose) polymerase (PARP) [70]. Sex hormones might therefore differentially activate intracellular signaling pathways in Mtb infected macrophages that can determine the course of disease.
Effect on adaptive immune responses
Adaptive immunity to TB is apparent 2–6 weeks after infection and T cells play a pivotal role in providing anti-TB immunity [47]. Cells involved in Th1 responses against Mtb are well characterized, and known to secrete IFN-γ, TNF-α, and IL-2. ERα-deficient mice have decreased IFNγ+-secreting cells in lymph nodes, suggesting that ERα-mediated signaling is required for Th1 cell responsiveness [71]. 17β-estradiol can stimulate both pro- and anti-inflammatory cytokine synthesis by CD4+ T cells depending on the hormone dose, as revealed by examining the effect of estrogens on cytokine secretion by human T cell clones [72]. High estradiol concentrations promote Th2-biased immunity (during pregnancy or follicular phase of the menstrual cycle), while basal/low concentrations trigger Th1 polarization, macrophage activation, and stimulation of pro-inflammatory cytokines such as IFN-γ and TNF-α [46,48,73]. As Th1 responses are key for Mtb containment, basal estradiol concentrations in the non-pregnant female might be a mechanism by which females are better protected against Mtb disease, although this remains conjectural. A recent study in which Mtb-infected human bronchial epithelial cells were treated with exogenous estradiol in vitro, revealed that estrogen inhibited intracellular Mtb proliferation, as examined by release of LDH [74]. Conversely, as a host-susceptibility factor, testosterone can promote growth of both Mtb and NTM in murine models [31,37,38]. High testosterone concentrations have an immunosuppressive effect by inhibiting B and T cell proliferation and maturation, impairing macrophage activation, reducing IFN-γ, TNF, iNOS, NO, and TLR4 concentrations, and elevating IL-4 amounts [73,75,76]. Moreover, testosterone signaling via the androgen receptor (AR) on CD4+ T lymphocytes leads to increased IL-10 production [77]. Addition of IL-10 blocks phagosome-maturation in Mtb-infected human macrophages [78]. IL-10 also reduces NF-κB activation, Th1 cytokine production, and ROS/NOS concentrations in murine macrophages [78–80]. Thus, we speculate that elevated IL-10 in males might play a causal role in late-stage TB disease progression. While, estradiol has been shown to inhibit IL-10 production in rat spinal cord, higher blood concentrations (as seen during pregnancy), promote IL-10 synthesis [73,81,82]. Also, the pro-inflammatory cytokine IL-23 shows sex-dependent variation, with higher amounts being present in female lungs during TB, compared to male mice [34]. As IL-23 is involved in long-term containment of Mtb and lymphoid follicle formation [83], the reduced concentrations of this cytokine in male lungs might also play a causal role in the reported sex bias, although this remains to be tested. Notably, AR signaling is known to reduce IL-17A and IL-23R production from Th17 cells [84], while estrogen increases both, suggesting that Th17 helper function might be reduced in males during TB, but again, this remains untested [34,84,85]. These findings provide evidence to strongly suggest that adaptive immune responses following Mtb infection might also be influenced by sex hormones.
Genetic determinants of sex differences in TB
While sex hormones are likely to play important roles in the TB sex bias, genetic factors may also contribute significantly, since chromosome complement has key effects on human immune function (Box 4, Figure 2). The X chromosome encodes 16 immunologic receptors, 15 immune response associated proteins, over 25 regulatory genes, and a larger number of microRNAs (miRNAs) and long non-coding RNA (lncRNAs) with immunomodulatory functions [86]. Among the X-encoded protein-coding genes are TLR7, TLR8, CYBB, NEMO, CD40L, and FOXP3 (Table 2) [87]. In contrast, the small ~60 Mb Y chromosome contains only 77 genes plus the sex-determining SRY gene which governs the development of the testes (Box 4) [88]. GTEx-generated RNA-Seq data, revealed that out of 34 genes with sex-biased expression in the human lung, 31 mapped to the Y chromosome; however except for KDM5D and DDX3Y, the immunostimulatory properties of other gene products is unknown [89].
Box 4 |. Sex chromosomes and X chromosome inactivation (XCI).
Human females are homogametic with two copies of the X chromosome (XX), while males with two different sex chromosomes (XY) are heterogametic. The ~155 million bp (Mb) X chromosome encodes over 800 proteins and contains 600 non-coding genes; many of which regulate immune function, including pattern recognition receptors (e.g., TLR7 and TLR8), cytokine receptors (e.g., IL13RA1, IL13RA2), regulatory proteins (NEMO, BTK, CYBB) and transcription factors (e.g., FOXP3) [86]. The acrocentric human Y chromosome harbors ~200 genes, at least 78 of which are protein coding and include SRY, azoospermia factor A and B (AZFA; AZFB), lysine-specific demethylase 5D (KDM5D), and histone demethylase (UTY) [88]. The Y chromosome is crucial for hosting genes relevant to autoimmune diseases and immune responses against infections; also, polymorphisms in Y-chromosome encoded genes can influence sex-dependent susceptibility to certain viral infections [160].
To nullify allele dose differences in X-linked genes between sexes, one of the X chromosomes is epigenetically silenced (XCI) randomly by the Xist lncRNA into an inactive Barr body, during early embryonic development in females [144]. Consequently, males with a single X chromosome are more likely to have X-linked disorders, while females with two X chromosomes have a biological advantage owing to XCI-mediated cellular mosaicism. However, 15–30% of the genes on the inactivated X chromosome may escape transcriptional silencing (“escape from XCI”), many with immunomodulatory potential, thus adding to sexually dimorphic traits of X-linked genes [161]. Moreover, since proximity to the X-chromosome’s pseudoautosomal region 1 (PAR-1) near one telomere is associated with highest likelihood of escape from XCI, immune function genes such as TLR7, TLR8, and CYBB are predicted to have a high probability of escaping XCI [144]. Much heterogeneity is observed in the number of genes which escape XCI, their expression across different tissues within an individual, and between sexes. Genetic imprinting and skewed XCI may also impart immunological advantages to females [162]. The immunologic impact of the X chromosome is exemplified by males with Klinefelter syndrome (XXY) which have low testosterone and gonadotrophins, but elevated estrogen concentrations, and hence exhibit female-like higher circulating IgG titers, B and CD4+ T cell counts, compared with XY males. Similarly, females with Turner syndrome (X0) show immunologic responses similar to those in XY males [48].
Figure 2. Various genetic events that may regulate male sex bias in anti-tuberculosis immunity.
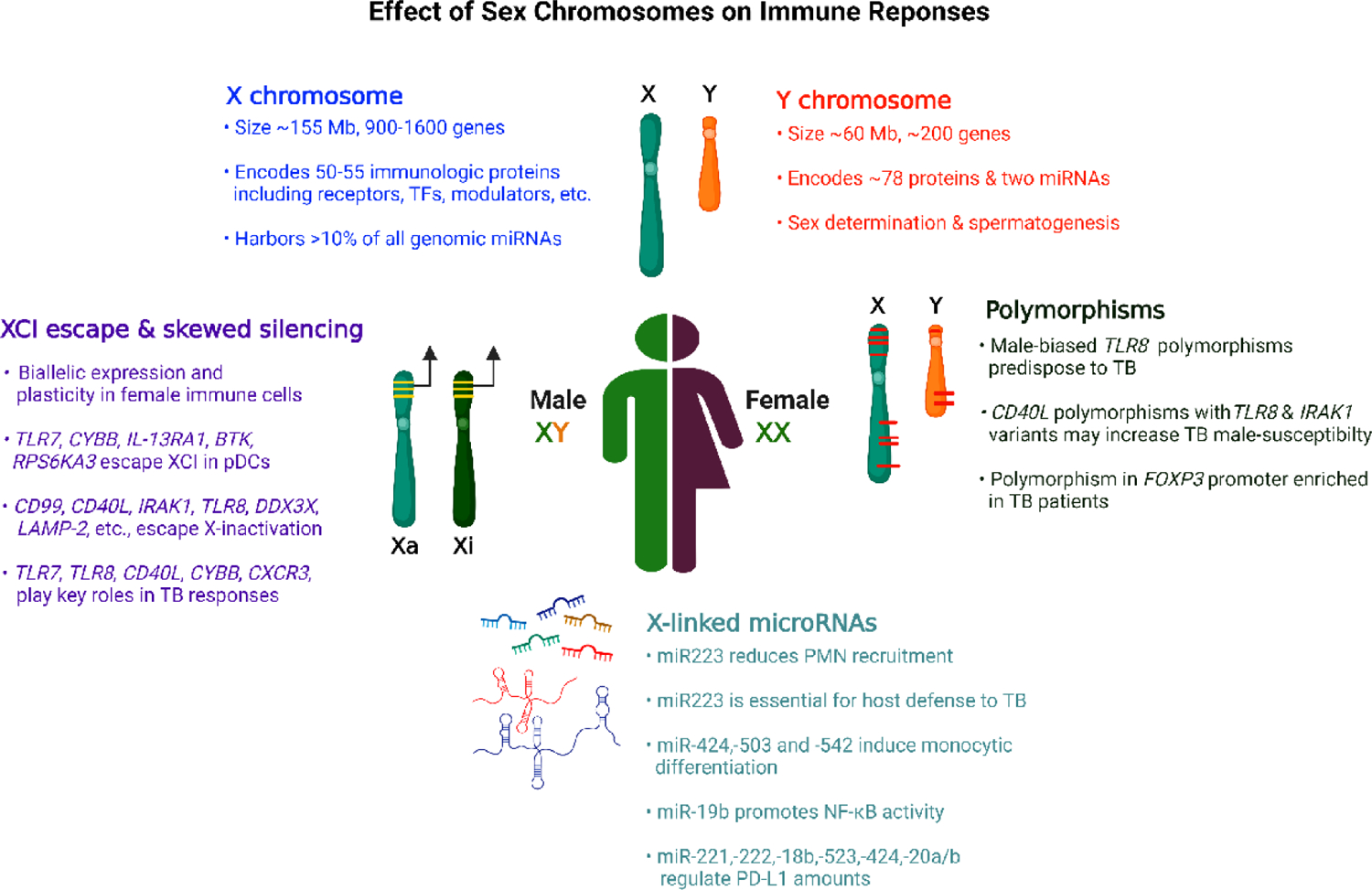
Key genetic determinants on sex chromosomes with potential roles in susceptibility to TB infection, as reported in mice or human studies. Schematic representation, images not scaled. Abbreviations: Mb, million base pairs; TFs, transcription factors; CD40L, CD40 ligand; FOXP3, Forkhead box P3; IRAK1, Interleukin-1 receptor-associated kinase 1; CYBB, Cytochrome b-245 beta chain; IL-13RA1, interleukin 13 receptor alpha 1; BTK, Bruton’s tyrosine kinase; RPS6KA3, Ribosomal Protein S6 Kinase A3; DDX3X, DEAD-Box Helicase 3 X-Linked; CXCR3, C-X-C Motif Chemokine Receptor 3; LAMP-2, lysosomal associated membrane protein-2; PMN, Polymorphonuclear neutrophils; PD-L1, Programmed death-ligand 1.
Table 2.
X-linked genes or miRNAs with a potential role in anti-TB immunity
| Gene Product | Location | Function | Human polymorphism phenotype | Mutant mouse TB phenotype | Ref. |
|---|---|---|---|---|---|
| TLR7 $ | Xp22 |
|
↑ HCV and HIV-1 | - | [97–99] |
| TLR8 $ | Xp22 |
|
↑ TB susceptibility | - | [90,91] |
| CYBB $ | Xp21 |
|
MSMD with CGD | Susceptible | [100,101] |
| CD40L $ | Xq26 |
|
Together with TLR8 & IRAKI polymorphic variants, ↑ male-susceptibility to pulmonary TB | Similar to wild type but impaired granuloma formation | [102,103] |
| IRAK1 $ | Xq28 |
|
Autoimmune diseases such as SLE, systemic sclerosis, rheumatoid arthritis, etc. | - | [104] |
| CXorf21 $ | Xp21 |
|
Sexual dimorphism in SLE | - | [105] |
| NEMO | Xq28 |
|
MSMD | - | [106] |
| FOXP3 | Xp11 |
|
↑ female susceptibility to TB, ↑ viral susceptibility | - | [107,108] |
| IL13RA1$ & RA2 | Xq24 |
|
Atopic disease | Unknown* | [109–112] |
| CXCR3 $ | Xq13 |
|
Male-bias and pleuritis in SLE | Resistant to TB | [113,114] |
| KDM6a $ | Xp11 |
|
Bladder cancer, B cell lymphoma | - | [115] |
| miR-223 | Xq13 |
|
Rheumatoid arthritis | Susceptible to TB | [53,116–118] |
Respective genes are known to escape from XCI
Transgenic IL-13 overexpressing C57BL/6 mice show necrosis and ↑ TB susceptibility
Abbreviations: CGD, Chronic granulomatous disease; MSMD, Mendelian susceptibility to mycobacterial disease; HCV, Hepatitis C virus; HIV-1, Human immunodeficiency virus −1; TLR, Toll like receptors; CYBB, Cytochrome B-245 Beta Chain; CD40L: CD40 ligand; IRAK1, Interleukin 1 receptor associated kinase 1; miR, MicroRNA PMN, Polymorphonuclear neutrophils, NEMO, Nuclear factor-κB (NF-κB) essential modulator; FOXP3, Forkhead box P3; IL13RA1 & RA2, Interleukin 13 receptor subunit alpha 1 and alpha 2; CXorf21, chromosome X open reading frame 21; SLC15A4, solute carrier family 15 member 4; SLE, Systemic Lupus Erythematosus. Upward arrow: increase; ss, single strand
Polymorphisms in X-linked genes may account for sex differences in immune function
The human genome wide association studies provide strong evidence about multiple X-linked genes influencing sex-specific susceptibility to TB. Functional polymorphisms in TLR7 and TLR8 are linked with TB susceptibility in humans [90–92], owed to impaired TNF-α production and increased Mtb phagocytosis in monocytes, particularly in male pulmonary TB patients, in comparison to those with the wild-type allele [92]. Six different studies performed in 7 different countries have found SNPs in TLR8 that are associated with statistically significant, sex-based differences in developing AT [90–94], some with male odds-ratios as high as 4.04 [92]. The CD40 ligand (CD40L), a TNF superfamily member expressed primarily on activated T cells, promotes B cell maturation and immunoglobulin class switching [95]. A recent case report on X-linked hyper IgM syndrome caused by mutations in CD40L, was associated with recurrent TB infection [96]. Also, polymorphisms in X-linked CD40L in combination with TLR8 and IRAK1 variants, increased male-susceptibility to pulmonary TB, in the Han Chinese population [102]. Lastly, a promoter polymorphism in the X-linked FOXP3 gene that encodes the master regulator of regulatory T cell development and function, was significantly enriched in TB patients versus healthy controls, and females with this polymorphism expressed ~5-fold more FOXP3 RNA than healthy control females [107]. Mutations in immunomodulatory X-linked alleles conferring TB susceptibility, would show a greater impact among gonadal males as they possess just one X chromosome. Thus, the significant association of these polymorphisms particularly in pulmonary TB males, further supports the importance of genetic variability in sex-disaggregated TB data (Figure 2).
Incomplete dosage compensation due to X chromosome inactivation (XCI) escape in females
Escape from XCI (Box 4) may account for sex differences in immune responses and disease incidence. Indeed, using the tumor–germline exome sequencing data obtained from 4,126 human patients across 21 different tumors, a study mapping the genetic determinants involved in ≥2:1 M/F ratio for certain cancer types, found escape of X-linked tumor-suppressor genes accountable for the male sex bias [119]. A large number of X-linked immunocompetent genes such as TLR7, TLR8, CYBB, IL-13RA1/RA2, CXCR3, BTK, KDM6a, CD99, CD40L, IRAK1, DDX3X, LAMP-2, KDM5c, etc., are known to escape XCI (Figure 2) [120,121], thereby generating a female-specific heterogeneous population with bi-allelic expression. The resulting cellular mosaicism is likely to impart functional plasticity within female immune cells. While XCI escape as a potential cause for male bias in TB has not been studied, the immunomodulatory functions of genes known to escape XCI is well established and hence might prove to be relevant in TB (Table 2). For example, TLR7 escapes XCI in plasmacytoid dendritic cells (pDCs), monocytes and B cells of normal women and Klinefelter syndrome males, and is known to exhibit a strong sex bias in humans and mice [122,123]. TLR7 signaling inhibits intracellular Mtb proliferation by triggering autophagy in macrophages [124]. Together with TLR9, TLR7 activation is also known to downregulate MARCH1 ubiquitin ligase and reduce IL-10 secretion, while inducing MHC-II expression in primary BMDMs of C57BL/6 mice [125]. The induction of class switch recombination (CSR) and the generation of antigen-specific antibodies rely on TLR7 engagement in B cells [126]. Indeed, type I IFN production in pDCs, induction of CSR, and the generation of antigen-specific antibodies in B cells following TLR7 signaling are all significantly higher in human females than in males [122,123,127,128]. Second, the CD40 ligand in association with IFN-γ is known to activate vitamin D-dependent antimicrobial responses and induction of autophagy against intracellular Mtb infection in human monocytes in vitro [129]. Third, the XCI escaping gene CYBB, which encodes cytochrome b-245 of NADPH oxidase, is the primary component of the ROS generating NADPH oxidase complex of phagocytes, and defects in the CYBB gene are known to cause mendelian susceptibility to mycobacterial diseases (MSMD) [130]. Mutations in CYBB lead to X-linked chronic granulomatous disease immunodeficiency (X-CGD), and while these patients have recurrent skin and soft tissue infections, they are also known to have a higher incidence of AT than healthy controls, suggesting that the oxidative burst is perhaps crucial for host resistance to mycobacterial infections [100]. Phagocytes extracted from CGD patients have shown no intracellular killing and have failed to limit replication upon infection with BCG under in vitro conditions [131]. Also, Cybb−/− knockout mice are more susceptible to TB than WT, although sex differences among these mice have not been investigated [101]. However, because CYBB is as an X-linked gene affecting more males than females [132,133], and is also associated with an elevated risk of TB [100], we argue that CYBB mutations that cause X-CGD are likely genetic contributors to the sex-bias in TB, even though the overall numbers of X-CGD patients is globally low. In contrast to the above examples, the X-inactivation escaping C-X-C motif chemokine receptor 3 gene (CXCR3) product is known to attenuate anti-TB immune responses in mice by altering T cell priming and granuloma composition; hence CXCR3-deficient BALB/c mice show more resistance to Mtb infection than the wild-type mice [113]. Lastly, the IL-13 receptor proteins α1 (IL13RA1) is also known to escape XCI in human pDCs [134]. While knockout mice for the IL13RA1 and RA2 genes are not available, it is known that transgenic C57BL/6 mice overexpressing IL-13 develop a more aggressive form of TB with large necrotizing granulomas than wild-type, similar to those seen in the C3HeB/FeJ “Kramnik” mice [111,112]. Thus, excess signaling through IL-13 and its receptor are deleterious in certain mouse models of TB, and mutations or altered expression of X-linked IL-13 receptor-encoding genes in humans might play a protective role in TB, although this certainly needs further investigation.
X-linked microRNAs (miRNAs)
MicroRNAs are negative regulators of gene expression (Figure 2). The X chromosome accounts for ~10% of all genomic miRNAs, while the Y chromosome harbors only two [86]. To date, miRNA-223 is the most studied X-linked small non-coding RNA known to be involved in immune regulation. Compared to healthy controls with LTBI, miRNA-223 is abundantly expressed in the peripheral blood of patients with active TB infection [101]. Notably, miRNA-223 as an anti-inflammatory microRNA has been found to downregulate chemoattractants like CXCL2 and CCL3 and thereby reduce polymorphonuclear neutrophil (PMN) recruitment – PMN recruitment being strongly associated with lung damage in TB [53,116,117]. Indeed, miR223−/− C57BL/6 mice were found to be highly susceptible to TB infection in comparison to the wild type, which is known to show resistance [117]. Thus, miRNA-223, in addition to its role in regulation of myelopoietic differentiation under homeostasis [135], appears to be crucial for immune responses and containment of TB infection; however, differential expression of miRNA-223 amongst males and females, and its correlation with sex differences in TB mortality, if any, remains uninvestigated. Overexpression of other X-linked microRNAs such as, miRNA-424 and 503, causes cell cycle arrest and promotes differentiation of THP-1 monocytes [86]; while miRNA-19b potentiates NF-κB activity in both mouse and human cells [136].
The programmed death-ligand 1 (PD-L1) is regulated by hypoxia-inducible factor-1α (HIF1α) and STAT3 [137]. Activation of the PD-1/PD-L1 immune checkpoint pathway leads to T cell dysfunction, and anti-PD-1 anitbody immunotherapy has led to reactivation of TB in a 3-dimensional cell culture model of human TB, with concomitant TNFα release and accelerated Mtb growth [138]. Notably, a large number of X-linked miRNAs can directly or indirectly regulate the expression of PD-L1. For example, miRNA-221 and 222 can potentially regulate PD-L1 expression as they regulate the transcription activator STAT3 [139], while miRNA-18a/b is known to regulate TF HIF1α in human cancer cells [140,141]. MicroRNAs-513, 424, 20a/b and 106a/b appear to modify PD-L1 expression by directly targeting the PD-L1 mRNA, as validated in various human cancer models [142]. Thus, sex-biased miRNA expression might influence PD-1/PD-L1 expression, which may in turn modulate TB susceptibility. However, such possibilities will require robust and extensive demonstration. Also, immunologic functions of only a few X-linked microRNAs are known in context to anti-TB immunity, necessitating additional studies to envisage the role of other microRNAs in sex differences to TB.
Concluding Remarks
Sex differences in TB are likely to be multifactorial, and elucidating the underlying mechanisms will require a thorough understanding of the social, behavioral, hormonal, and genetic factors that mediate differing immune responses in males and females. Significant gaps remain in our knowledge of how sex chromosomes complement, how sex hormones influence innate and adaptive immune responses during TB, especially during transition from latent to active TB, and whether there are sex differences in response to anti-TB therapy (see outstanding questions). Future studies in humans and in animal model systems evaluating the influence of sex hormone and identification of sex-linked biomarkers at different stages of TB infection will be valuable in understanding male sex bias in TB and the development of specific therapeutics. New tools such as the FCG mouse model combined with advanced ‘omic’ techniques hold significant promise in this effort (Box 5). Similarly, comparative analyses on gene expression across blood and pulmonary samples from males and females with latent and advanced TB disease will be needed to validate findings from animal models. Finally, it is important to recognize that the greater biological susceptibility to TB is observed in a number of viral, bacterial, and parasitic infections in humans that lead to diseases such as COVID-19, Ebola disease, syphilis, and leishmaniasis, to name a few [48,143,144]. However, it remains to be determined whether the male bias in these different infections has a common biologic basis or whether disease-specific mechanisms apply (see outstanding questions). A better understanding of sex-based differences in these disparate infections offers greater insight into infectious disease pathogenesis, mechanisms of immunity, and may enable development of new and sex-specific interventions and therapies.
Outstanding questions.
What are the specific hormonal mechanisms underlying female resistance to TB infection in humans? While the sex hormones are known to have different immunologic effects (see Fig. 1), the specific pathways modulated by the sex hormones following TB infections in males and females remains unknown.
Do X-linked genes, microRNAs and/or genes that escape XCI, account for the observed male-susceptibility and female resistance to TB in humans? While there is credible evidence that polymorphisms in the X-linked gene TLR8 are associated with increased TB susceptibility, we still do not know whether TLR8 polymorphisms contribute to the male bias in TB incidence. Likewise, there are other X-linked immunologically important genes and microRNAs that may contribute to the sex bias. While escape from XCI has been shown to account for the male bias of certain cancers, it has not been evaluated in the context of TB.
Is there a sex bias in the progression from LTBI to AT in humans? If so, then what are the anti-TB immune response biomarkers that differ in the sexes? Despite dozens of publications on human transcriptomic signatures in AT and LTBI, sex differences in the signatures remains unclear.
Are there sex differences in response to TB therapy, especially host-directed therapies, and in treatment outcomes?
Why do most human infectious diseases exhibit a male sex bias? Are there generalizable sexually divergent mechanisms that account for this male bias or are there different mechanisms for different infections? The question of whether a common set of endocrine or genetic factors regulate this broader male bias to infection disease severity and lethality remains unanswered.
Box 5. Murine models to evaluate sex differences in TB.
Traditionally, gonadal male or female C57BL/6 mice or Gdx BALB/c animals–with appropriate sham controls and with and without hormonal supplementation–have been the mainstay of modeling sex differences, and this has been shown for the TB male sex-bias [31,32,34]. However, sex-based phenotypes are influenced throughout life, including embryonic development, and thus reflect a combinatorial effect of sex hormone organizational (prenatal) and activational (post-pubertal) effects, alongside genetic factors [163]. Indeed, transient testosterone surges occur twice in the developmental stage of most mammals including rodents, one in the prenatal phase to masculinize the gonad [164] and the second postnatal surge referred as ‘mini-puberty’ to stimulate male behavior [165]. Use of adult Gdx animals, therefore, fails to include the impact of these developmental effects.
Recently, new models have emerged to discriminate the impact of genetics and sex steroids on disease, which also consider embryonic and pre-adulthood development, have been established. These include the Four Core Genotype (FCG) and the XY* mouse models. The FCG model involves deletion of ‘Sry’ from ChrY (Y−) and a translocation of it onto Chr3, resulting in XY−Sry males. Mating XY−Sry gonadal males with XX females generates XY mice with ovaries (XYF) or testes (XYM) and XX mice with ovaries (XXF) or testes (XXM) [166,167]. These FCG mice can be studied in a 2×2 factorial design, and 3 different comparisons can be made: (1) comparing the phenotypic effect of gonads/sex steroids (XXF vs. XXM and XYF vs. XYM); (2) testing the effect of sex chromosome complement (XXM vs. XYM, and XXF vs. XYF); and (3) the cumulative effect of 1 and 2 (XXF vs. XYM). In the C57BL/6 background, FCG mice of the same gonadal sex, have comparable concentrations of circulating sex steroids [163], thus making them an ideal background for investigating the effect of sex chromosome complementation. Gdx-FCG mice (with or without specific hormonal supplementation) can be employed to completely rule out the activation effects of sex hormones, although their long-term developmental effects cannot be controlled [163].
Phenotypic differences in gonadally identical XX vs. XY FCG mice may have various genetic explanations: (i) presence of the Y chromosome in XY mice, (ii) overexpression of genes that escape XCI, (iii) allelic mosaicism, or (iv) X-linked gene silencing in XX mice. In such situations, the second XY* model can be used to further map the genetic cause to the X or Y chromosome followed by identification of the allele itself and related downstream pathways [166]. In this model, the Y* chromosome harbors the usual Sry gene but also an aberrant pseudoautosomal region that leads to an abnormal recombination with the X chromosome during meiotic division. Mating such XY* males to XX females, generates XX females, XO females, XY males and XXY males [168]. Consequently, the effect of X chromosome complementation and escape from XCI on sexual dimorphic traits can be inferred by comparing XO vs. XX females, or XY vs. XXY males. Likewise, the effect of one or no Y chromosome can be inferred by testing XO vs. XY, and XX vs. XXY mice. In addition, the Trisomy model yields 8 different genotypes, gonadal male and female mice with the chromosomal complements XX, XY, XXY, and XYY. This model may also be employed to validate the effect of sex chromosome dosage [169].
Significance box.
Tuberculosis (TB) continues to remain one of the world’s most important medical challenges with a significant male bias. Elucidating the underlying mechanisms that mediate differences in immune responses to Mycobacterium tuberculosis in males and females will not only enlighten our understanding of TB pathogenesis, but also aid in developing novel sex-specific vaccines and therapies.
Highlights.
Human adult males are 1.7 times more likely than females to develop active TB disease. Recent studies indicate that reporting biases and differential healthcare access do not fully explain the higher TB incidence in males worldwide.
Sex steroid hormones, sex chromosome-encoded genes, and microRNAs differentially modulate innate and adaptive immune responses to TB in males and females, both in humans and in animal models of TB.
Research addressing the biologic bases for the male bias is likely to provide novel insights into the pathogenesis of active TB disease and latent TB infection.
Understanding the immunological basis for the male bias might enable the development of precision medicine interventions for TB, including host-directed therapies that are sex-specific.
Dissecting the molecular and immunological mechanisms of the male bias in TB will benefit from improved animal models and sex-linked biomarkers that can predict the risk of developing active disease.
Acknowledgments
The authors gratefully acknowledge the support of NIH grants AI167750 and AI155346.
Glossary
- Active TB
multiorgan disease caused by Mycobacterium tuberculosis (Mtb) primary infection or due to reactivation of its latent infection (~90% of the cases), in an immunocompromised background. Unlike the asymptomatic latent form, active TB is highly contagious and causes symptoms depending on whether it is pulmonary or extrapulmonary
- Autophagy:
conserved lysosome-dependent degradation of a cell to remove dysfunctional components. It regulates inflammation and generates cell-autonomous defense against intracellular pathogens such as Mtb. Autophagy is often looked upon as an adaptive stress response for survival; however, in some diseases, it appears to promote cell death and morbidity
- B-cell follicles
complex network of co-localized cells comprised of B cells, follicular dendritic cells, T cell subsets, and adjoining macrophages. These cells reside in primary and secondary follicles of lung, spleen, and lymph nodes and participate in T-cell–dependent antibody responses mediated by follicular B cells
- Bone marrow-derived macrophages
Cells extracted from mammalian bone marrow cells and differentiated under ex vivo settings into mature macrophages by stimulation with growth factors and signaling molecules
- Class switch recombination
complex genetic process in B cells in which DNA-rearrangement reactions of immunoglobulin heavy chains causes sa witch from IgM antibody production to that of IgG, IgE or IgA antibodies
- C3HeB/FeJ ‘Kramnik’ mice
transgenic C3H mice, carrying a normal allele at the Tlr4 locus, but is homozygous for Pde6brd1. C3HeB/FeJ inbred mice are extremely susceptible to Mtb and unlike BALB/c and C57BL/6 develop necrotic lung lesions following aerosol challenge
- Genetic imprinting
refers to the differential epigenetic marking of paternally and maternally inherited alleles of specific genes or chromosome regions during gametogenesis, leading to monoallelic expression depending on parental origin. Female mammals possess two X chromosomes, one from each parent, while in males, the single X chromosome is invariably of maternal origin
- Genotype-tissue expression (GTEx) project
open-access public domain for comprehensive analysis of tissue-specific gene expression and regulation (https://gtexportal.org/home). GTEx helps correlate between genotypes and differential gene expression in context to genetic or sex hormone-related factors
- Host-directed therapy
In the context of infectious diseases, refers to treating infections with agents that alter host responses rather than kill or inhibit microbial proliferations
- Hormone response elements
short DNA sequence in a gene promoter to which specific steroid hormone-receptor complexes bind and regulate transcription
- Hyper IgM syndrome
rare genetic defects with an inability to switch from IgM antibody production to the IgG, IgA or IgE types. HIGM patients are susceptible to recurrent and severe infections, risk of cancer, and opportunistic infections
- Latent TB infection
asymptomatic Mtb infection with immunologic evidence of responsiveness to Mtb antigens. Typical immunologic diagnostics for LTBI are tuberculin skin test or interferong release assay (IGRA) positivity
- Mendelian susceptibility to mycobacterial diseases
rare genetic disorder associated with susceptibility to infections caused by weakly virulent mycobacteria, such as BCG and NTMs. Defects in 9 genes, 7 autosomal (STAT1, IFNGR1, IFNGR2, IL12B, IL12RB1, ISG15, and IRF8) and 2 X-linked (NEMO and CYBB), are known to cause MSMD in humans
- PD-1/PD-L1 immune checkpoint
PD-L1 is a transmembrane protein involved in suppressing the adaptive arm of immunity; PD-1 is a checkpoint receptor abundantly expressed by activated T cells, B cells, dendritic cells, and NK cells. Binding of PD-L1 to PD1, triggers a series of pathways leading to inhibition of T cell proliferation and regulatory T cell apoptosis
- Sex-determining region on chromosome Y (SRY)
SOX gene family transcription factor (‘testis-determining factor’) encoded by the Y chromosome; required for the development of male gonads. In mammals, sry/Sry presence leads to development of testis; in its absence, ovaries develop
- Toll like receptors
innate immune receptors expressed by macrophages, dendritic cells, NK cells, T and B cells; TLRs recognize pathogen-associated molecular patterns and activate innate immune cell responses
- Treg cells
subpopulation of CD4+ T cells with immunosuppressive functions involved in sustaining homeostasis and self-tolerance
- XCI
X-chromosome inactivation (Lyonization or Barr body formation); normal physiologic process in females by which one copy of the X chromosome is inactivated and becomes transcriptionally inactive heterochromatin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeremiah C et al. (2022) The WHO Global Tuberculosis 2021 Report–not so good news and turning the tide back to End TB. International Journal of Infectious Diseases, S1201–9712(22)00149–7. doi: 10.1016/j.ijid.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton KC et al. (2016) Sex differences in tuberculosis burden and notifications in low-and middle-income countries: a systematic review and meta-analysis. PLoS medicine 13, e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worl Health Organization, (2015) Gear up to end TB: introducing the end TB strategy, https://www.who.int/publications/i/item/gear-up-to-end-tb-introducing-the-end-tb-strategy
- 4.Long NH et al. (1999) Different tuberculosis in men and women: beliefs from focus groups in Vietnam. Social Science & Medicine 49, 815–822 [DOI] [PubMed] [Google Scholar]
- 5.Li T et al. (2019) Under-reporting of diagnosed tuberculosis to the national surveillance system in China: an inventory study in nine counties in 2015. BMJ open 9, e021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledesma JR et al. (2022) Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990–2019: results from the Global Burden of Disease Study 2019. The Lancet Infectious Diseases 22, 222–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgdorff M et al. (2000) Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. The International Journal of Tuberculosis and Lung Disease 4, 123–132 [PubMed] [Google Scholar]
- 8.Fox GJ et al. (2016) Tuberculosis in newborns: the lessons of the “Lübeck Disaster”(1929–1933). PLoS pathogens 12, e1005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton JB and Mestler GE (1969) Mortality and survival: comparison of eunuchs with intact men and women in a mentally retarded population. Journal of Gerontology 24, 395–411 [DOI] [PubMed] [Google Scholar]
- 10.Chidambaram V et al. (2021) Male sex is associated with worse microbiological and clinical outcomes following tuberculosis treatment: A retrospective cohort study, a systematic review of the literature, and meta-analysis. Clinical Infectious Diseases 73, 1580–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bothamley GH (2021) Male Sex Bias in Immune Biomarkers for Tuberculosis. Frontiers in immunology 12, 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aktogu S et al. (1996) Clinical spectrum of pulmonary and pleural tuberculosis: a report of 5,480 cases. European Respiratory Journal 9, 2031–2035 [DOI] [PubMed] [Google Scholar]
- 13.Imperial MZ et al. (2021) Precision-enhancing risk stratification tools for selecting optimal treatment durations in tuberculosis clinical trials. American journal of respiratory and critical care medicine 204, 1086–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully EP and Bryson BD (2021) Unlocking the complexity of HIV and Mycobacterium tuberculosis coinfection. Journal of Clinical Investigation 131, e154407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agudelo CA et al. (2021) Outcomes and complications of hospitalised patients with HIV-TB co-infection. Tropical Medicine & International Health 26, 82–88 [DOI] [PubMed] [Google Scholar]
- 16.Bock P et al. (2018) Incidence of Tuberculosis amongst HIV positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. Journal of acquired immune deficiency syndromes (1999) 77, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golub JE et al. (2009) Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS (London, England) 23, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresges C et al. (2021). Early Empirical Tuberculosis Treatment in HIV-Positive Patients Admitted to Hospital in South Africa: An Observational Cohort Study. Open Forum Infectious Diseases. 8(7):ofab162. doi: 10.1093/ofid/ofab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino RJ et al. (2020) Characteristics indicative of tuberculosis/HIV coinfection in a high-burden setting: lessons from 13,802 incident tuberculosis cases in Harare, Zimbabwe. The American journal of tropical medicine and hygiene 103, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos JM et al. (2020) Sex differences and HIV status of tuberculosis in adults at a rural hospital in southern Ethiopia: an 18-year retrospective cross-sectional study. African Health Sciences 20, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baluku JB et al. (2021) Gender differences among patients with drug resistant tuberculosis and HIV co-infection in Uganda: a countrywide retrospective cohort study. BMC Infectious Diseases 21, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ting W-Y et al. (2014) Gender disparities in latent tuberculosis infection in high-risk individuals: a cross-sectional study. PloS one 9, e110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizza FN et al. (2015) Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC infectious diseases 15, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barry M (2021) Prevalence of latent tuberculosis infection in the Middle East and North Africa: a systematic review. Pulmonary medicine 2021:6680651. doi: 10.1155/2021/6680651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva V et al. (2003) Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. The International Journal of Tuberculosis and Lung Disease 7, 478–484 [PubMed] [Google Scholar]
- 26.Guerra-Silveira F and Abad-Franch F (2013) Sex bias in infectious disease epidemiology: patterns and processes. PloS one 8, e62390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddon JA et al. (2018) The wonder years: what can primary school children teach us about immunity to Mycobacterium tuberculosis? Frontiers in immunology, 9: 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stival A et al. (2014) Sexual dimorphism in tuberculosis incidence: children cases compared to adult cases in Tuscany from 1997 to 2011. PLoS One 9, e105277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S. j. et al. (2021) Population aging and trends of pulmonary tuberculosis incidence in the elderly. BMC Infectious Diseases 21, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izzo R and Cicardo V (1947) Gonads and experimental tuberculosis. Nature 160, 155–155 [DOI] [PubMed] [Google Scholar]
- 31.Bini EI et al. (2014) The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PloS one 9, e93831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dibbern J et al. (2017) Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Scientific Reports 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrichs T et al. (2004) Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 204, 217–228 [DOI] [PubMed] [Google Scholar]
- 34.Hertz D et al. (2020) Increased male susceptibility to Mycobacterium tuberculosis infection is associated with smaller B cell follicles in the lungs. Scientific Reports 10, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieuwenhuizen NE et al. (2021) Weaker protection against tuberculosis in BCG-vaccinated male 129 S2 mice compared to females. Vaccine 39, 7253–7264 [DOI] [PubMed] [Google Scholar]
- 36.López-Olvera JR et al. (2013) Sex-related differences in body condition and serum biochemical parameters in red deer (Cervus elaphus) naturally infected with Mycobacterium bovis. The Veterinary Journal 198, 702–706 [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y et al. (1990) Sex differences in the susceptibility of mice to infection induced by Mycobacterium intracellulare. American Journal of Respiratory and Critical Care Medicine 142, 430–433 [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto Y et al. (1991) Sex differences in host resistance to Mycobacterium marinum infection in mice. Infection and immunity 59, 4089–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuyuguchi K et al. (2001) Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clinical & Experimental Immunology 123, 428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown I and Glynn A (1987) The Ity/Lsh/Bcg gene significantly affects mouse resistance to Mycobacterium lepraemurium. Immunology 62, 587. [PMC free article] [PubMed] [Google Scholar]
- 41.Cowman S et al. (2019) Non-tuberculous mycobacterial pulmonary disease. European Respiratory Journal 54(1):1900250 [DOI] [PubMed] [Google Scholar]
- 42.Ratnatunga CN et al. (2020) The rise of non-tuberculosis mycobacterial lung disease. Frontiers in immunology, 11, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirsaeidi M and Sadikot RT (2015) Gender susceptibility to mycobacterial infections in patients with non-CF bronchiectasis. International journal of mycobacteriology 4, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y et al. (2012) Tuberculosis and sexual inequality: the role of sex hormones in immunity. Critical Reviews in Eukaryotic Gene Expression 22, (3):233–41. [DOI] [PubMed] [Google Scholar]
- 45.Oliva M et al. (2020) The impact of sex on gene expression across human tissues. Science 369, eaba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding AT and Heaton NS (2022) The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 14, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Martino M et al. (2019) Immune response to Mycobacterium tuberculosis: a narrative review. Frontiers in pediatrics, 7: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein SL and Flanagan KL (2016) Sex differences in immune responses. Nature Reviews Immunology 16, 626–638 [DOI] [PubMed] [Google Scholar]
- 49.Scotland RS et al. (2011) Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood, The Journal of the American Society of Hematology 118, 5918–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribas V et al. (2011) Myeloid-specific estrogen receptor α deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences 108, 16457–16462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borkute RR et al. (2021) Neutrophils in tuberculosis: Cell biology, cellular networking and multitasking in host defense. International Journal of Molecular Sciences 22, 4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dallenga T and Schaible U Neutrophils in tuberculosis—first line of defence or booster of disease and targets for host-directed therapy? Pathog Dis. 2016; 74 (3): ftw012. [DOI] [PubMed] [Google Scholar]
- 53.Martineau AR et al. (2007) Neutrophil-mediated innate immune resistance to mycobacteria. The Journal of clinical investigation 117, 1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan BH et al. (2006) Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. The Journal of Immunology 177, 1864–1871 [DOI] [PubMed] [Google Scholar]
- 55.Yang C-T et al. (2012) Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell host & microbe 12, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stygar D et al. (2006) Identification of wild type and variants of oestrogen receptors in polymorphonuclear and mononuclear leucocytes. Clinical endocrinology 64, 74–81 [DOI] [PubMed] [Google Scholar]
- 57.Saikia TC et al. (2003) Phagocytic activities of neutrophilic leukocytes in women in various phases of menstrual cycle, and in pregnancy. Southeast Asian journal of tropical medicine and public health 34, 877–880 [PubMed] [Google Scholar]
- 58.Gideon HP et al. (2019) Neutrophils express pro-and anti-inflammatory cytokines in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Mucosal immunology 12, 1370–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravesloot-Chávez MM et al. (2021) The innate immune response to Mycobacterium tuberculosis infection. Annual review of immunology 39, 611–637 [DOI] [PubMed] [Google Scholar]
- 60.Scalerandi MV et al. (2018) Inefficient N2-like neutrophils are promoted by androgens during infection. Frontiers in immunology 9, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chackerian A et al. (2002) Activation of NKT cells protects mice from tuberculosis. Infection and immunity 70, 6302–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothchild AC et al. (2014) iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS pathogens 10, e1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gourdy P et al. (2005) Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-γ production by invariant natural killer T cells. Blood 105, 2415–2420 [DOI] [PubMed] [Google Scholar]
- 64.Lotter H et al. (2013) Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PLoS One 8, e55694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song C-H et al. (2003) Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-α, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. Journal of clinical immunology 23, 194–201 [DOI] [PubMed] [Google Scholar]
- 66.Zhang X et al. (2017) Inhibition of the PI3K-Akt-mTOR signaling pathway in T lymphocytes in patients with active tuberculosis. International Journal of Infectious Diseases 59, 110–117 [DOI] [PubMed] [Google Scholar]
- 67.Brace PT et al. (2017) Mycobacterium tuberculosis subverts negative regulatory pathways in human macrophages to drive immunopathology. PLoS Pathogens 13, e1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malik ZA et al. (2000) Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. The Journal of experimental medicine 191, 287–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moriarty K et al. (2006) Estrogen receptor-mediated rapid signaling. Endocrinology 147, 5557–5563 [DOI] [PubMed] [Google Scholar]
- 70.Jin L et al. (2006) Testosterone induces apoptosis via Fas/FasL-dependent pathway in bone marrow-derived macrophages. Methods and findings in experimental and clinical pharmacology 28, 283–294 [DOI] [PubMed] [Google Scholar]
- 71.Maret A et al. (2003) Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor α expression in hematopoietic cells. European journal of immunology 33, 512–521 [DOI] [PubMed] [Google Scholar]
- 72.Gilmore W et al. (1997) Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. The Journal of Immunology 158, 446–451 [PubMed] [Google Scholar]
- 73.Nhamoyebonde S and Leslie A (2014) Biological differences between the sexes and susceptibility to tuberculosis. The Journal of infectious diseases 209, S100–S106 [DOI] [PubMed] [Google Scholar]
- 74.Gan Y et al. (2022) Estradiol inhibits autophagy of Mycobacterium tuberculosis-infected 16HBE cells and controls the proliferation of intracellular Mycobacterium tuberculosis. Molecular Medicine Reports 25, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrivastava P and Bagchi T (2021) Testosterone in pathogenesis of tuberculosis. Chemical Biology Letters 8, 238–247 [Google Scholar]
- 76.Furman D et al. (2014) Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences 111, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liva SM and Voskuhl RR (2001) Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. The Journal of Immunology 167, 2060–2067 [DOI] [PubMed] [Google Scholar]
- 78.O’Leary S.n. et al. (2011) IL-10 blocks phagosome maturation in Mycobacterium tuberculosis–infected human macrophages. American journal of respiratory cell and molecular biology 45, 172–180 [DOI] [PubMed] [Google Scholar]
- 79.Redford P et al. (2011) The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal immunology 4, 261–270 [DOI] [PubMed] [Google Scholar]
- 80.Dokka S et al. (2001) Interleukin-10-mediated inhibition of free radical generation in macrophages. American Journal of Physiology-Lung Cellular and Molecular Physiology 280, L1196–L1202 [DOI] [PubMed] [Google Scholar]
- 81.Shivers K-Y et al. (2015) Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine 72, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matalka KZ and Ali DA (2005) Stress-induced versus preovulatory and pregnancy hormonal levels in modulating cytokine production following whole blood stimulation. Neuroimmunomodulation 12, 366–374 [DOI] [PubMed] [Google Scholar]
- 83.Khader SA et al. (2011) IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. The Journal of Immunology 187, 5402–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwinge D et al. (2015) Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. The Journal of Immunology 194, 2522–2530 [DOI] [PubMed] [Google Scholar]
- 85.Newcomb DC et al. (2015) Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. Journal of Allergy and Clinical Immunology 136, 1025–1034. e1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinheiro I et al. (2011) X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 33, 791–802 [DOI] [PubMed] [Google Scholar]
- 87.Fish EN (2008) The X-files in immunity: sex-based differences predispose immune responses. Nature Reviews Immunology 8, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bachtrog D (2013) Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nature Reviews Genetics 14, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melé M et al. (2015) The human transcriptome across tissues and individuals. Science 348, 660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salie M et al. (2015) Association of toll-like receptors with susceptibility to tuberculosis suggests sex-specific effects of TLR8 polymorphisms. Infection, genetics and evolution 34, 221–229 [DOI] [PubMed] [Google Scholar]
- 91.Davila S et al. (2008) Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS genetics 4, e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lai Y-F et al. (2016) Functional polymorphisms of the TLR7 and TLR8 genes contribute to Mycobacterium tuberculosis infection. Tuberculosis 98, 125–131 [DOI] [PubMed] [Google Scholar]
- 93.Dalgic N et al. (2011) Relationship between toll-like receptor 8 gene polymorphisms and pediatric pulmonary tuberculosis. Disease markers 31, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashemi-Shahri SM et al. (2014) Association between TLR8 and TLR9 gene polymorphisms and pulmonary tuberculosis. Gene Cell Tissue 1, e18316 [Google Scholar]
- 95.Elgueta R et al. (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological reviews 229, 152–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krishnan V et al. (2020) X-Linked Hyper IgM Syndrome Presenting with Recurrent Tuberculosis—a Case Report. Journal of Clinical Immunology 40, 531–533 [DOI] [PubMed] [Google Scholar]
- 97.Wang C-H et al. (2011) TLR7 and TLR8 gene variations and susceptibility to hepatitis C virus infection. PloS one 6, e26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azar P et al. (2020) TLR7 dosage polymorphism shapes interferogenesis and HIV-1 acute viremia in women. Jci Insight 5(12):e136047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koval TI et al. (2018) TLR4 and TLR7 genetic polymorphism in patients with HIV/HCV-coinfection: prevalence and gender-related features. Wiad Lek 71(8):1566–1570. [PubMed] [Google Scholar]
- 100.Lee PP et al. (2008) Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. The Pediatric infectious disease journal 27, 224–230 [DOI] [PubMed] [Google Scholar]
- 101.Olive AJ et al. (2018) The phagocyte oxidase controls tolerance to Mycobacterium tuberculosis infection. The Journal of Immunology 201, 1705–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu C et al. (2015) Polymorphism of X-linked CD40 ligand gene associated with pulmonary tuberculosis in the Han Chinese population. Genes & Immunity 16, 399–404 [DOI] [PubMed] [Google Scholar]
- 103.Saunders BM and Britton WJ (2007) Life and death in the granuloma: immunopathology of tuberculosis. Immunology and cell biology 85, 103–111 [DOI] [PubMed] [Google Scholar]
- 104.Shi Z et al. (2020) IRAK1 polymorphisms are associated with susceptibility to neuromyelitis optica spectrum disorder. Multiple Sclerosis and Related Disorders 37, 101438. [DOI] [PubMed] [Google Scholar]
- 105.Odhams CA et al. (2019) Interferon inducible X-linked gene CXorf21 may contribute to sexual dimorphism in Systemic Lupus Erythematosus. Nature communications 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filipe-Santos O et al. (2006) X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. The Journal of experimental medicine 203, 1745–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beiranvand E et al. (2017) G allele at− 924 A> G position of FoxP3 gene promoter as a risk factor for tuberculosis. BMC infectious diseases 17, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pereira LMS et al. (2020) Sex and FOXP3 gene rs2232365 polymorphism may be associated with the clinical and pathological aspects of chronic viral diseases. BMC immunology 21, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguirre CA et al. (2020) Two single nucleotide polymorphisms in IL13 and IL13RA1 from individuals with idiopathic Parkinson’s disease increase cellular susceptibility to oxidative stress. Brain, Behavior, and Immunity 88, 920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konstantinidis A et al. (2007) Genetic association studies of interleukin-13 receptor α1 subunit gene polymorphisms in asthma and atopy. European Respiratory Journal 30, 40–47 [DOI] [PubMed] [Google Scholar]
- 111.Heitmann L et al. (2014) The IL-13/IL-4R α axis is involved in tuberculosis-associated pathology. The Journal of pathology 234, 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hölscher C et al. (2016) A mutation in IL4RA is associated with the degree of pathology in human TB patients. Mediators of inflammation 2016, 4245028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chakravarty SD et al. (2007) The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. The Journal of Immunology 178, 1723–1735 [DOI] [PubMed] [Google Scholar]
- 114.Im CH et al. (2014) CXCR3 polymorphism is associated with male gender and pleuritis in patients with systemic lupus erythematosus. Human immunology 75, 466–469 [DOI] [PubMed] [Google Scholar]
- 115.Chen X et al. (2021) Significance of KDM6A mutation in bladder cancer immune escape. BMC cancer 21, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eum S-Y et al. (2010) Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorhoi A et al. (2013) MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. The Journal of clinical investigation 123, 4836–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khalifa O et al. (2016) X-linked miRNAs associated with gender differences in rheumatoid arthritis. International journal of molecular sciences 17, 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dunford A et al. (2017) Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nature genetics 49, 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Youness A et al. (2021) Escape from X chromosome inactivation and the female predominance in autoimmune diseases. International Journal of Molecular Sciences 22, 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sauteraud R et al. (2021) Inferring genes that escape X-Chromosome inactivation reveals important contribution of variable escape genes to sex-biased diseases. Genome research 31, 1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Souyris M et al. (2018) TLR7 escapes X chromosome inactivation in immune cells. Science immunology 3, eaap8855. [DOI] [PubMed] [Google Scholar]
- 123.Souyris M et al. (2019). Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Seminars in immunopathology. 41(2):153–164. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen H et al. (2020) A role of intracellular Toll-like receptors (3, 7, and 9) in response to Mycobacterium tuberculosis and co-infection with HIV. International Journal of Molecular Sciences 21, 6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bakhru P et al. (2014) BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cellular immunology 287, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hua Z and Hou B (2013) TLR signaling in B-cell development and activation. Cellular & molecular immunology 10, 103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu B et al. (2021) B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 184, 1790–1803. e1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fink AL et al. (2018) Biological sex affects vaccine efficacy and protection against influenza in mice. Proceedings of the National Academy of Sciences 115, 12477–12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klug-Micu GM et al. (2013) CD40 ligand and interferon-γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 139, 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vermot A et al. (2021) NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 10, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lamhamedi-Cherradi S et al. (1999) Growth of Mycobacterium bovis, Bacille Calmette-Guerin, within human monocytes-macrophages cultured in serum-free medium. Journal of immunological methods 225, 75–86 [DOI] [PubMed] [Google Scholar]
- 132.Lent-Schochet D and Jialal I (2021) Chronic Granulomatous Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–.. [PubMed] [Google Scholar]
- 133.Roos D (2016) Chronic granulomatous disease. British medical bulletin 118, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hagen SH et al. (2020) Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell reports 33, 108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rajasekhar M et al. (2018) Identifying microRNA determinants of human myelopoiesis. Scientific reports 8, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gantier MP et al. (2012) A miR-19 regulon that controls NF-κB signaling. Nucleic acids research 40, 8048–8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zerdes I et al. (2018) Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene 37, 4639–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tezera LB et al. (2020) Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. Elife 9, e52668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu S et al. (2014) A microRNA 221–and 222–mediated feedback loop maintains constitutive activation of NFκB and STAT3 in colorectal Cancer cells. Gastroenterology 147, 847–859. e811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krutilina R et al. (2014) MicroRNA-18a inhibits hypoxia-inducible factor 1α activity and lung metastasis in basal breast cancers. Breast Cancer Research 16, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y et al. (2016) MicroRNA-18b inhibits the growth of malignant melanoma via inhibition of HIF-1α-mediated glycolysis Retraction in/ 10.3892/or.2021.8161. Oncology reports 36, 471–479 [DOI] [PubMed] [Google Scholar]
- 142.Carè A et al. (2018) Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death & Differentiation 25, 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peckham H et al. (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature communications 11, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schurz H et al. (2019) The X chromosome and sex-specific effects in infectious disease susceptibility. Human genomics 13, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dockrell HM and Butkeviciute E (2022) Can what have we learnt about BCG vaccination in the last 20 years help us to design a better tuberculosis vaccine? Vaccine 40, 1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andersen P and Doherty TM (2005) The success and failure of BCG—implications for a novel tuberculosis vaccine. Nature Reviews Microbiology 3, 656–662 [DOI] [PubMed] [Google Scholar]
- 147.Roth A et al. (2006) Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology, 17(5):562–8 [DOI] [PubMed] [Google Scholar]
- 148.Darboe F et al. (2017) Minimal sex-differential modulation of reactivity to pathogens and toll-like receptor ligands following infant bacillus Calmette–Guérin Russia vaccination. Frontiers in immunology 8, 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koeken VA et al. (2020) BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. The Journal of clinical investigation 130, 5591–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prabowo SA et al. (2019) Impact of individual-level factors on Ex vivo mycobacterial growth inhibition: Associations of immune cell phenotype, cytomegalovirus-specific response and sex with immunity following BCG vaccination in humans. Tuberculosis 119, 101876. [DOI] [PubMed] [Google Scholar]
- 151.Robinson GA et al. (2019) Sex differences in autoimmunity could be associated with altered regulatory T cell phenotype and lipoprotein metabolism. bioRxiv, 760975 https://www.biorxiv.org/content/10.1101/760975v1.full [Google Scholar]
- 152.Jaron B et al. (2008) Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PloS one 3, e2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ordway DJ et al. (2011) Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clinical and vaccine Immunology 18, 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qvist T et al. (2015) Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. Journal of Cystic Fibrosis 14, 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maggioncalda EC et al. (2020) A mouse model of pulmonary Mycobacteroides abscessus infection. Scientific reports 10, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aswathy T et al. (2018). Evaluation of hormones in the menstrual cycle of women with pulmonary tuberculosis. Eur Respiratory Journal 52: PA2703; DOI: 10.1183/13993003.congress-2018.PA2703 [DOI] [Google Scholar]
- 157.Erbay G et al. (2016) Relationship between tuberculosis and female hormone levels in post-menopausal women. Southeast Asian Journal of Tropical Medicine and Public Health 47, 78. [PubMed] [Google Scholar]
- 158.Del Rey A et al. (2007) Endocrine and cytokine responses in humans with pulmonary tuberculosis. Brain, behavior, and immunity 21, 171–179 [DOI] [PubMed] [Google Scholar]
- 159.Moulton VR (2018) Sex hormones in acquired immunity and autoimmune disease. Frontiers in immunology 9, 2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kloc M et al. (2020) The role of genetic sex and mitochondria in response to COVID-19 infection. International archives of allergy and immunology 181, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Posynick BJ and Brown CJ (2019) Escape from X-chromosome inactivation: an evolutionary perspective. Frontiers in cell and developmental biology 7, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Migliore L et al. (2021) Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines 9, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arnold AP (2014) Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Experimental neurology 259, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richards G and Browne WV (2022) Prenatal testosterone and sexually differentiated childhood play preferences: a meta-analysis of amniotic fluid studies. Current Psychology, 1–14 [Google Scholar]
- 165.WINTER JS et al. (1976) Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. The Journal of Clinical Endocrinology & Metabolism 42, 679–686 [DOI] [PubMed] [Google Scholar]
- 166.Arnold AP (2020) Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neuroscience & Biobehavioral Reviews 119, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arnold AP and Chen X (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in neuroendocrinology 30, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burgoyne PS and Arnold AP (2016) A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biology of sex differences 7, 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen X et al. (2013) The Sex Chromosome Trisomy mouse model of XXY and XYY: metabolism and motor performance. Biology of sex differences 4, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]


