Abstract
Study Design:
Post hoc comparison using single-site data from 4 multicenter randomized controlled trials.
Objectives:
Discogenic back pain is associated with significant morbidity and medical cost. Several terminated, unreported randomized controlled trials have studied the effect of intradiscal biologic injections. Here we report single-center outcomes from these trials to determine if there is clinical improvement associated with these intradiscal injections.
Methods:
Post hoc comparison was performed using single-site data from 4 similar multi-center randomized controlled trials. All trials evaluated an injectable therapy (growth factor, fibrin sealant, or stem cells) for symptomatic lumbar disc disease with near-identical inclusion and exclusion criteria. Demographics and patient reported outcomes were analyzed across treatment arms postinjection.
Results:
A total of 38 patients were treated with biologic agents and 12 were treated with control saline injections. There was a significant decrease in visual analogue score (VAS) pain for both the investigational and saline groups up to 12 months postinjection (P < .01). There was no significant difference in VAS scores between the saline and investigational groups at 12 months. Similarly, there was significant improvement in patient-reported disability scores in both the investigational and saline groups at all time points. There were no significant differences in disability score improvement between the saline and investigational treatment groups at 12 months postinjection.
Conclusions:
A single-center analysis of 4 randomized controlled studies demonstrated no difference in outcomes between therapeutic intradiscal agents (growth factor, fibrin sealant, or stem cells) and control saline groups. In all groups, patient reported pain and disability scores decreased significantly. Future studies are needed to evaluate the therapeutic benefit of any intradiscal injections.
Keywords: degenerative disc disease, discogenic back pain, intradiscal therapy, saline
Introduction
Low back pain is a leading cause of disability and morbidity in the adult population, affecting approximately 80% of adults within their lifetime.1,2 Up to 40% of all low back pain is believed to be primarily caused by degeneration of the intervertebral disc, also known as discogenic pain.3,4 Currently, there are no Food and Drug Administration–approved methods for preventing or reversing intervertebral disc degeneration and associated discogenic pain. Surgical interventions such as arthrodesis or arthroplasty for discogenic pain are invasive and costly, leading to efforts to develop effective and safe nonoperative treatment modalities.
Direct injection of active substances to slow or even reverse intervertebral disc degeneration is a popular topic of research. 5 Multiple trials studying biologic, cell-based, or scaffold-based injectable therapies to treat discogenic back pain have been initiated in the past decade. 6 Many investigational products have had early promising results from preclinical and phase I/II studies, prompting the initiation of large multicenter randomized controlled studies.7-10 To date, none of these biological treatments for discogenic back pain have demonstrated clinical superiority over placebo in these large randomized controlled studies with regards to patient pain or function, and many trials have been terminated. This may be related to inadequate efficacy of the investigational product, but it could also be due to an “any-treatment” effect where outcomes are improved with either the control or investigational agent. Unfortunately, to our knowledge, no data from these terminated trials has ever been published in the scientific literature for analysis.
In this study, we utilize single-site data from 4 multicenter, randomized, controlled trials to report patient outcomes after intradiscal injection of saline placebo versus biologic investigational treatments for discogenic low back pain. The purpose of the study was to report saline-related outcomes from intradiscal injection studies, as it is possible that the saline “placebo” may have a therapeutic effect that has not been previously quantified. Given that saline is used as a standard control in ongoing and future studies, this study also aims to report saline-related effects to help determine a minimum clinical effectiveness threshold for future investigational agents to surpass.
Methods
Study Design
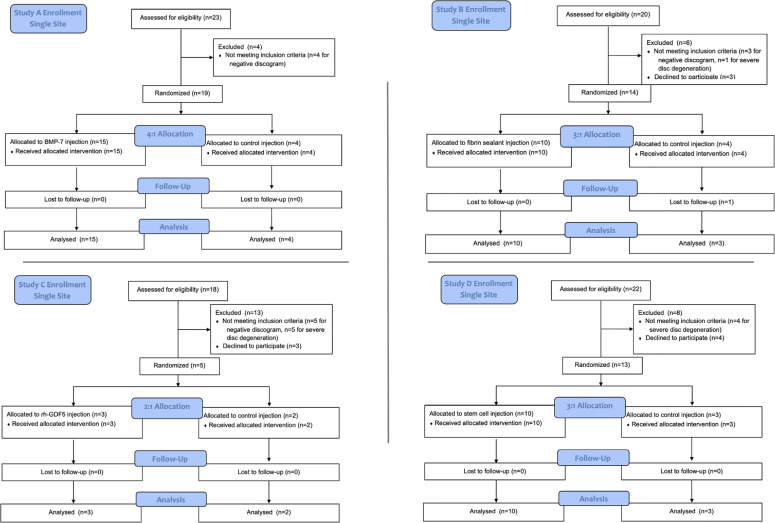
A single-site post hoc comparison was performed utilizing patient self-reported outcomes derived from 4 similarly designed multicenter US FDA–approved studies with enrollment from 2006 to 2012. All four studies were prospective, randomized, controlled, and double-blinded. Full multicenter data was requested from each company behind the four trials, but no company was willing to provide data, citing intellectual property, legal, and fiscal concerns. Standard across the studies, patients were only included if they had symptomatic degenerative disc disease at lumbar levels of L1 to L5/S1, had a positive provocative discography, and failed at least 3 months of nonoperative treatment. The discography was performed by 1 of 2 board-certified physicians at a single institution who participated in all four trials. Patients, both male and female, ranged from 18 to 65 years of age, and were randomized into placebo (saline) or investigational treatment (target growth factor, cell based, or fibrin-based allograft) intervertebral disc injection groups. All injections were performed under fluoroscopic guidance by an experienced physician blinded to the treatment assignment. Each study protocol was approved by the institutional review board at the study site. Both patients and providers were blinded throughout the study period until study termination. Individual study characteristics are detailed in Figure 1 and Table 1.
Figure 1.
Single-center flow diagrams for the 4 reported randomized control studies: A, B, C, and D.
Table 1.
Individual Single-Center Study Characteristics for 4 Intradiscal Randomized Controlled Studies.
| Characteristics | Experimental (SD) | Saline (SD) |
|---|---|---|
| Mean age, years | 39.1 (11.3) | 43.3 (11.5) |
| % males | 81.5% (39.3) | 60% (51.6) |
| Mean body mass index, kg/m2 | 26.0 (4.8) | 25.9 (5.3) |
| Mean levels treated | 1.1 (0.3) | 1.0 (0.0) |
| Mean baseline visual analogue score | 68.5 (17.1) | 74.7 (19.8) |
| Mean baseline disability % | 45.1% (17.6) | 50.1% (11.9) |
Study A involved a 0.5 mL injection of a growth factor treatment (BMP-7/OP-1, Stryker), at concentrations of 0.1, 0.5, 1, and 2 mg/0.5 mL or control for 1-level lumbar discogenic pain. The randomization scheme was 4:1 of experimental to control. Inclusion criteria included 6 months of chronic low back pain, 3 months of pain unresponsive to nonoperative care, and a positive provocative discography. Visual analogue scale for low back pain (VAS) and Oswestry Disability Index Questionnaires (ODI) were administered preinjection and at follow-up visits conducted up to 104 weeks (not listed in ClinicalTrials.gov).
Study B involved injection of up to 4 mL of an allograft biologically active fibrin sealant treatment (Biostat, Spinal Restoration) or control into 1-level intervertebral lumbar discogenic pain. The randomization scheme was 3:1 of experimental to control. Inclusion criteria included 6 months of chronic low back pain, 3 months of pain unresponsive to nonoperative care, and a positive provocative discography. VAS and Roland-Morris Disability Questionnaire (RM) scores were designated as secondary endpoints at 26 weeks. Follow-up was conducted up to 78 weeks (ClinicalTrials.gov NCT01011816).
Study C involved injection of 1 mL of a 1.0 mg growth factor treatment (rhGDF-5, Depuy Synthes) or placebo for injection in 1-level degenerative disc disease. The randomization scheme was 2:1 of experimental to control. Inclusion criteria included persistent back pain with at least 3 months of nonsurgical therapy, and a positive provocative discography. Clinical outcome measures of VAS and ODI were administered preinjection and at follow-up up to 12 months (ClinicalTrials.gov NCT01124006).
Study D involved injection of 2 mL of cell-based stem cell treatment (MPC-06-ID, Mesoblast) of 1 of 2 cell quantities (6 million, 18 million cells) in hyaluronic acid (HA), HA carrier control, or saline control in 1-level degenerative disc disease. The randomization scheme was 3:1 of experimental to control. Inclusion criteria were 6 months of chronic low back pain, 3 months of failed nonoperative therapy, and radiologic changes in disc hydration at the symptomatic level. VAS and ODI were obtained at preinjection and at follow-ups of up to 36 months (ClinicalTrials.gov NCT01290367).
Data Collection
For each patient, details were prospectively recorded regarding patient demographics, medical history, surgical history, radiographic imaging, pain scores, and disability scores. A telephone interview was conducted to determine the current status on each patient regarding any additional treatment since the initial study injection. The telephone interview collected retrospective data and was not part of the original randomized controlled study designs. Across all studies, pain was assessed with the VAS (0-100 scale with 100 being the most severe pain). Patient disability was assessed according to either the ODI (0-50 scale with 50 indicating maximum disability) or the RM (0-24 scale with 24 indicating maximum disability). For the grouped cohort analysis, the ODI and RM scores were converted into a 0-100 percentage scale utilizing the “proportional recalculation” method. 11 The radiographic grading of disc disease was assessed according to the modified Pfirrmann grading scale. 12
Statistical Analysis
The Wilcoxon signed rank test was used to compare paired outcomes. Multiple variable analysis of variance (ANOVA) was applied to specific outcome score measures with a grouping factor for treatment (saline vs investigational treatment) and a repeated factor for outcome score over time (eg, 12 months vs pretreatment) controlling for age, gender, and specific study. Survivorship of the intradiscal injection as a function of time was expressed by using Kaplan-Meier estimates, with further surgery as the failure event. Statistical analyses were performed using SAS 9.4 (SAS Institute). For all analyses, probability values P ≤ .05 were considered statistically significant.
Results
As a single-site institution involved in all 4 described multicenter studies, there were 38 patients who were treated with investigational agents and 12 patients who were treated with a control saline injection into the targeted intervertebral disc. The saline-injected patients included 4 patients from study A, 3 from study B, 2 from study C, and 3 from study D. Preinjection patient demographics for the grouped saline and investigational treatment arms are presented in Table 2. There were no significant differences in patient demographics between the 2 cohorts.
Table 2.
Preinjection Patient Demographics for the Grouped Saline and Investigational Treatment Arms.
| Study A | Study B | Study C | Study D | |||||
|---|---|---|---|---|---|---|---|---|
| Growth factor BMP-7 | Control saline | Active fibrin sealant | Control saline | Growth factor rhGDF-5 | Control saline | Mesenchymal stem cells | Control saline | |
| Injected volume, mL | 0.5 | Up to 4 | 1 | 2 | ||||
| Patients, n | 15 | 4 | 10 | 3 | 3 | 2 | 10 | 3 |
| No. of levels injected | ||||||||
| 1 | 13 | 4 | 6 | 3 | 3 | 2 | 10 | 3 |
| 2 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Levels injected | ||||||||
| L3-4 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 0 |
| L4-5 | 7 | 3 | 5 | 0 | 2 | 0 | 4 | 2 |
| L5-S1 | 10 | 1 | 7 | 2 | 1 | 0 | 5 | 1 |
Individual Study Results
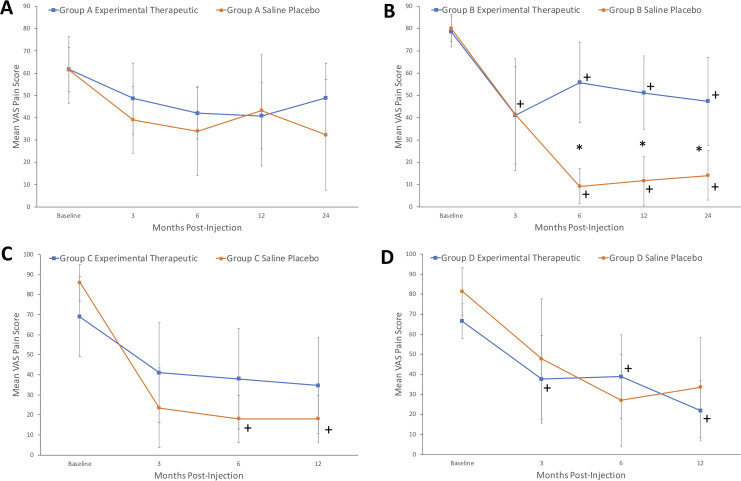
In study A, there were 15 patients injected with an experimental growth factor treatment and 4 patients injected with saline. Over 24 months, the mean VAS decreased from 61.7 to 48.8 (P = .17) in the experimental group and decreased from 61.5 to 32.3 (P = .37) in the saline group (Figure 2a). The mean ODI increased from 19.4 to 19.7 (P = .92) in the experimental group and decreased from 22.0 to 17.3 (P = .57) in the saline group (Figure 3a). There was no significant improvement seen in pain or disability scores within 2 years with either injection.
Figure 2.
Mean visual analogue scale (VAS) pain score changes (and 95% CIs) over time for the experimental therapeutic and saline placebo groups for study A, B, C, and D. Asterisk (*) denotes statistical significance between arms at certain time points. Plus (+) denotes statistical significance compared to baseline value.
Figure 3.
Mean disability score changes (and 95% CIs) over time for the experimental therapeutic and saline placebo groups for study A, B, C, and D. Asterisk (*) denotes statistical significance between arms at certain time points. Plus (+) denotes statistical significance compared to baseline value.
In study B, there were 10 patients injected with an experimental fibrin sealant and 3 patients injected with saline. Over 24 months, the mean VAS significantly decreased from 78.6 to 47.3 (P < .01) in the experimental group and decreased from 80.2 to 14.0 (P < .01) in the control group (Figure 2b). Compared with the fibrin sealant group, patients in the control group reported significantly improved pain scores at 6 months (9.2 vs 55.8, P = .02), 12 months (11.7 vs 51.1, P = .03), and 24 months (14.0 vs 47.3, P = .04). The mean RM significantly decreased from 14.2 to 8.9 (P = .05) in the experimental group and decreased from 14.3 to 5.0 (P < .01) in the saline group (Figure 3b). The control group reported significantly improved disability scores compared with the investigational group at 6 months (3.3 vs 9.9, P = .13) and 12 months (2.3 vs 9.6, P < .14), but this effect was not sustained by 24 months.
In study C, there were 3 patients injected with an experimental growth factor treatment and 2 patients injected with placebo. Over 12 months, the mean VAS decreased from 69.0 to 34.7 (P = .19) in the experimental group and decreased from 86.0 to 18.0 (P = .02) in the placebo group (Figure 2c). The mean ODI decreased from 25.0 to 13.7 (P = .09) in the experimental group and decreased from 24.5 to 9.0 (P = .14) in the control injection group (Figure 3c). There was no significant difference in the amount of pain or disability improvement between the experimental and control groups.
In study D, there were 10 patients injected with an experimental mesenchymal precursor cell solution and 3 patients injected with saline control. Over 12 months, the mean VAS decreased significantly from 66.5 to 21.8 (P < .01) in the experimental group and decreased from 81.3 to 33.5 (P = .06) in the saline injection group (Figure 2d). The mean ODI decreased significantly from 20.0 to 9.3 (P < .01) in the experimental group and decreased from 26.7 to 12.0 (P = .05) in the saline injection group (Figure 3d). There was no significant difference in the amount of pain or disability improvement between the mesenchymal injection and control groups.
Combined Cohort Analysis
In the combined cohort analysis of all patients across the 4 studies, there was a significant decrease in VAS pain in both the investigational (baseline 68.5, 3-month 47.4, 6-month 44.9, 12-month 40.88, all Ps < .01) and saline-injected control groups (baseline 74.7, 3-month 40.6, 6-month 24.3, 12-month 29.6, all Ps < .01, Figure 4). The saline-injected cohort reported significantly more VAS pain improvement at 6 months compared with the investigational group (67.5% decrease vs 34.5%, P = .02), but by 12 months there was no significant difference in pain reduction between the saline and investigational treatment groups (60.3% decrease vs 40.3%, P = .29).
Figure 4.
Mean visual analogue scale (VAS) pain score changes (and 95% CIs) over time for the grouped experimental therapeutic cohort and grouped saline cohort. Asterisk (*) denotes statistical significance between arms at certain time points. Plus (+) denotes statistical significance compared with baseline value.
Similarly, there was significant proportional improvement in adjusted patient-reported disability scores in both the investigational (baseline 45.1, 3-month 35.4, 6-month 31.4, 12-month 31.5, all Ps < .03) and saline-injected control groups (baseline 50.1, 3-month 30.6, 6-month 22.1, 12-month 20.9, all Ps < .01, Figure 5). There were no significant differences in disability score improvement between the saline and investigational treatment groups up to 12 months postinjection (58.2% decrease vs 30.3%, P = .19).
Figure 5.
Mean adjusted disability score changes (and 95% CIs) over time for the grouped experimental therapeutic cohort and grouped saline cohort. Asterisk (*) denotes statistical significance between arms at certain time points. Plus (+) denotes statistical significance compared with baseline value.
Analysis of sustained treatment was obtained by follow-up phone calls to determine if any further treatment had occurred after study completion (Figure 6). At 2-year follow-up, 100% of saline-injected patients demonstrated sustained treatment without requiring further interventions, while investigational-injected patients were at 84% sustained. Between 3 and 4 years, both the saline-injected (75%) and investigational (74%) groups had similar survival rates, while between 7 and 8 years, the saline-injected patients were 75% surgery-free, while the rate for investigational-treated patients declined to 50%.
Figure 6.
Kaplan-Meier survival graph demonstrating survival time after injection to further interventional treatment for discogenic pain in the grouped experimental therapeutic cohort and grouped saline cohort.
Discussion
Low back pain due to intervertebral disc disease represents a significant health and societal burden. 13 The treatment of discogenic back pain remains challenging, leading toward novel regenerative strategies to reduce pain and improve quality of life.14,15 Current efforts in developing injectable intradiscal therapies can be broadly categorized into 2 strategies: disc augmentation and disc repair. 7 Disc augmentation involves adding material to the diseased nucleus, which can be biologically inert (eg, synthetic polymer) or biologically active (eg, fibrin sealant).8,16 Disc repair strategies focus on enhancing the repair and regenerative capability of the diseased nucleus, and encompass growth factor therapy, gene therapy, and cell-based therapy (eg, chondrocytes and stem cells).6,10 To date, there are no FDA-approved intradiscal therapies for discogenic back pain, and there are no large randomized trials that have shown clinically significant improvement with any investigational biological injectable.
In this study, we utilized single-site data to report data from 4 multicenter, randomized, controlled trials investigating experimental intradiscal injectable therapies. Experimental treatments investigated in these 4 studies included fibrin sealants, growth factors, and stem cells. Each of these studies were terminated and results have not previously been reported in the literature, presumably due to nonsuperiority of the investigational agent compared with control. Our results indicate that patients reported significantly improved pain and disability scores after intradiscal injection, regardless of injection with experimental therapeutic or saline control solution. From these four studies there is an apparent “any-treatment” effect after intradiscal injection, where outcomes are similarly improved with both the control and investigational agent.
Here, we report for the first time, control outcomes for injectable biologic intradiscal therapies, which are important to understand and may affect future study design. Control groups are critical in assessing new therapies and establishing a “null” baseline. While the patients treated with experimental therapeutics achieved impressive reductions in pain (40.3%) and disability scores (30.3%) at 1-year follow-up, the patients injected with saline control also demonstrated robust improvements in pain (60.3%) and disability (58.2%). Independent of the underlying reasons for this finding, understanding the response of the saline-injected patients may help future investigational studies define a threshold of improvement needed to exceed. Recently published preliminary pilot studies of multiple investigational intradiscal therapies under investigation have declared success with pain and disability improvements from 30% to 62%.7,8,17,18 Future phase I/II studies may need to demonstrate pain and disability score improvements in excess of 60% as a new threshold for success, prior to initiating large phase III trials with saline-injected controls.
A more thorough understanding of this “any-treatment” effect is needed. Whether this is due to placebo effect, therapeutic effect of saline, or simply due to the natural history of discogenic back pain needs to be determined by future studies. One possible explanation is that the vast majority of low back pain, especially acute pain, resolves without invasive treatment. 19 However, as the 4 studies in this trial involved patients with chronic discogenic pain refractory to conservative treatment, the natural history of chronic discogenic back pain is not well understood. Peng et al 20 reported that only 13% of patients with chronic discogenic pain reported improvement after 4-year follow-up. In contrast, Smith et al 21 reported that 68% of patients with chronic discogenic pain improved at a mean follow-up of 5 years. The median age in these studies was relatively low, likely due to lumbar spondylosis as an exclusion criterion, which may have selected a younger population that has a higher chance of natural resolution of pain. Future intradiscal studies would be wise to include a no-treatment arm or a sham-injection arm, to help differentiate between these possible effects.
While speculative, it is possible that intradiscal saline has a therapeutic effect that has not been previously quantified in the literature. One hypothesis is the dilution of pro-inflammatory mediators within the degenerated intervertebral disc.22,23 Saline may also dilute the presence of growth factors such as FGF-2 and TGF-β, which promote proliferation and extracellular matrix synthesis linked to painful disc fibrosis and degeneration.23,24 Intradiscal injections of saline in a rabbit model have been shown to decrease intradiscal pressure, potentially providing another mechanism behind the therapeutic effect of saline. 25 The inherent analgesic effect of saline has been postulated in many other fields, including studies on intra-articular injections for knee osteoarthritis and intrathecal therapy for neuropathic pain.26-28
Another potential explanation for this “any-treatment” effect is that the improvements in pain and disability are simply due to a placebo effect. In fact, the magnitude of the placebo response in randomized controlled studies has seemingly increased over the past decade, making it increasingly difficult to interpret the effect of new medications and treatments.29-31 The placebo mechanism is complex and multifactorial, stemming from an interplay of social, psychological, and patient-specific factors. Intradiscal injection trials may be at inherent risk of increased placebo response, as high patient baseline pain and invasive procedures are known risk factors for placebo response.29,32 Numerous studies involving lumbar injections for radicular back pain, which share similar baseline patient pain scores and procedures to discogenic pain studies, demonstrate significant improvement in the control groups.33-36 Future studies should include a no-treatment arm to help differentiate between the placebo effect, therapeutic effect of saline, and the natural history of discogenic back pain.
The authors acknowledge the limitations of this study. First, this is a combined analysis of single-site data across 4 separate but similar multicenter, randomized, controlled trials. Ideally, the entire dataset from each trial would be available for reporting to improve the sample size and avoid statistical errors. However, this was the only way to report this important data from these 4 studies, as none of the companies behind these studies would allow the entire datasets to be made available. Further, although the sample size is relatively large in comparison with other reports on intradiscal therapy, the study may be underpowered for statistical significance in some variables owing to the small patient cohorts. We encourage the investigators of each trial to report their entire trial results for future clarification. Furthermore, while we demonstrate improvements in patient reported outcomes in both the investigational and control cohorts, the reason behind this improvement cannot be determined at this time. The strengths of the study include the use of patients with a single diagnosis, at a single institution, enrolled in prospective randomized trials with near-identical inclusion criteria, treated in a blinded fashion.
In conclusion, a single-center analysis of 4 randomized controlled studies demonstrated no difference in outcomes between therapeutic intradiscal agents (growth factor, fibrin sealant, or stem cells) and the control saline arm. In all groups, patient reported pain and disability scores decreased significantly. This is a single-center analysis and may be underpowered, and the authors encourage the investigators of each trial to release full results for complete analysis. Future studies are needed to evaluate the therapeutic benefit of any intradiscal injections.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Derek G. Ju, MD  https://orcid.org/0000-0003-4860-2281
https://orcid.org/0000-0003-4860-2281
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. [DOI] [PubMed] [Google Scholar]
- 2.Frank JW, Kerr MS, Brooker AS, et al. Disability resulting from occupational low back pain. Part I: What do we know about primary prevention? A review of the scientific evidence on prevention before disability begins. Spine (Phila Pa 1976). 1996;21:2908–2917. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Phila Pa 1976). 1994;19:801–806. [DOI] [PubMed] [Google Scholar]
- 4.de Schepper EI, Damen J, van Meurs JB, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976). 2010;35:531–536. [DOI] [PubMed] [Google Scholar]
- 5.An HS, Masuda K, Cs-Szabo G, et al. Biologic repair and regeneration of the intervertebral disk. J Am Acad Orthop Surg. 2011;19:450–452. [PubMed] [Google Scholar]
- 6.Moriguchi Y, Alimi M, Khair T, et al. Biological treatment approaches for degenerative disk disease: a literature review of in vivo animal and clinical data. Global Spine J. 2016;6:497–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coric D, Pettine K, Sumich A, Boltes MO. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013;18:85–95. [DOI] [PubMed] [Google Scholar]
- 8.Yin W, Pauza K, Olan WJ, Doerzbacher JF, Thorne KJ. Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: results of a prospective multicenter pilot study with 24-month follow-up. Pain Med. 2014;15:16–31. [DOI] [PubMed] [Google Scholar]
- 9.Akeda K, Ohishi K, Masuda K, et al. intradiscal injection of autologous platelet-rich plasma releasate to treat discogenic low back pain: a preliminary clinical trial. Asian Spine J. 2017;11:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H, Ha DH, Lee EJ, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther. 2017;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent P, Lauridsen HH. Managing missing scores on the Roland Morris Disability Questionnaire. Spine (Phila Pa 1976). 2011;36:1878–1884. [DOI] [PubMed] [Google Scholar]
- 12.Griffith JF, Wang YX, Antonio GE, et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2007;32:E708–E712. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21–24. [DOI] [PubMed] [Google Scholar]
- 14.Buckley CT, Hoyland JA, Fujii K, Pandit A, Iatridis JC, Grad S. Critical aspects and challenges for intervertebral disc repair and regeneration—harnessing advances in tissue engineering. JOR Spine. 2018;1:e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basso M, Cavagnaro L, Zanirato A, et al. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet Surg. 2017;101:93–104. [DOI] [PubMed] [Google Scholar]
- 16.Berlemann U, Schwarzenbach O. An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. Eur Spine J. 2009;18:1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noriega DC, Ardura F, Hernández-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101:1945–1951. [DOI] [PubMed] [Google Scholar]
- 18.Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33:146–156. [DOI] [PubMed] [Google Scholar]
- 19.Will JS, Bury DC, Miller JA. Mechanical low back pain. Am Fam Physician. 2018;98:421–428. [PubMed] [Google Scholar]
- 20.Peng B, Fu X, Pang X, et al. Prospective clinical study on natural history of discogenic low back pain at 4 years of follow-up. Pain Physician. 2012;15:525–532. [PubMed] [Google Scholar]
- 21.Smith SE, Darden BV, Rhyne AL, Wood KE. Outcome of unoperated discogram-positive low back pain. Spine (Phila Pa 1976). 1995;20:1997–2000. [DOI] [PubMed] [Google Scholar]
- 22.Fukui S, Iwashita N, Nitta K, Tomie H, Nosaka S. The results of percutaneous intradiscal high-pressure injection of saline in patients with extruded lumbar herniated disc: comparison with microendoscopic discectomy. Pain Med. 2012;13:762–768. [DOI] [PubMed] [Google Scholar]
- 23.Peng BG. Pathophysiology, diagnosis, and treatment of discogenic low back pain. World J Orthop. 2013;4:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine (Phila Pa 1976). 1995;20:1972–1978. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Nagata K, Ariyoshi M, Hirohashi T, Inoue A. Intradiscal pressure after intradiscal injection of hypertonic saline: an experimental study. Eur Spine J. 2000;9:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiphart JW, Dills CV, Levy RM. The analgesic effects of intrathecally pumped saline and artificial cerebrospinal fluid in a rat model of neuropathic pain. Neuromodulation. 2002;5:214–220. [DOI] [PubMed] [Google Scholar]
- 27.Saltzman BM, Leroux T, Meyer MA, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45:2647–2653. [DOI] [PubMed] [Google Scholar]
- 28.Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016;46:151–159. [DOI] [PubMed] [Google Scholar]
- 29.Vase L, Vollert J, Finnerup NB, et al. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain. 2015;156:1795–1802. [DOI] [PubMed] [Google Scholar]
- 30.Häuser W, Bartram-Wunn E, Bartram C, Reinecke H, Tölle T. Systematic review: placebo response in drug trials of fibromyalgia syndrome and painful peripheral diabetic neuropathy-magnitude and patient-related predictors. Pain. 2011;152:1709–1717. [DOI] [PubMed] [Google Scholar]
- 31.Vase L, Skyt I, Hall KT. Placebo, nocebo, and neuropathic pain. Pain. 2016;157(suppl 1):S98–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irizarry MC, Webb DJ, Ali Z, et al. Predictors of placebo response in pooled lamotrigine neuropathic pain clinical trials. Clin J Pain. 2009;25:469–476. [DOI] [PubMed] [Google Scholar]
- 33.Gogan WJ, Fraser RD. Chymopapain. A 10-year, double-blind study. Spine (Phila Pa 1976). 1992;17:388–394. [DOI] [PubMed] [Google Scholar]
- 34.Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Efficacy of steroid and nonsteroid caudal epidural injections for low back pain and sciatica: a prospective, randomized, double-blind clinical trial. Spine (Phila Pa 1976). 2009;34:1441–1447. [DOI] [PubMed] [Google Scholar]
- 35.Fraser RD. Chymopapain for the treatment of intervertebral disc herniation. The final report of a double-blind study. Spine (Phila Pa 1976). 1984;9:815–818. [DOI] [PubMed] [Google Scholar]
- 36.Bromley JW, Varma AO, Santoro AJ, Cohen P, Jacobs R, Berger L. Double-blind evaluation of collagenase injections for herniated lumbar discs. Spine (Phila Pa 1976). 1984;9:486–488. [DOI] [PubMed] [Google Scholar]