Abstract
Study design:
Retrospective study at a unique center.
Objective:
The aim of this study is twofold, to develop a virtual patients model for lumbar decompression surgery and to evaluate the precision of an artificial neural network (ANN) model designed to accurately predict the clinical outcomes of lumbar decompression surgery.
Methods:
We performed a retrospective study of complete Electronic Health Records (EHR) to identify potential unfavorable criteria for spine surgery (predictors). A cohort of synthetics EHR was created to classify patients by surgical success (green zone) or partial failure (orange zone) using an Artificial Neural Network which screens all the available predictors.
Results:
In the actual cohort, we included 60 patients, with complete EHR allowing efficient analysis, 26 patients were in the orange zone (43.4%) and 34 were in the green zone (56.6%). The average positive criteria amount for actual patients was 8.62 for the green zone (SD+/- 3.09) and 10.92 for the orange zone (SD 3.38). The classifier (a neural network) was trained using 10,000 virtual patients and 2000 virtual patients were used for test purposes. The 12,000 virtual patients were generated from the 60 EHR, of which half were in the green zone and half in the orange zone. The model showed an accuracy of 72% and a ROC score of 0.78. The sensitivity was 0.885 and the specificity 0.59.
Conclusion:
Our method can be used to predict a favorable patient to have lumbar decompression surgery. However, there is still a need to further develop its ability to analyze patients in the “failure of treatment” zone to offer precise management of patient health before spinal surgery.
Keywords: machine learning, lumbar decompression surgery, retrospective study, synthetic electronic medical record, ROC curve
Introduction
Lumbar spinal disorders are among the most disabling conditions, particularly in developed countries, due to the increase in sedentary lifestyles and aging populations. 1
When conservative treatment is insufficient or pharmaceutical options show too many secondary effects (dependency, misuse), surgery is a valid option to relieve pain and improve function.2-4
However, patient selection remains very complex and the benefits of surgical interventions sometimes uncertain. 5 Indeed, between 2 and 23% of patients having back surgery will present an adverse event or a complication after surgery.6,7
Around 30% to 50% of patients will not be—or only slightly—relieved—by the surgical act, and will maintain their intake of morphine, with the side effects and the costs that this entails 8
Surgery success is well evaluated by validated indicators such as patient-reported outcomes measures (PROMS). 9 This protocol is based on the standardized collection of patient well-being and health status after a surgical procedure. It is used on large cohorts to study a set of factors participating in clinical outcomes after surgical treatment (see Table 1.).
Table 1.
Predictors.
| Author | Year | Significant predictor | Positive predictive factor | Negative predictive factor | Area |
|---|---|---|---|---|---|
| Katz et al 10 | 1999 | Low cardiovascular comorbidity | * | GREEN ZONE | |
| Hägg et al 11 | 2003 | Severe disc degeneration, Neuroticism, Pre-operative sick leave | * | ORANGE ZONE | |
| Kohlboeck et al 12 | 2004 | Straight leg raise test, Depression, Sensory pain | * | ORANGE ZONE | |
| Trief et al 13 | 2006 | Better emotional health | * | GREEN ZONE | |
| Slover et al 14 | 2006 | Active compensation case, Self-rated poor health, Smoking, Headaches, Depression, Nervous system disorders | * | ORANGE ZONE | |
| Braybrooke et al 15 | 2007 | Time to surgery | * | ORANGE ZONE | |
| Mannion et al 16 | 2007 | Pain duration, Re-operations, Multilevel surgery, Depression, FABQ Score | * | ORANGE ZONE | |
| Park et al 17 | 2008 | Minimally invasive surgery | * | GREEN ZONE | |
| Park et al 17 | 2008 | Age, BMI > 25, Hypertension, Coronary artery diseases, Diabetes | * | RED ZONE | |
| Garcia et al 18 | 2008 | Weight reduction program | * | GREEN ZONE | |
| Vaidya et al 19 | 2009 | Obesity, Multiple level fusions | * | RED ZONE | |
| Chen et al 20 | 2009 | Diabetes | * | RED ZONE | |
| Abbott et al 21 | 2011 | Catastrophizing, Pain intensity, Bad expectations | * | ORANGE ZONE | |
| Senker et al 22 | 2011 | Minimally invasive surgery | * | GREEN ZONE | |
| Chaichana et al 23 | 2011 | Depression, Decreased perception scale anxiety | * | ORANGE ZONE | |
| Sinikallio et al 24 | 2011 | Depression | * | ORANGE ZONE | |
| Kalanithi et al 25 | 2012 | Morbid obesity | * | RED ZONE | |
| Sørlie et al 26 | 2012 | MODIC type 1 smoking | * | ORANGE ZONE | |
| Hellum et al 28 | 2012 | Long duration Low back pain high fear avoidance for work, MODIC changes | * | ORANGE ZONE | |
| Gaudelli and Thomas 29 | 2012 | Instrumented fusion | * | RED ZONE | |
| Mehta et al 30 | 2012 | Obesity | * | RED ZONE | |
| Sharma et al 31 | 2013 | Diabetes | * | RED ZONE | |
| Takahashi et al 32 | 2013 | Diabetes of more than 20 years | * | RED ZONE | |
| Bekelis et al 33 | 2014 | Age, Extensive operations, Medical deconditioning (weight loss, dialysis, peripheral vascular disease) BMI, Neurologic deficit, Bleeding disorders | * | RED ZONE | |
| Lee et al 34 | 2014 | Opioid consumption, Modified somatic perception, Depression | * | ORANGE ZONE | |
| Pakarinen et al 27 | 2014 | Depression | * | ORANGE ZONE | |
| Kim et al 35 | 2018 | Back pain, Pain sensitivity | * | ORANGE ZONE | |
| Coronado et al 36 | 2015 | Increased pain sensitivity Increased pain catastrophizing | * | ORANGE ZONE | |
| McGirt et al 37 | 2015 | Functional score opioid use, Hypertension, Atrial fibrillation, extremity pain, myocardial infarction, Diabetes, Osteoporosis, Smoking | * | ORANGE ZONE | |
| Anderson et al 38 | 2015 | Chronic opioid therapy, Additional lumbar surgery, depression, work loss | * | ORANGE ZONE | |
| Chotai et al 39 | 2015 | Insurance status, Functional score, BP/NP Scores | * | ORANGE ZONE | |
| Schöller et al 40 | 2016 | Re-operation, Duration of pain, Spondylisthesis, Smoking, gender, Age, BMI | * | ORANGE ZONE | |
| Archer et al 41 | 2016 | Cognitive-behavioral based physical therapy (CBPT) |
* | GREEN ZONE | |
| Asher et al 42 | 2017 | ASA score, disability, education, Unemployment, Insurance status | * | ORANGE ZONE | |
| Mummaneni et al 43 | 2017 | Open surgery | * | ORANGE ZONE | |
| Crawford et al 44 | 2017 | Discopathy | ORANGE ZONE | ||
| Suri et al 45 | 2017 | Smoking, Depression | * | ORANGE ZONE | |
| McGirt et al 5 | 2017 | Education, Employment status, Baseline EQ5D, Fusion | * | ORANGE ZONE | |
| Sharma et al 46 | 2018 | Prior opioid dependence, Younger age | * | ORANGE ZONE | |
| Dunn et al 47 | 2018 | Catastrophizing, depression | * | ORANGE ZONE | |
| Chan et al 48 | 2018 | Symptom duration | * | ORANGE ZONE | |
| O’Donnell et al 49 | 2018 | Opioid use, Time to surgery, Legal representation, Psychiatric comorbidity | * | ORANGE ZONE | |
| Khor et al 50 | 2018 | Age, Gender, Ethnic, Insurance Status, ASA Score, functional score | * | ORANGE ZONE | |
| Dobran et al 51 | 2019 | Age, BMI | * | RED ZONE | |
| Staub et al 52 | 2020 | Obesity, Re-operation, insurance status | * | ORANGE ZONE | |
| Mauro et al 53 | 2020 | BMI | * | ORANGE ZONE | |
| Rudolfsen et al 54 | 2020 | Quality of life score, Functional score | * | GREEN ZONE |
Most of these studies are based on the analysis of electronic medical records (EHR) in single-institution or in large national Database, describing statistically relevant risk factors of adverse event or surgery failure on a population.5,55 There is a growing interest about predictive factors influencing individual response after surgery, especially in terms of individual PROM. Furthermore, some promising predictive models in disk herniation recurrence or fusion50,56,57 exist but there is a lack of practical models for lumbar spine decompression in general.
“4P” (predictive, preventive, personalized and participative) medicine benefits from the support of artificial intelligence 58 (AI) machine learning and synthetic patient models.59,60 Regarding spine surgery, tools are already capable of improving the quality of the spine diagnosis. 61
Some algorithms allow to determine the average duration of sick leave, 62 the risks of opioids dependence for prolonged periods post-operatively 63 and to predict postoperative adverse events up to 30 days after spinal surgery64-66 (see Table 2.).
Table 2.
Predictive Model for Spine Surgery.
| Author | Year | Data collection (center) | Number of patients | Classifier used | Prediction / AUC |
|---|---|---|---|---|---|
| Azimi et al 67 | 2014 | Database (single-center) |
168 | ANN, Logistic regression analysis | 2-year surgical satisfaction (AUC 0.80) |
| Azimi et al 68 | 2014 | Database (single-center) |
203 | ANN, Logistic regression analysis | Successful surgery outcome for disk herniation (AUC 0.82) |
| Azimi et al 69 | 2015 | Database (single-center) |
402 | ANN, Logistic regression analysis | Successful ANN model to predict recurrent lumbar disk herniation (AUC 0.84) |
| Ratliff et al 70 | 2016 | Database (National) |
279 135 | LASSO (GLMnet), multivariate logistic regression | Adverse events (AUC 0.61) |
| Azimi et al 56 | 2017 | Database (single-center) |
346 | ANN | Optimal treatment choice for LSCS patients (AUC 0.89) |
| Oh et al 71 | 2017 | Database (Multi-center) |
234 | C5.0 algorithm (type of decision tree model) | Post-operative improvement AUC (0.96) |
| Scheer et al 72 | 2017 | Database (Multi-center) |
557 | C5.0 algorithm (type of decision tree model) | Major intra- or perioperative complications (AUC 0.89) |
| Staarjes et al 73 | 2018 | Registry (single-center) |
422 | TensorFlow ANN | Favorable outcome (AUC 0.87) |
| Khor et al 50 | 2018 | Database (Multi-center) |
1 965 | Multivariate analysis | Predicting lower ODI: nonprivate insurance workers’ compensation (0.20), current smoking (0.43) or previous smoking (0.66), asthma (0.54), and a lower baseline score (1.05) |
| Iderberg et al 62 | 2018 | Registry (Multi-center) |
19 131 | Multivariate, regression analysis / GLM | Predicting Clinical outcomes: Odds ratios: Social welfare (1.34) / Living Alone (1.14) / Educational level (-2.39) / Disposable income (-2.58) |
| Kim et al 35 | 2018 | Registry (Multi-center) |
22 629 | ANNs and multivariate logistic regression | Wound complications and mortality (AUC 0.6 to 0.71) |
| Karhade et al 74 | 2018 | Registry (Multi-center) |
26 364 | SVM, ANN | Prediction of anormal discharges (AUC 0.82) |
| Kuo et al 75 | 2018 | Database (Single-center) |
532 | SVMs, logistic regression, C4.5 decision tree | Medical costs (AUC 0.90) |
| Kalagara et al 65 | 2018 | Registry (Multi-center) |
26 869 | R Foundation for statistical computing/ GBM | Readmission (AUC 0.69) |
| Goyal et al 76 | 2019 | Registry (Multi-center) |
59 145 | GLM/ GMB/ ANN/ RF / pLDA/ VarBayes | Discharge to non-home facility (AUC >0.80) |
| Han et al 66 | 2019 | MarketScan & Medicaid Databases (Multi-center) |
1 106 234 | Multivariate logistic regression analysis | Predicting the risk of a pulmonary complication (AUC 0.76) |
| Siccoli et al 64 | 2019 | Registry | 635 | Random forests, extreme gradient boosting (XGBoost), Bayesian generalized linear models (GLMs), boosted trees, k-nearestneighbor, simple GLMs, artificial neural networks with a single hidden layer | Extended hospital stay with an accuracy of 77% (AUC 0.58) |
| Shah et al 77 | 2019 | Database (single-center) |
367 | Logistic regression analysis, Stochastic gradient boosting, Random Forest, Support Vector machine | Failure of nonoperative management. Random Forest (AUC 0.56) Logistic Regression (AUC 0.79) |
| Karhade et al 78 | 2019 | Database (single-center) |
1 053 | Logistic regression analysis, Stochastic gradient boosting, Random Forest, Support Vector machine | Prediction of 90-day mortality in spinal epidural abscess (AUC 0.89) |
| Hopkins et al 79 | 2019 | Registry (Multi-center) |
23 264 | ANN (7 layers) | Readmissions (AUC > 0.60) |
| Nelson et al 80 | 2019 | Database (Single-center) |
22 318 appointments |
ANN, Logistic regression analysis, Support vector machine, Random Forest | Scheduled appointment attendance in healthcare ANN AUC (0.81) |
| Karhade et al 63 | 2019 | Database (Multi-center) |
5 413 | Logistic regression analysis, Stochastic gradient boosting, Random Forest, Support Vector machine | Prolonged postoperative opioid prescription (AUC 0.81) |
| Hopkins et al 81 | 2020 | Database (single-center) |
4046 | ANN (9 layers deep neural network) | Prediction of infections (AUC 0.78) |
Notes: ACC = accuracy; ACS-NSQIP = American College of Surgeons National Surgical Quality Improvement Program; ANN = artificial neural networks; AUC = area under the receiver operating characteristic curve; COPD = chronic obstructive pulmonary disease; DNN = deep neural networks; EHR = electronic health records; GBM = gradient boosting machine; GLM = generalized linear model; GLMnet = elastic-net GLM; LSS = lumbar spinal stenosis; MCID = minimum clinically important difference; ML = machine learning; NPV = negative predictive value; NRS = numeric rating scale; NRS-BP = NRS for back pain; NRS-LP = NRS for leg pain; ODI = Oswestry Disability Index; PHC = predictive hierarchical clustering; PPV = positive predictive value; PROMs = patient-reported outcome measures; RF = random forest; ROC = receiver operating characteristic
Among these machine learning methods, we found multivariate logistic regression, stochastic gradient boosting or support vector machine methods and recently artificial neural networks and their improvement in deep neural networks60,77 to support decision-making activities.
Despite the current focus using EHR as the standard for development of machine learning algorithms, it can be very difficult to gather all the data needed to train such models. Likewise, for technical reasons (interoperability, data exchange, and ability of the operator to use information technologies) or legal and ethical issues, 82 it is difficult to access the full records in academic and industrial research.
The generation of synthetic patients from the exploitation of EHR solves many problems related to the processing of real patients data. 83 Therefore data-driven methods were developed based on synthetic EHR 84 in 3 different ways: using synthetic health data records to help overcome confidentiality issues,62,85 modeling disease progression and interventions for prospective analysis of large scale virtual cohorts 86 ; and completing EHR data for imbalanced cohorts (cf. Table 3).
Table 3.
Synthetic Patient Models.
| Study | Authors | Patient synthetic model and technology | Keypoint |
|---|---|---|---|
| He et al 87 | 2008 | Adaptive Synthetic Sampling Method for Imbalanced Data (ADASYN) | Reducing the bias introduced by the class imbalance, and promote recognition of complex patients |
| Teutonico et al 88 | 2015 | Discrete re-sampling and multivariate normal distribution (MVND) methodologies in the creation of virtual patient population | The multivariate distribution method produces realistic covariate correlations, comparable to the real population. Moreover, it allows simulation of patient characteristics beyond the limits of inclusion and exclusion criteria in historical protocols. |
| McLachlan et al 89 | 2016 | The CoMSER method takes a constraint-based approach
involving: (1) formalizing clinical practice guidelines into the CareMap constraint and the CareMap into the State Transition Machine (STM), (2) incorporating published Health Incidence Statistics based constraints into the STM, and (3) exploiting domain expertise in verifying domain knowledge and creating the reusable library of clinical notes |
Production of synthetic EHR that is considered realistic. The main contribution of this work is the approach that uses a CareMap for generating synthetic EHR with neither access to the real EHR nor using anonymized EHR. . |
| Kim et al 90 | 2018 | ADASYN | Adaptive synthetic sampling approach to imbalanced learning (ADASYN) was used to generate positive synthetic complications for training model |
| Kim et al 35 | 2018 | ADASYN | ADASYN utilizes examples from the minority class that are difficult to learn and generates synthetic new cases based on these examples to improve model learning and generalizability |
| Baowaly et al 83 | 2019 | MedWGAN / MedBGAN (modified Generating Adversarial network) |
Learn the distribution of real-world EHRs and exhibit remarkable performance in generating realistic synthetic EHRs for both binary and count variables. |
| Pollack et al 91 | 2019 | 5 Steps Generating Synthetic Patient Data* | Steps to generate EHR for testing and evaluation of Health information technology |
Objective
The aim of this study is twofold, to develop a virtual patients model for lumbar decompression surgery and to evaluate the precision of an artificial neural network (ANN) model designed to accurately predict the clinical outcomes of lumbar decompression surgery.
Materials and Methods
A transparent reporting of a multivariable prediction model for individual prognosis was used for reporting our model of machine learning in Biomedical Research.
Institutional Review Board
The EHR screening was approved by the department review board from the Department of Neurosurgery, Pitié-Salpêtrière University Hospital, all other data was anonymously reported and there is no specific approval.
Population
Any patient who underwent lumbar decompression surgery from January 2019 to April 2019 in the Department of Neurosurgery, Pitié-Salpêtrière University Hospital was included. We exploited retrospectively the local EHR.
Data Collection
Data collection was carried out through the automated request of EHR patients from our center (Orbis, Agfa Healthcare).
Pre-operative criteria were collected, including the patient’s age, sex, body mass index (BMI), demographic, radiological criteria, as well as the presence of comorbidities (diabetes, sleep apnea syndrome, kidney disease.), the type of work and the duration of sick leave, socio-professional problems, psychological disorders (anxiety or depressive syndrome) drugs consumption (NSAIDs, opioids), and immediate post-operative criteria such as: radiological criteria, sleep or food improvement, return to work, or rehabilitation inpatients center.
Patients were classified into 3 categories according to their surgery outcome: Green (significant improvement of pain and function without level 2 or 3 analgesics or other symptom) Orange (no significant improvement and/or significant medication intake anxiety-depression and/or persistent lumbar pain) and Red (early adverse event or complication)
Predictors
The potential predictive factors were identified based on a comprehensive literature review (see Table 1.) on PubMed central library using the following MESH terms combined to the screening of preoperative data available in our EHRs (see Table 4.):
“Machine Learning”[Mesh] OR “Artificial Intelligence”[Mesh] OR “Natural Language Processing”[Mesh] OR “Neural Networks (Computer)”[Mesh] OR “Support Vector Machine”[Mesh] OR Machine learning[Title/Abstract] OR Artificial Intelligence[Title/Abstract] OR Neural network[Title/Abstract] OR Neural networks[Title/Abstract] OR Natural language processing[Title/Abstract] OR deep learning[Title/Abstract] OR machine intelligence[Title/Abstract] OR computational intelligence[Title/Abstract] OR computer reasoning[Title/Abstract]))) AND (((“Neurosurgery”[Mesh] OR “Neurosurgical Procedures”[Mesh] OR “Intervertebral Disc Displacement”[Mesh] OR “Spinal Stenosis”[Mesh] OR neurosurgery[Title/Abstract] OR neurosurgeries[Title/ Abstract] OR neurosurgical[Title/Abstract] OR neurosurgically[Title/Abstract] OR spinal [Title/Abstract] OR lumbar[Title/Abstract] AND (“Surgical Procedures, operative”[Mesh] OR “Postoperative Complications”[Mesh] OR “surgery” [Subheading] OR “Postoperative Period”[Mesh] OR “Perioperative Period”[Mesh] OR “Preoperative Period”[Mesh] OR surgery[Title/Abstract] OR surgeries[Title/Abstract] OR surgical[Title/Abstract] OR postoperative*[Title/Abstract] OR post-operative*[Title/Abstract] OR preoperative*[Title/Abstract] OR preoperative*[Title/Abstract] OR perioperative*[Title/Abstract] OR peri-operative*[Title/Abstract] OR operative procedure*[Title/Abstract])))) NOT (Comment[Publication Type] OR editorial[Publication Type] OR letter[Publication Type] OR case reports[Publication Type]).”
Table 4.
Patient Baseline Predictors.
| Variable | Binary criteria (1;0) | Baseline Strength established |
|---|---|---|
| Day of surgery | Same day; day before | 0% |
| Length of stay (LOS) | > 4 days: < 4 days | 10% |
| Timing for procedure (1st,2nd,3 rd, 4th, 5th positioning in the day) | 3 rd, 4th, 5th in the day; 1st, 2nd,3rd | 10% |
| Type of job: sedentary | Presence; absence | 30% |
| Type of job: heavy worker | Presence; absence | 30% |
| Work stopping duration before surgery-sedentary >1, 0 | < 1 day | 10% |
| Work stopping duration before surgery-heavy worker >3, 0 | < 3 days | 10% |
| Work stopping duration before surgery-moderate >14, 0 | < 14 days | 10% |
| Work stopping duration before surgery-light worker >35, 0 | < 35 days | 10% |
| Sleep disorder | Presence; absence | 15% |
| Professional conflict | Presence; absence | 30% |
| Family conflict | Presence; absence | 15% |
| Specific physical activity | Presence; absence | 30% |
| General physical activity | Absence; presence | 30% |
| Appetite | Absence; presence | 5% |
| Age | > 65 ans | 15% |
| BMI | > 30 | 50% |
| Smoking | > 10 pack-year | 10% |
| Pre-operative walking distance reduction | Presence; absence | 15% |
| Prior to surgery opioid consumption | Presence; absence | 20% |
| Cauda equina syndrome | Presence; absence | 30% |
| Transit disorders | Presence; absence | 5% |
| Pre-operative motor deficit | Presence; absence | 20% |
| Pre-operative sensitive deficit | Presence; absence | Indication |
| Impulsive movement or pushing effort | Presence; absence | 30% |
| Pre-operative inflammatory pain | Presence; absence | 30% |
| Limp | Presence; absence | 10% |
| Acute lumbar pain | Presence; absence | 5% |
| Chronic lumbar pain | Presence; absence | 30% |
| Lumbar stifness | Presence; absence | 20% |
| Sphincter dysfunction | Presence; absence | 40% |
| Diabete | Presence; absence | 10% |
| Pre-operative anxiety or depressive syndrome | Presence; absence | 20% |
| Sleep apnea syndrome | Presence; absence | 10% |
| COPD | Presence; absence | 5% |
| Pneumopathy | Presence; absence | 20% |
| Liver disorder | Presence; absence | 15% |
| Atheroma | Presence; absence | 15% |
| Kidney Disease | Presence; absence | 5% |
| Pre-operative MODIC Images | Presence; absence | 30% |
| Pre-operative Calcification | Presence; absence | 30% |
| Pre-operative stenosis | Presence; absence | Indication |
| Pre-operative protrusion | Presence; absence | 0% |
| Pre-operative excluded disc herniation | Absence; presence | 50% |
| Pre-operative disc herniation | Presence; absence | Discrete |
| L1L2 Level | Presence; absence | 30% |
| L2L3 Level | Presence; absence | 30% |
| L3L4 Level | Presence; absence | 30% |
| Pre-operative arthritis | Presence; absence | 0% |
| Pre-operative hypertrophic facet disease | Presence; absence | 0% |
| Pre-operative osteophyte | Presence; absence | 0% |
| Pre-operative spondylolysis | Presence; absence | 0% |
| Explicit pre-operative explanations | Absence; Presence | 50% |
| Favorable operator experience | Absence;presence | 70% |
| Food intake improvement | > 3 days | 10% |
| Sleep improvement | > 2 days | 20% |
| Return to work sedentary >42 | > 42 days | 30% |
| Return to work light >42 | > 42 days | 30% |
| Return to work moderate >75 | > 75 days | 30% |
| Return to work heavy workers >90 | > 90 days | 30% |
| Infection | Presence; absence | 15% |
| Autonomous walking recovery | > 2 days | 20% |
| Anti-inflammatory drugs post-operatively | Presence; absence | 10% |
| Post-operative anxiety or depressive syndrome | Presence; absence | 20% |
| Post-operative disc calcification | Presence; absence | 20% |
| Post-operative stenosis | Presence; absence | 40% |
| Post-operative fibrosis | Presence; absence | 50% |
| Rehabilitation inpatients center | Convalescent home; home | 20% |
| Operative recurrence | Presence; absence | 50% |
From Predictors to Criteria Tables
The potential predictors had to be usable in a neural network algorithm (see part Training and validation of the model). In the input table each criterion was a binary value (1 or 0) that represents the presence or absence. So, each predictor was transformed into discrete criterium to fill the binary values tables.
Statistical Analysis
Criteria for real and synthetic patients were compared. The mean percentage of presence for each criterion for each zone (green and orange), as well as the mean number of criteria for each category of patients and each zone were reported.
Synthetic Patient Model
Our synthetic patient model allows us to generate as many virtual patients as we desire in order to train the classifier without the need of real patients. The model that we propose can help in bootstrapping a new model without long and costly data collection, it could also be used to boost under represented categories in classification problem. 35
It is a statistical approach designed to create a virtual model, statistically representative of real patients’ population. Our method was to create patients that fall in the 2 zones that we defined (orange or green). To do so, we generated tables of random pre-op symptoms based on the input data defined before. Each input data (criteria) has a probability of presence, either 1 or 0 (present or not) based on a uniform distribution.
Then, each criterium was associated with a strength. The strength of each criteria was determined by a cross-professional group including spine surgeons, clinical register experts and statisticians.
In the input table, each criterium strength was added to the total strength of the table. This total strength was compared to a threshold, classifying patient in the orange zone (superior to the threshold) or the green zone (inferior)
Tables are generated for 10000 virtual patients, of which 5000 are green and 5000 are orange.
Artificial Neural Network Architecture
Our classifier is an artificial neural network, which architecture is based on our criteria (see Figure 1). Each input neuron represents a pre-operative criterium and the value associated is the presence or the absence of it.
Figure 1.
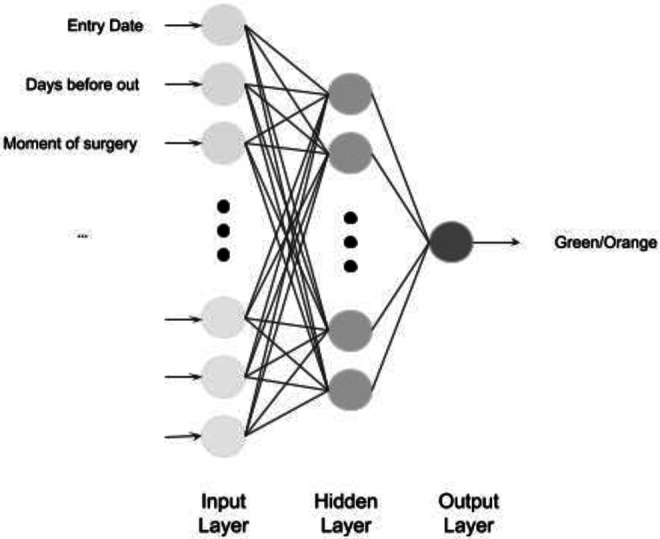
Architecture of our artificial neural network.
Activation functions for input and hidden layers are Rectified Linear Unit (ReLU). The activation function of the output layer is a sigmoid, the output value is then a Boolean: 1 if green, 0 if orange (See Figure 1). We use Keras Tensorflow framework for the construction and training of our model.
Training and Validation of the Model
The training of the classifier is done using 80% of the data set of virtual patients and 20% were used for testing purposes. The sets are randomly chosen in the virtual patient’s dataset, but we keep the 50% green and orange repartition. The algorithm chosen for loss calculation is binary cross entropy and Adam optimizer for back propagation.
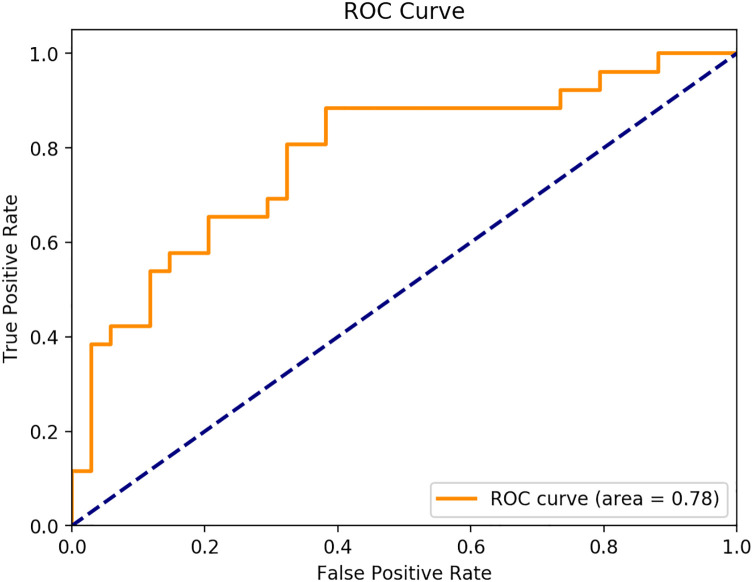
The indicator that we use for real data is twofold: accuracy of the model—i.e. classification in either green or orange zone for a given table, and the ROC curve—i.e. the percentage of true positive on false positive at different thresholds. Validation of the ANN is done against real patient tables using the Receiver Operating Characteristic Curve (AUC).
Results
Population and EHR Data Set
In the actual cohort, we included 60 patients, with complete EHR allowing sufficient analysis, 26 patients are in the orange zone constituting (43.4%) and 34 are in the green zone (56.6%) (See Figure 2). The average positive criteria amount for actual patients is 8.5 for the green zone (SD+/- 3.09) and 10.47 for the orange zone (SD 3.38). Results are presented in Figures 2 and 3.
Figure 2.
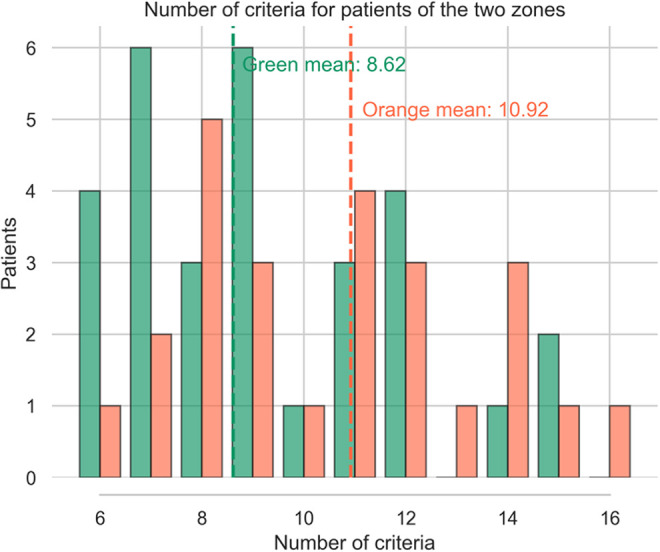
Real patient distribution according the number of pre operative criteria and their outcome (green: success/orange: failure).
Figure 3.
Statistical presence of criteria for each group orange / green (EHR).
Predictors
A total of 68 unfavorable predictors were collected and included in the initial training of the predictive model (See Table 4.). Those 68 criteria are used (58 “type of criteria” and their variants). Among the 68 criteria, 54 are pre-operative criteria and 14 are peri-operative criteria (from surgery to 1-month follow-up). Missing criteria are also counted.
5 other criteria are related to Patient-Related Outcome and allow us to assess the improvement of the quality of life (See Table 5.). The presence of one of these criteria defines the patient’s outcome as falling into the orange zone. Our machine learning model was then evaluated through the correct patient classification in the orange zone.
Table 5.
Patient’s Clinical Outcomes (orange zone).
| Clinical characteristic evaluated | Binary criteria (1;0) | Area |
|---|---|---|
| Walking distance still limited at 1 month | Presence; absence | Orange zone |
| Partial recovery from post-operatively motor deficit at 1 month | Presence; absence | Orange zone |
| Partial recovery from post-operatively sensory deficit at 1 month | Presence; absence | Orange zone |
| Post-operative neuropathic pain at 1 month | Presence; absence | Orange zone |
| Post-operative anxiety-depression syndrome at 1 month | Presence; absence | Orange zone |
Synthetic Data Set
We generated 10000 virtual patients for training our classifier, 5000 were allocated to the green zone, 5000 to the orange zone. We chose a 50/50 split in order not to introduce a bias of distribution between the 2 zones during the algorithm training. We also generated 2000 tables for testing (20% of the training set).
Figure 4 shows a Gaussian distribution of the number of criteria for the 2 zones.
Figure 4.
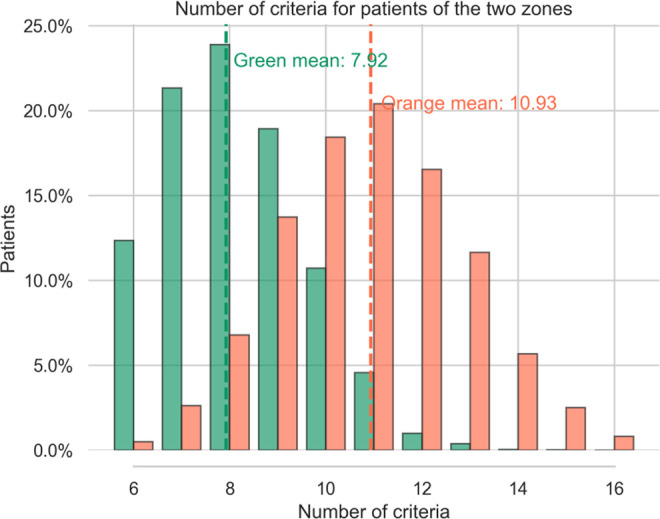
Number of patient criteria for the 2 zones (syn-EHRS).
For patients in the green zone we found a mean of 7.92 symptoms per table, (median: 9, SD +/- 1.71), for patients in the orange zone the mean is 10.93, (median: 11, SD +/- 1.81). These numbers are coherent with what we observe in real patient distributions (see Figure 2.). Submitting the number of criteria to a Welch’s test we get a value of -71.31 715 with a p-value of 0.0, confirming that the difference in number of criteria for the 2 zones is significantly different.
Indeed, patients in the orange zone tend to have more criteria. Moreover, the higher the strength of a criteria the higher the probability of presence is for that symptom in the orange category. For instance, the predictor “BMI >30” is more represented in orange tables (16.88%) than in green ones (1.84%). Conversely, most of the criteria with low strength are represented with nearly the same proportion in the 2 categories (<2%): age, appetite, COPD, transit disorders, Sleep apnea work stopping duration before surgery-light worker>35, kidney disease and diabetes.
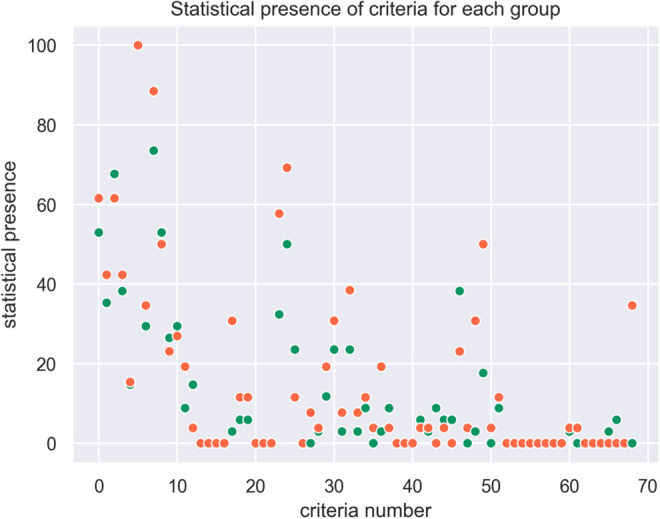
The statistical presence of each criteria in each zone is plotted in Figure 5.
Figure 5.
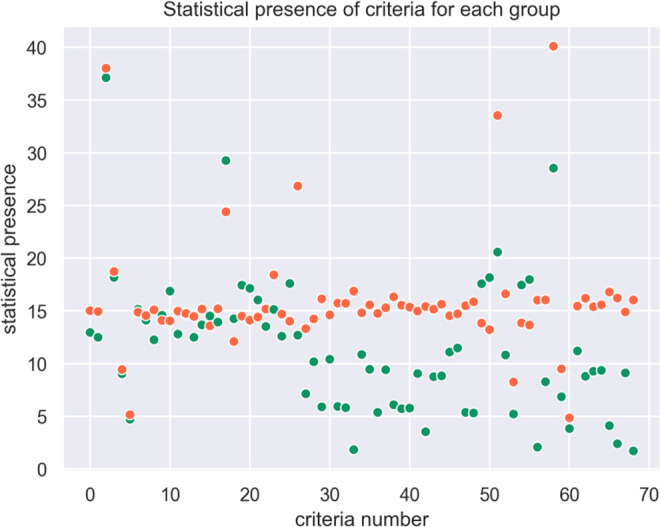
Statistical presence of criteria for each group (syn-EHRs).
The combination of several criteria leads from green to orange zone, i.e, the presence of 1 or 2 criteria is not significant in itself to classify the patient outcome. In our synthetic population, 5 criteria are present more than 20% of the time, but these criteria alone do not determine the zone.
Comparison of Criteria Between Real Patient and Synthetic Patient
The criteria proportions in each cohort are compared in Table 6. In order to assess the relevance of the virtually generated patients and their representativeness, we used an open-clustering approach.
Table 6.
Real and Synthetics Patient’s Predictors Distribution (%).
| Criteria | Green_real (%) | Orange_real (%) | Green_synth (%) | Orange_synth (%) | |
|---|---|---|---|---|---|
| 0 | Day of surgery | 52.94 | 61.54 | 17.6 | 14.02 |
| 1 | Length of stay (LOS) | 35.29 | 42.31 | 12.96 | 15.02 |
| 2 | Timing for procedure (1st, 2nd,3 rd, 4th, 5th in the day) | 67.65 | 61.54 | 12.5 | 14.94 |
| 3 | Type of job sedentary | 8.82 | 19.23 | 12.7 | 26.84 |
| 4 | Type of job worker | 14.71 | 3.85 | 7.14 | 13.32 |
| 5 | Work stopping duration before surgery-sedentary>1 | 0 | 0 | 37.12 | 38.02 |
| 6 | Work stopping duration before surgery-heavy worker>3 | 0 | 0 | 18.18 | 18.74 |
| 7 | Work stopping duration before surgery-moderate>14 | 0 | 0 | 9.04 | 9.44 |
| 8 | Work stopping duration before surgery-light worker>35 | 0 | 0 | 4.72 | 5.16 |
| 9 | Sleep disorder | 2.94 | 30.77 | 10.18 | 14.24 |
| 10 | Professional conflict | 5.88 | 11.54 | 5.9 | 16.14 |
| 11 | Family conflict | 5.88 | 11.54 | 10.42 | 14.62 |
| 12 | Specific physical activity | 0 | 0 | 5.94 | 15.74 |
| 13 | General physical activity | 0 | 0 | 5.82 | 15.72 |
| 14 | Appetite | 0 | 0 | 15.16 | 14.88 |
| 15 | Age | 32.35 | 57.69 | 14.12 | 14.56 |
| 16 | BMI | 50 | 69.23 | 1.84 | 16.88 |
| 17 | Smoking | 23.53 | 11.54 | 12.26 | 15.1 |
| 18 | Pre-operative walking distance | 38.24 | 42.31 | 10.86 | 14.82 |
| 19 | Prior to surgery opioid consumption | 0 | 0 | 9.46 | 15.58 |
| 20 | Cauda equina syndrome | 0 | 7.69 | 5.38 | 14.76 |
| 21 | Transit disorders | 2.94 | 3.85 | 14.58 | 14.1 |
| 22 | Pre-operative motor deficit | 11.76 | 19.23 | 9.42 | 15.3 |
| 23 | Pre-operative sensitive deficit | 23.53 | 30.77 | 16.88 | 14.06 |
| 24 | Impulsive movement or pushing effort | 14.71 | 15.38 | 6.1 | 16.34 |
| 25 | Pre-operative inflammatory pain | 2.94 | 7.69 | 5.72 | 15.54 |
| 26 | Limp | 100 | 100 | 12.8 | 14.98 |
| 27 | Acute lumbar pain | 29.41 | 34.62 | 14.64 | 14.76 |
| 28 | Chronic lumbar pain | 73.53 | 88.46 | 5.78 | 15.36 |
| 29 | Lumbar stiffness | 23.53 | 38.46 | 9.06 | 14.98 |
| 30 | Sphincter dysfunction | 2.94 | 7.69 | 3.54 | 15.42 |
| 31 | Diabetes | 8.82 | 11.54 | 12.5 | 14.48 |
| 32 | Pre-operative anxiety or depressive syndrome | 0 | 3.85 | 8.76 | 15.16 |
| 33 | Sleep apnea syndrome | 2.94 | 19.23 | 13.68 | 15.18 |
| 34 | COPD | 8.82 | 3.85 | 14.52 | 13.58 |
| 35 | Pneumopathy | 0 | 0 | 8.84 | 15.64 |
| 36 | Liver disorder | 0 | 0 | 11.1 | 14.54 |
| 37 | Atheroma | 0 | 0 | 11.48 | 14.72 |
| 38 | Kidney Disease | 5.88 | 3.85 | 13.94 | 15.2 |
| 39 | Pre-operative MODIC Images | 2.94 | 3.85 | 5.38 | 15.5 |
| 40 | Pre-operative Calcification | 8.82 | 0 | 5.32 | 15.86 |
| 41 | Pre-operative stenosis | 52.94 | 50 | 17.58 | 13.84 |
| 42 | Pre-operative protrusion | 5.88 | 3.85 | 18.16 | 13.22 |
| 43 | Pre-operative excluded disc herniation | 5.88 | 0 | 29.26 | 24.4 |
| 44 | Pre-operative disc herniation | 38.24 | 23.08 | 14.26 | 12.1 |
| 45 | L1L2 Level | 0 | 3.85 | 20.58 | 33.54 |
| 46 | L2L3 Level | 2.94 | 30.77 | 10.82 | 16.62 |
| 47 | L3L4 Level | 17.65 | 50 | 5.22 | 8.26 |
| 48 | Pre-operative arthrosis | 26.47 | 23.08 | 17.44 | 14.5 |
| 49 | Pre-operative hypertrophic facet disease | 29.41 | 26.92 | 17.14 | 14.12 |
| 50 | Pre-operative osteophyte | 0 | 3.85 | 17.46 | 13.86 |
| 51 | Pre-operative spondylolysis | 8.82 | 11.54 | 17.98 | 13.66 |
| 52 | Explicit pre-operative explanations | 0 | 0 | 2.08 | 16.02 |
| 53 | Operator experience (years of practice) | 0 | 0 | 16.04 | 14.42 |
| 54 | Food intake improvement | 0 | 0 | 13.52 | 15.18 |
| 55 | Sleep improvement | 0 | 0 | 8.28 | 16.04 |
| 56 | Return to work sedentary >42 | 0 | 0 | 28.54 | 40.1 |
| 57 | Return to work light >42 | 0 | 0 | 15.14 | 18.42 |
| 58 | Return to work moderate >75 | 0 | 0 | 6.86 | 9.5 |
| 59 | Return to work heavy workers >90 | 0 | 0 | 3.84 | 4.86 |
| 60 | Infection | 2.94 | 3.85 | 11.2 | 15.46 |
| 61 | Autonomous walking recovery | 0 | 3.85 | 8.8 | 16.2 |
| 62 | Anti-inflammatory drugs | 0 | 0 | 12.6 | 14.7 |
| 63 | Post-operative anxiety or depressive syndrom | 0 | 0 | 9.28 | 15.4 |
| 64 | Post-operative disc calcification | 0 | 0 | 9.36 | 15.58 |
| 65 | Post-operative stenosis | 2.94 | 0 | 4.12 | 16.8 |
| 66 | Post-operative fibrosis | 5.88 | 0 | 2.4 | 16.22 |
| 67 | Rehabilitation inpatients center | 0 | 0 | 9.12 | 14.9 |
| 68 | Operative recurrence | 0 | 34.62 | 1.72 | 16.04 |
As we are conscious of the lack of exhaustive data in the real patients cohort criteria, we presume that several non-significantly different criteria could be finally relevant if correctly assessed. Therefore, we preserve them to keep a maximum of meaningful data for the training of our machine learning and increase the reliability of our synthetic population.
Training and Validation of the Model (ANN Results)
The classifier is trained using 10000 patients from the training set and 2000 patients from the test set. The batch size is 2000 and the model is trained for 100 epochs. The loss decreases rapidly, and the accuracy is growing also quickly. After 50 epochs the model is already close to convergence (see Figure 6.).
Figure 6.
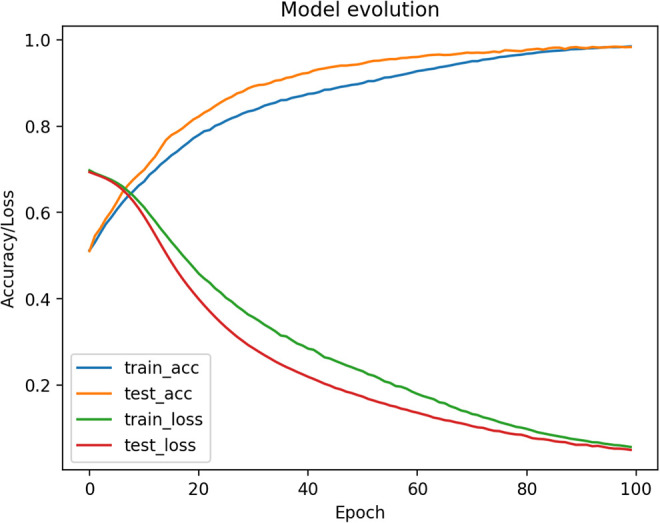
Training model evolution (Accuracy and loss / Number of epochs).
The test set is also synthetic and does not provide a solid way of stopping the model before overfitting because it has the same convergence as the training set. Thus, we use the real data to test our model and stop training.
After 100 epochs the test on real data gives an accuracy of 72% and the ROC curve is as follows with a ROC score of 0.78 (See Figure 6). The sensitivity of our model is then 88,5%, specificity is 58%, PPV is 62% an NPV 87%, these numbers for each zone are reported in Table 7.
Table 7.
ANN Model for Predict Successful Spine Surgery.
| Precision | Recall | f1-score | Support | |
|---|---|---|---|---|
| Orange Zone | 0.62 | 0.885 | 0.73 | 26 |
| Green Zone | 0.87 | 0.59 | 0.70 | 34 |
| Accuracy | 0.72 | 60 | ||
| Macro average | 0.75 | 0.74 | 0.72 | 60 |
| Weighted avg | 0.76 | 0.72 | 0.71 | 60 |
| ANN Model global performance | ||||
| ROC AUC Score | Sensitivity | Specificity | PPV | NPV |
| 0.78 | 0.885 | 0.59 | 0.62 | 0.87 |
Notes: PPV = Positive Predictive Value; NPV = Negative Predictive Values
Discussion
Our results show similar risk factors identified in other cohorts. 92 In our real patients cohort, age > 65 years, BMI> 30, surgery same day of hospital entry, chronic low back pain are strongly predictive of the orange zone. In our virtual cohorts, sedentary job, L1L2 level, return to work to sedentary job >42 days, work stopping duration before surgery-sedentary>1, are the strongest predictors for the orange zone, ie. treatment failure or poor improvement.
However, on their own, they cannot determine one outcome or the other. This illustrates the need for an individual predictive tool based on several predictors, having multiple degrees of influence (strength) on the outcome.
Our model was statistically representative of the real data. We also used the real data as the validation set of the classifier, in order to better fit the real world.
Our machine learning model can classify the orange population in 88,5% of cases, whereas our green zone is correctly classified in 59% of the cases. The overall precision, calculated by the area under the ROC curve (AUC) is 0.78 (see Figure 7).35,56,63,74,67-76,78-81 This model is particularly suitable for screening patients who react negatively to lumbar surgery, with similar sensitivity to other predicting tools recently published. Nevertheless, there is still a lack of specificity, maybe due to the 23 missing criteria from the database, which prevent our model to evaluate their impact as clinical predictors. Although ANNs show very promising performance, it was trained using virtual patients generated by our model, thus limiting the precision of the response in real cases. Moreover the study sample of real patients was small, and therefore this study will need to be repeated with larger, multicentre datasets and external validation to convincingly demonstrate its validity and predictive power.
Figure 7.
AUC of our ANN-models using EHRs and syn-EHRs.
The goal of our method was to obtain a reproducible, repeatable, and usable tool, that can fit with various databases, deal with missing data and can be applied to similar stakes. Indeed, the missing complete electronic patient data, the difficulty to access it and the inability to standardize and exploit this data make the development of an omniscient prediction tool challenging.
Thus, we increase the number of exploitable variables (below the significance threshold) to obtain an individual response, we generate virtual patients to increase the size of our training cohort, and we use medical know-how as a tool for architecture of our virtual patients to answer a data quantity problem.
Our algorithm is based on deep learning, which goal is to use as much data as possible to increase its accuracy and precision. The more intensive the use of the algorithm, the better the accuracy in cases statistically farther and farther from the center of the Gaussian. Indeed, the amount of data influences the variability of this data. This increases the number of “rare” cases far from the median value, making it less necessary to use techniques to boost their number (data augmentation). The real cases collected by retro-analysis of the data will gradually replace the data augmentation of the training set and the model will increase its robustness. This method is used in all machine learning algorithms whose training is supervised. Successive versions are improved by increasing the dataset as the actual data is captured. 93
As we move toward personalized medicine and value-based care, there is an increasing need to collect and use PRO scores not just in research settings, but also in routine clinical care or quality improvement activities. 50 The progressive digital transformation in the healthcare facilities should allow us to collect more precise and valuable clinical data.
Conclusion
Our method can be used to predict outcome lumbar decompression surgery. There is still a need to further develop its ability to analyze patients in the “failure of treatment” zone in order to offer precise management of patient health before spinal surgery. Through the exploitation of a larger database more representative over time, we think that our model will be capable of improving classification of the orange zone. This model is in concordance with already published machine-learning tools in spine surgery, successfully allowing to predict the improvement of post-operative symptomatology64,94 and reduction of drug consumption.38,95,96 Thus, it will be possible to administer the patient’s health monitoring to reduce the post-operative risks and above all to promote its recovery after surgery with appropriate therapies. In addition, a software suite could help surgical practice by reducing the surgical gesture to its anatomical usefulness by avoiding the psychological or iatrogenic undesirable effects inherent in the medico-social framework of the intervention.
Abbreviations
- ACC
accuracy
- ACS-NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- ANN
artificial neural networks
- AUC
area under the receiver operating characteristic curve
- COPD
chronic obstructive pulmonary disease
- DNN
deep neural networks
- HER
electronic health records
- GBM
gradient boosting machine
- GLM
generalized linear model
- GLMnet
elastic-net GLM
- LSS
lumbar spinal stenosis
- MCID
minimum clinically important difference
- ML
machine learning
- NPV
negative predictive value
- NRS
numeric rating scale
- NRS-BP
NRS for back pain
- NRS-LP
NRS for leg pain
- ODI
Oswestry Disability Index
- PHC
predictive hierarchical clustering
- PPV
positive predictive value
- PROMs
patient-reported outcome measures
- RF
random forest
- ROC
receiver operating characteristic
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Arthur André, MD, MSc  https://orcid.org/0000-0002-0075-5257
https://orcid.org/0000-0002-0075-5257
Jean-Jacques Vignaux, MSc  https://orcid.org/0000-0002-2767-772X
https://orcid.org/0000-0002-2767-772X
References
- 1.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2015;40(2):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CS, Rasoulinejad P, Taylor D, et al. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med. 2020;382(12):1093–1102. [DOI] [PubMed] [Google Scholar]
- 5.McGirt MJ, Bydon M, Archer KR, et al. An analysis from the Quality Outcomes Database, Part 1. Disability, quality of life, and pain outcomes following lumbar spine surgery: predicting likely individual patient outcomes for shared decision-making. J Neurosurg Spine. 2017;27(4):357–369. [DOI] [PubMed] [Google Scholar]
- 6.Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144–157. [DOI] [PubMed] [Google Scholar]
- 7.Yeramaneni S, Robinson C, Hostin R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr Rev Musculoskelet Med. 2016;9(3):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalakoti P, Hendrickson NR, Bedard NA, Pugely AJ. Opioid utilization following lumbar arthrodesis: trends and factors associated with long-term use. Spine (Phila Pa 1976). 2018;43(17):1208–1216. [DOI] [PubMed] [Google Scholar]
- 9.Austevoll IM, Gjestad R, Grotle M, et al. Follow-up score, change score or percentage change score for determining clinical important outcome following surgery? An observational study from the Norwegian Registry for Spine Surgery evaluating patient reported outcome measures in lumbar spinal stenosis and lumbar degenerative spondylolisthesis. BMC Musculoskelet Disord. 2019;20(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24(21):2229. [DOI] [PubMed] [Google Scholar]
- 11.Hägg O, Fritzell P, Ekselius L, Nordwall A.Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish Lumbar Spine study. Eur Spine J. 2003;12(1):22–33. [DOI] [PubMed] [Google Scholar]
- 12.Kohlboeck G, Greimel KV, Piotrowski WP, et al. Prognosis of multifactorial outcome in lumbar discectomy: a prospective longitudinal study investigating patients with disc prolapse. Clin J Pain. 2004;20(6):455–461. [DOI] [PubMed] [Google Scholar]
- 13.Trief PM, Ploutz-Snyder R, Fredrickson BE. Emotional health predicts pain and function after fusion: a prospective multicenter study. Spine. 2006;31(7):823–830. [DOI] [PubMed] [Google Scholar]
- 14.Slover J, Abdu WA, Hanscom B, Weinstein JN. The impact of comorbidities on the change in Short-Form 36 and Oswestry scores following lumbar spine surgery. Spine. 2006;31(17):1974–1980. [DOI] [PubMed] [Google Scholar]
- 15.Braybrooke J, Ahn H, Gallant A, et al. The impact of surgical wait time on patient-based outcomes in posterior lumbar spinal surgery. Eur Spine J. 2007;16(11):1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannion AF, Elfering A, Staerkle R, et al. Predictors of multidimensional outcome after spinal surgery. Eur Spine J. 2007;16(6):777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park P, Upadhyaya C, Garton HJL, Foley KT. The impact of minimally invasive spine surgery on perioperative complications in overweight or obese patients. Neurosurg. 2008;62(3):693–699. [DOI] [PubMed] [Google Scholar]
- 18.Garcia RM, Messerschmitt PJ, Furey CG, Bohlman HH, Cassinelli EH. Weight loss in overweight and obese patients following successful lumbar decompression. JBJS. 2008;90(4):742–747. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya R, Carp J, Bartol S, Ouellette N, Lee S, Sethi A. Lumbar spine fusion in obese and morbidly obese patients. Spine. 2009;34(5):495–500. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Anderson MV, Cheng WK, Wongworawat MD. Diabetes associated with increased surgical site infections in spinal arthrodesis. Clin Orthop Relat Res. 2009;467(7):1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott AD, Tyni-Lenné R, Hedlund R. Leg pain and psychological variables predict outcome 2–3 years after lumbar fusion surgery. Eur Spine J. 2011;20(10):1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senker W, Meznik C, Avian A, Berghold A. Perioperative morbidity and complications in minimal access surgery techniques in obese patients with degenerative lumbar disease. Eur Spine J. 2011;20(7):1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaichana KL, Mukherjee D, Adogwa O, Cheng JS, McGirt MJ. Correlation of preoperative depression and somatic perception scales with postoperative disability and quality of life after lumbar discectomy. J Neurosurg Spine. 2011;14(2):261–267. [DOI] [PubMed] [Google Scholar]
- 24.Sinikallio S, Aalto T, Airaksinen O, Lehto SM, Kröger H, Viinamäki H. Depression is associated with a poorer outcome of lumbar spinal stenosis surgery: a two-year prospective follow-up study. Spine. 2011;36(8):677–682. [DOI] [PubMed] [Google Scholar]
- 25.Kalanithi PA, Arrigo R, Boakye M. Morbid obesity increases cost and complication rates in spinal arthrodesis. Spine. 2012;37(11):982–988. [DOI] [PubMed] [Google Scholar]
- 26.Sørlie A, Moholdt V, Kvistad KA, et al. Modic type I changes and recovery of back pain after lumbar microdiscectomy. European Spine J., 2012;21(11): 2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pakarinen M, Vanhanen S, Sinikallio S, et al. Depressive burden is associated with a poorer surgical outcome among lumbar spinal stenosis patients: a 5-year follow-up study. Spine J. 2014;14(10):2392–2396. [DOI] [PubMed] [Google Scholar]
- 28.Hellum C, Johnsen LG, Gjertsen Ø, et al. Predictors of outcome after surgery with disc prosthesis and rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up. Eur Spine J. 2012;21(4):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudelli C, Thomas K. Obesity and early reoperation rate after elective lumbar spine surgery: a population-based study. Evid Based Spine Care J. 2012;3(02):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta AI, Babu R, Karikari IO, et al. 2012 Young investigator award winner: the distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine. 2012;37(19):1652–1656. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Muir R, Johnston R, Carter E, Bowden G, Wilson-MacDonald J. Diabetes is predictive of longer hospital stay and increased rate of Clavien complications in spinal surgery in the UK. Ann R Coll Surg Engl. 2013;95(4):275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi S, Suzuki A, Toyoda H, et al. Characteristics of diabetes associated with poor improvements in clinical outcomes after lumbar spine surgery. Spine. 2013;38(6):516–522. [DOI] [PubMed] [Google Scholar]
- 33.Bekelis K, Desai A, Bakhoum SF, Missios S. A predictive model of complications after spine surgery: the National Surgical Quality Improvement Program (NSQIP) 2005–2010. Spine J. 2014;14(7):1247–1255. [DOI] [PubMed] [Google Scholar]
- 34.Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. JBJS. 2014;96(11):e89. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Arvind V, Oermann EK, et al. Predicting surgical complications in patients undergoing elective adult spinal deformity procedures using machine learning. Spine Deform. 2018;6(6):762–770. [DOI] [PubMed] [Google Scholar]
- 36.Coronado RA, George SZ, Devin CJ, Wegener ST, Archer KR. Pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery. Arch Phys Med Rehabil. 2015;96(10):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGirt MJ, Sivaganesan A, Asher AL, Devin CJ. Prediction model for outcome after low-back surgery: individualized likelihood of complication, hospital readmission, return to work, and 12-month improvement in functional disability. Neurosurg Focus. 2015;39(6):E13. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JT, Haas AR, Percy R, Woods ST, Ahn UM, Ahn NU. Chronic opioid therapy after lumbar fusion surgery for degenerative disc disease in a workers’ compensation setting. Spine (Phila Pa 1976). 2015;40(22):1775–1784. [DOI] [PubMed] [Google Scholar]
- 39.Chotai S, Sivaganesan A, Parker SL, McGirt MJ, Devin CJ. Patient-specific factors associated with dissatisfaction after elective surgery for degenerative spine diseases. Neurosurg. 2015;77(2):157–163. [DOI] [PubMed] [Google Scholar]
- 40.Schöller K, Steingrüber T, Stein M, et al. Microsurgical unilateral laminotomy for decompression of lumbar spinal stenosis: long-term results and predictive factors. Acta Neurochir. 2016;158(6):1103–1113. [DOI] [PubMed] [Google Scholar]
- 41.Archer KR, Devin CJ, Vanston SW, et al. Cognitive-behavioral–based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. 2016;17(1):76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asher AL, Devin CJ, McCutcheon B, et al. Patient characteristics of smokers undergoing lumbar spine surgery: an analysis from the Quality Outcomes Database. J Neurosurg Spine. 2017;27(6):661–669. [DOI] [PubMed] [Google Scholar]
- 43.Mummaneni PV, Bisson EF, Kerezoudis P, et al. Minimally invasive versus open fusion for grade I degenerative lumbar spondylolisthesis: analysis of the Quality Outcomes Database. Neurosurg Focus. 2017;43(2):E11. [DOI] [PubMed] [Google Scholar]
- 44.Crawford CH, Carreon LY, Bydon M, Asher AL, Glassman SD. Impact of preoperative diagnosis on patient satisfaction following lumbar spine surgery. J Neurosurg Spine. 2017;26(6):709–715. [DOI] [PubMed] [Google Scholar]
- 45.Suri P, Pearson AM, Zhao W, et al. Pain recurrence after discectomy for symptomatic lumbar disc herniation. Spine. 2017;42(10):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma M, Ugiliweneza B, Aljuboori Z, Nuño MA, Drazin D, Boakye M. Factors predicting opioid dependence in patients undergoing surgery for degenerative spondylolisthesis: analysis from the MarketScan databases. J Neurosurg Spine. 2018;29(3):271–278. [DOI] [PubMed] [Google Scholar]
- 47.Dunn LK, Durieux ME, Fernández LG, et al. Influence of catastrophizing, anxiety, and depression on in-hospital opioid consumption, pain, and quality of recovery after adult spine surgery. J Neurosurg Spine. 2018;28(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan AK, Bisson EF, Bydon M, et al. Laminectomy alone versus fusion for grade 1 lumbar spondylolisthesis in 426 patients from the prospective Quality Outcomes Database. J Neurosurg Spine. 2018;30(2):234–241. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell JA, Anderson JT, Haas AR, et al. Preoperative opioid use is a predictor of poor return to work in workers’ compensation patients after lumbar diskectomy. Spine. 2018;43(8):594–602. [DOI] [PubMed] [Google Scholar]
- 50.Khor S, Lavallee D, Cizik AM, et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg. 2018;153(7):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobran M, Nasi D, Paracino R, et al. Analysis of risk factors and postoperative predictors for recurrent lumbar disc herniation. Surg Neurol Int. 2019;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staub LP, Aghayev E, Skrivankova V, Lord SJ, Haschtmann D, Mannion AF. Development and temporal validation of a prognostic model for 1-year clinical outcome after decompression surgery for lumbar disc herniation. Eur Spine J. 2020;29(7):1742–1751. [DOI] [PubMed] [Google Scholar]
- 53.Mauro D, Nasi D, Paracino R, et al. The relationship between preoperative predictive factors for clinical outcome in patients operated for lumbar spinal stenosis by decompressive laminectomy. Surg Neurol Int. 2020;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudolfsen JH, Solberg TK, Ingebrigtsen T, Olsen JA. Associations between utilization rates and patients’ health: a study of spine surgery and patient-reported outcomes (EQ-5D and ODI). BMC Health Serv Res. 2020;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asher AL, Devin CJ, Archer KR, et al. An analysis from the quality outcomes database, part 2. Predictive model for return to work after elective surgery for lumbar degenerative disease. J Neurosurg Spine. 2017;27(4):370–381. [DOI] [PubMed] [Google Scholar]
- 56.Azimi P, Mohammadi HR, Benzel EC, Shahzadi S, Azhari S. Use of artificial neural networks to decision making in patients with lumbar spinal canal stenosis. J Neurosurg Sci. 2017;61(6):603–611. [DOI] [PubMed] [Google Scholar]
- 57.Azimi P, Mohammadi HR, Benzel EC, Shahzadi S, Azhari S. Use of artificial neural networks to predict recurrent lumbar disk herniation. J Spinal Disord Tech. 2015;28(3):E161–165. [DOI] [PubMed] [Google Scholar]
- 58.André A. The information technology revolution in health care. In: Arthur A, ed. Digital Medicine. Springer International Publishing; 2019:1–7. [Google Scholar]
- 59.Dillavou ED, Sun Z, Peiris P, Huang E, Benrashid E, Chang B.IP145. Deep learning-based risk model for best management of closed surgical incisions following vascular surgery. J Vasc Surg. 2019;69(6):e149–e150. [DOI] [PubMed] [Google Scholar]
- 60.Shahid N, Rappon T, Berta W. Applications of artificial neural networks in health care organizational decision-making: a scoping review. PLoS One. 2019;14(2):e0212356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal I. Developing new machine learning ensembles for quality spine diagnosis. Knowl-Based Syst. 2015;73(1):298–310. [Google Scholar]
- 62.Iderberg H, Willers C, Borgström F, et al. Predicting clinical outcome and length of sick leave after surgery for lumbar spinal stenosis in Sweden: a multi-register evaluation. Eur Spine J. 2019;28(6):1423–1432. [DOI] [PubMed] [Google Scholar]
- 63.Karhade AV, Ogink PT, Thio QCBS, et al. Development of machine learning algorithms for prediction of prolonged opioid prescription after surgery for lumbar disc herniation. Spine J. 2019;19(11):1764–1771. [DOI] [PubMed] [Google Scholar]
- 64.Siccoli A, Marlies P, Schröder ML, Staartjes VE. Machine learning–based preoperative predictive analytics for lumbar spinal stenosis. Neurosurg Focus. 2019;46(5):E5. [DOI] [PubMed] [Google Scholar]
- 65.Kalagara S, Eltorai AEM, Durand WM, DePasse JM, Daniels AH. Machine learning modeling for predicting hospital readmission following lumbar laminectomy. J Neurosurg Spine. 2018;30(3):344–352. [DOI] [PubMed] [Google Scholar]
- 66.Han SS, Azad TD, Suarez PA, Ratliff JK. A machine learning approach for predictive models of adverse events following spine surgery. Spine J. 2019;19(11):1772–1781. [DOI] [PubMed] [Google Scholar]
- 67.Azimi P, Benzel EC, Shahzadi S, Azhari S, Mohammadi HR. Use of artificial neural networks to predict surgical satisfaction in patients with lumbar spinal canal stenosis. J Neurosurg Spine. 2014;20(3):300–305. [DOI] [PubMed] [Google Scholar]
- 68.Azimi P, Benzel EC, Shahzadi S, Azhari S, Zali AR. Prediction of successful surgery outcome in lumbar disc herniation based on artificial neural networks. Global Spine J. 2014;4(1_suppl):s–0034. [PubMed] [Google Scholar]
- 69.Azimi P, Mohammadi HR, Benzel EC, Shahzadi S, Azhari S. Use of artificial neural networks to predict recurrent lumbar disk herniation. Clin Spine Surg. 2015;28(3):E161–E165. [DOI] [PubMed] [Google Scholar]
- 70.Ratliff JK, Balise R, Veeravagu A, et al. Predicting occurrence of spine surgery complications using “big data” modeling of an administrative claims database. JBJS. 2016;98(10):824–834. [DOI] [PubMed] [Google Scholar]
- 71.Oh T, Scheer JK, Smith JS, et al. Potential of predictive computer models for preoperative patient selection to enhance overall quality-adjusted life years gained at 2-year follow-up: a simulation in 234 patients with adult spinal deformity. Neurosurg Focus. 2017;43(6):E2. [DOI] [PubMed] [Google Scholar]
- 72.Scheer JK, Smith JS, Schwab F, et al. Development of a preoperative predictive model for major complications following adult spinal deformity surgery. J Neurosurg Spine. 2017;26(6):736–743. [DOI] [PubMed] [Google Scholar]
- 73.Staartjes VE, Marlies P, Vandertop WP, Schröder ML. Deep learning-based preoperative predictive analytics for patient-reported outcomes following lumbar discectomy: feasibility of center-specific modeling. Spine J. 2019;19(5):853–861. [DOI] [PubMed] [Google Scholar]
- 74.Karhade AV, Ogink P, Thio Q, et al. Development of machine learning algorithms for prediction of discharge disposition after elective inpatient surgery for lumbar degenerative disc disorders. Neurosurg Focus. 2018;45(5):E6. [DOI] [PubMed] [Google Scholar]
- 75.Kuo C-Y, Yu L-C, Chen H-C, Chan C-L. Comparison of models for the prediction of medical costs of spinal fusion in Taiwan Diagnosis-Related Groups by machine learning algorithms. Healthc Inform Res. 2018;24(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goyal A, Ngufor C, Kerezoudis P, McCutcheon B, Storlie C, Bydon M. Can machine learning algorithms accurately predict discharge to nonhome facility and early unplanned readmissions following spinal fusion? Analysis of a national surgical registry. J Neurosurg Spine. 2019;1–11. [DOI] [PubMed] [Google Scholar]
- 77.Shah AA, Karhade AV, Bono CM, Harris MB, Nelson SB, Schwab JH. Development of a machine learning algorithm for prediction of failure of nonoperative management in spinal epidural abscess. Spine J. 2019;19(10):1657–1665. [DOI] [PubMed] [Google Scholar]
- 78.Karhade AV, Shah AA, Bono CM, et al. Development of machine learning algorithms for prediction of mortality in spinal epidural abscess. Spine J. 2019;19(12):1950–1959. [DOI] [PubMed] [Google Scholar]
- 79.Hopkins BS, Yamaguchi JT, Garcia R, et al. Using machine learning to predict 30-day readmissions after posterior lumbar fusion: an NSQIP study involving 23,264 patients. J Neurosurg Spine. 2019;1(aop):1–8. [DOI] [PubMed] [Google Scholar]
- 80.Nelson A, Herron D, Rees G, Nachev P. Predicting scheduled hospital attendance with artificial intelligence. NPJ Digit Med. 2019;2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins BS, Mazmudar A, Driscoll C, et al. Using artificial intelligence (AI) to predict postoperative surgical site infection: a retrospective cohort of 4046 posterior spinal fusions. Clin Neurol Neurosurg. 2020;192:105718. [DOI] [PubMed] [Google Scholar]
- 82.Yoon J, Drumright LN, Van Der Schaar M. Anonymization through data synthesis using Generative Adversarial Networks (ADS-GAN). IEEE Journal of Biomedical and Health Informatics; 2020. [DOI] [PubMed] [Google Scholar]
- 83.Baowaly MK, Lin C-C, Liu C-L, Chen K-T. Synthesizing electronic health records using improved generative adversarial networks. J Am Med Inform Assoc. 2019;26(3):228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buczak AL, Babin S, Moniz L. Data-driven approach for creating synthetic electronic medical records. BMC Med Inform Decis Mak. 2010;10(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yale A, Dash S, Dutta R. Privacy preserving synthetic health data. F1000Res. 2019;8:724. [Google Scholar]
- 86.Walonoski J, Kramer M, Nichols J, et al. Synthea: an approach, method, and software mechanism for generating synthetic patients and the synthetic electronic health care record. J Am Med Inform Assoc. 2017;25(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He H, Bai Y, Garcia EA, Li S. ADASYN: Adaptive synthetic sampling approach for imbalanced learning. In: 2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence), Hong Kong, China, 1-8 June 2008. IEEE; 2008. [Google Scholar]
- 88.Teutonico D, Musuamba F, Maas HJ, et al. Generating virtual patients by multivariate and discrete re-sampling techniques. Pharm Res. 2015;32(10):3228–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McLachlan S, Dube K, Gallagher T.Using the caremap with health incidents statistics for generating the realistic synthetic electronic healthcare record. In: 2016 IEEE International Conference on Healthcare Informatics (ICHI), Chicago, IL, 4-7 October 2016. IEEE; 2016. [Google Scholar]
- 90.Kim JS, Merrill RK, Arvind V, et al. Examining the ability of artificial neural networks machine learning models to accurately predict complications following posterior lumbar spine fusion. Spine (Phila Pa 1976). 2018;43(12):853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pollack AH, Simon TD, Snyder J, Pratt W. Creating synthetic patient data to support the design and evaluation of novel health information technology. J Biomed Inform. 2019;95:103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lønne G, Fritzell P, Hägg O, et al. Lumbar spinal stenosis: comparison of surgical practice variation and clinical outcome in three national spine registries. Spine J. 2019;19(1):41–49. [DOI] [PubMed] [Google Scholar]
- 93.Hestness J, Narang S, Ardalani N, et al. Deep learning scaling is predictable, empirically. arXiv. arXiv preprint arXiv:171200409 . 2017.
- 94.Malik AT, Khan SN. Predictive modeling in spine surgery. Ann Transl Med. 2019;7(suppl 5):S173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hills JM, Pennings JS, Archer KR, et al. Preoperative opioids and 1-year patient-reported outcomes after spine surgery. Spine (Phila Pa 1976). 2019;44(12):887–895. [DOI] [PubMed] [Google Scholar]
- 96.Oleisky ER, Pennings JS, Hills J, et al. Comparing different chronic preoperative opioid use definitions on outcomes after spine surgery. Spine J. 2019;19(6):984–994. [DOI] [PubMed] [Google Scholar]