Abstract
Study Design:
Retrospective study.
Objective:
The aim of this study is to evaluate the clinical, neurological, and radiological outcomes of posterior vertebral column resection (PVCR) technique for treatment of thoracic and thoracolumbar burst fractures.
Methods:
Fifty-one patients (18 male, 33 female) with thoracic/thoracolumbar burst fractures who had been treated with PVCR technique were retrospectively reviewed. Preoperative and most recent radiographs were evaluated and local kyphosis angle (LKA), sagittal and coronal spinal parameters were measured. Neurological and functional results were assessed by the American Spinal Injury Association (ASIA) Impairment Scale, visual analogue scale score, Oswestry Disability Index, and Short Form 36 version 2.
Results:
The mean age was 49 years (range 22-83 years). The mean follow-up period was 69 months (range 28-216 months). Fractures were thoracic in 16 and thoracolumbar in 35 of the patients. AO spine thoracolumbar injury morphological types were as follows: 1 type A3, 15 type A4, 4 type B1, 23 type B2, 8 type C injuries. PVCR was performed in a single level in 48 of the patients and in 2 levels in 3 patients. The mean operative time was 434 minutes (range 270-530 minutes) and mean intraoperative blood loss was 520 mL (range 360-1100 mL). The mean LKA improved from 34.7° to 4.9° (85.9%). For 27 patients, the initial neurological deficit (ASIA A in 8, ASIA B in 3, ASIA C in 5, and ASIA D in 11) improved at least 1 ASIA grade (1-3 grades) in 22 patients (81.5%). Solid fusion, assessed with computed tomography at the final follow-up, was achieved in all patients.
Conclusion:
Single-stage PVCR provides complete spinal canal decompression, ideal kyphosis correction with gradual lengthening of anterior column together with sequential posterior column compression. Anterior column support, avoidance of the morbidity of anterior approach and improvement of neurological deficit are the other advantages of the single stage PVCR technique in patients with thoracic/thoracolumbar burst fractures.
Keywords: thoracic/thoracolumbar, burst fracture, PVCR
Introduction
Thoracic and thoracolumbar burst fractures are the most common spine injuries, constituting approximately 10% to 20% of all spinal fractures. 1 Burst fracture is defined as the fracture of the anterior and middle columns with or without posterior column involvement of the spine and with or without vertebral body fragments in the spinal canal.2,3 Moreover, burst fracture is defined as a vertebral body fracture involving both endplates as well as the posterior wall. 4 Selection of the treatment method for thoracic and thoracolumbar burst fractures mainly depends on the stability of the burst fracture and presence of neurological deficit. 2 However, there are many factors that influence selection of the treatment method. The degree of kyphotic angle, global sagittal alignment, comminution of the fracture, disc and/or endplate injuries, posterior ligamentous complex injury, presence of neurological deficit, need for proper decompression, severity of osteoporosis, and patient-related comorbidities are the main factors affecting the treatment approach.
Surgery is generally the preferred treatment in unstable fractures while stable fractures are managed conservatively, and there is also a small gray area in between.2,3,5-8 There are controversies on how to treat burst fractures that are not associated with neurologic deficits and have an intact posterior column. Two randomized controlled studies have investigated the management of these latter types of fractures and they found contradictory results.9,10 Wood et al 9 reported better outcomes with conservative treatment for those fractures. On the other hand, Siebenga et al 10 reported a randomized controlled study, which concluded that type A3 burst fractures should be managed with short segment posterior stabilization rather than conservatively.
There is also no consensus on the ideal surgical treatment method for unstable thoracic and thoracolumbar burst fractures. Suggested surgical options include posterior instrumentation with or without decompression, anterior vertebrectomy, or combined surgery.2,3,5-8,11
The goals of surgery in unstable burst fractures are to reduce kyphotic deformity, maintain spinal stability, and decompress neural structures when necessary. Each of these is crucial for early mobilization. The advantages and disadvantages of each surgical technique should be carefully considered. An anterior approach provides direct and more complete decompression of the spinal canal, which could potentially yield better neurological outcomes. 12 However, the anterior approach is associated with a longer operative time, greater blood loss, and potential injury to the great vessels. Another surgical procedure for unstable thoracic and thoracolumbar burst fractures is posterior vertebral column resection (PVCR). This technique is demanding and can be associated with dural tears and iatrogenic neurologic injury. The benefit of PVCR is that it allows decompression and stabilization from a single approach which is more familiar to the majority of spine surgeons. There is limited information in the literature about PVCR for treatment of thoracic/thoracolumbar burst fractures.13-15 The aim of this study therefore is to evaluate the clinical, neurological, and radiological outcomes of PVCR technique in the treatment of thoracic/thoracolumbar burst fractures.
Material and Methods
We retrospectively reviewed our database for traumatic thoracic or thoracolumbar burst fractures operated with PVCR. The study was approved by the institutional review board. Verbal and written consents were received from all patients. The inclusion criteria were presence of a traumatic thoracic or thoracolumbar burst fracture with or without neurological deficit, in a skeletally mature individual (age >18 years), presentation within 10 days of the time of injury, and having a single-stage PVCR technique within 15 days of the injury and having a follow-up period of at least 2 years. AO type B and type C fractures that were obviously mechanically unstable with or without neurological deficit and some of selected type A3 and type A4 fractures that had symptomatic anterior spinal cord compression from retropulsed bone, symptomatic traumatic disc herniation, or severe local kyphosis were included in the study. Exclusion criteria were pathologic fractures due to primary malignancy or spinal metastasis, history of previous spinal intervention or surgery, spinal infection, incomplete radiologic workup, or unwillingness of the patient to complete the clinical questionnaires.
From 2000 to 2017, a total of 51 patients (33 female, 18 male) with thoracic and thoracolumbar burst fractures who met the criteria mentioned above were enrolled in this single-center, single-surgeon study. Burst fractures were caused by motor vehicle accidents in 34 patients (66.7%) and fall from a high altitude in 17 (33.3%). Fifteen patients (29.4%) had polytrauma and early total care was performed in those patients. Mean age was 49 years (range 22-83 years). Mean follow-up period was 69 months (range 28-216 months). Twenty-seven patients had neurologic deficits on initial American Spinal Injury Association (ASIA) assessment 16 : ASIA A in 8, ASIA B in 3, ASIA C in 5, and ASIA D in 11 patients. The remaining 24 patients did not have any neurological deficits.
Charts of the patients and radiological studies were evaluated retrospectively. For radiological evaluation preoperative and final follow-up of direct radiographs, computed tomography (CT) scans, and magnetic resonance imaging (MRI) were reviewed in all patients. In the 23 patients who were able to stand prior to surgery, upright posterior-anterior and lateral radiographs were obtained of the entire spine. Preoperative direct radiographic assessments of the remaining 28 patients who were not able to stand or sit were performed in supine position. Preoperative CT scan was used to assess the osseous detail of the fracture, evaluate canal compromise, and the presence of laminar fractures or posterior bony injuries.17,18 Preoperative MRI was used to evaluate the spinal cord, dura mater, adjacent disc spaces, and the posterior ligamentous complex.19,20 Patients underwent surgery on a radiolucent spine frame and intraoperative whole spine radiographs were obtained to assess the sagittal and coronal alignment along with screw and cage placement.
The follow-up radiographs were obtained in the immediate postoperative period, 6 months after surgery and at the most recent follow-up. Eight patients were not able to stand at the final follow-up and the final radiographs of these patients were assessed in the sitting position. The remaining 43 patients were able to stand at the final follow-up. CT scan was performed six months following surgery to evaluate fusion status (Figure 1). Radiographic outcome parameters measured were mean local kyphosis, global kyphosis (T2-T12), lumbar lordosis (T12-S1), thoracolumbar kyphosis (T10-L2), sagittal and coronal balance before surgery and at the final follow-up.21,22 Clinical outcome parameters were composed of Oswestry Disability Index (ODI), visual analogue scale (VAS), and Short Form–36 version 2 (SF36-v2) at final follow-up.23-25 Statistical analysis was performed with the Wilcoxon test. All complications related to surgery were reviewed in the hospital files retrospectively.
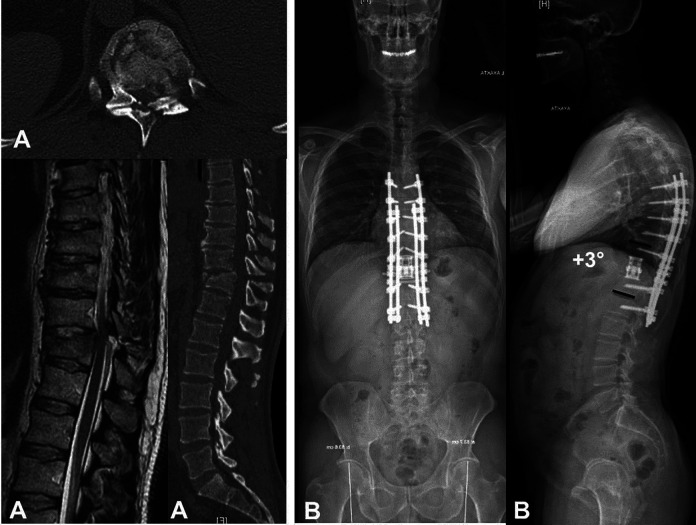
Figure 1.
(A, B) Preoperative computed tomography (CT) and magnetic resonance images a of 46-year-old male patient with T12 burst fracture. (C) Postoperative anteroposteior and lateral standing radiographs. (D) Postoperative CT image confirmed the solid union after 18 months.
Surgical Technique
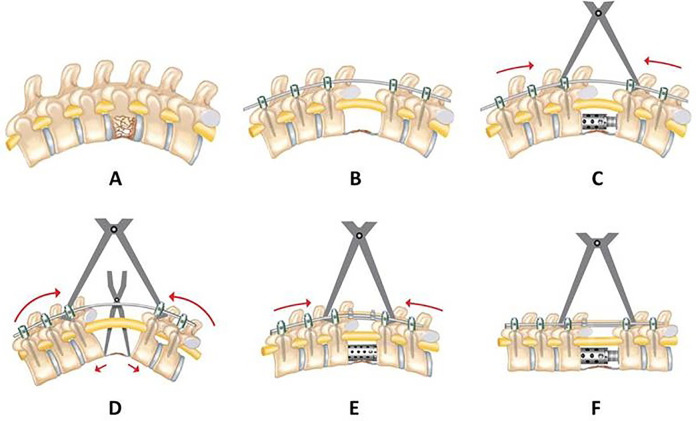
All operations were performed by the senior author (A.H). All patients underwent neuromonitoring and general anesthesia and were positioned prone on a spine frame. Prior to making a posterior longitudinal incision, posterior-anterior, and lateral radiographs were obtained of the entire spine using cassette holders. After the longitudinal incision, a midline approach was used, placing pedicle screws at least 2 levels cephalad and caudal to the fracture to minimize the length of the fusion. Occasionally in the thoracic spine a longer pedicle screw construct was used. In cases where the T-score was less than −2.5 on DEXA (dual-energy X-ray absorptiometry) or quantitative CT scan or with additional compression fractures adjacent to the burst fracture, instrumentation was performed 3 levels above and below the main fracture. For 8 of the patients with a preoperative history of osteoporosis who also had a T-score of less than −2.5, cement augmentation was performed with fenestrated pedicle screws and prophylactic vertebroplasty was done 1 level above the proximal instrumented vertebra and one level below the lowest instrumented vertebra to avoid proximal or distal junctional kyphosis or adjacent vertebral compression fracture that might possibly occur due to stress rising. 26 A temporary rod was placed on one side to avoid translation during vertebrectomy. Wide laminectomy was performed at the level of the fracture and 1 level above and below. The corresponding ribs and transverse processes were resected in 31 cases unilaterally and in 21 cases bilaterally to expose the lateral wall of the pedicles. In the thoracic spine, the nerve roots were sacrificed to facilitate exposure and osteotomy, but nerve roots below T11 were preserved for abdominal muscle innervation. The pedicles of the fractured vertebra were burred using a high-speed burr. A malleable retractor was used to protect the pleura, vessels, and sympathetic chain. With the retractor in place, the discs above and below the fracture level were then removed, followed by resection of the pedicles and vertebral body using osteotomies and a high-speed burr. A thin shell of bone at the anterior part along with the anterior longitudinal ligament was preserved to protect translation (Figure 2A and B). Endplates were prepared properly under direct vision. Lateral radiographs were taken to evaluate endplate inclination for matching with the cage end caps. Ideal sagittal alignment was achieved by gradual anterior column lengthening and posterior column compression, which also prevented iatrogenic neurological deficit (Figure 2C and D). A titanium mesh cage or an expandable cage was filled with autograft ± allograft and then was placed between the endplates of the adjacent vertebrae and the position was checked with fluoroscopy (Figure 2E and F). Following placement of the final rods, additional posterior compression was performed to achieve ideal sagittal alignment and increase stability of the cage. A femoral strut allograft was shaped in the form of letter H by using burr and was placed over the laminectomy defect to prevent spinal cord compression that might possibly occur due to hematoma or scar tissue formation (Figure 3).
Figure 2.
(A) Correction of kyphotic deformity with sequential posterior compression and simultaneous anterior column lengthening technique. (B) Placement of initial temporal rod, resection of the fractured vertebral body with preservation of anterior longitudinal ligament and anterior cortex. (C, D) Gradual anterior column lengthening using a spreader and expandable cage. (E, F) Simultaneous posterior column compression.
Figure 3.
(A) H-shaped femoral strut allograft and the laminectomy defect following posterior vertebral column resection. (B, C) Anteroposterior and lateral view of the H-shaped strut allograft stabilized in place by rod-cross links and autograft in upper and lower ends.
Results
There were 16 thoracic and 35 thoracolumbar fractures; the AO morphologic classification were type A3 in 1, type A4 in 15, type B1 in 4, type B2 in 23, and type C in 8 patients. PVCR was performed on a single level in 48 patients, and on 2 levels in 3 patients (Table 1). The mean operative time was 434 minutes (range 270-535 minutes). Mean intraoperative blood loss was 520 mL (range 360-1100 mL). A titanium mesh cage was used in 25 patients and an expandable cage was used in 26 patients. Prior to surgery, the local kyphotic angle (LKA) averaged 34.7° (range 8° to 83°) and was 4.9° at final follow-up (range −2° to 27°) with a mean improvement of 85.9%. This difference in preoperative and final kyphotic values was statistically significant (P < .05).
Table 1.
Summary of Preoperative and Follow-up Data in All Patients.
| No. | Sex | Age, y | AO class | PVCR level | Cage type | ASIA Class | LKA, deg | ||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Follow-up | Preoperative | Follow-up | ||||||
| 1 | M | 37 | C | T12 | Expandable | ASIA A | ASIA A | 23 | 5 |
| 2 | M | 45 | C | T4-T5 | Titanium | ASIA A | ASIA A | 83 | 27 |
| 3 | F | 22 | B2 | T12 | Titanium | ASIA B | ASIA E | 25 | 4 |
| 4 | F | 64 | A4 | T12 | Expandable | ASIA D | ASIA E | 16 | 1 |
| 5 | F | 72 | A4 | T12 | Expandable | ASIA E | ASIA E | 59 | 2 |
| 6 | M | 66 | B2 | L1 | Expandable | ASIA E | ASIA E | 25 | 0 |
| 7 | M | 25 | B1 | T7 | Titanium | ASIA B | ASIA E | NA | 13 |
| 8 | M | 34 | B2 | L1 | Expandable | ASIA E | ASIA E | 14 | 2 |
| 9 | F | 30 | C | L1 | Expandable | ASIA A | ASIA C | 32 | -2 |
| 10 | F | 68 | A4 | L1 | Expandable | ASIA E | ASIA E | 31 | 0 |
| 11 | F | 27 | A4 | T12 | Expandable | ASIA A | ASIA C | 36 | 0 |
| 12 | F | 68 | B2 | T12 | Expandable | ASIA E | ASIA E | 36 | 3 |
| 13 | F | 38 | B1 | T7 | Titanium | ASIA E | ASIA E | 46 | 15 |
| 14 | F | 62 | B2 | T11 | Titanium | ASIA E | ASIA E | 46 | 3 |
| 15 | F | 66 | B2 | T12 | Expandable | ASIA E | ASIA E | 28 | 2 |
| 16 | F | 27 | A3 | L2 | Expandable | ASIA D | ASIA E | 32 | -2 |
| 17 | F | 65 | B2 | T12 | Expandable | ASIA E | ASIA E | 55 | 5 |
| 18 | F | 24 | B2 | T8 | Titanium | ASIA B | ASIA E | 34 | 9 |
| 19 | M | 45 | B2 | T12 | Expandable | ASIA E | ASIA E | 41 | 3 |
| 20 | F | 22 | C | T5 | Titanium | ASIA A | ASIA C | 35 | 8 |
| 21 | F | 47 | B2 | T2-T3 | Titanium | ASIA E | ASIA E | 32 | 9 |
| 22 | F | 80 | B1 | T11 | Expandable | ASIA E | ASIA E | 41 | 0 |
| 23 | M | 46 | A4 | T12 | Expandable | ASIA C | ASIA E | 33 | -2 |
| 24 | F | 55 | B2 | L1 | Titanium | ASIA E | ASIA E | 23 | 0 |
| 25 | M | 66 | A4 | L1 | Expandable | ASIA D | ASIA E | 28 | 0 |
| 26 | M | 36 | C | T7 | Titanium | ASIA A | ASIA A | 33 | 9 |
| 27 | F | 22 | B2 | T4 | Titanium | ASIA E | ASIA E | 30 | 18 |
| 28 | F | 73 | B2 | T7 | Titanium | ASIA E | ASIA E | 25 | 16 |
| 29 | F | 67 | A4 | T12 | Expandable | ASIA E | ASIA E | 37 | 3 |
| 30 | F | 79 | A4 | T2 | Titanium | ASIA E | ASIA E | 65 | 16 |
| 31 | M | 37 | B2 | T11 | Expandable | ASIA E | ASIA E | 23 | 0 |
| 32 | M | 56 | A4 | L1 | Expandable | ASIA D | ASIA E | 27 | 0 |
| 33 | M | 45 | C | T6 | Expandable | ASIA A | ASIA A | 35 | 9 |
| 34 | F | 75 | A4 | L1 | Titanium | ASIA C | ASIA D | 26 | 0 |
| 35 | F | 29 | B2 | T3 | Titanium | ASIA C | ASIA E | 34 | 6 |
| 36 | F | 83 | A4 | T12 | Titanium | ASIA D | ASIA E | 8 | 0 |
| 37 | F | 28 | B2 | L1 | Expandable | ASIA D | ASIA E | 26 | -1 |
| 38 | M | 65 | A4 | T12 | Titanium | ASIA D | ASIA E | 25 | 0 |
| 39 | M | 37 | A4 | T12 | Expandable | ASIA D | ASIA E | 25 | 0 |
| 40 | F | 34 | B2, | L1 | Expandable | ASIA E | ASIA E | 52 | 0 |
| 41 | F | 54 | B2 | L1 | Expandable | ASIA E | ASIA E | 22 | 0 |
| 42 | M | 43 | B2 | L1 | Titanium | ASIA E | ASIA E | 39 | 0 |
| 43 | F | 64 | B2 | T12 | Expandable | ASIA E | ASIA E | 22 | 3 |
| 44 | M | 23 | C | T4 | Titanium | ASIA A | ASIA A | 40 | 14 |
| 45 | M | 25 | B2 | T5-T6 | Titanium | ASIA C | ASIA E | 52 | 20 |
| 46 | F | 57 | B2 | T5 | Titanium | ASIA E | ASIA E | 36 | 8 |
| 47 | F | 48 | B1 | T5 | Titanium | ASIA E | ASIA E | 65 | 20 |
| 48 | F | 61 | A4 | T12 | Titanium | ASIA D | ASIA E | 36 | 3 |
| 49 | F | 42 | B2 | L1 | Expandable | ASIA D | ASIA E | 40 | 0 |
| 50 | M | 59 | A4 | L1 | Titanium | ASIA D | ASIA E | 12 | 0 |
| 51 | F | 44 | C | L1 | Titanium | ASIA C | ASIA D | 46 | 2 |
Abbreviations: ASIA, American Spinal Injury Association; AO class, AO Spine injury classification; LKA, local kyphosis angle; F, female; M, male; PVCR, posterior vertebral column resection; NA, not applicable.
The expandable cages were associated a 95% improvement in the LKA compared with the mesh cages improvement of 76%. The mean preoperative thoracic kyphosis (TK) angle between T2 and T12 was 56.6° (range 14° to 122°), which improved to 45.1° (range 26° to 64°) at final follow-up. The mean sagittal vertical axis (SVA) improved from 21.3 mm (range −87 to +191 mm) preoperatively to 3.1 mm (range −38 to +66 mm) at final follow-up. The mean T10-L2 thoracolumbar kyphosis improved from +22.5° (range −15° to +60°) prior to surgery to 4.8° (range −4° to +19°) at final follow-up. Mean lumbar lordosis was 48.7° (range 22° to 78°) before surgery and 49.9° (range 36° to 64°) at final follow-up, without significant change. Coronal balance did not change significantly with a preoperative value of 2.9 mm (range −29 to 36 mm) and 0.63 mm (range −23 to 36 mm) at final follow-up (Table 2).
Table 2.
The Mean Values of Radiographic Measurements of Coronal and Sagittal Parameters. Mean (Min. to Max.)
| Local kyphotic angle, deg | |
| Preoperative | 34.7 (8 to 83) |
| Follow-up | 4.9 (−2 to 27) |
| Thoracic kyphosis (T2-T12), deg | |
| Preoperative | 56.6 (14 to 122) |
| Follow-up | 45.1 (26 to 64) |
| Sagittal vertical axis, mm | |
| Preoperative | 21.3 (−87 to 191) |
| Follow-up | 3.1 (−38 to 66) |
| Thoracolumbar kyphosis (T10-L2), deg | |
| Preoperative | 22.5 (−15 to 60) |
| Follow-up | 4.8 (−4 to 19) |
| Lumbar lordosis (L1-L5), deg | |
| Preoperative | 48.7 (22 to 78) |
| Follow-up | 49.9 (36 to 64) |
| Coronal balance, mm | |
| Preoperative | 2.9 (−29 to 47) |
| Follow-up | 0.6 (−23 to 36) |
Preoperative CT scan and MRI findings showed that posterior ligamentous complex injury occurred in 28 patients (54.9%), laminar fractures in 13 patients (25%), endplate fractures in 45 patients (88.2%) and disc space injuries in 11 patients (21.5%). Six patients (11.5%) showed dural tear findings at preoperative MRI.
Six months after the surgery, a CT scan was performed to assess the fusion. Solid fusion was defined as the consolidation of the bone graft in the cage as confirmed by a musculoskeletal radiologist. There was no subsidence or pseudarthrosis in any of the cases. The CT scan also showed complete canal clearance of the bony fragments related to the burst fracture (Figures 4–6).
Figure 4.
(A) Preoperative computed tomography and magnetic resonance images of a 37-year-old male patient with T11 burst fracture. (B) Postoperative anteroposterior and lateral Standing radiographs after T11 posterior vertebral column resection using an expandable cage.
Figure 5.
(A, B) Preoperative computed tomography and magnetic resonance images of a 24-year-old female patient with T7 burst fracture. (C) Postoperative anteroposterior and lateral standing radiographs after T7 posterior vertebral column resection using a mesh cage.
Figure 6.
(A, B) Preoperative radiographs, computed tomography scans, and magnetic resonance images of a 27-year-old female patient with L2 burst fracture. (C) Postoperative anteroposterior and lateral standing radiographs after L2 posterior vertebral column resection using an expandable cage.
Neurological Recovery Results
Prior to surgery, 24 patients did not have any neurologic deficit (ASIA E) and this did not change following surgery. In the 27 patients with a neurologic deficit prior to surgery, 22 patients improved at least 1 ASIA grade (range 1-3 grades) and 17 improved to a normal neurologic status (ASIA E). Eight patients presented with complete neurologic injuries (ASIA A) and 3 of these patients improved to ASIA C following surgery. Three ASIA B patients recovered completely following surgery and 5 of the ASIA C patients improved to ASIA E (3 patients) or ASIA D (2 patients). Among the patients who showed neurological recovery, the greatest improvement was observed in 11 patients who were neurologically in ASIA D class preoperatively, all ended up as ASIA E. Five patients did not have any neurologic improvement following surgery and initially presented as ASIA A (Table 1).
The mean VAS score was 1.74 (range 0-7) for back pain, and 1.77 (range 0-9) for leg pain at final follow-up. The mean ODI was 15.5 (range 0-60) at final follow-up. Mean SF-36v2 PCS (physical component score)/MCS (mental component score) was 44.50/5170.The other subdomains of SF36-v2 are shown in Table 3.
Table 3.
The Mean Values of Clinical Outcome Measures of the Patients. Mean ± SD.
| Score at follow-up, mean ± SD | |
|---|---|
| SF-36v2 | |
| Physical function | 65.43 ± 29.54 |
| Role: physical | 61.79 ± 27.90 |
| Bodily pain | 67.14 ± 23.52 |
| General health | 61.60 ± 23.78 |
| Vitality | 61.25 ± 17.27 |
| Social functioning | 72.50 ± 26.91 |
| Role: emotional | 82.86 ± 22.04 |
| Mental health | 74.86 ± 11.97 |
| PCS | 44.50 ± 10.18 |
| MCS | 51.70 ± 6.64 |
| VAS | |
| Back pain | 1.74 ± 2.24 |
| Leg pain | 1.77 ± 2.80 |
| ODI | 15.50 ± 17.30 |
Abbreviations: SF-36v2, Short Form 36 version 2; PCS, physical component summary; MCS, mental component summary; VAS, visual analogue scale; ODI, Oswestry Disability Index.
We found 16 early complications due to surgery. Two iatrogenic dural tears occurred during surgery and these were primarily repaired without sequela along with the other 6 patients identified as having a dural tear prior to surgery. Three patients had transient neuropraxia of nerve roots in the operative field which did not require additional intervention and recovered completely 3 months after surgery. Six patients with hematoma formation or skin wound problems underwent debridement or skin revision before discharge. Cultures taken during revision surgery remained negative and there were no late infections. There were 5 patients who had hemopneumothorax due to laceration of the pleura and needed a chest tube in the same session. All 5 patients recovered without any pulmonary dysfunction at final follow-up.
Discussion
The goals for surgical treatment of unstable thoracic/thoracolumbar burst fractures are several: (1) to restore immediate stability to allow early mobilization and rehabilitation and avoid complications of prolonged immobilization and (2) decompress the spinal canal and potentially improve any neurologic deficit. The shortest instrumentation construct to achieve this and the optimal surgical technique and approach remain controversial. Burst fractures are quite varied in presentation and different injuries in the endplates or discs, presence of osteoporosis or neurologic deficit, fracture type and location, amount of spinal canal compromise, sagittal alignment, vertebral body height and nonspine factors such as age of the patient and medical comorbidities all likely affect the postinjury recovery.
According to biomechanical considerations when the anterior column is compromised from a burst fracture, the tensile loads are increased over posterior instrumentations. 27 Many studies have suggested a high failure rate of the posterior instrumentation when the anterior column is not supported with anterior stabilization and/or reconstruction.28-31 Carl et al 32 reported that 9 of 38 patients who underwent posterior fixation due to thoracolumbar burst fractures and dislocations developed either broken or bent screws. However, many authors report satisfactory outcomes with posterior-only constructs/fixation with or without spinal canal decompression; these authors suggest that these techniques allow adequate spinal alignment and neurologic recovery with low complications.33-35
Kallemeier et al 36 reported a biomechanical study that showed the superiority of circumferential fixation to posterior-only or anterior-only stabilization. We were able to reproduce Kallemeier et al’s biomechanical findings in our clinical study and did not find any screw loosening, breakage, or fracture, which we attribute to the anterior column support in this PVCR study.
It is a well-known fact that anterior column reconstruction provides better sagittal alignment and correction of kyphosis in thoracolumbar burst fractures. A retrospective study among 46 patients with thoracolumbar burst fractures was performed by Been and Bouma. 37 They reported that the loss of reduction and failure of instrumentation is significantly higher in posterior fixation group than anterior-posterior stabilization group. In our study, we observed local kyphosis improvement from preoperative to final follow-up in radiographs with anterior column supporting by using PVCR technique. This is similar to the findings of Hitchon et al 38 who analyzed patients that have undergone either a posterior or a lateral approach for thoracolumbar burst fractures and found that preoperative local kyphosis improved more in the anterior approach group than the posterior group. In our study, the local kyphosis improved from +34.7° to +4.9° with 85.9% correction rate with anterior reconstruction by using the PVCR technique.
Advantages of using anterior approach in cases of unstable thoracolumbar burst fractures are providing good exposure and visualization for direct decompression of the spinal canal, facilitating good reconstruction of the anterior and middle portions of the spinal column and reestablishing the normal sagittal contour of the fractured vertebra to achieve a solid fusion. Anterior approaches without posterior fixation are indicated in cases where the posterior longitudinal ligament and posterior column is intact; however, the complications are different for the 2 approaches.12,39
When we compare the posterior and anterior approaches; posterior approach is more familiar to most spine surgeons, decreases the risk of damaging the vital visceral-vascular structures that may exist in anterior approach, and allows safe surgical reexplorations in case of necessity. In order to maintain anterior support for rigid stabilization and improvement of kyphosis while avoiding anterior approach–related complications, vertebral column resection via posterior approach in thoracolumbar fractures has more advantages.
In their systemic review study, Oprel et al 40 compared combined anterior-posterior group with single posterior approach group and reported that significantly higher kyphotic correction and improvement of vertebral height were provided with combined anterior-posterior approach. However, they found more blood loss, longer operation times, longer hospital stays, higher costs, and a potentially higher intraoperative and postoperative complication rate requiring reoperation in this combined approach. Altogether, the presented combined approach might be a good therapeutic choice in case of unstable thoracic/thoracolumbar burst fractures; the main concerns are the higher complication rate and the destruction of the anterior longitudinal ligament with anterior cortex, which affects the stability of the spine. Although complete canal decompression is certainly best obtained by the direct anterior approach due to direct access to the fragments of the fracture and posterior wall, sacrifice of the anterior longitudinal ligament and anterior cortex is required.3,6,12,41 Furthermore, equal neurological improvement between anterior and posterior corpectomy are reported. 12 In a meta-analysis, Xu et al 42 concluded that the anterior approach is not significantly superior to the posterior approach in terms of recovery of neurological function and return to work. They also found that an anterior approach has disadvantages like operative time, blood loss, and cost when compared with the posterior approach. 42
Treatment of the unstable thoracic/thoracolumbar burst fractures with corpectomy and posterior fixation by using only the posterior approach was mentioned by Ayberk et al, 13 Sasani et al, 14 and Haiyun et al. 15 Our study compares favorably with those studies: We had a larger sample size (51 patients) compared with Haiyun et al (37 patients), Sasani et al (14 patients), and Ayberk et al (8 patients). In addition, our study had an average blood loss of 520 mL compared with 1086 mL by Haiyun et al 15 and Ayberk et al 13 who reported a blood loss of 6 units. That could be due to hemostatic measures that we used in our surgical technique such as intravenous tranexamic acid (TXA), optimization of mean blood pressure, meticulous cauterization of bleeding vessels, and high-speed burring performed to control bleeding from bone. We also had longer follow-up with an average 69 months. In their studies, Haiyun et al 15 and Sasani et al 14 reported mean follow-up periods of 24 months.
We achieved a fusion rate of 100% similar to Haiyun et al in spite of cage subsidence in one patient in their series. Sasani et al had 1 patient with pseudoarthrosis. When we compare restoration of LKA in our study, which improved from +34.7° to +4.9°, with the results of Haiyun et al, 15 which decreased from +25.5° to +4.4°, the final LKAs achieved were almost equal.
Although the initial deformity was greater in our case series with a larger initial average LKA, we were able to maintain the correction better compared to these other authors. The LKA correction was not maintained as well in the studies by Haiyun et al 15 ; Sasani et al 14 reported a final LKA of 17.1° corrected from 24.6°. These differences may be related to our surgical techniques and vigorous preparation of the endplates. We also aggressively treated osteoporosis (T-score <2.5) when appropriate with a specialized team and by using double-threaded and cement-augmented pedicle screws.
While our mean ASIA grade improvement of 1.55 was similar to Haiyun et al 15 (1.54) and higher than Sasani et al 14 (1.16), we had a greater percentage of patients with neurologic improvement compared with Haiyun et al and a similar percentage compared to Sasani et al. We had 22 of 27 patients improve (81.5%) compared with 65.3% (17 of 26 patients) by Haiyun et al and 85.7% (6 of 7 patients) by Sasani et al. Our superior neurologic recovery could possibly be related to the longer follow-up period, which may allow more complete recovery with longer rehabilitation.
The other superiority of our series is the neurological improvement of 3 patients who were in ASIA class A and improved to ASIA class C at final follow-up. This improvement can be related to the relatively younger ages (22, 27, and 30 years) and longer period of the rehabilitation available to our patients.
Haiyun et al 15 reported a surgical-site infection in one patient, whereas we did not encounter infection in any of our patients. This may be attributed to meticulous wound closure with routine local application of vancomycin powder inside the wound before closure. Postoperative average VAS score was 1.74 for back pain and 1.77 for leg pain in our study and these scores compare favorably with Sasani et al 14 who reported a mean VAS score of 2.66. Haiyun et al did not report a postoperative mean VAS score in their study. We also measured health-related quality-of-life measures with the ODI and SF-36v2 in our studies which were not done in the other studies.
Limitations of our study include the retrospective nature and the lack of a comparative or a control group (eg, comparison with an anterior/posterior approach or an only anterior approach or a posterior approach without the PVCR). The various morphologic types of the burst fractures in our study may also be a limitation, although this reflects the heterogenous nature of burst fractures. Finally, the cage types used in our study were not consistent, although the expandable cage predominated compared with the mesh-type cage.
The strengths of our study include the single surgeon, single institution treatment and the long follow-up period in this standardized PVCR approach to burst fractures; we find that the PVCR technique achieved a stable and ideal correction of the kyphotic deformity in thoracic and thoracolumbar burst fractures, with no loss of correction during healing and with solid fusion in the final follow-up with a low complication rate.
Conclusion
Single-stage PVCR is technically demanding and has a long learning curve but allows 360° spinal canal decompression and optimizes kyphosis correction and sagittal alignment with anterior support when treating thoracic and thoracolumbar burst fractures. The morbidity and potential complications of an additional anterior approach are avoided, and earlier mobilization and rehabilitation are possible, which improves the outcome in spine trauma patients with thoracic and thoracolumbar burst fractures.
However, while patients who underwent PVCR performed well in our single-surgeon, single-center study, the decision to surgically treat A3-, A4-, B-, and C-type burst fractures should be made on a case-by-case basis and should reflect the surgeon’s experience and institution support. We suggest the need for prospective comparative studies to identify the optimal surgery for the treatment of thoracic/thoracolumbar burst fractures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mustafa Elsadig, MD  https://orcid.org/0000-0003-1248-0251
https://orcid.org/0000-0003-1248-0251
Sinan Kahraman, MD  https://orcid.org/0000-0003-3408-7389
https://orcid.org/0000-0003-3408-7389
References
- 1.Korovessis P, Baikousis A, Zacharatos S, Petsinis G, Koureas G, Iliopoulos P. Combined anterior plus posterior stabilization versus posterior short-segment instrumentation and fusion for mid-lumbar (L2-L4) burst fractures. Spine (Phila Pa 1976). 2006;31:859–868. [DOI] [PubMed] [Google Scholar]
- 2.Alanay A, Acaroglu E, Yazici M, et al. Short-segment pedicle instrumentation of thoracolumbar burst fractures: does transpedicular intracorporeal grafting prevent early failure. Spine (Phila Pa 1976). 2001;26:213–217. [DOI] [PubMed] [Google Scholar]
- 3.Alvine GF, Swain JM, Asher MA, Burton DC. Treatment of thoracolumbar burst fractures with variable screw placement or Isola instrumentation and arthrodesis: case series and literature review. J Spinal Disord Tech. 2004;17:251–264. [DOI] [PubMed] [Google Scholar]
- 4.Vaccaro AR, Oner C, Kepler CK, et al. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976). 2013;38:2028–2037. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Wang WK, Li KC, Chen TH. Biomechanical effects of the body augmenter for reconstruction of the vertebral body. Spine (Phila Pa 1976). 2004;29:E382–E387. [DOI] [PubMed] [Google Scholar]
- 6.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79:69–83. [DOI] [PubMed] [Google Scholar]
- 7.Sasso RC, Best NM, Reilly TM, McGuire RA, Jr. Anterior-only stabilization of three column thoracolumbar injuries. J Spinal Disord Tech. 2005;18(suppl):S7–S14. [DOI] [PubMed] [Google Scholar]
- 8.Li KC, Hsieh CH, Lee CY, Chen TH. Transpedicle body augmenter: a further step in treating burst fractures. Clin Orthop Relat Res. 2005;436:119–125. [PubMed] [Google Scholar]
- 9.Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V.Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85:773–781. [DOI] [PubMed] [Google Scholar]
- 10.Siebenga J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine (Phila Pa 1976). 2006;31:2881–2890. [DOI] [PubMed] [Google Scholar]
- 11.Verlaan JJ, Diekerhof CH, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine (Phila Pa 1976). 2004;29:803–814. [DOI] [PubMed] [Google Scholar]
- 12.Lin B, Chen ZW, Guo ZM, Liu H, Yi ZK. Anterior approach versus posterior approach with subtotal corpectomy, decompression, and reconstruction of spine in the treatment of thoracolumbar burst fractures: a prospective randomized controlled study. J Spinal Disord Tech. Published online June 1, 2011. doi:10.1097/BSD.0b013e3182204c53 [DOI] [PubMed] [Google Scholar]
- 13.Ayberk G, Ozveren MF, Altundal N, et al. Three column stabilization through posterior approach alone: transpedicular placement of distractable cage with transpedicular screw fixation. Neurol Med Chir (Tokyo). 2008;48:8–14. [DOI] [PubMed] [Google Scholar]
- 14.Sasani M, Ozer AF. Single-stage posterior corpectomy and expandable cage placement for treatment of thoracic or lumbar burst fractures. Spine (Phila Pa 1976). 2009;34:E33–E40. [DOI] [PubMed] [Google Scholar]
- 15.Haiyun Y, Rui G, Shucai D, et al. Three-column reconstruction through single posterior approach for the treatment of unstable thoracolumbar fracture. Spine (Phila Pa 1976). 2010;35:E295–E302. [DOI] [PubMed] [Google Scholar]
- 16.American Spinal Injury Association. Standards for Neurological Classification of Spinal Injury Patients. American Spinal Injury Association; 1982. [Google Scholar]
- 17.Dai LY, Wang XY, Jiang LS. Evaluation of traumatic spinal canal stenosis in thoracolumbar burst fractures. Eur J Radiol. 2008;67:526–530. [DOI] [PubMed] [Google Scholar]
- 18.Brant-Zawadzki M, Jeffrey RB, Jr, Minagi H, Pitts LH. High resolution CT of thoracolumbar fractures. AJR Am J Roentgenol. 1982;138:699–704. doi:10.2214/ajr.138.4.699 [DOI] [PubMed] [Google Scholar]
- 19.Petersilge CA, Pathria MN, Emery SE, Masaryk TJ. Thoracolumbar burst fractures: evaluation with MR imaging. Radiology. 1995;194:49–54. [DOI] [PubMed] [Google Scholar]
- 20.Haba H, Taneichi H, Kotani Y, et al. Diagnostic accuracy of magnetic resonance imaging for detecting posterior ligamentous complex injury associated with thoracic and lumbar fractures. J Neurosurg. 2003;99(1 suppl):20–26. [DOI] [PubMed] [Google Scholar]
- 21.Gamanagatti S, Rathinam D, Rangarajan K, Kumar A, Farooque K, Sharma V. Imaging evaluation of traumatic thoracolumbar spine injuries: radiological review. World J Radiol. 2015;7:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farcy JP, Weidenbaunn M, Glassman SD. Sagittal index in management of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1990;15:958–965. [DOI] [PubMed] [Google Scholar]
- 23.Funke F. Vergleich Visueller Analogskalen mit Kategorialskalen in Offline- und Online-Design [master’s thesis]. Justus-Liebig-Universität Gießen; 2004. [Google Scholar]
- 24.Flynn D, van Schaik P, van Wersch A. A comparison of multi-item Likert and visual analogue scales for the assessment of transactionally defined coping. Eur J Psychol Assess. 2004;20:49–58. doi:10.1027/1015-5759.20.1.49 [Google Scholar]
- 25.Fairbank J, Pynsent P. The Oswestry Disablility Index. Spine (Phila Pa 1976). 2000;25:2940–2953. [DOI] [PubMed] [Google Scholar]
- 26.Ghobrial GM, Eichberg DG, Kolcun JPG, et al. Prophylactic vertebral cement augmentation at the uppermost instrumented vertebra and rostral adjacent vertebra for the prevention of proximal junctional kyphosis and failure following long segment fusion for adult spinal deformity. Spine J. 2017;17:1499–1505. [DOI] [PubMed] [Google Scholar]
- 27.Branson WH, Vaccaro AR. Is there a role for anterior augmentation in thoracolumbar burst fractures? Indian Spinal J. 2018;1:86–93. [Google Scholar]
- 28.McKinley LM, Obenchain TG, Roth KR. Loss of correction: late kyphosis in short segment pedicle fixation in cases of posterior transpedicular decompression. In: Proceedings of the Sixth International Congress on Cotrel-Dubousset Instrumentation. Sauramps Medical; 1989:37–39. [Google Scholar]
- 29.Sasso RC, Cotler HB, Reuben JD. Posterior fixation of thoracic and lumbar spine fractures using DC plates and pedicle screws. Spine (Phila Pa 1976). 1991;16(3 suppl):S134–S139. [DOI] [PubMed] [Google Scholar]
- 30.Stephens GC, Devito DP, McNamara MJ. Segmental fixation of lumbar burst fractures with Cotrel-Dubousset instrumentation. J Spinal Disord. 1992;5:344–348. [DOI] [PubMed] [Google Scholar]
- 31.Ebelke DK, Asher MA, Neff JR, Kraker DP. Survivorship analysis of VSP spine instrumentation in the treatment of thoracolumbar and lumbar burst fractures. Spine (Phila Pa 1976). 1991;16(8 suppl):S428–S432. [PubMed] [Google Scholar]
- 32.Carl AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for thoracolumbar burst fractures and fracture dislocations. Spine (Phila Pa 1976). 1992;17(8 suppl):S317–S324. [DOI] [PubMed] [Google Scholar]
- 33.Sjostrom L, Karlstrom G, Pech P, Rauschning W. Indirect spinal canal decompression in burst fractures treated with pedicle screw instrumentation. Spine (Phila Pa 1976). 1996;21:113–123. [DOI] [PubMed] [Google Scholar]
- 34.Cresswell TR, Marshall PD, Smith RB. Mechanical stability of the AO internal spinal fixation system compared with that of the Hartshill rectangle and sublaminar wiring in the management of unstable burst fractures of the thoracic and lumbar spine. Spine (Phila Pa 1976). 1998;23:111–115. [DOI] [PubMed] [Google Scholar]
- 35.Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine (Phila Pa 1976). 2000;25:1157–1170. [DOI] [PubMed] [Google Scholar]
- 36.Kallemeier PM, Beaubien BP, Buttermann GR, Polga DJ, Wood KB. In vitro analysis of anterior and posterior fixation in an experimental unstable burst fracture model. J Spinal Disord Tech. 2008;21:216–224. [DOI] [PubMed] [Google Scholar]
- 37.Been HD, Bouma GJ. Comparison of two types of surgery for thoracolumbar burst fractures: Combined anterior and posterior stabilisation vs posterior instrumentation only. Acta Neurochir (Wien). 1999;141:349–357. [DOI] [PubMed] [Google Scholar]
- 38.Hitchon PW, Torner JC, Haddad SF, Follett KA. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49:619–627. [DOI] [PubMed] [Google Scholar]
- 39.Zheng GQ, Wang Y, Tang PF, et al. Early posterior spinal canal decompression and circumferential reconstruction of rotationally unstable thoracolumbar burst fractures with neurological deficit. Chin Med J (Engl). 2013;126:2343–2347. [PubMed] [Google Scholar]
- 40.Oprel PP, Tuinebreijer WE, Patka P, den Hartog D. Combined anterior-posterior surgery versus posterior surgery for thoracolumbar burst fractures: a systematic review of the literature. Open Orthop J. 2010;4:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang ST, Ma HL, Liu CL, Yu WK, Chang MC, Chen TH. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine? A prospective, randomized study. Spine (Phila Pa 1976). 2006;31:2646–2652. [DOI] [PubMed] [Google Scholar]
- 42.Xu GJ, Li ZJ, Ma JX, Zhang T, Fu X, Ma XL. Anterior versus posterior approach for treatment of thoracolumbar burst fractures: a meta-analysis. Euro Spine J. 2013;22:2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]