Abstract
Study Design:
Systematic review and meta-analysis.
Objectives:
Arthrodesis has been a valid treatment option for spinal diseases, including spondylolisthesis and lumbar spinal stenosis. Posterolateral and posterior lumbar interbody fusion are amongst the most used fusion techniques. Previous reports comparing both methods have been contradictory. Thus, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to establish substantial evidence on which fusion method would achieve better outcomes.
Methods:
Major databases including PubMed, Embase, Web of Science and CENTRAL were searched to identify studies comparing outcomes of interest between posterolateral fusion (PLF) and posterior lumbar interbody fusion (PLIF). We extracted data on clinical outcome, complication rate, revision rate, fusion rate, operation time, and blood loss. We calculated the mean differences (MDs) for continuous data with 95% confidence intervals (CIs) for each outcome and the odds ratio with 95% confidence intervals (CIs) for binary outcomes. P < 0.05 was considered significant.
Results:
We retrieved 8 studies meeting our inclusion criteria, with a total of 616 patients (308 PLF, 308 PLIF). The results of our analysis revealed that patients who underwent PLIF had significantly higher fusion rates. No statistically significant difference was identified in terms of clinical outcomes, complication rates, revision rates, operation time or blood loss.
Conclusions:
This systematic review and meta-analysis provide a comparison between PLF and PLIF based on RCTs. Although PLIF had higher fusion rates, both fusion methods achieve similar clinical outcomes with equal complication rate, revision rate, operation time and blood loss at 1-year minimum follow-up.
Keywords: lumbar, fusion, lumbar interbody fusion, stenosis, spondylolisthesis
Introduction
Spinal fusion surgery or spinal arthrodesis has been widely used to treat different spinal conditions, including isthmic spondylolisthesis (IS), degenerative spondylolisthesis (DS), lumbar spinal stenosis (LSS) and other diseases involving surgical decompression or discectomy.1,2 Spinal fusion has proven to be an effective, safe, and reliable treatment option. Compared to conservative management, surgical intervention achieved more significant pain relief and better function.3,4
Several fusion modalities have been proposed, including posterolateral fusion (PLF), anterior lumbar interbody fusion (ALIF), transforaminal interbody fusion (TLIF), and posterior lumbar interbody fusion (PLIF). The 2 most commonly used choices are PLF and PLIF. Since their first introduction by Watkins in the 1950s and Cloward in the 1960s, both techniques have been modified.
Although several studies compared PLF and PLIF, it is still not possible to draw a solid conclusion on which procedure is better. Some previous reports showed that PLIF was superior to PLF in terms of clinical outcome and fusion rate,5,6 while others suggested no significant difference between the 2 procedures.7,8 Moreover, even existing meta-analyses were unable to reach definite conclusions.9-14
Through a comprehensive literature review, we found that studies included in previous meta-analyses had inadequate or limited randomization. More concrete evidence (i.e., analysis using randomized controlled studies only) is required to reach more accurate results. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials comparing PLF and PLIF to investigate differences between fusion techniques in clinical outcomes, complication rate, reoperation rate, fusion rate, operation time and blood loss.
Methods
Search Protocol and Information Sources
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. 15 PubMed, Embase, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched from inception until January 2021 using the following search terms: posterolateral fusion OR PLF, posterior lumbar interbody fusion OR PLIF AND spondylolisthesis OR spondylolistheses OR lumbar stenosis OR spondylolysis OR spondylolyses.
Eligibility Criteria, Study Selection, and Data Items
Retrieved results were imported into Endnote X9 software (Thomson Reuters, New York, NY, USA), where a check for duplicates was conducted. The titles and abstracts of the remaining articles were then screened with the following exclusion criteria:
Articles published in languages other than English.
Reviews, guidelines, or classifications.
Letters to the editor or case reports, small case series or conference papers
In vitro and animal experiment studies
Irrelevant studies.
Subsequently, full-text articles of potentially relevant studies were obtained and assessed for eligibility. We included studies that met the following inclusion criteria:
Randomized controlled studies comparing PLF and PLIF in patients with spondylolisthesis or lumbar spinal stenosis, or studies from which data could be extracted independently.
A Minimum follow-up period of 12 months.
The ability to extract data related to the outcomes used for comparison.
The following information was extracted from studies that met the inclusion criteria: the name of the first author, year of publication, country, diagnosis, number of participants in each group, participants’ age and gender, length of follow-up time and outcomes of interest including fusion rate, Oswestry Disability Index (ODI), Visual Analogue Scale (VAS) for back pain, VAS for leg pain, clinical satisfaction, complication rate, revision rate, operation time and blood loss.
Data Collection Process, and Risk of Bias in Individual Studies
Two independent reviewers reviewed the list of potential references and the extraction of data, and a third reviewer was consulted, when necessary, to decide any uncertainties regarding eligibility.
To assess the risk of bias among included RCTs, we used the Cochrane Collaboration’s quality assessment tool. 16 Two reviewers independently carried out the assessment, and dissimilarities were settled by discussing with the senior author.
Summary Measures, Synthesis of Results, and Risk of Bias Across Studies
We performed all data analyses using Review Manager version 5.4.1. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We calculated the odds ratio (OR) with a 95% confidence interval (CI) for binary outcomes, while the mean difference (MD) with a 95% CI for continuous outcomes was calculated. To calculate the overall effect estimate with a 95% CI, we used a fixed-effect model with the method of Mantel-Haenszel when there is no evidence of heterogeneity between studies. Otherwise, a random-effect model with the method of DerSiomonian and Laird was chosen. Heterogeneity between studies was evaluated using the Q statistic and I2 test, which describes the percentage of variability in the effect estimates. A P value of < 0.05 was considered significant. Publication bias was assessed using funnel plots. Sensitivity analysis was also carried out to assess if the results were affected by a single study.
Results
Study Selection
The electronic search yielded 1292 references from the 4 databases. After excluding 558 duplicates, 734 records remained for title/abstract screening. We had 18 relevant articles for full-text screening, 7 fulfilled the inclusion criteria, and 11 were excluded for not comparing between PLF and PLIF or lack of randomization or full-text unavailability. The manual search of references imported 1 additional article. Eight studies were ultimately included in the qualitative and quantitative analyses. Figure 1 shows the flow diagram of the study selection process.
Figure 1.
Flow diagram of the study selection process.
Study Characteristics
Details for included studies are summarized in Table 1. Eight studies1,5,17-22 were included in the analysis, with a total of 616 patients: 308 patients underwent PLF, and 308 patients received PLIF. All included studies were randomized controlled trials. Across studies, the mean age ranged from 44.1 to 58.6 years in the PLF group and from 41.4 to 58.35 years in the PLIF group. The follow-up period ranged from 12 to 48 months. Six studies1,5,17,19,20,22 included patients with spondylolisthesis, either isthmic or degenerative or both. One study 18 included patients with lumbar stenosis and degenerative instability, and 1 study 21 included patients with either lumbar stenosis or spondylolisthesis.
Table 1.
Baseline Characteristics of Included Studies.
| Study | Country | Diagnosis | Follow up (months) | Sample size | Age (mean, SD/range) | Sex (PLF) | Sex (PLIF) | Reported outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | PLF | PLIF | PLF (yrs) | PLIF (yrs) | M | F | M | F | |||||
| Inamdar et al 2006 | India | DS/IS | 12 | 20 | 10 | 10 | 44.7 | 41.4 | 15 | 47 | 17 | 40 | ①④⑤⑦⑧⑨ |
| Kim 2006 | Korea | LSS/DS/IS | 36 | 119 | 62 | 57 | 58.6 (42-47) | 55.2 (38-79) | NA | NA | NA | NA | ①②③④⑤⑥⑦⑧⑨ |
| Cheng et al 2009 | China | DS/IS | 48 | 138 | 68 | 70 | 48 (38-63) | 49 (36-62) | 36 | 32 | 39 | 31 | ①④⑤⑥⑦⑧ |
| Musluman et al 2011 | Turkey | IS | 39.6 | 50 | 25 | 25 | 47.3 | 50.6 | 9 | 16 | 8 | 17 | ①②③④⑤⑥⑦⑧⑨ |
| Farrokhi et al 2012 | Iran | IS | 12 | 80 | 40 | 40 | 49.66 ± 9.01 | 50.35 ± 11.3 | 10 | 30 | 9 | 31 | ①②③⑤⑦⑨ |
| Lee 2014 | Korea | IS | 24 | 81 | 39 | 42 | 53.4 ± 2.3 | 53.7 ± 2.1 | 21 | 18 | 23 | 19 | ①②③⑤⑥⑦⑧⑨ |
| Farrokhi et al 2018 | Iran | LSS with DS | 24 | 88 | 44 | 44 | 57.76 ± 8.82 | 58.35 ± 9.03 | 10 | 34 | 12 | 32 | ①②③⑤⑥⑦⑧⑨ |
| Gad 2018 | Egypt | IS | 24 | 40 | 20 | 20 | 44.1 ± 7.34 | 44.15 ± 6.9 | 5 | 15 | 6 | 14 | ⑤⑦⑧⑨ |
①Oswestry Disability Index, ②VAS for back pain, ③VAS for leg pain, ④Clinical satisfaction, ⑤Complication rate, ⑥Revision rate, ⑦Fusion rate, ⑧Operative time, ⑨Blood loss.
Abbreviations: DS, degenerative spondylolisthesis; IS, isthmic spondylolisthesis; TS, traumatic spondylolisthesis; LSS, lumbar spine stenosis; PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; SD, standard deviation.
Risk of Bias Within Studies
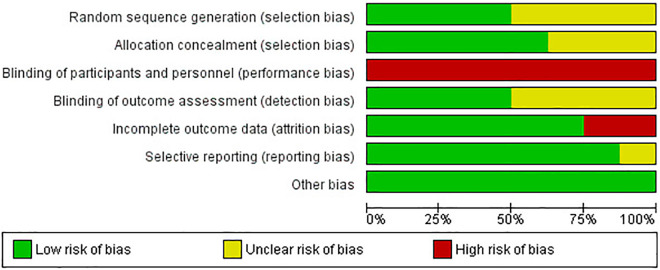
Figures 2 and 3 show risk of bias across included studies in terms of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. As the obvious differences of operative procedure between PLIF and PLF, high risk of blinding of participants and personnel was observed. Other risk of bias parameters showed either low or unclear risk except for studies by Cheng et al 1 and kim et al 21 which showed high risk of attrition bias.
Figure 2.

Risk of bias summary.
Figure 3.
Risk of bias graph.
Synthesis of Results
Clinical Outcomes
ODI
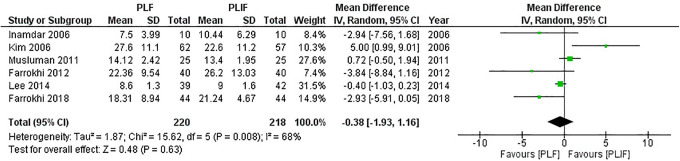
In all, 7 studies1,5,17,18,20-22 reported differences in the ODI, but only 6 studies5,17,18,20-22 were suitable for analysis due to incomplete data, with 220 patients in the PLF groups and 218 patients in the PLIF groups. We used the random-effect model for analysis because significant heterogeneity was detected (I2 = 68%, P = 0.008). The combined MD and 95% CIs was–0.38 (–1.93 to 1.16). This demonstrates no statistical difference in the ODI with either PLF or PLIF (Z = 0.48, P = 0.63). The result of meta-analysis is shown in Figure 4. After removing the study conducted by Kim et al, 21 heterogeneity was significantly reduced (I2 = 55%, P = 0.06). The result remained insignificant (pooled MD,–0.74 [95% CI,–2.02 to 0.55]; Z = 1.12, P = 0.26).
Figure 4.
Forest plot of ODI demonstrates no statistically significant difference between PLF and PLIF.
VAS for back pain
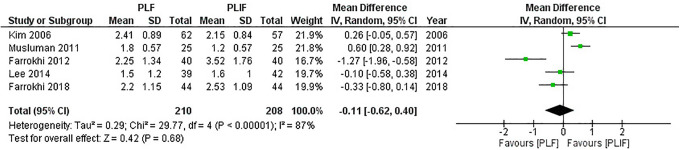
In all, 5 studies5,17,18,21,22 reported differences in the VAS score for back pain with 210 patients in the PLF groups and 208 patients in the PLIF groups. We used the random-effect model for analysis because significant heterogeneity was detected (I2 = 87%, P < 0.01). The combined MD and 95% CIs was–0.11 (–0.62 to 0.40). This demonstrates no statistical difference in the back pain scale with either PLF or PLIF (Z = 0.42, P = 0.68). The result of meta-analysis is shown in Figure 5. After removing studies conducted by Musluman et al 5 and Farrokhi et al, 17 heterogeneity was significantly reduced (I2 = 57%, P = 0.10). The result remained insignificant (pooled MD,–0.02 [95% CI,–0.39 to 0.35]; Z = 0.11, P = 0.91).
Figure 5.
Forest plot of VAS for back pain demonstrates no statistically significant difference between PLF and PLIF.
VAS for leg pain
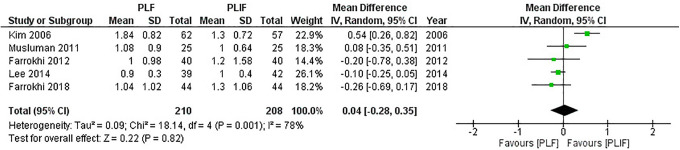
In all, 5 studies5,17,18,21,22 reported differences in the VAS score for leg pain with 210 patients in the PLF groups and 208 patients in the PLIF groups. We used the random-effect model for analysis because significant heterogeneity was detected (I2 = 78%, P = 0.001). The combined MD and 95% CIs was 0.04 (–0.28 to 0.35). This demonstrates no statistical difference in the leg pain scale with either PLF or PLIF (Z = 0.22, P = 0.82). The result of meta-analysis is shown in Figure 6. After removing the study conducted by Kim et al, 21 heterogeneity was significantly reduced (I2 = 0%, P = 0.73). The result remained insignificant (pooled MD,–0.1 [95% CI,–0.24 to 0.03]; Z = 1.52, P = 0.13).
Figure 6.
Forest plot of VAS for leg pain demonstrates no statistically significant difference between PLF and PLIF.
Clinical satisfaction
In all, 4 studies1,5,20,21 reported differences in the patient clinical satisfaction with 162 patients in the PLF group and 159 patients in the PLIF group, but the assessment methods used were different. Cheng et al 1 used the global outcome, Kirkaldy-Willis Criteria were used by Kim et al, 21 and the 2 remaining studies5,20 evaluated the improvement in scores of the Oswestry Disability Index (ODI). In the current meta-analysis, clinical satisfaction was defined as global outcome assessed by patients as “much better” or “better,” Kirkaldy-Willis Criteria graded as “excellent” or “good” and ODI classified as “excellent” or “better.” No significant heterogeneity was detected (I2 = 0%, P = 0.97), using the fixed-effect model for analysis. The combined OR and 95% CIs was 0.53 (0.27 to 1.04). This demonstrates no statistical difference in clinical satisfaction with either PLF or PLIF (Z = 1.86, P = 0.06). The result of meta-analysis is shown in Figure 7.
Figure 7.
Forest plot of clinical satisfaction demonstrates no statistically significant difference between PLF and PLIF.
Complication Rate
All 8 studies1,5,17-22 reported postoperative complications. The complication rate was assessed in 308 patients in each group. We used the random-effect model for analysis as significant heterogeneity was detected (I2 = 52%, P = 0.04). The combined OR and 95% CIs was 0.09 (0.0 to 1.84). This demonstrates no statistical difference in complication rate with either PLF or PLIF (Z = 0.38, P = 0.70). The result of meta-analysis is shown in Figure 8. After removing the study conducted by Inamdar et al, 20 heterogeneity was significantly reduced (I2 = 23%, P = 0.25). The result remained insignificant (pooled OR, 1.07 [95% CI, 0.54 to 2.15]; Z = 0.20, P = 0.84).
Figure 8.
Forest plot of complication rate demonstrates no statistically significant difference between PLF and PLIF.
Revision Rate
In all, 5 studies1,5,18,21,22 reported differences in the revision rate with 238 patients in each group. No significant heterogeneity was detected (I2 = 0%, P = 0.53), using the fixed-effect model for analysis. The combined odds ratio and 95% CIs was 0.88 (0.44 to 1.77). This demonstrates no statistical difference in reoperation rate with either PLF or PLIF (Z = 0.36, P = 0.72). The result of meta-analysis is shown in Figure 9.
Figure 9.
Forest plot of revision rate demonstrates no statistically significant difference between PLF and PLIF.
Fusion Rate
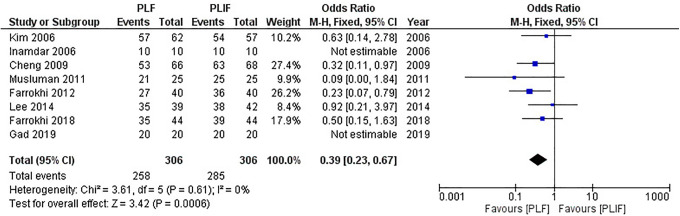
All 8 studies1,5,17-22 reported differences in the fusion rate with 306 patients in the PLF group and 306 patients in the PLIF group. No significant heterogeneity was detected (I2 = 0%, P = 0.61), using the fixed-effect model for analysis. The combined OR and 95% CIs was 0.09 (0.0 to 1.84). The combined result suggested that the PLIF group had a significantly higher fusion rate than did the PLF group (Z = 3.42, P = 0.0006). The result of meta-analysis is shown in Figure 10.
Figure 10.
Forest plot of fusion rate demonstrates a statistically significant difference in favor of PLIF.
Operation Time
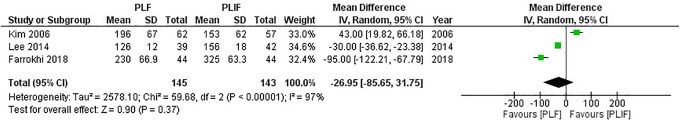
In all, 7 studies1,5,18-22 reported differences in the operation time between PLF and PLIF (Table 2), but only 3 studies18,21,22 were suitable for analysis due to incomplete data, with 145 patients in the PLF group and 143 patients in the PLIF group. We used random-effect model for analysis as significant heterogeneity was detected (I2 = 97%, P < 0.01). The combined MD and 95% CIs was–26.95 (–85.65 to 31.75). This demonstrates no statistical difference in operation time with either PLF or PLIF (Z = 0.9, P = 0.37). The result of meta-analysis is shown in Figure 11. Despite the significant heterogeneity, subgroup analysis was not performed due to the limited number of studies.
Table 2.
The Operation Time and Blood Loss of Included Studies.
| Study | Operation time (min) | Blood loss (ml) | ||
|---|---|---|---|---|
| PLF | PLIF | PLF | PLIF | |
| Inamdar et al 2006 | 180 | 240 | 500 | 500 |
| Kim et al 2006 | 196 ± 67 | 153 ± 62 | 1082 ± 320 | 738 ± 205 |
| Cheng et al 2009 | 192 | 210 | NA | NA |
| Musluman et al 2011 | 146 (105-300) | 168 (120-310) | 1100 ± 280 | 830 ± 215 |
| Farrokhi et al 2012 | NA | NA | 748 ± 439 | 873 ± 370 |
| Lee 2014 | 126 ± 12 | 156 ± 18 | 350 ± 25 | 360 ± 30 |
| Farrokhi et al 2018 | 230 ± 66.9 | 325 ± 63.3 | 768 ± 450 | 883 ± 390 |
| Gad 2018 | 95 | 105 | 1000 | 1100 |
Abbreviations: PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; NA, not available.
Figure 11.
Forest plot of operation time demonstrates no statistically significant difference between PLF and PLIF.
Blood Loss
In all, 7 studies5,17-22 reported differences in the blood loss between PLF and PLIF (Table 2), but only 5 studies5,17,18,21,22 were suitable for analysis due to incomplete data, with 210 patients in PLF group and 208 patients in PLIF group. We used random-effect model for analysis as significant heterogeneity was detected (I2 = 94%, P < 0.01). The combined MD and 95% CIs was 79.21 (–103.58 to 262.01). This demonstrates no statistical difference in blood loss with either PLF or PLIF (Z = 0.85, P = 0.40). The result of meta-analysis is shown in Figure 12. After removing studies by Kim et al 21 and Musluman et al, 5 heterogeneity was significantly reduced (I2 = 32%, P = 0.23). The result remained insignificant (pooled MD,–40.97 [95% CI,–114.84 to 32.91]; Z = 1.09, P = 0.28).
Figure 12.
Forest plot of blood loss demonstrates no statistically significant difference between PLF and PLIF.
Subgroup Analysis
Table 3 shows a subgroup analysis based on the diagnosis. Spondylolisthesis, either isthmic or degenerative, was responsible for the significantly higher fusion rate in the PLIF group.
Table 3.
Subgroup Analysis Based on Diagnosis.
| Diagnosis | Parameter | No. of studies | No. of patients | Pooled effect estimates | Heterogeneity | Analysis model | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| PLF | PLIF | I2 (%) | P value | ||||||
| IS | ODI | 3 | 104 | 107 | –0.15 (–1.37, 1.08) * | 57 | 0.1 | Fixed | 0.81 |
| VAS back pain | 3 | 104 | 107 | –0.22 (–1.19, 0.76) * | 92 | < 0.01 | Random | 0.66 | |
| VAS leg pain | 3 | 104 | 107 | –0.09 (–0.23, 0.05) * | 0 | 0.69 | Fixed | 0.22 | |
| Clinical satisfaction | 2 | 35 | 35 | 0.40 (0.10, 1.60) ** | 0 | 0.85 | Fixed | 0.2 | |
| Complication rate | 4 | 124 | 127 | 1.01 (0.41, 2.52) ** | 0 | 0.56 | Fixed | 0.98 | |
| Revision rate | 4 | 64 | 67 | 1.03 (0.17, 6.21) ** | 0 | 0.39 | Fixed | 0.97 | |
| Fusion rate | 4 | 124 | 127 | 0.33 (0.14, 0.77) ** | 31 | 0.23 | Fixed | 0.01 | |
| Blood loss | 3 | 104 | 107 | 46.88 (–142.24 236.00) * | 88 | < 0.01 | Random | 0.63 | |
| Operation time | 1 | 39 | 42 | –30.00 (–36.62,–23.38) * | - | - | - | < 0.01 | |
| Mixed (DS/IS) | ODI | 4 | 114 | 117 | –0.32 (–1.53, 0.89) * | 49 | 0.11 | Fixed | 0.60 |
| VAS back pain | 3 | 104 | 107 | –0.22 (–1.19, 0.76) * | 92 | < 0.01 | Random | 0.66 | |
| VAS leg pain | 3 | 104 | 107 | –0.09 (–0.23, 0.05) * | 0 | 0.69 | Fixed | 0.22 | |
| Clinical satisfaction | 3 | 100 | 102 | 0.49 (0.20, 1.20) ** | 0 | 0.91 | Fixed | 0.12 | |
| Complication rate | 6 | 202 | 207 | 0.79 (0.19, 3.19) ** | 64 | 0.02 | Random | 0.74 | |
| Revision rate | 3 | 132 | 127 | 1.39 (0.30, 6.38) ** | 0 | 0.59 | Fixed | 0.67 | |
| Fusion rate | 6 | 200 | 205 | 0.33 (0.17, 0.64) ** | 0 | 0.4 | Fixed | 0.001 | |
| Blood loss | 3 | 104 | 107 | 46.88 (–142.24 236.00) * | 88 | < 0.01 | Random | 0.63 | |
| Operation time | 1 | 39 | 42 | –30.00 (–36.62,–23.38) * | - | - | - | < 0.01 | |
| LSS with DS | ODI | 1 | 44 | 44 | –2.93 (–5.91, 0.05) * | - | - | - | 0.05 |
| VAS back pain | 1 | 44 | 44 | –0.33 (–0.80, 0.14) * | - | - | - | 0.17 | |
| VAS leg pain | 1 | 44 | 44 | –0.26 (–0.69, 0.17) * | - | - | - | 0.24 | |
| Clinical satisfaction | - | - | - | - | - | - | - | - | |
| Complication rate | 1 | 44 | 44 | 0.60 (0.24, 1.46) ** | - | - | - | 0.26 | |
| Revision rate | 1 | 44 | 44 | 0.63 (0.27, 1.47) ** | - | - | - | 0.28 | |
| Fusion rate | 1 | 44 | 44 | 0.50 (0.15, 1.63) ** | - | - | - | 0.25 | |
| Blood loss | 1 | 44 | 44 | –115.00 (–290.95, 60.95) * | - | - | - | 0.2 | |
| Operation time | 1 | 44 | 44 | –95.00 (–122.21,–67.79) * | - | - | - | < 0.01 | |
| Mixed (DS/IS/LSS) | ODI | 1 | 62 | 57 | 5.00 (0.99, 9.01) * | - | - | - | 0.01 |
| VAS back pain | 1 | 62 | 57 | 0.26 (–0.05, 0.57) * | - | - | - | 0.1 | |
| VAS leg pain | 1 | 62 | 57 | 0.54 (0.26, 0.82) * | - | - | - | < 0.01 | |
| Clinical satisfaction | 1 | 62 | 57 | 0.58 (0.21, 1.60) ** | - | - | - | 0.3 | |
| Complication rate | 1 | 62 | 57 | 1.24 (0.27, 5.80) ** | - | - | - | 0.78 | |
| Revision rate | 1 | 62 | 57 | 4.75 (0.22 101.12) ** | - | - | - | 0.32 | |
| Fusion rate | 1 | 62 | 57 | 0.63 (0.14, 2.78) ** | - | - | - | 0.54 | |
| Blood loss | 1 | 62 | 57 | 344.00 (248.20 439.80) * | - | - | - | < 0.01 | |
| Operation time | 1 | 62 | 57 | 43.00 (19.82, 66.18) * | - | - | - | < 0.01 | |
Abbreviations: PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; ODI, Oswestry Disability Index; DS, degenerative spondylolisthesis, IS, isthmic spondylolisthesis; LSS, lumbar spinal stenosis.
* Data is presented as combined mean difference (95% confidence interval); ** Data is presented as combined odds ratio (95% confidence interval).
Risk of Bias Across Studies
On visual inspection of the funnel plots, there was a possibility of publication bias found in the published studies measuring ODI, back pain, leg pain, complication rate, operation time and blood loss. No other variables showed obvious asymmetry. Funnel plots for each variable are displayed in Supplement 2.
Discussion
PLF and PLIF are the most widely used fusion techniques in spine surgery. Instrumented PLF used to be the most popular fusion method to manage lumbar spine instability, where a bone graft is placed between transverse processes, over the intertransverse membrane and adjacent facet joints. Satisfactory short term outcomes could be accomplished, but the curative effect does not seem to be permanent.23,24 PLF does not support the anterior spine,25,26 or entail removing of the degenerated disc, which may result in postoperative back pain and recurrent instability.
Therefore, interbody fusion was introduced to address these disadvantages. From a biomechanical point of view, PLIF could achieve superior mechanical strength via immediate stabilization, maintenance of intervertebral disc height, support to the anterior column, and better sagittal balance.21,27 Previous reports28,29 suggested that restoration of the anterior column support which bears the majority of weight relieves strain from the hardware used to augment lumbar fusion and widens the intervertebral foramen achieving indirect nerve root decompression.30-32 Furthermore, the disc space’s evacuation and distraction reduce vertebral slippage, restore lumbar segmental lordosis, provide good stability to allow solid fusion, and alleviates back pain caused by the degenerating disc.33,34 However, the risks of dural laceration or nerve root injury and the operative technique’s complexity suppress the practical application of PLIF. 14
Despite each fusion method’s theoretical advantages, studies have reported controversial results regarding the superiority of 1 technique over the other. Up-to-date meta-analyses were not capable of solving this controversy neither. Results of previous meta-analyses comparing both methods are summarized in Table 4. We postulated that these meta-analyses’ contradictory results could be attributed to the limited randomization in the selected studies. Thus, we conducted a systematic review and meta-analysis of randomized controlled trials to reach high-quality evidence for future surgical practice guidance.
Table 4.
Summary of Previous Meta-Analyses Comparing PLF and PLIF.
| Study | No. of included studies | Study design | Clinical outcome | Complication rate | Revision rate | Fusion rate | Operation time | Blood loss |
|---|---|---|---|---|---|---|---|---|
| Zhou 2011 | 9 | 3 RCT 6 Observational | NS | NS | NS | PLIF > PLF | NS | NS |
| Ye 2013 | 5 | 2 RCT 2 nRCT 1 Retrospective | NS | NS | - | PLIF > PLF | - | - |
| Liu 2014 | 9 | 4 RCT 5 Comparative | PLIF > PLF | NS | PLF > PLIF | PLIF > PLF | NS | NS |
| Luo 2017 | 9 | 2 RCT 3 Retrospective 4 Prospective | NS | NS | - | PLIF > PLF | PLIF > PLF | NS |
| Campbell 2017 | 6 | 2 Prospective 4 Retrospective | NS | NS | NS | NS | NS | NS |
| Chen 2018 | 11 | RCT CCT Cohort | NS | NS | - | NS | - | NS |
| Li 2020 | 8 | 4 RCT 2 CCT 2 Prospective nRCT | PLIF > PLF | NS | - | PLIF > PLF | - | NS |
| Our study | 8 | RCT only | NS | NS | NS | PLIF > PLF | NS | NS |
Abbreviations: PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; NS, not significant; RCT, randomized controlled trials; nRCT, nonrandomized controlled trials; CCT, controlled clinical trials.
Our analysis compared PLF and PLIF in terms of postoperative ODI, VAS for back and leg pain, and patient satisfaction. Both techniques achieved satisfactory clinical outcomes and significant pain relief. However, we were not able to detect a statistically significant difference between fusion approaches.
There were no consistent criteria for defining complications among the included trials. For instance, some studies reported nonunion as a postoperative complication while others did not. In our analysis, we defined complications as a combination of any of the following: deep infection, transient or permanent nerve injury, persistent back/leg pain, pain at the graft donor site, hardware failure (screw breakage, cage dislocation, screw loosening), deep venous thrombosis, dural tear with or without CSF leakage or adjacent segment disease.
On the 1 hand, PLF usually requires more extensive exposure of the paravertebral muscles, leading to more severe low-back pain postoperatively. 14 Complications associated with PLF are usually related to implant failure. Macki et al 35 found that all reoperations in the PLF group were due to instrumentation failure.
On the other hand, PLIF is often associated with complications resulting from its invasive nature, such as accidental dural injury. 13 There is a high risk of postoperative leg pain due to retraction of the nerve root and thecal sac when inserting interbody grafts.36-38 Besides, extensive dissection can lead to prolonged operation time and more blood loss, resulting in a higher complication rate.7,27 However, the continuous modification and refinement in surgical techniques, the development of transpedicular screw instrumentation and engineered interbody devices are supposed to lower the operative risks for PLIF. 39
Our study found a similar complication rate of 16.5% and 17.8% in PLF and PLIF groups, respectively, with no statistically significant difference. Subsequently, both approaches had insignificantly different reoperation rates. Indications of revision surgery in both groups in included studies are summarized in Table 5.
Table 5.
Indications of Reoperation in Included Studies.
| Study | PLF | PLIF |
|---|---|---|
| Inamdar et al 2006 | NA | NA |
| Kim et al 2006 | 1 Nonunion | No revision |
| 1 Aggravated symptoms | ||
| Cheng et al 2009 | 2 Nonunion with increasing low back pain | No revision |
| Musluman et al 2011 | No revision | 1 Cage dislocation |
| Farrokhi et al 2012 | NA | NA |
| Lee 2014 | No revision | 1 Deep infection followed by neurological deterioration |
| Farrokhi et al 2018 | 7 Screw loosening | 12 Screw loosening |
| 3 Pseudoarthrosis | 3 Pseudoarthrosis | |
| 3 Durotomy | 4 Durotomy | |
| 5 Adjacent segment disease | 4 Adjacent segment disease | |
| Gad 2018 | NA | NA |
Abbreviations: PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; NA: not available.
Solid fusion is the primary target of spine surgeons. For successful fusion to be achieved, appropriate fusion site and well-prepared tissue bed are fundamental. In theory, PLIF is more likely to achieve higher fusion rates as the cancellous bone of the vertebral body offer a superior fusion bed and provide a larger surface area to support the fusion. 1 Our results showed significantly higher fusion rates in the PLIF group of 93% compared to 84% in the PLF group. This difference was more pronounced in patients with spondylolisthesis rather than spinal stenosis as demonstrated by subgroup analysis.
Some authors believe that the successful fusion of the unstable segment reduces mechanical back pain caused by a pars defect, degenerated intervertebral disc, or facet arthropathy resulting in favourable functional outcomes.40-43 In contrast, nonunion and its associated complications such as fatigue failure of the construct, may result in postoperative recurrent back pain or even failure of the surgery and reoperation if necessary, thus preventing a satisfactory outcome.21,44,45 In contrast, our analysis concluded that significantly higher fusion rates in the PLIF group do not seem to translate into a more satisfactory clinical outcome or a lower complication rate.
The operation time and amount of blood loss were quite different among included studies. Two studies5,21 reported more blood loss with PLF procedure, while the other 3 studies17,18,22 did not find a significant difference. As for operation time, 2 studies18,22 showed significantly longer operative time in the PLIF group, whereas another study 21 reported longer surgical time in PLF. Overall, we did not find a significant difference in blood loss or operation time between both fusion techniques.
Limitations
There are some inherent limitations to our analysis. The first limitation is the unstandardized use of various measurements used to report clinical outcomes. Future trials should adhere to the North American Spine Society’s outcome measurements, including the ODI, VAS, and SF-36, to assess spinal conditions. 46 Secondly, some studies had relatively small sample sizes and short follow-up periods. Inamdar et al 20 and Farrokhi et al 17 reported mid-term outcomes at only a-12 month follow-up. Larger sample size trials with longer follow-up periods are required to assess long term outcomes of both fusion techniques. The third limitation is the significant heterogenicity among included studies. Heterogeneity could be due to varying experience of each surgical team, nonstandardized approaches, different bone graft material, inconsistent operative, rehabilitation and hospitalization protocols or different diagnoses. In an attempt to eliminate such significant heterogeneity, we used a random-effect model and conducted subgroup analyses. However, these results should be interpreted with caution as the small number of included trials may decrease the power of the subgroup analyses.
Conclusions
This systematic review and meta-analysis provide evidence based on RCTs comparing PLF and PLIF. The results showed that at 1-year minimum follow-up, PLIF achieved higher fusion rates with no significant difference in terms of clinical outcomes, complication rate, revision rate, operation time or blood loss compared with PLF.
Supplemental Material
Supplemental Material, sj-docx-1-gsj-10.1177_21925682211016426 for Posterolateral Fusion Versus Posterior Lumbar Interbody Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials by Elsayed Said, Mohamed E. Abdel-Wanis, Mohamed Ameen, Ali A. Sayed, Khaled H. Mosallam, Ahmed M. Ahmed and Hamdy Tammam in Global Spine Journal
Supplemental Material, sj-docx-2-gsj-10.1177_21925682211016426 for Posterolateral Fusion Versus Posterior Lumbar Interbody Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials by Elsayed Said, Mohamed E. Abdel-Wanis, Mohamed Ameen, Ali A. Sayed, Khaled H. Mosallam, Ahmed M. Ahmed and Hamdy Tammam in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ahmed M. Ahmed, MBBCh  https://orcid.org/0000-0003-4819-2542
https://orcid.org/0000-0003-4819-2542
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Cheng L, Nie L, Zhang L. Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis: a prospective controlled study in the Han nationality. Int Orthop. 2009;33(4):1043-1047. doi:10.1007/s00264-008-0588-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantas FL, Prandini MN, Ferreira MA. Comparison between posterior lumbar fusion with pedicle screws and posterior lumbar interbody fusion with pedicle screws in adult spondylolisthesis. Arq Neuropsiquiatr. 2007;65(3B):764–770. doi:10.1590/s0004-282x2007000500006 [DOI] [PubMed] [Google Scholar]
- 3.Möller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis—a prospective randomized study: part 1. Spine (Phila Pa 1976). 2000;25(13):1711–1715. doi:10.1097/00007632-200007010-00016 [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–2270. doi:10.1056/NEJMoa070302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müslüman AM, Yılmaz A, Cansever T, et al. Posterior lumbar interbody fusion versus posterolateral fusion with instrumentation in the treatment of low-grade isthmic spondylolisthesis: midterm clinical outcomes. J Neurosurg Spine. 2011;14(4):488-496. doi:10.3171/2010.11.SPINE10281 [DOI] [PubMed] [Google Scholar]
- 6.Yijian Z, Hao L, Huilin Y, Bin P. Comparison of posterolateral fusion and posterior lumbar interbody fusion for treatment of degenerative spondylolisthesis: analysis of spino-pelvic sagittal balance and postoperative chronic low back pain. Clin Neurol Neurosurg. 2018;171:1–5. doi:10.1016/j.clineuro.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 7.Ekman P, Moller H, Tullberg T, Neumann P, Hedlund R. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine. 2007;32(20):2178–2183. doi:org/10.1097/BRS.0b013e31814b1bd8 [DOI] [PubMed] [Google Scholar]
- 8.Urquhart JC, Alnaghmoosh N, Gurr KR, et al. Posterolateral versus posterior interbody fusion in lumbar degenerative spondylolisthesis. Clin Spine Surg. 2018;31(9):E446–E452. doi:10.1097/bsd.0000000000000698 [DOI] [PubMed] [Google Scholar]
- 9.Ye Y-P, Xu H, Chen D. Comparison between posterior lumbar interbody fusion and posterolateral fusion with transpedicular screw fixation for isthmic spondylolithesis: a meta-analysis. Arch Orthop Trauma Surg. 2013;133(12):1649–1655. doi:10.1007/s00402-013-1868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Wang Y, Qiu G, Weng X, Yu B. A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J. 2014;23(1):43–56. doi:10.1007/s00586-013-2880-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Cao K, Yu T, et al. Comparison of posterior lumbar interbody fusion versus posterolateral fusion for the treatment of isthmic spondylolisthesis. Clin Spine Surg. 2017;30(7):E915–E922. doi:10.1097/bsd.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 12.Campbell RC, Mobbs RJ, Lu VM, Xu J, Rao PJ, Phan K. Posterolateral fusion versus interbody fusion for degenerative spondylolisthesis: systematic review and meta-analysis. Global Spine J. 2017;7(5):482–490. doi:10.1177/2192568217701103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Zhang L, Li EN, et al. Comparison of posterolateral fusion and posterior lumbar interbody fusion in the treatment of lumbar spondylolithesis: a meta-analysis. J Invest Surg. 2019;32(4):290–297. doi:10.1080/08941939.2017.1411543 [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Wu Z, Guo D, You H, Fan X. A comprehensive comparison of posterior lumbar interbody fusion versus posterolateral fusion for the treatment of isthmic and degenerative spondylolisthesis: a meta-analysis of prospective studies. Clin Neurol Neurosurg. 2020;188:105594. doi:10.1016/j.clineuro.2019.105594 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e10000.97. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrokhi MR, Rahmanian A, Masoudi MS. Posterolateral versus posterior interbody fusion in isthmic spondylolisthesis. J Neurotrauma. 2012;29(8):1567-1573. doi:10.1089/neu.2011.2167 [DOI] [PubMed] [Google Scholar]
- 18.Farrokhi MR, Yadollahikhales G, Gholami M, Mousavi SR, Mesbahi AR, Asadi-Pooya AA. Clinical outcomes of posterolateral fusion versus posterior lumbar interbody fusion in patients with lumbar spinal stenosis and degenerative instability. Pain Physician. 2018;21(4):383–405. [PubMed] [Google Scholar]
- 19.Gad Abdelkader S, El Zahlawy HN, Elkhateeb TM. Interbody fusion versus posterolateral fusion in treatment of low grade lytic spondylolisthesis. Acta Orthop Belg. 2019;85(3):269-273. [PubMed] [Google Scholar]
- 20.Inamdar DN, Alagappan M, Shyam L, Devadoss S, Devadoss A. Posterior lumbar interbody fusion versus intertransverse fusion in the treatment of lumbar spondylolisthesis. J Orthop Surg (Hong Kong). 2006;14(1):21-26. doi:10.1177/230949900601400106 [DOI] [PubMed] [Google Scholar]
- 21.Kim KT, Lee SH, Lee YH, Bae SC, Suk KS. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006;31(12):1351–1357; discussion 1358. doi:10.1097/01.brs.0000218635.14571.55 [DOI] [PubMed] [Google Scholar]
- 22.Lee GW, Lee SM, Ahn MW, Kim HJ, Yeom JS. Comparison of posterolateral lumbar fusion and posterior lumbar interbody fusion for patients younger than 60 years with isthmic spondylolisthesis. Spine. 2014;39(24):E1475-E1480. doi:10.1097/BRS.0000000000000596 [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Wang L, Gong H, Zhu D. Biomechanical evaluation of three surgical scenarios of posterior lumbar interbody fusion by finite element analysis. Biomed Eng Online. 2012;11:31. doi:10.1186/1475-925X-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hägg O, Fritzell P, Ekselius L, Nordwall A.Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish Lumbar Spine Study. Eur Spine J. 2003;12(1):22–33. doi:10.1007/s00586-002-0465-z [DOI] [PubMed] [Google Scholar]
- 25.Vaccaro AR, Ring D, Scuderi G, Cohen DS, Garfin SR. Predictors of outcome in patients with chronic back pain and low-grade spondylolisthesis. Spine (Phila Pa 1976). 1997;22(17):2030–2034; discussion 2035. doi:10.1097/00007632-199709010-00018 [DOI] [PubMed] [Google Scholar]
- 26.de Loubresse CG, Bon T, Deburge A, Lassale B, Benoit M. Posterolateral fusion for radicular pain in isthmic spondylolisthesis. Clin Orthop Relat Res. 1996;(323):194–201. doi:10.1097/00003086-199602000-00027 [DOI] [PubMed] [Google Scholar]
- 27.van Dijk M, Smit TH, Sugihara S, Burger EH, Wuisman PI. The effect of cage stiffness on the rate of lumbar interbody fusion: an in vivo model using poly(l-lactic acid) and titanium cages. Spine (Phila Pa 1976). 2002;27(7):682–688. doi:10.1097/00007632-200204010-00003 [DOI] [PubMed] [Google Scholar]
- 28.Enker P, Steffee AD. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994;(300):90–101. [PubMed] [Google Scholar]
- 29.Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J. 2002;11(6):594–598. doi:10.1007/s00586-002-0469-8 [DOI] [PubMed] [Google Scholar]
- 30.Fleischer GD, Hart D, Ferrara LA, Freeman AL, Avidano EE. Biomechanical effect of transforaminal lumbar interbody fusion and axial interbody threaded rod on range of motion and S1 screw loading in a destabilized L5-S1 spondylolisthesis model. Spine (Phila Pa 1976). 2014;39(2):E82–E88. doi:10.1097/brs.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 31.McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976). 2005;30(6 suppl):S60–S65. doi:10.1097/01.brs.0000155578.62680.dd [DOI] [PubMed] [Google Scholar]
- 32.Suk SI, Lee CK, Kim WJ, Lee JH, Cho KJ, Kim HG. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 1997;22(2):210–219; discussion 219-20. doi:10.1097/00007632-199701150-00016 [DOI] [PubMed] [Google Scholar]
- 33.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976). 2000;25(7):853–857. doi:10.1097/00007632-200004010-00014 [DOI] [PubMed] [Google Scholar]
- 34.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995;20(3):356–361. doi:10.1097/00007632-199502000-00018 [DOI] [PubMed] [Google Scholar]
- 35.Macki M, Bydon M, Weingart R, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. doi:10.1016/j.clineuro.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 36.Dai LY, Jia LS, Yuan W, Ni B, Zhu HB. Direct repair of defect in lumbar spondylolysis and mild isthmic spondylolisthesis by bone grafting, with or without facet joint fusion. Eur Spine J. 2001;10(1):78–83. doi:10.1007/s005860000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritzell P, Hägg O, Wessberg P, Nordwall A.2001 Volvo award winner in clinical studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26(23):2521–2532; discussion 2532-254. doi:10.1097/00007632-200112010-00002 [DOI] [PubMed] [Google Scholar]
- 38.Lin PM. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res. 1985;(193):90–102. [PubMed] [Google Scholar]
- 39.DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16(3):130–139. doi:10.5435/00124635-200803000-00004 [DOI] [PubMed] [Google Scholar]
- 40.Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976). 2002;27(23):2674–2683. doi:10.1097/00007632-200212010-00006 [DOI] [PubMed] [Google Scholar]
- 41.Vamvanij V, Fredrickson BE, Thorpe JM, Stadnick ME, Yuan HA. Surgical treatment of internal disc disruption: an outcome study of four fusion techniques. J Spinal Disord. 1998;11(5):375–382. [PubMed] [Google Scholar]
- 42.Wetzel FT, Brustein M, Phillips FM, Trott S.Hardware failure in an unconstrained lumbar pedicle screw system. A 2-year follow-up study. Spine (Phila Pa 1976). 1999;24(11):1138–1143. doi:10.1097/00007632-199906010-00014 [DOI] [PubMed] [Google Scholar]
- 43.Yu CH, Wang CT, Chen PQ. Instrumented posterior lumbar interbody fusion in adult spondylolisthesis. Clin Orthop Relat Res. 2008;466(12):3034–3343. doi:10.1007/s11999-008-0511 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O’Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976). 2010;35(2):219–226. doi:10.1097/BRS.0b013e3181c91180 [DOI] [PubMed] [Google Scholar]
- 45.Raizman NM, O’Brien JR, Poehling-Monaghan KL, Yu WD. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 2009;17(8):494–503. doi:10.5435/00124635-200908000-00003 [DOI] [PubMed] [Google Scholar]
- 46.Kreiner DS, Baisden J, Mazanec DJ, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of adult isthmic spondylolisthesis. Spine J. 2016;16(12):1478–1485. doi:10.1016/j.spinee.2016.08.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-gsj-10.1177_21925682211016426 for Posterolateral Fusion Versus Posterior Lumbar Interbody Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials by Elsayed Said, Mohamed E. Abdel-Wanis, Mohamed Ameen, Ali A. Sayed, Khaled H. Mosallam, Ahmed M. Ahmed and Hamdy Tammam in Global Spine Journal
Supplemental Material, sj-docx-2-gsj-10.1177_21925682211016426 for Posterolateral Fusion Versus Posterior Lumbar Interbody Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials by Elsayed Said, Mohamed E. Abdel-Wanis, Mohamed Ameen, Ali A. Sayed, Khaled H. Mosallam, Ahmed M. Ahmed and Hamdy Tammam in Global Spine Journal