Abstract
Study Design:
Systematic review.
Objective:
The authors aimed to systematically compare the effectiveness and safety of endoscopic discectomy (ED) with non-endoscopic discectomy (NED) for treatment of symptomatic lumbar disc herniation (LDH).
Methods:
A systematic search was performed on PubMed, EMBASE, the Cochrane Library and China National Knowledge Infrastructure for randomized controlled trial from inception until August 13, 2020. Trials which investigated multiple operative approaches on lumbar disc herniation were identified without language restrictions.
Results:
In total, 25 trials involving 2258 patients with symptomatic LDH were included. Twenty trials performed the comparison between ED and NED. Five trials performed the comparison between percutaneous endoscopic transforaminal discectomy (PETD) and percutaneous endoscopic interlaminar discectomy (PEID). The operative time of micro-endoscopic discectomy (MED) was longer than open discectomy (OD). The length of hospital stay of percutaneous endoscopic lumbar discectomy (PELD) was shorter than fenestration discectomy (FD). Significant differences in intraoperative blood loss volumes were found between PELD with FD and MED with OD. The complication rate of PELD was lower than FD (PELD: 4.3%; FD: 14.6%) and the complication rate of full-endoscopic discectomy (FE) was lower than microscopic discectomy (MD) (FE: 13.4%; MD: 32.1%).
Conclusions:
PELD and FE have the advantage of limiting intraoperative damages. ED and NED can be both considered sufficient to achieve good clinical outcomes. PETD and PEID are able to achieve similar results but the learning curve of PETD was steeper. More independent high-quality RCTs with sufficiently large sample sizes performing cost-effectiveness analyzes are needed.
Keywords: symptomatic lumbar disc herniation, endoscopic discectomy, non-endoscopic discectomy, meta-analysis, systematic review
Introduction
Globally, 20 percent of low back and leg pain are caused by lumbar disc herniation (LDH) 1 and LDH is also the most common cause of adults’ sciatica. 2 Though the low back pain of LDH can be self-limiting, it still can incur significant financial costs and physical disabilities. 3 The majority of patients can recover by conservative treatment without the necessity of surgery. But after the conservative treatment has failed, surgery is indicated for symptomatic LDH. The first surgical treatment of symptomatic LDH was described by Mixter and Barr in 1934. 4 A minimally invasive surgery for treating symptomatic LDH was reported by Caspar and Yasargil with the introduction of the microscope in 1977.5,6 Micro-endoscopic discectomy (MED) was described by Foley and Smith as a minimally invasive trans-muscular approach using advanced optics. 7 Uni-portal arthroscopic microdiscectomy was reported by Kambin and bi-portal lumbar nucleotomy was reported by Schreiber and Leu in 1991.8,9 Percutaneous endoscopic lumbar discectomy (PELD) was introduced by Mayer in 1992. 10 Thomas Hoogland Endoscopic Spine System (TESSYS) was developed by Hoogland in 1994 11 and the Yeung Endoscopic Spine System (YESS) was developed by Yeung in 1997. 12 PELD can be classified to percutaneous endoscopic transforaminal discectomy (PETD) or percutaneous endoscopic interlaminar discectomy (PEID) according to the surgical approach. More recently, full-endoscopic discectomy (FE) was introduced by Ruetten as a minimally invasive access to the spinal canal under continuous visualization, either via a transforaminal or interlaminar corridor. 13
The first meta-analysis comparing the effectiveness and safety of endoscopic discectomy with open discectomy (OD) for symptomatic LDH was performed by us in 2015. 14 Due to the lack of data at that time, we put these kinds of discectomy (MED, PELD and FE) together into 1 group named as endoscopic discectomy and compared them with open discectomy in the meta-analysis of 2015, which was actually not scientific and fastidious enough. Since then, many meta-analyzes similar to our previous study have appeared performing the comparison between endoscopic discectomy (ED) and non-endoscopic discectomy (NED). However, the conclusions remain inconsistent. Now that there is enough data for us to perform a series of brand-new comparisons and subgroup analyzes based on each surgical procedures of MED, PELD and FE for conducting more scientific and comprehensive results. Considering whether any kind of ED is more effective and safer than NED is still unclear, we performed this study to systematically compare the effectiveness and safety of endoscopic discectomy with non-endoscopic discectomy for treatment of symptomatic LDH. The findings of this study could provide surgeons and patients with not only the choice of open discectomy or endoscopic discectomy, but also a more thorough and accurate selection of each surgical procedures on discectomy.
Materials and Methods
Search Methods and Selection Criteria
A systematic search was performed on PubMed, EMBASE, the Cochrane Library and China National Knowledge Infrastructure (CNKI) for randomized controlled trial from inception until August 13, 2020. Randomized controlled trials which investigated multiple operative approaches on lumbar disc herniation were identified without language restrictions. Endoscopic discectomy, percutaneous endoscopic transforaminal discectomy, micro-endoscopic discectomy and lumbar disc herniation were used as key words. The review protocols were registered on PROSPERO (International Prospective Register of Systematic Reviews number, CRD42020209478).
Trials were included according to the following criteria: (1) performed the comparison between ED (PELD or MED or FE) and NED (OD or MD or FD or MD); (2) the interventions of trials were PETD and PEID; (3) participants were adults who suffer lumbar disc herniation and failed with conservative treatment; (4) contained at least 1 outcome of interest. Trials were excluded if: Interventions were different from the previous description; Or original data was lost after confirmation with corresponding author.
Data Extraction and Statistical Analyzes
Two researchers extracted the data independently. Characteristics of trials and outcomes of interest were extracted and checked carefully. The primary outcomes were operative time, length of hospital stay, blood loss volume and complication rate between ED and NED. Secondary outcomes were clinical outcomes evaluated by the Macnab criteria, reoperation rate, recurrence rate, visual analog scale (VAS), Oswestry Disability Index (ODI), Japanese Orthopedic Association back pain evaluation questionnaire (JOA) between ED and NED and fluoroscopy times, operative time, postoperative bedrest time, clinical outcomes evaluated by the Macnab criteria, complication rate, recurrence rate, ODI and VAS between PETD and PEID. To compare the effect of multiple surgical techniques more precisely, subgroup analyzes were performed based on the interventions of trials. The continuous outcomes were analyzed using mean difference (MD) and 95% confidence interval (CI). Odds ratio (OR) and 95% CI were used for dichotomous outcomes. Standardized mean difference (SMD) was used when a continuous outcome is presented with different units. All analyzes were performed by RevMan software (version 5.3). Between study heterogeneity were evaluated using Chi-squared test and I2. If the P value was < .05, statistical heterogeneity exists. In this situation, a random-effects model was utilized. P < .05 was considered to be statistically significant.
Assessment of Risk of Bias
The Cochrane Collaboration’s risk-of-bias criteria was used for evaluating the risk of bias in each included trial. The classifications of bias were based on 7 items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Each item was rated as low risk, unclear risk, or high risk.
Results
Study Selection and Characteristics
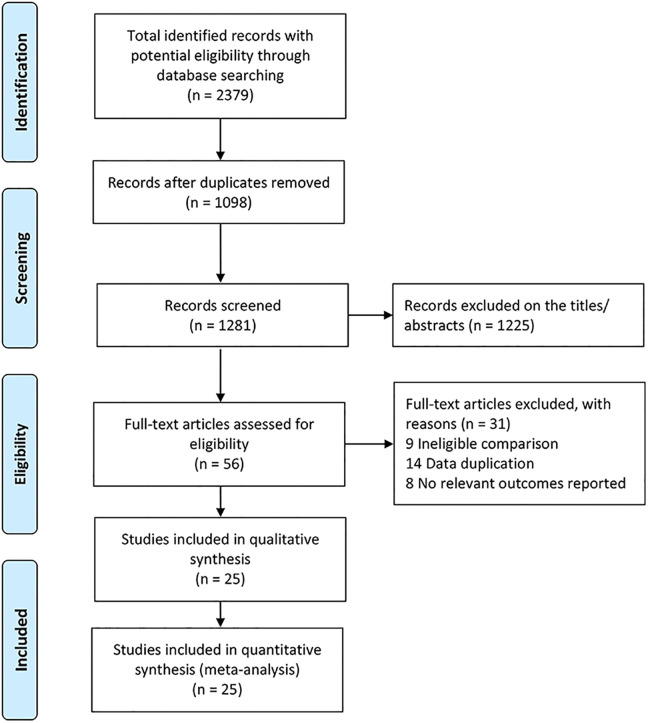
A total of 2379 studies were identified yielding 1281 studies after removal of duplications. Title and abstract screening excluded 1225 trials. After removing duplications and full-text screening, 31 trials were eliminated. In the end, 25 trials which met the eligibility criteria were included in this study (Figure 1).
Figure 1.
The flow-diagram showing the selection process of RCTs for meta-analysis.
Twenty-five trials involving 2,258 patients with symptomatic LDH were included in this study. Twenty trials performed the comparison between ED and NED.15-34 Five trials performed the comparison between PETD and PEID.35-39 Among those trials comparing ED with NED, PELD was performed in 10 trials,16,20,22-26,31,33,34 FE in 4 trials21,28-30 and MED in 6 trials.15,17-19,27,32 The characteristics of the included trials were shown in Table 1 and Table 2.
Table 1.
Characteristics of the Included Trials.
| Interventions | Sample size | Mean age | Female | Mean duration of symptom | Mean follow-up | |
|---|---|---|---|---|---|---|
| Trial | (Exp/ Clt) | (Exp/ Clt) | (Year, Exp/ Clt) | (%, Exp/ Clt) | (months, Exp/ Clt) | (months) |
| Garg, 15 2011 | MED/ OD | 112 (55/ 57) | 37.5 (37·0/38·0) | 34.5/ 22.8 | 11.6 ± 9.5/16.7 ± 15.2 | 12.0 |
| Gibson, 16 2016 | PETD/ MD | 140 (70/ 70) | 40.5 (42.0/39.0) | 57.0/ 43.0 | 18.0 (4.0-120.0)/ 15.0 (3.0-120.0) | 24.0 |
| Hermantin, 17 1999 | VAMD/ OD | 60 (30/ 30) | 39.5 (39.0/40.0) | 26.7/ 43.3 | NA | 24.0 |
| Huang, 29 2005 | MED/ OD | 22 (10/ 12) | 39.5 (39.2/39.8) | 40.0/ 25.0 | NA | 18.9 |
| Hussein, 18 2014 | MED/ OD | 185 (95/ 90) | 30.8 (30.2/31.5) | 44.2/ 51.1 | 3.0/ 3.5 | 102.8 |
| Jin, 34 2017 | PETD/ FD | 90 (45/ 45) | 41.0 (40.1/41.9) | 40.0/ 44.4 | 24.5 ± 13.1/ 25.5 ± 12.8 | 13.0 |
| Lee, 20 2006 | PELD/ MD | 60 (30/ 30) | 39.5 (39.3/39.6) | 26.7/ 26.7 | NA | 37.5 |
| Liu, 33 2014 | PETD/ FD | 80 (40/ 40) | 41.1 (39.8/42.4) | 40.0/ 47.5 | 25.4 ± 12.8/ 23.7 ± 12.5 | 19.0 |
| Mayer, 21 1993 | PELD/ MD | 40 (20/ 20) | 41.3 (39.8/42.7) | 40.0/ 30.0 | 6.9/ 7.3 | 24.0 |
| Pan, 22 2014 | PELD/ OD | 20 (10/ 10) | NA | NA | NA | 0.1 |
| Pan, 23 2016 | PETD/ FD | 106 (48/ 58) | 41.3 (39.5/42.8) | 45.8/ 46.6 | 15.5 (5.0-72.0)/ 22.3 (0.2-84.0) | 17.0 |
| Righesso, 24 2007 | MED/ OD | 40 (21/ 19) | 43.9 (42.0/46.0) | 52.4/ 31.6 | 2.0 (1.0-7.0)/ 2.0 (1.0-6.0) | 36.1 |
| Ruetten, 25 2008 | FE/ MD | 200 (100/ 100) | 43.0 (NA) | NA | 2.7 | 24.0 |
| Ruetten, 26 2009 | FE/ MD | 100 (50/ 50) | 39.0 (NA) | NA | 2.3 | 24.0 |
| Komp, 19 2015 | FE/ MD | 160 (80/ 80) | 62.0 (NA) | NA | 17.0 | 24.0 |
| Tacconi, 27 2020 | FE/ MD | 50 (25/ 25) | 44.0 (43.0/45.0) | 48.0/52.0 | NA | 22.0 |
| Tang, 32 2012 | PETD/ FD | 80 (40/ 40) | 64.7 (NA) | NA | 122.4 ± 21.6 | 24.0 |
| Teli, 28 2010 | MED/ OD&MD | 212 (70/ 142) | 39.3 (39.0/39.5) | 35.7/ 33.8 | 2.8 ± 1.3/ 2.9 ± 1.4 | 26.0 |
| Wang, 31 2015 | PETD/ FD | 96 (48/ 48) | 45.0 (42.8/47.2) | 41.7/ 45.8 | NA | 12.0 |
| Wu, 30 2016 | PETD/ FD | 50 (25/ 25) | 45.2 (46.3/44.1) | 48.0/ 40.0 | NA | 17.3 |
| Chen, 35 2015 | PETD/PEID | 76 (40/ 36) | 48.7 (49.5/47.9) | 50/ 41.7 | 9.0 | 15.6 |
| Huang, 36 2017 | PETD/PEID | 82 (41/ 41) | 41.3 (41.8/40.8) | 26.8/ 36.6 | 7.12 ± 0.72/ 7.08 ± 0.49 | 12.0 |
| Mo, 39 2019 | PETD/PEID | 40 (20/ 20) | 42.1 (40.9/43.3) | 38.5/ 56.1 | NA | 16.7 |
| Nie, 38 2016 | PETD/PEID | 60 (30/ 30) | 37.4 (36.6/38.2) | 40.0/ 33.3 | NA | 27.7 |
| Xu, 37 2013 | PETD/PEID | 68 (31/ 37) | 47.3 (46.6/47.9) | 45.2/ 29.7 | 7.0 | 3.0 |
Abbreviations: PELD, percutaneous endoscopic lumbar discectomy; PETD, percutaneous endoscopic transforaminal discectomy; PEID, percutaneous endoscopic interlaminar discectomy; MED, micro-endoscopic discectomy; OD, open discectomy; MD, microscopic discectomy; FD, fenestration discectomy; FE, full-endoscopic discectomy; NA, not available; Exp, experimental group; Clt, control group.
Table 2.
Outcomes of the Included Trials.
| Trial | Outcomes |
|---|---|
| Garg, 15 2011 | Operative time, length of hospital stay, blood loss volume, complication, reoperation, recurrence |
| Gibson, 16 2016 | Operative time, complication, reoperation |
| Hermantin, 17 1999 | Complication, reoperation, satisfaction |
| Huang, 29 2005 | Operative time, length of hospital stay, blood loss volume, Macnab criteria |
| Hussein, 18 2014 | Operative time, length of hospital stay, blood loss volume, Macnab criteria, complication, reoperation, recurrence |
| Jin, 34 2017 | Operative time, length of hospital stay, Macnab criteria, complication, VAS, ODI |
| Lee, 20 2006 | Macnab criteria |
| Liu, 33 2014 | Operative time, length of hospital stay, blood loss volume, Macnab criteria, VAS, ODI, JOA |
| Mayer, 21 1993 | Operative time |
| Pan, 22 2014 | Macnab criteria |
| Pan, 23 2016 | Operative time, length of hospital stay, blood loss volume, Macnab criteria, complication, ODI, JOA |
| Righesso, 24 2007 | Operative time, complication, reoperation, recurrence |
| Ruetten, 25 2008 | Complication, reoperation, recurrence, satisfaction |
| Ruetten, 26 2009 | Complication, reoperation, recurrence, satisfaction |
| Komp, 19 2015 | Complication, reoperation |
| Tacconi, 27 2020 | Complication, reoperation |
| Tang, 32 2012 | Operative time, length of hospital stay, blood loss volume, VAS, ODI |
| Teli, 28 2010 | Operative time, length of hospital stay, complication, reoperation, recurrence |
| Wang, 31 2015 | Operative time, length of hospital stay, blood loss volume, VAS, ODI, JOA |
| Wu, 30 2016 | Operative time, length of hospital stay, blood loss volume, VAS, ODI, JOA |
| Chen, 35 2015 | Operative time, Macnab criteria, VAS |
| Huang, 36 2017 | Fluoroscopy times, recurrent disc herniation, operative time, VAS, ODI |
| Mo, 39 2019 | Fluoroscopy times, operative time, postoperative bed time, Macnab criteria, complication, VAS, ODI |
| Nie, 38 2016 | Fluoroscopy times, operative time, postoperative bed time, Macnab criteria, complication, recurrent disc herniation |
| Xu, 37 2013 | Fluoroscopy times, operative time, Macnab criteria, VAS |
Abbreviations: VAS, visual analog scale; ODI, Oswestry disability index; JOA, Japanese Orthopaedic Association back pain evaluation questionnaire.
ED VS NED
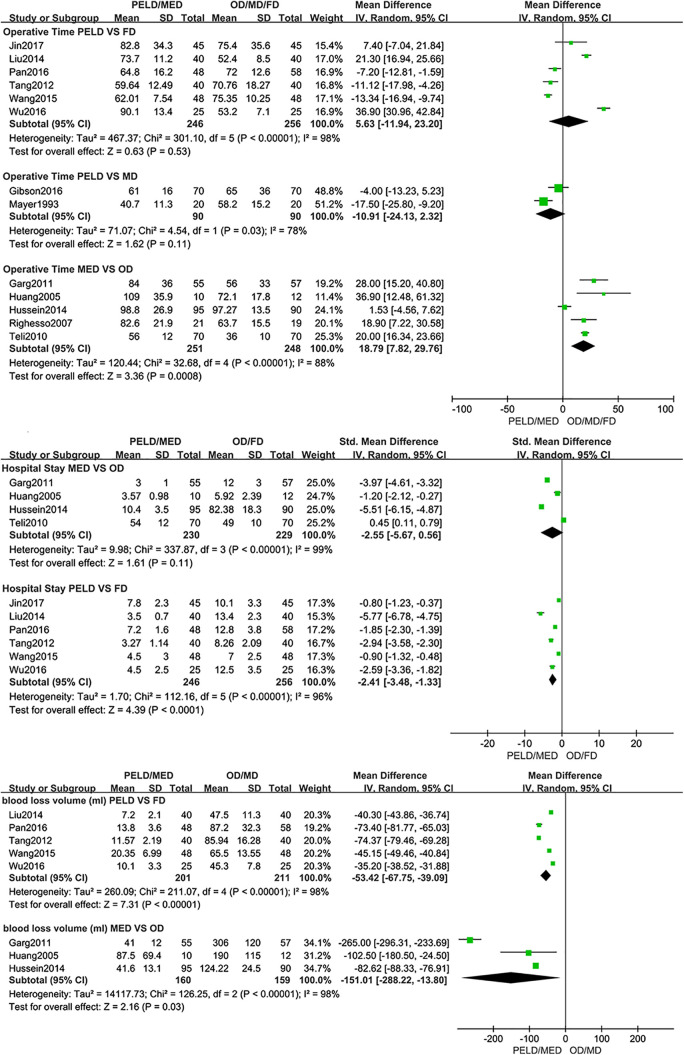
The operative time of MED was longer than OD (open discectomy) (MD: 18.79; 95% CI: [7.82, 29.76], P < .001, I2=88%). No significant differences were found in operative time between PELD with MD (microscopic discectomy) (MD: −10.91; 95% CI: [−24.13, 2.32], P = .11, I2=78%) and PELD with FD (fenestration discectomy) (MD: 5.63; 95% CI: [−11.94, 23.20], P = .53, I2=98%). The length of hospital stay of PELD was shorter than FD (SMD: −2.41; 95% CI: [−3.48, −1.33], P < .001, I2=96%). And there was no significant difference between MED with OD in the length of hospital stay (SMD: −2.55; 95% CI: [−5.67, 0.56], P = .11, I2=99%). Significant differences were found in intraoperative blood loss volume between PELD with FD (MD: −53.42; 95% CI: [−67.75, −39.09], P < .001, I2=98%) and MED with OD (MD: −151.01; 95% CI: [−288.22, −13.80], P = .03, I2=98%) (Figure 2).
Figure 2.
Pooling results of the ED group and the NED group. The results were shown as follows: Operative Time, Hospital Stay and Intraoperative Blood Loss.
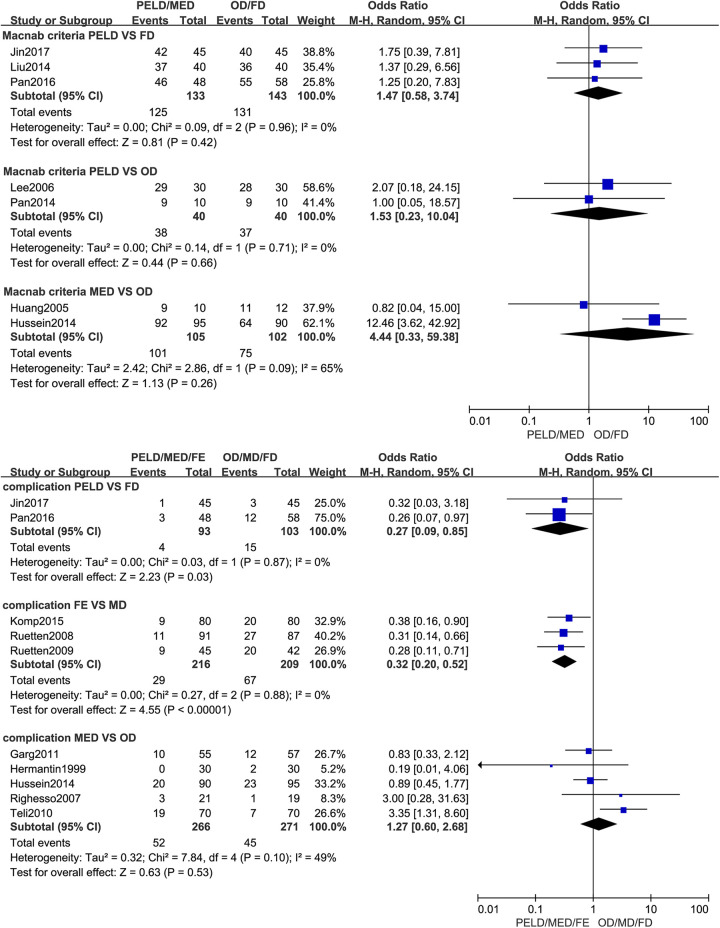
No statistical significance was found in clinical outcomes evaluated by the Macnab criteria between MED with OD (OR: 4.44; 95% CI: [0.33, 59.38], P = .26, I2=65%) and PELD with FD (OR: 1.47; 95% CI: [0.58, 3.74], P = .42, I2=0%) and PELD with OD (OR: 1.53; 95% CI: [0.23, 10.04], P = .66, I2=0%). The complication rate of PELD was lower than FD (PELD: 4.3%; FD: 14.6%; OR: 0.27; 95% CI: [0.09, 0.85], P = .03, I2=0%) and the complication rate of FE was lower than MD (FE: 13.4%; MD: 32.1%; OR: 0.32; 95% CI: [0.20, 0.52], P < .001, I2=0%). The complication rate of MED was slightly higher than OD however this was not statistically significant (MED: 19.5%; OD: 16.6%; OR: 1.27; 95% CI: [0.60, 2.68], P = .53, I2=49%). There was no significant difference in the rate of reoperation between MED with OD (MED: 6.3%; OD: 6.0%; OR: 1.03; 95% CI: [0.51, 2.06], P = .93, I2=14%) and FE with MD (FE: 6.1%; MD: 7.0%; OR: 0.86; 95% CI: [0.42, 1.76], P = .69, I2=0%). And no significance in the rate of recurrence was found between MED with OD (MED: 5.0%; OD: 2.5%; OR: 1.93; 95% CI: [0.74, 5.04], P = .18, I2=0%) and FE with MD (FE: 6.6%; MD: 5.4%; OR: 1.24; 95% CI: [0.45, 3.42], P = .68, I2=0%). (Figure 3).
Figure 3.
Pooling results of the ED group and the NED group. The results were shown as follows: Clinical Outcomes Evaluated by the Macnab Criteria, Complication Rate, Reoperation Rate and Recurrence Rate.
Significant difference was found between PETD with FD in VAS at 1 day after operation (MD: −1.27; 95% CI: [−2.47, −0.07], P = .04, I2=96%). And there was no significant difference between PETD with FD in VAS at 3 days (MD: −1.56; 95% CI: [−4.29, 1.18], P = .26, I2=99%), 3 months (MD: −0.10; 95% CI: [−0.29, 0.09], P = .31, I2=0%) and 1 year (MD: −0.14; 95% CI: [−0.34, 0.06], P = .17, I2=33%) after operation. No significant difference was found between PETD with FD in ODI at 1 month (MD: −0.74; 95% CI: [−1.59, 0.11], P = .09, I2=91%), 3 months (MD: 0.03; 95% CI: [−0.22, 0.28], P = .81, I2=2%), 6 months (MD: −1.01; 95% CI: [−2.66, 0.63], P = .23, I2=97%) and 1 year (MD: −0.42; 95% CI: [−0.98, 0.13], P = .13, I2=87%) after operation. No significant difference was found between PETD with FD in JOA (MD: 0.11; 95% CI: [−0.38, 0.60], P = .65, I2=36%) (Table 3).
Table 3.
Pooling Results of the ED Group and the NED Group. The Results Were Shown as Follows: VAS Between PETD and FD, VAS Between PELD and FD and JOA Between PELD and FD.
| Outcomes | Trials | Participants | Mean difference (95%CI) | P valuea |
|---|---|---|---|---|
| PETD VS FD | ||||
| VAS after 1 day | 3 | 250 | −1.27 [−2.47 to −0.07] | .04 |
| VAS after 3 day | 2 | 160 | −1.56 [−4.29 to 1.18] | .26 |
| VAS after 3 month | 2 | 140 | −0.10 [−0.29 to 0.09] | .31 |
| VAS after 1 year | 3 | 226 | −0.14 [−0.34 to 0.06] | .17 |
| ODI after 1 month | 3 | 266 | −0.74 [−1.59 to 0.11] | .09 |
| ODI after 3 month | 3 | 246 | 0.03 [−0.22 to 0.28] | .81 |
| ODI after 6 month | 3 | 276 | −1.01 [−2.66 to 0.63] | .23 |
| ODI after 1 year | 5 | 412 | −0.42 [−0.98 to 0.13] | .13 |
| JOA | 4 | 332 | 0.11 [−0.38 to 0.60] | .65 |
Abbreviations: PETD, percutaneous endoscopic transforaminal discectomy; FD, fenestration discectomy; VAS, visual analog scale; ODI, Oswestry disability index; JOA, Japanese Orthopaedic Association back pain evaluation questionnaire.
a P value for heterogeneity between interventions calculated by using mixed-effects models.
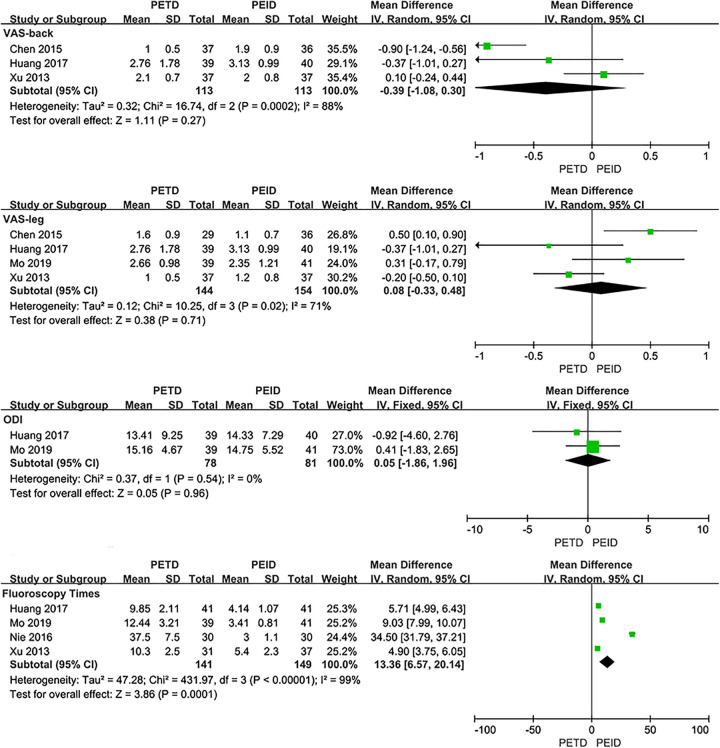
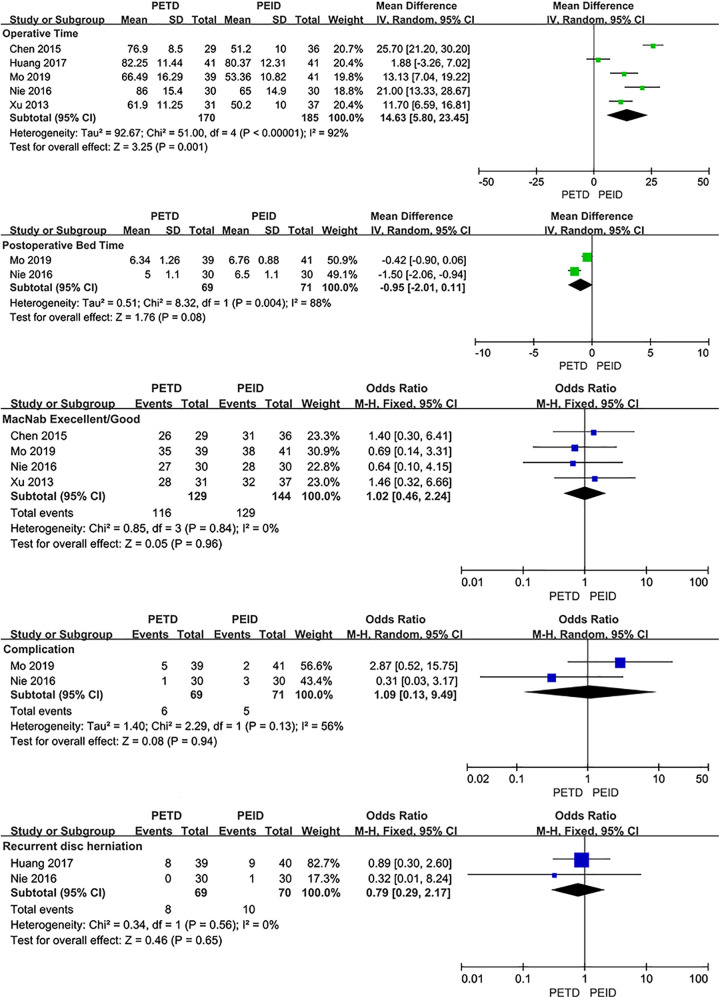
PETD VS PEID
No significant difference was found between PETD with PEID in VAS-back (MD: −0.39; 95% CI: [−1.08, 0.30], P = .27, I2=88%), VAS-leg (MD: −0.08; 95% CI: [−0.45, 0.29], P = .68, I2=49%), ODI (MD: 0.05; 95% CI: [−1.86, 1.96], P = .96, I2=0%) and postoperative bedrest time (MD: −0.95; 95% CI: [−2.01, 0.11], P = .08, I2=88%). The fluoroscopy time of PETD was more than PEID (MD: 13.36; 95% CI: [6.57, 20.14], P < .001, I2=99%). And the operative time of PETD was longer than PEID (MD: 14.63; 95% CI: [5.80, 23.45], P = .001, I2=92%). No statistical significance was found between PETD with PEID in clinical outcomes evaluated by the Macnab criteria (OR: 1.02; 95% CI: [0.46, 2.24], P = .96, I2=0%), complication rates (PETD: 8.7%; PEID: 7.0%; OR: 1.09; 95% CI: [0.13, 9.49], P = .94, I2=56%) and recurrence rates (PETD: 11.6%; PEID: 14.3%; OR: 0.79; 95% CI: [0.29, 2.17], P = .65, I2=0%) (Figure 4).
Figure. 4.
Pooling results of the PETD group and the PEID group. The results were shown as follows: VAS, ODI, Fluoroscopy Times, Operative Time, Postoperative Bedrest Time, Clinical Outcomes Evaluated by the Macnab Criteria, Complication Rate and Recurrence Rate.
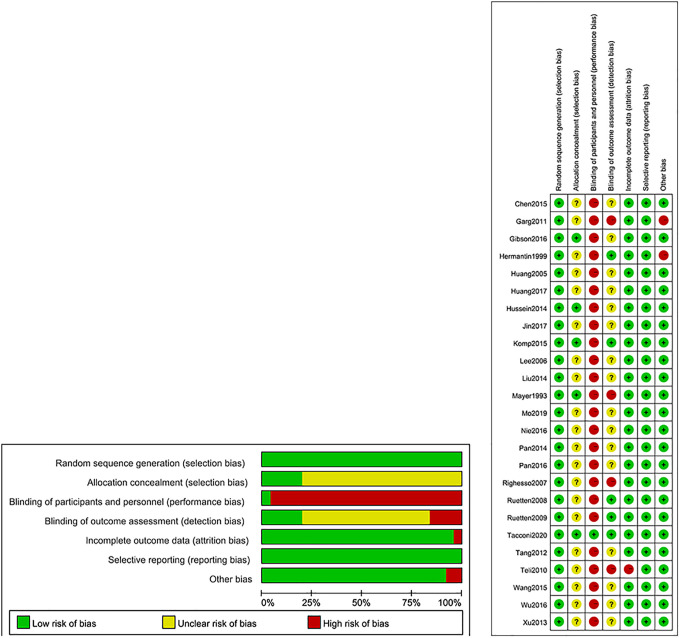
Risk of Bias
All trials described the appropriate random sequence generation and 5 trials reported the allocation concealment.16,19,21,24,30 One trial was a double-blind randomized controlled trial among participants. 30 Trial of Teli 32 et al failed to report some of the original data. We tried to contact the corresponding author but was unsuccessful. Dural leaks occurred in earlier patients in Grag et al 15 and physicians were able to override decision for type of surgery in the trial of Hermantin et al, 17 which are the reasons why the other bias of these trials were high risk (Figure 5).
Figure 5.
Risk of bias summary.
Discussion
In this study, we included 20 RCTs that performed the comparison between ED and NED. In MED, air is the medium. A small incision is performed and a tubular retractor (16- or 18-mm in diameter) is used to approach the herniated disc posteriorly using an endoscope. 27 PELD is a minimally invasive discectomy with water as medium. With patients under local anesthesia in PETD or general anesthesia in PEID a needle is used to locate the herniated disc under fluoroscopic imaging. An endoscope is then introduced along the needle to perform decompression under direct visualization.22,25 FE evolved from PELD but access the herniated disc posterolaterally. 28 Significant differences were found between PELD with FD and MED with OD in intraoperative blood loss volume. Nakagawa et al concluded that MED was superior to OD in the control of intraoperative injury. 40 However, most of included studies (5/6) were non-randomized controlled trials in the study of Nakagawa et al, 40 which could lead to biases of results and weakened the reliability of that conclusion. And we believed that intraoperative injury should be a composite result of multiple factors and should be reflected not only in terms of intraoperative bleeding, but also in other aspects such as operative time and hospital stay. The results of our study suggest that MED is superior to OD in controlling the amount of intraoperative blood loss. As for PELD and FE, drainage systems were not placed intraoperatively. Water pressure can promote hemostasis, and the surgeon does not need to spend more time and energy on hemostasis during operation. For operative time, no significant differences were found between PELD with MD and PELD with FD. And the operative time of MED was longer than OD. The operation time is related to the age and physical condition of the patient, the surgeon’s proficiency with the procedure and the cooperation of the surgical team, so it is difficult to compare the operative time as a separate variable. Ruan et al suggested that the operative time of PELD was shorter than that of OD. 41 In their study, 5 of the 6 trials included in the comparison of operative time were non-randomized controlled trials and only 1 was randomized controlled trial, which increased the risk of bias. Greater operative time will correspondingly increase the risk of intraoperative damage. Ondeck et al suggested that longer operative time was associated with higher risk of overall postoperative adverse events and multiple individual adverse outcomes. 42 Therefore, we believe that MED may be able to achieve better control of intraoperative hemorrhage rather than intraoperative damage.
Shorter hospital stay means that patients could recover more quickly after surgery and return to normal work and life earlier, which can indirectly reflect less surgical damage from the surgery. Zhang et al reported that transforaminal endoscopic discectomy was superior to open microdiscectomy in the length of hospital stay. 43 In this study, the length of hospital stay of PELD was shorter than FD. And there was no significant difference between MED and OD in the length of hospital stay. In several countries, however, the length of hospital stay is also associated with reimbursement issues. 14 Thus, shorter hospital stay is supposed to reduce the cost of treatment. Due to lack of enough RCTs comparing the cost-effectiveness between different surgical techniques, further research is needed to determine which surgical technique is more cost-effective. Phana et al reported that the length of hospital stay of MED was shorter than OD. 44 We believe that this difference in results may be due to the conversion of units used for comparison. We compared the data according to the standardized mean difference instead of converting the data into uniform units and the original format of the data was preserved, which would increase the statistical reliability.
The complication rate of PELD was lower than FD (PELD: 4.3%; FD: 14.6%) and the complication rate of FE was lower than MD (FE: 13.4%; MD: 32.1%), both with statistical significance. And the complication rate of MED was higher than OD without statistical significance (MED: 19.5%; OD: 16.6%) in this study. To a certain extent, postoperative complications could reflect the intraoperative damage. Phana et al suggested that no statistical significance was found in complication rate between FE with OD and MED with OD. 44 We believe that this difference in results is mainly due to the different types of trials selected, and our studies are all based on pure randomized controlled trials for the comparison between MED and OD. Moreover, we included more high-quality RCTs comparing the complications of FE and OD. These findings on operative time, intraoperative blood loss, hospital stay and complication rate in our study suggested that PELD and FE are more advantageous in controlling intraoperative damage
In this study, we performed the comparison of VAS and ODI between PETD and FD at various time points during the follow-up period, as well as JOA and clinical outcomes evaluated by the Macnab criteria between PELD and FD, PELD and OD, lastly, MED and OD. Visual analog scale (VAS) is widely used to measure pain relief in spinal surgery. Oswestry Disability Index (ODI) and Japanese Orthopedic Association back pain evaluation questionnaire (JOA) are questionnaires evaluating dysfunction. And the Macnab criteria is mainly used to evaluate postoperative working and living conditions. The only statistically significant outcome in these comparisons was the VAS at 1 day after operation between PETD with FD. We believed that the reason for this result may be that PELD is more advantageous in controlling intraoperative damage and less intraoperative damage allows patients to recover more quickly, leading pain relief to come sooner. At present, none of these surgical techniques has been abandoned for symptomatic LDH. Many studies suggested that open surgery, minimally invasive surgery and spinal endoscopic surgery are considered as sufficient and safe techniques with good clinical outcomes.14,41,44-46 Open discectomy is still considered as the gold standard treatment for symptomatic LDH.47,48 The findings of this study suggested that there was no difference in achieving pain relief, functional recovery, and quality of life improvement among these surgical techniques. All of these surgical techniques can be considered sufficient to achieve good clinical outcomes.
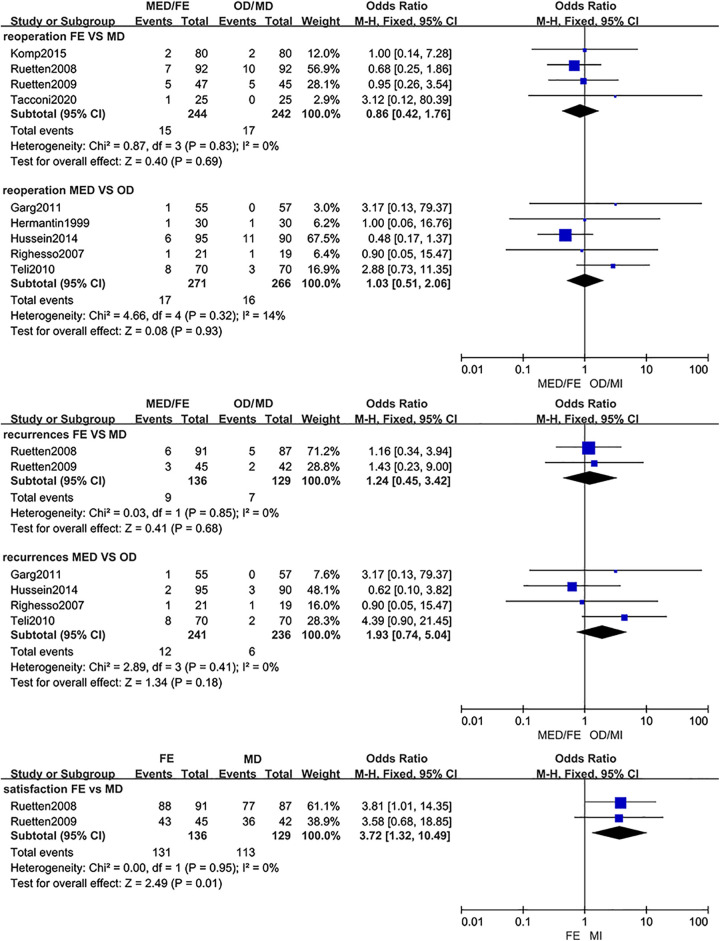
Some studies suggested that the reoperation rate of ED was higher than that of NED due to the steep learning curve and the limited operative field of ED.12,49-51 The study of Qin et al 52 and Ruan et al 41 reported that there was no statistical significance between PELD and OD in the rate of reoperation. But it is worth mentioning that misclassifications of trials appeared in this comparison in both studies. The trial of Ruetten et al published in 2008 28 included in the study of Qin et al 52 and the trial of Ruetten et al published in 2009 53 included in the study of Ruan et al 41 were misclassified. The interventions of these 2 trials were full-endoscopic discectomy rather than percutaneous endoscopic lumbar discectomy. After accurate classification in this study, there was no significant difference in the rate of reoperation between MED and OD (MED: 6.3%; OD: 6.0%) or FE and MD (FE: 6.1%; MD: 7.0%) and no significant significance in the rate of recurrence was found between MED and OD (MED: 5.0%; OD: 2.5%) or FE and MD (FE: 6.6%; MD: 5.4%). The findings of this study suggested that ED (FE and MED) and NED could achieve similar results in the rate of reoperation and recurrence. Due to lack of high-quality RCTs, comparison of the rate of reoperation and recurrence between PELD and OD needs further research.
PELD can be classified as percutaneous endoscopic transforaminal discectomy (PETD) or percutaneous endoscopic interlaminar discectomy (PEID) according to the surgical approach. Some studies suggested that the indications for these 2 approaches were different. Since PEID is not affected by the height of the iliac crest, patients with high iliac crest are suitable for the interlaminar approach but the nerve roots are more easily stimulated during surgery, resulting in poor intraoperative tolerance.35,36 PETD is suitable for patients with interlaminar stenosis or tension phenotype. 39 In this study with 5 RCTs introduced, we performed the comparisons of VAS, ODI, postoperative bed time, fluoroscopy times, operative time, clinical outcomes evaluated by the Macnab criteria, complication rate and recurrence rate between PETD with PEID. The fluoroscopy time of PEID was less than PETD and the operative time of PEID was statistically shorter than PETD with statistical significance but no significance was found in the remaining comparisons. The reasons for these results may be that the anatomical structure and technique during PEID are very similar to traditional open discectomy, which let the surgeons adapt to this approach very quickly and make the introduction of endoscope relatively simple. Based on these findings, we believe that both PETD and PEID are able to achieve similar results but the learning curve of PETD was steeper.
The objective of this study was to systematically compare the effectiveness and safety of endoscopic discectomy with non-endoscopic discectomy for the treatment of symptomatic LDH. Many published studies have performed the comparison of the same topic without pure RCTs included.41,43-46 The advantage of this study is greater number of high-quality RCTs are available that compared ED and NED allowing more accurate classification of interventions. And we performed a series of comparisons and subgroup analyzes based on each surgical procedures of MED, PELD (PETD and PEID) and FE for conducting more scientific and comprehensive results. We believed that these findings of this study could provide surgeons and patients with not only the choice of open discectomy or endoscopic discectomy, but also a more thorough and accurate selection of each surgical procedures on discectomy. By considering all included trials without language restrictions, this study could avoid outcomes distorted by language bias. But there were still several limitations in this study. First, the number of trials involved in some comparisons are relatively small. The cost-effectiveness of discectomy for symptomatic LDH has rarely been reported in studies, despite its need. 54 Only 1 trial included in this study reported that MED was more expensive than OD with statistical significance. 32 And due to the lack of high-quality RCTs, comparisons could not be performed for all surgical approaches for some outcomes. Second, differences existed in the inclusion criteria and patient characteristics between some trials and the follow-up period in the trial of Hussein et al 19 significantly longer than other trials, resulting in statistically significant heterogeneity in some results. Third, clear allocation concealment and complete outcome data were not presented in some trials. And some trials failed to fully describe the baseline characteristics of patients.
Conclusion
PELD and FE are more advantageous in controlling intraoperative damage. Both ED and NED can be considered sufficient to achieve good clinical outcomes. In subgroup analyzes, ED (FE and MED) could achieve similar results in the rate of reoperation and recurrence compared with NED. Both PETD and PEID are able to achieve similar results but the learning curve of PETD was steeper. More independent high-quality RCTs using sufficiently large sample sizes and performing cost-effectiveness analyzes are needed.
Abbreviations
- 1. ED
endoscopic discectomy
- 2. NED
non-endoscopic discectomy
- 3. PELD
percutaneous endoscopic lumbar discectomy
- 4. PETD
percutaneous endoscopic transforaminal discectomy
- 5. PEID
percutaneous endoscopic interlaminar discectomy
- 6. MED
micro-endoscopic discectomy
- 7. OD
open discectomy
- 8. MD
microscopic discectomy
- 9. FD
fenestration discectomy
- 10. FE
full-endoscopic discectomy
- 11. LDH
lumbar disc herniation
- 12. ODI
Oswestry disability index
- 13. JOA
Japanese Orthopedic Association back pain evaluation questionnaire
- 14. VAS
visual analog scale
- 15. MD
mean difference
- 16. OR
odds ratios
- 17. 95% CI
95% confidence interval
- 18. SMD
standardized mean difference
- 19. RCT
randomized controlled trials
- 20. Exp
experimental group
- 21. Clt
control group
- 22. NA
not available
Footnotes
Authors’ Note: Contributors WSL did study concept and design, data acquisition and interpretation, and drafted the manuscript; QY did data acquisition, data interpretation and language editing; LC did study concept and design, study supervision, and critical review of the manuscript. All authors reviewed the study findings and read and approved the final version before submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (NO: 81871803). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Lin Cong, PhD  https://orcid.org/0000-0001-9951-5239
https://orcid.org/0000-0001-9951-5239
References
- 1.Gadjradj PS, Arts MP, van Tulder MW, Rietdijk WJR, Peul WC, Harhangi BS. Management of symptomatic lumbar disk herniation: an international perspective. Spine (Phila Pa 1976). 2017;42(23):1826–1834. [DOI] [PubMed] [Google Scholar]
- 2.Kerr D, Zhao W, Lurie JD. What are long-term predictors of outcomes for lumbar disc herniation? A randomized and observational study. Clin Orthop Relat Res. 2015;473(6):1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318(5):291–300. [DOI] [PubMed] [Google Scholar]
- 4.Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211(5):210–215. [Google Scholar]
- 5.Iwa H, Caspar W. A microsurgery operation for lumbar disc herniation (author’s transl). No Shinkei Geka. 1978;6(7):657–662. [PubMed] [Google Scholar]
- 6.Yasargil MG, Vise WM, Bader DC. Technical adjuncts in neurosurgery. Surg Neurol. 1977;8(5):331–336. [PubMed] [Google Scholar]
- 7.Foley KT, Smith MM, Rampersaud YR. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus. 1999;7(5):e5. [DOI] [PubMed] [Google Scholar]
- 8.Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991;58(2):159–164. [PubMed] [Google Scholar]
- 9.Leu H, Schreiber A. Percutaneous nucleotomy with disk endoscopy—a minimally invasive therapy in non-sequestrated intervertebral disk hernia. Schweiz Rundsch Med Prax. 1991;80(14):364–368. [PubMed] [Google Scholar]
- 10.Mayer HM, Brock M, Berlien HP, Weber B. Percutaneous endoscopic laser discectomy (PELD). A new surgical technique for non-sequestrated lumbar discs. Acta Neurochir Suppl (Wien). 1992;54:53–58. [DOI] [PubMed] [Google Scholar]
- 11.Hoogland T, Schubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976). 2006;31(24):E890–E897. [DOI] [PubMed] [Google Scholar]
- 12.Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976). 2002;27(7):722–731. [DOI] [PubMed] [Google Scholar]
- 13.Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine (Phila Pa 1976). 2005;30(22):2570–2578. [DOI] [PubMed] [Google Scholar]
- 14.Cong L, Zhu Y, Tu G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J. 2016;25(1):134–143. [DOI] [PubMed] [Google Scholar]
- 15.Garg B, Nagraja UB, Jayaswal A. Microendoscopic versus open discectomy for lumbar disc herniation: a prospective randomised study. J Orthop Surg (Hong Kong). 2011;19(1):30–34. [DOI] [PubMed] [Google Scholar]
- 16.Gibson JNA, Subramanian AS, Scott CEH. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J. 2017;26(3):847–856. [DOI] [PubMed] [Google Scholar]
- 17.Hermantin FU, Peters T, Quartararo L, Kambin P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am. 1999;81(7):958–965. [DOI] [PubMed] [Google Scholar]
- 18.Huang TJ, Hsu RW, Li YY, Cheng CC. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res. 2005;23(2):406–411. [DOI] [PubMed] [Google Scholar]
- 19.Hussein M, Abdeldayem A, Mattar MM. Surgical technique and effectiveness of microendoscopic discectomy for large uncontained lumbar disc herniations: a prospective, randomized, controlled study with 8 years of follow-up. Eur Spine J. 2014;23(9):1992–1999. [DOI] [PubMed] [Google Scholar]
- 20.Jin D, Xu N, Zhao G. Comparative study of percutaneous transforaminal endoscopic discectomy versus fenestration discectomy in patients with lumbar disc herniation. Chin J Min Inv Surg. 2017;17(6):491–494. [Google Scholar]
- 21.Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18(1):61–70. [PubMed] [Google Scholar]
- 22.Lee SH, Chung SE, Ahn Y, Kim TH, Park JY, Shin SW. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med. 2006;73(5):795–801. [PubMed] [Google Scholar]
- 23.Liu J, Zhen W, Gao G, et al. A prospective and controlled study of percutaneous transforaminal endoscopic discectomy vercus fenestration discectomy for lumbar disc herniation. Chinses J Bone Joint. 2014;3(4):245–250. [Google Scholar]
- 24.Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78(2):216–225. [DOI] [PubMed] [Google Scholar]
- 25.Pan L, Zhang P, Yin Q. Comparison of tissue damages caused by endoscopic lumbar discectomy and traditional lumbar discectomy: a randomised controlled trial. Int J Surg. 2014;12(5):534–537. [DOI] [PubMed] [Google Scholar]
- 26.Pan Z, Ha Y, Yi S, Cao K. Efficacy of transforaminal endoscopic spine system (TESSYS) technique in treating lumbar disc herniation. Med Sci Monit. 2016;22:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery. 2007;61(3):545–549. [DOI] [PubMed] [Google Scholar]
- 28.Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008;33(9):931–939. [DOI] [PubMed] [Google Scholar]
- 29.Ruetten S, Komp M, Merk H, Godolias G. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. 2009;22(2):122–129. [DOI] [PubMed] [Google Scholar]
- 30.Tacconi L, Signorelli F, Giordan E. Surgery is full endoscopic lumbar discectomy less invasive than conventional? A randomized MRI study. World Neurosurg. 2020;138:e867–e875. [DOI] [PubMed] [Google Scholar]
- 31.Tang G, Huang Q, Zhang W. Preliminary outcomes of percutaneous transformational endoscopic lumbar discectomy for elder patients with lumbar disc herniation. China J Endoscopy. 2012;18(12):1300–1303. [Google Scholar]
- 32.Teli M, Lovi A, Brayda-Bruno M, et al. Higher risk of Dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J. 2010;19(3):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Pan L, Huang B. Comparative study on percutaneous transforaminal endoscopic discectomy and small incision method for lumbar disc herniation. J Pract Orthop. 2015;21(4):293–296. [Google Scholar]
- 34.Wu J, Ge B, Wu D. A comparison between intervertebral fenestration and percutaneous transforaminal endoscopic discectomy for treatment of lumbar disc herniation. Orthop J China. 2016;24(21):1972–1976. [Google Scholar]
- 35.Chen Z. Comparison of percutaneous endoscopic through different surgical treatment for lumber disc herniation. J Medical Forum. 2015;36(6):39–42. [Google Scholar]
- 36.Huang H, Yang B, Song J. A comparison of surgical treatment effect of L5 / S1 herniated disc under different percutaneous endoscopy. Lab Med Clin. 2017;14(11):1651–1653. [Google Scholar]
- 37.Mo X, Shen J, Jiang W, et al. Percutaneous endoscopic lumbar diskectomy for axillar herniation at L5-S1 via the transforaminal approach versus the interlaminar approach: a prospective clinical trial. World Neurosurg. 2019;125:e508–e514. [DOI] [PubMed] [Google Scholar]
- 38.Nie H, Zeng J, Song Y, et al. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation via an interlaminar approach versus a transforaminal approach: a prospective randomized controlled study with 2-year follow up. Spine (Phila Pa 1976). 2016;41(suppl 19):B30–B37. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Shi L, Chu L, Chen L, Ke Z, Deng Z. Comparison of percutaneous endoscopic via interlaminar and transforaminal approach for lumbar disc herniation. J Spinal Surg. 2013;11(2):97–100. [Google Scholar]
- 40.Nakagawa H, Kamimura M, Uchiyama S, Takahara K, Itsubo T, Miyasaka T. Microendoscopic discectomy (MED) for lumbar disc prolapse. J Clin Neurosci. 2003;10(2):231–235. [DOI] [PubMed] [Google Scholar]
- 41.Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg. 2016;31:86–92. [DOI] [PubMed] [Google Scholar]
- 42.Ondeck NT, Bohl DD, McLynn RP, et al. Longer operative time is associated with increased adverse events after anterior cervical diskectomy and fusion: 15-minute intervals matter. Orthopedics. 2018;41(4):e483–e488. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, Liu S, Liu J, et al. Transforaminal endoscopic discectomy versus conventional microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. J Orthop Surg Res. 2018;13(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan K, Xu J, Schultz K, et al. Full-endoscopic versus micro-endoscopic and open discectomy: a systematic review and meta-analysis of outcomes and complications. Clin Neurol Neurosurg. 2017;154:1–12. [DOI] [PubMed] [Google Scholar]
- 45.Alvi MA, Kerezoudis P, Wahood W, Goyal A, Bydon M. Operative approaches for lumbar disc herniation: a systematic review and multiple treatment meta-analysis of conventional and minimally invasive surgeries. World Neurosurg. 2018;114:391–407.e392. [DOI] [PubMed] [Google Scholar]
- 46.Kim M, Lee S, Kim HS, Park S, Shim SY, Lim DJ. A comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for lumbar disc herniation in the Korean: a meta-analysis. Biomed Res Int. 2018;2018:9073460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casal-Moro R, Castro-Menéndez M, Hernández-Blanco M, Bravo-Ricoy JA, Jorge-Barreiro FJ. Long-term outcome after microendoscopic diskectomy for lumbar disk herniation: a prospective clinical study with a 5-year follow-up. Neurosurgery. 2011;68(6):1568–1575. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51(5 suppl):S129–136. [PubMed] [Google Scholar]
- 49.Goald HJ. Microlumbar discectomy: follow-up of 477 patients. J Microsurg. 1980;2(2):95–100. [DOI] [PubMed] [Google Scholar]
- 50.Kim MJ, Lee SH, Jung ES, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol. 2007;68(6):623–631. [DOI] [PubMed] [Google Scholar]
- 51.Schaffer JL, Kambin P.Percutaneous posterolateral lumbar discectomy and decompression with a 6.9-millimeter cannula. Analysis of operative failures and complications. J Bone Joint Surg Am. 1991;73(6):822–831. [PubMed] [Google Scholar]
- 52.Qin R, Liu B, Hao J, et al. Percutaneous endoscopic lumbar discectomy versus posterior open lumbar microdiscectomy for the treatment of symptomatic lumbar disc herniation: a systemic review and meta-analysis. World Neurosurg. 2018;120:352–362. [DOI] [PubMed] [Google Scholar]
- 53.Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine. 2009;10(5):476–485. [DOI] [PubMed] [Google Scholar]
- 54.Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane review. Spine (Phila Pa 1976). 2007;32(16):1735–1747. [DOI] [PubMed] [Google Scholar]