Abstract
Study Design:
Systematic review.
Objective:
Indirect decompression via lateral lumbar interbody fusion (LLIF) can ameliorate central and foraminal lumbar stenosis. In severe central stenosis, additional posterior direct decompression is utilized. The aim of this review is to synthesize existing literature on these 2 techniques and identify significant differences in outcomes between isolated indirect decompression via LLIF and combined indirect decompression supplemented with direct posterior decompression.
Methods:
A database search algorithm was utilized to query MEDLINE, COCHRANE, and EMBASE to identify literature reporting adult decompression study groups that involved an oblique or lateral fusion approach through September 2020. Improvement in outcomes measures and complication rates were pooled and tested for significance.
Results:
A total of 110 publications were assessed with 15 studies meeting inclusion criteria, including 557 patients and 1008 levels. Mean age was 63.1 years with BMI of 27.5 kg/m2. For the combined indirect and direct decompression cohort, lumbar lordosis (LL) increased 133.9%, from 22.8o to 48.7o, while the indirect decompression cohort LL increased 8.9%, from 41.9o to 45.5o. Difference in LL improvement between cohorts was insignificant (P > .05). Oswestry Disability Index (ODI) decreased from 36.5 to 19.4 in the combined indirect and direct decompression cohort, and from 44.4 to 23.1 in the indirect decompression cohort. ODI reduction was insignificant (P = .053).
Conclusions:
Prior studies of both indirect decompression as well as combined indirect and direct decompression of lumbar spine stenosis are limited by small samples, heterogeneous populations, and lack of direct comparisons. Both procedures result in improved function and pain postoperatively with direct decompression restoring more lordosis in patients with worse preoperative alignment.
Keywords: spine fusion, lateral approach, indirect decompression, direct decompression, posterior instrumentation, systematic review
Introduction
Indirect decompression via minimally invasive lateral lumbar interbody fusion (LLIF) can be utilized to treat both foraminal stenosis and mild central stenosis secondary to various degenerative spinal pathologies.1-5 Although direct visualization of the dural sac or nerve roots is not performed in LLIF, neural elements are decompressed indirectly by increasing disc height, foraminal area, and central canal area after placement of an interbody cage. Iterations of LLIF, which have been patented and described in the literature, include direct lateral interbody fusion (DLIF, Medtronic Sofamore Danek, Minneapolis, Minnesota, USA) and extreme lateral interbody fusion (XLIF, NuVasive, Inc., San Diego, California, USA). 6 Oblique lumbar interbody fusion (OLIF) enables achievement of the same therapeutic aims of LLIF through use of a very similar surgical approach developed to obviate the need for psoas muscle dissection, thereby minimizing risk for both post-operative leg weakness and symptoms secondary to iatrogenic genitofemoral nerve injury. 7 Together, lateral and oblique spine fusion have grown in popularity by avoiding the drawbacks of anterior interbody lumbar fusion (ALIF), a procedure which requires a significantly larger surgical incision and great vessel mobilization to enable visualization of the vertebral bodies, while yielding improved clinical outcomes and good fusion rates.1-8
In cases of severe central stenosis, additional posterior direct decompression may be performed in conjunction with LLIF.9-11 Resection of posterior compressive structures such as bony osteophytes, facet joints, and ligamentum flavum cannot be performed via the lateral approach to the lumbar spine.12,13 Today, posterior decompression techniques may be performed using midline open, mini-open, or minimally invasive surgery (MIS) techniques. MIS posterior lumbar decompression utilizes a tubular retractor system to minimize dissection and destabilization of posterior midline structures such as the paraspinal musculature and stabilizing ligaments.1,2,14
The aim of this review is to analyze the existing literature and identify any significant differences in outcomes or complications between isolated indirect decompression from lateral interbody fusion and combined indirect decompression with additional direct posterior decompression surgery. As this type of analysis has not yet been performed, better understanding of the relative outcomes of indirect decompression and indirect decompression with additional direct posterior decompression may allow for an expansion of indications for isolated lateral interbody fusion as well as a better understanding for when additional posterior decompression is warranted.
Methods
Protocol
Methodological workflow for this review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes Protocols (PRISMA-P) approach. 15
Eligibility Criteria, Information Sources, and Search Strategy
A database search algorithm was utilized to individually query 3 databases (MEDLINE, COCHRANE, and EMBASE) and identify all relevant literature reporting decompression study groups that involved a lateral or oblique spinal fusion approach. The cutoff date for publications examined for inclusion was September 1, 2020. The database search algorithm utilized Boolean logic to search for literature which included the following terminology and their associated acronyms: “lateral lumbar interbody fusion,” “oblique lumbar interbody fusion,” “indirect decompression,” “direct decompression,” “posterior decompression,” “laminectomy,” and “laminotomy.” Excluded from the query results were all non-human, cadaveric, and non-English literature.
Study Selection
All publications assessed in this review were collected and managed using a systematic review platform (Covidence Software, Veritas Health Innovation, Melbourne, Australia, available at www.covidence.org). These publications were examined at the title and abstract level independently by 3 reviewers (AMS, KWM, KAS). Full manuscript review was conducted by 2 reviewers (MKM and AMS). Discordance of study inclusion recommendations between the independent reviewers were reviewed by the senior author (SI) who individually examined each disagreement and finalized the study inclusion list. Table 1 defines the full criteria used to screen the 110 publications considered in this review for inclusion and exclusion.
Table 1.
Review Inclusion/Exclusion Criteria.
| Review criteria | Inclusion | Exclusion |
|---|---|---|
| Patient population | Age ≥18 years old Indicated pathologies:
|
Age <18 Years Old Out-of-scope pathologies:
|
| Intervention | DLIF, LLIF, LIF, OLIF, or XLIF ± (stand-alone, PSF, and/or open decompression) | ALIF, AxiaLIF, PLIF, TLIF |
| Outcomes | Reported PROMs (VAS, ODI, SF-12/36), cross-sectional areas, vertebral angles, complications, revisions | Unreported outcomes |
| Level of evidence | I/II—RCT III—non-random trials IV—Case-Control/Cohort |
Case reports Reviews |
| Publication types | English language Peer reviewed journal |
Letters & abstracts Duplicate patient population studies Feasibility studies Radiologic diagnostic studies Cadaveric studies In Vitro studies Non-Human studies Non-English studies |
Abbreviations: ALIF, anterior lumbar interbody fusion; AxiaLIF, axial interbody fusion (Baxano Surgical, Raleigh, NC, USA); DLIF, direct lateral interbody fusion (Medtronic Sofamor Danek, Memphis, TN, USA); LIF, lateral interbody fusion; LLIF, lateral lumbar interbody fusion; ODI, Oswestry disability index; OLIF, oblique lumbar interbody fusion (Medtronic Sofamor Danek, Memphis, TN, USA); PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion; PROMs, patient reported outcomes measurements; SF-12, short form-12; SF-36, short form-36; TLIF, transforaminal lumbar interbody fusion; VAS, visual analog scale; XLIF, extreme lateral interbody fusion (NuVasive, San Diego, CA, USA).
Data Collection Process and Data Items
Each study group included in this review was assessed across 110 individually extracted data points. Data extracted from each study spanned the following categories: surgical approach, group size, levels, patient demographics, radiological assessment approach, as well as all reported surgical and clinical outcomes.
Risk of Bias Assessment
The Newcastle-Ottawa Scale (NOS) was utilized to assess the methodological quality of all publications included in this review. Several aspects were considered: subject selection (patient diagnoses and study population source), data comparability, radiological assessment approach (radiograph vs. advanced imaging), follow-up length (months), and loss-to-follow-up rate.
Summary Measures and Synthesis of Results
Statistical analysis involved examination of data for significant difference across 2 study cohorts (indirect and direct decompression) for the included study groups summarized in this review. Means reported in this review were pooled with weighting by group size. Meta-analysis was determined to not be feasible in this review because of relatively small sample sizes as well as a high degree of variability in outcomes assessment methodology across the included studies. Statistical difference was assessed using Student t tests (two-tail, unpaired, unequal variance assumed) with secondary post-hoc Tukey’s HSD testing for validation. Significance was assigned if P < .05. All data analysis was completed using Microsoft Excel 365 Version 2011 (Microsoft Corporation, Redmond, Washington, USA).
Results
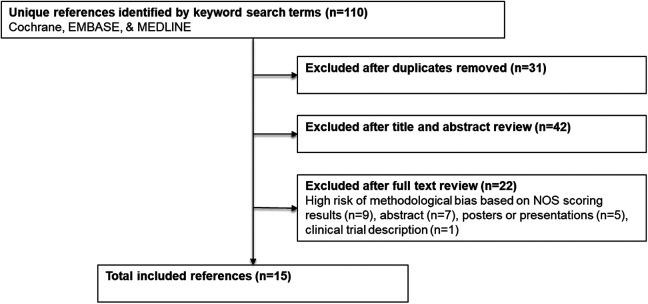
A total of 79 publications were assessed for inclusion at the title and abstract level after excluding 31 duplicate publications (Figure 1). Overall, 15 studies satisfied the inclusion criteria after full review. With regard to study design, there were 10 retrospective studies, 5 prospective studies, and no randomized control trials. Three studies analyzed lateral interbody fusion combined with additional direct posterior decompression surgery (direct cohort) across 42 patients and 161 vertebral levels (Table 2). Mean subject age was 67.2 ± 5.8 years, 76.3% were female, and the mean follow-up period was 15.6 ± 7.6 months (range, 6.0-21.0 months). Fifteen studies analyzed isolated indirect decompression approach across 557 patients and 1008 vertebral levels with a mean fusion rate of 95.1% ± 4.5% (range, 89.2%-100.0%). Mean subject age was 63.1 ± 11.6 years, 59.2% were female, and the mean follow-up period was 17.6 ± 9.6 months (range, 6.0-34.8 months). There was a statistically significant difference in age (P < .01) and female to male ratio (P < .01) between cohorts.
Figure 1.
Workflow for identifying included references. The systematic review workflow utilized in this review closely followed the PRISMA-P best-practice recommendations.
Table 2.
Demographics of Included Studies.
| Study | Subjects | Levels | Age (years) | BMI (kg/m2) | F/U Period (Months) | Duration (minutes) | Blood loss (mL) | LOS (Days) | Female % | Smoker % |
|---|---|---|---|---|---|---|---|---|---|---|
| Direct | ||||||||||
| Attenello et al, 201816 | 13 | 37 | 68.0 | NR | 21.0 | NR | NR | NR | 84.6% | 0.0% |
| Lee et al, 201617 | 21 | 113 | 67.1 | NR | 15.9 | 680.0 | 2127.1 | 24.6 | 81.3% | NR |
| Lim et al, 201914 | 8 | 11 | 66.0 | NR | 6.0 | NR | NR | 6.0 | 50.0% | 0.0% |
| Total or pooled mean | 42 | 161 | 67.2 | - | 15.6 | 680.0 | 2127.1 | 19.5 | 76.3% | 0.0% |
| Standard deviation | - | - | 5.8 | - | 7.6 | - | - | 13.2 | 19.1% | 0.0% |
| Indirect | ||||||||||
| Ahmadian et al, 201310 | 31 | 31 | 61.5 | NR | 18.2 | NR | 94.0 | 3.5 | 71.0% | NR |
| Attenello et al, 201816 | 9 | 25 | 68.0 | NR | 24.0 | NR | NR | NR | 66.7% | 0.0% |
| Campbell et al, 20185 | 18 | 20 | 64.0 | 34.0 | 6.2 | 165.0 | 113.0 | NR | 61.0% | NR |
| Castellvi et al, 201411 | 44 | 117 | 66.0 | 29.4 | 12.0 | 410.0 | 217.0 | NR | 47.7% | NR |
| Fujibayashi et al, 201526 | 28 | 52 | 65.3 | NR | NR | 72.5 | 32.7 | NR | 64.3% | NR |
| Lee et al, 201617 | 11 | 59 | 67.1 | NR | 15.9 | 648.0 | 809.1 | 17.4 | 81.3% | NR |
| Lim et al, 201914 | 42 | 55 | 64.0 | NR | 6.0 | NR | NR | 3.0 | 60.0% | 16.7% |
| Lin et al, 20183 | 25 | 25 | 64.0 | 24.1 | 29.0 | 96.0 | 106.4 | 8.5 | 68.0% | 4.0% |
| Louie et al, 201828 | 25 | 100 | 61.9 | NR | 34.8 | 108.1 | 63.0 | 1.8 | 28.0% | 8.0% |
| Malham et al, 201613 | 40 | 40 | 58.5 | 28.3 | 34.1 | NR | NR | NR | 22.0% | 0.0% |
| Marchi et al, 201327 | 74 | 98 | 56.7 | 24.7 | 12.0 | 77.3 | 50.0 | NR | 64.9% | NR |
| Nemani et al, 20144 | 117 | 239 | 63.6 | 27.4 | 15.6 | 146.0 | 144.0 | NR | 70.9% | NR |
| Pereira et al, 201725 | 23 | 42 | 61.0 | NR | 12.0 | 222.0 | NR | 7.7 | 52.2% | 13.0% |
| Sato et al, 20171 | 20 | 20 | 69.0 | NR | 12.0 | NR | NR | NR | 55.0% | 10.0% |
| Scherman et al, 20192 | 50 | 84 | 68.2 | 29.0 | 25.2 | NR | NR | 6.9 | 62.0% | 10.0% |
| Total or pooled mean | 557 | 1008 | 63.1 | 27.5 | 17.6 | 173.1 | 173.1 | 5.8 | 59.2% | 8.5% |
| Standard deviation | - | - | 11.6 | 4.1 | 9.6 | 192.9 | 241.9 | 5.3 | 15.8% | 6.0% |
| P-value | - | - | .01* | - | .24 | - | - | .27 | .17 | .00* |
Abbreviations: BMI, body mass index; F/U, follow-up; LOS, average length of stay; NR, not reported.
* Significant difference.
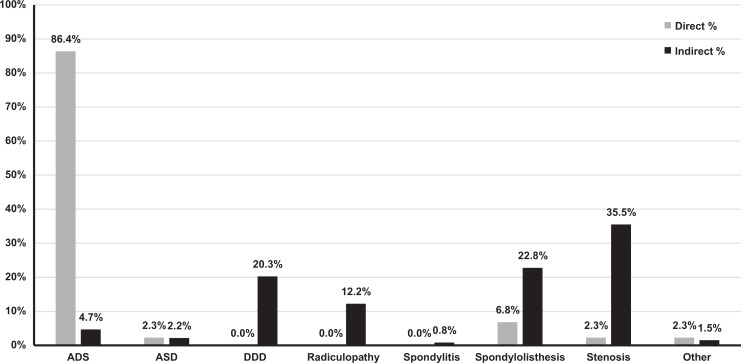
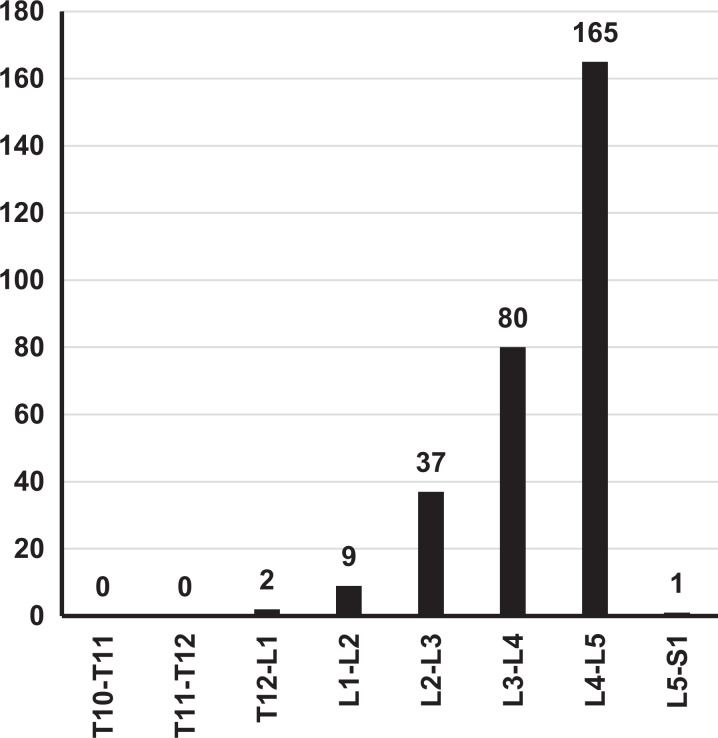
Indication for surgical intervention and operative segment level varied across the included studies. For the direct decompression cohort, a diagnosis of adult degenerative scoliosis (ADS) was the primary indication in 86.4% of patients (Figure 2). For the indirect decompression cohort, stenosis was the most common diagnosis (36.1%), followed by spondylolisthesis (23.1%), degenerative disc disease (DDD, 20.6%), radiculopathy (12.4%), ADS (4.8%), and adjacent segment disorder (ASD, 2.2%). With respect to the indirect decompression cohort, the most commonly operated spinal segment level was L4-L5 (56.1%) followed by L3-L4 (27.2%), L2-L3 (12.6%), and L1-L2 (3.1%) (Figure 3). Publications reporting a combined direct and indirect decompression group did not report operative level data.
Figure 2.
Percentage of cases by indication. ASD, adjacent segment disorder, ADS, adult degenerative scoliosis, DDD, degenerative disc disease. Indication of cases varied by decompression approach.
Figure 3.
Indirect decompression operative spinal segment levels. Operative spinal segment levels were generally located in the lumbar spine, most frequently involving L4-L5.
Radiological outcomes of surgery were assessed utilizing radiographs in 11 out of 15 (73.3%) studies summarized by this review with the rest utilizing advanced imaging techniques (computed tomography [CT] or magnetic resonance imaging [MRI]). All 3 studies reporting direct decompression groups utilized radiographs.14,16,17 For the direct decompression cohort, lumbar lordosis (LL) increased by 133.9% from 22.8o to 48.7o (Table 3). For the indirect decompression cohort, LL increased by 8.9% from 41.9o to 45.5o. LL improvement difference between cohorts was insignificant (P > .05). Fusion rate was reported for only 1 of the 3 direct decompression groups (87.5%). 14 Pooled fusion rate reported for 9 indirect decompression groups was higher (95.1%); however, it was not possible to assess this difference for statistical significance.
Table 3.
Lumbar Lordosis Radiological Measurements Reported.
| Direct | Indirect | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | Mean | SD | Studies | Mean | SD | Studies | P-value |
| Pre-Op lumbar lordosis | 22.8 | 8.9 | 2 | 41.9 | 9.1 | 8 | .09 |
| Post-Op lumbar lordosis | 48.7 | 8.0 | 2 | 45.5 | 9.4 | 8 | .38 |
| Lumbar lordosis difference | 25.9 | 16.9 | 2 | 3.7 | 2.8 | 8 | .18 |
| Lumbar lordosis difference % | 133.9% | 111.9% | 2 | 8.9% | 6.9% | 8 | .20 |
Abbreviations: Pre-Op, pre-operation; Post-Op, post-operation.
Patient reported outcome measures (PROMs) were reported using 10 individual scales across all 15 studies included in this review. The most consistently utilized PROM scales (≥2 studies) were considered for comparison across the indirect and direct decompression cohorts. Direct decompression Oswestry Disability Index (ODI) mean pre- and post-operative scoring decreased from 36.5 to 19.4 (Table 4). The indirect decompression ODI mean pre- and post-operative scoring decreased from 44.4 to 23.1. This difference in ODI improvement between groups was not statistically significant (P = .053). Mean decrease in Visual Analog Scale (VAS) back pain between pre- and post-operative scoring was 7.0 to 2.7 for the direct decompression cohort and 7.1 to 2.9 for the indirect decompression cohort This difference in VAS-back pain score improvement was also not statistically significant between groups (P = .46). VAS-leg pain reduction was from 4.9 to 2.3 for the direct decompression cohort and 6.8 to 2.2 for the indirect decompression cohort (P = .27).
Table 4.
Patient-Reported Outcomes Measures of Included Studies.
| Direct | Indirect | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | Mean | SD | # of studies | Mean | SD | # of studies | P-value |
| ODI | |||||||
| Pre-Op ODI | 36.5 | 12.8 | 3 | 44.4 | 8.8 | 12 | .17 |
| Post-Op ODI | 19.4 | 10.3 | 3 | 23.1 | 8.3 | 12 | .30 |
| ODI difference | −17.1 | 3.5 | 3 | −21.3 | 7.2 | 12 | .05 ^ |
| VAS back pain | |||||||
| Pre-Op VAS back pain | 7.0 | 1.8 | 3 | 7.1 | 0.8 | 13 | .40 |
| Post-Op VAS back pain | 2.7 | 0.6 | 3 | 2.9 | 1.0 | 13 | .34 |
| VAS back pain difference | −4.3 | 1.5 | 3 | −4.2 | 0.9 | 13 | .46 |
| VAS leg pain | |||||||
| Pre-Op VAS leg pain | 4.9 | 2.9 | 2 | 6.8 | 1.6 | 8 | .37 |
| Post-Op VAS leg pain | 2.3 | 0.8 | 2 | 2.2 | 0.5 | 8 | .34 |
| VAS leg pain difference | −2.7 | 2.0 | 2 | −4.6 | 1.6 | 8 | .27 |
Abbreviations: ODI, Oswestry disability index; VAS, visual analog scale.
^ Insignificant Difference.
All-complications and revision rates varied across the direct and indirect decompression groups (Table 5). Direct decompression studies reported a mean all-complication rate of 16.7%. Indirect decompression studies reported a mean all-complication rate of 29.0%. Revision rate for direct decompression cases was 9.5% while the revision rate for indirect decompression cases was 8.1%. Relative to direct decompression, increased odds of all-complications and revision surgery after isolated indirect decompression were not statistically significant. Overall, the reported revision and all-complications rates in this systematic review are consistent with observed means across the spine fusion and decompression literature. 18
Table 5.
Post-Operative Clinical Indicators of Included Studies.
| Direct | Indirect | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | Mean | SD | # of Studies | Mean | SD | # of studies | P-value |
| Complication rate | 16.7% | 8.4% | 3 | 29.0% | 17.6% | 14 | .06 |
| Revision rate | 9.5% | 7.8% | 3 | 8.3% | 9.9% | 11 | .34 |
Twelve studies were assessed to have medium risk of bias due to methodological quality based on their NOS scores of 5, 6, or 7, with an average of 6.9 ± 0.5 out of 9.0 possible points (Table 6). The remaining 3 papers were assigned NOS scores of 8 or 9, with an average of 8.0 ± 0.0 out of 9.0 possible points, indicating low risk of methodological bias. Primary sources of bias identified during this assessment included study population selection, limited follow-up duration, and radiological evaluation approach. All included papers involved patients older on average relative to the patient demographics observed among all spine fusion surgery cases in the United States. 19 Radiological follow-up periods of less than 12 months typically required for bony fusion to occur, unreported fusion rate, unspecified graft approach, or limited patient demographic details were additional aspects that led to assignment of lower NOS scores. Overall, the studies included in this review have relatively limited risk of bias due to quality of approach.
Table 6.
Newcastle-Ottawa Scale (NOS) Risk of Bias Scoring of Included Studies.
| Study | NOS selection | NOS comparable | NOS outcomes | NOS total | Risk of bias |
|---|---|---|---|---|---|
| Ahmadian et al, 201310 | 3 | 1 | 3 | 7 | Medium |
| Attenello et al, 201816 | 2 | 1 | 3 | 6 | Medium |
| Campbell et al, 20185 | 3 | 1 | 2 | 6 | Medium |
| Castellvi et al, 201411 | 3 | 2 | 3 | 8 | Low |
| Fujibayashi et al, 201526 | 3 | 2 | 2 | 7 | Medium |
| Lee et al, 201617 | 2 | 1 | 3 | 6 | Medium |
| Lim et al, 201914 | 3 | 1 | 2 | 6 | Medium |
| Lin et al, 20183 | 3 | 1 | 3 | 7 | Medium |
| Louie et al, 201828 | 3 | 1 | 3 | 7 | Medium |
| Malham et al, 201613 | 3 | 2 | 3 | 8 | Low |
| Marchi et al, 201327 | 3 | 2 | 3 | 8 | Low |
| Nemani et al, 20144 | 3 | 1 | 3 | 7 | Medium |
| Pereira et al, 201725 | 3 | 1 | 3 | 7 | Medium |
| Sato et al, 20171 | 3 | 1 | 3 | 7 | Medium |
| Scherman et al, 20192 | 3 | 1 | 3 | 7 | Medium |
| Average | 2.9 | 1.3 | 2.8 | 6.9 | Medium |
Discussion
Prior studies of outcomes after lumbar indirect decompression via lateral interbody fusion with or without additional posterior direct decompression are limited by small sample sizes and heterogeneous patient populations. In addition, few studies directly compare these 2 techniques, with most studies reporting outcomes after either indirect or direct decompression procedures. Therefore, the current study aims to synthesize this existing literature via a systematic review. While significant differences in clinical and radiographic outcomes were not identified, both cohorts demonstrated improvements in patient reported outcome measures postoperatively. In addition, there was a notable trend toward a greater decrease in ODI with isolated indirect decompression and greater improvement in lumbar lordosis with additional direct posterior decompression.
Of the 15 studies included in this review, the most consistently comparable patient reported outcomes were ODI, VAS-leg pain, and VAS-back pain. Of those, statistically significant differences in improvement were not identified between the 2 cohorts. There was a trend toward greater improvement in ODI after isolated indirect decompression cohort (−21.3 vs. −17.1; P = .053). The limited number of patients in the current analysis likely limits the statistical power of this comparison. However, suggestion of a difference in functional outcomes between these procedures is novel, and the reason for this is unclear. While it may be assumed that patients requiring additional direct decompression have more severe preoperative stenosis and worse baseline function, this was not the case. In fact, patients undergoing additional direct posterior decompression actually had less preoperative disability compared with isolated indirect decompression patients, although this difference was not statistically significant. Another possible explanation is that posterior decompression results in additional approach-related pain and longer postoperative recovery. While studies of indirect decompression in isolation show good postoperative outcomes overall, it is often difficult to compare these studies with populations undergoing direct decompression due to differences in the underlying degenerative pathology being managed. However, it appears that in many cases, isolated indirect decompression is in indeed sufficient for treating lumbar degenerative pathology.
Interestingly, in terms of radiographic parameters, no significant differences were noted between indirect and direct decompression groups. While a greater increase in lumbar lordosis was seen in patients undergoing direct posterior decompression (+25.9° versus +3.7°), this difference was not statistically significant (P = .18). We hypothesize that this difference is likely due to additional posterior column bony resection and facetectomies that can be performed with direct posterior decompression, thereby achieving decompression across posterior instrumentation. In addition, patients that underwent an additional direct posterior decompression had lower mean preoperative lumbar lordosis (22.8° vs. 41.9°, P = .09), suggesting that restoration of lordosis was a defined goal of the fusion surgery and potentially 1 reason for the additional posterior decompression. For example, Lee et al retrospectively studied an adult degenerative scoliosis population and found that preoperative lordosis was lower in patients indicated for direct posterior decompression (18.0°) versus indirect decompression (35.0°) while these patients had similar lordosis postoperatively (53.0° versus 49.0°). 17 Attenello et al similarly studied adult spinal deformity population. They demonstrated a greater postoperative improvement in lumbar lordosis with direct decompression (11.1°) versus indirect decompression (3.8°), despite a similar mean preoperative lordosis. 16 In the studies considering only indirect decompression, limited improvement in lordosis was seen. Pereira et al followed 23 patients, 8 of which had a diagnosis of degenerative scoliosis and found a postoperative improvement in lumbar lordosis of only 3.9°. 25 Fujibayashi et al similarly found a 4.5° increase in segmental lordosis (total lumbar lordosis was not reported) in 28 patients, 25.0% of whom had degenerative scoliosis. 26 Therefore, despite the lack of statistically significant results, the current data does suggest that addition of direct posterior decompression may aid in restoration of lumbar lordosis.
Overall, complication and revision rates were not statistically different between groups. However, there was a trend toward increased complications with isolated indirect decompression at 29.0% versus 16.7% for combined indirect and direct decompression (P = .06). As complications could only be assessed from 2 of the 3 studies of direct decompression, the true complications rates may be similar with a varied distribution of complication types. Higher rates of intraoperative nerve root injuries are seen with a posterior approach to the lumbar spine. In 1 systematic review of 6,669 MIS transforaminal lumbar interbody fusions (TLIF), the rate of postoperative radiculitis or neurological deficits was 5.9%. 20 While posterior dissection and nerve root manipulation for MIS TLIF is more extensive than for a posterior decompression, these risks are largely avoided with an isolated lateral approach. However, the lateral approach also introduces the possibility of different risks associated with spine-adjacent structures including the lumbar plexus and psoas muscle. In a series of 55 patients undergoing either MIS LLIF or TLIF, 31.0% of LLIF patients had postoperative hip flexion weakness compared with 0.0% of TLIF patients. 21
Similar reoperation rates were observed between cohorts. Lee et al demonstrated a 14.3% revision rate among 21 patients undergoing direct decompression. 17 This included 2 cases of epidural hematoma requiring evacuation as well as 1 deep infection requiring irrigation and debridement. There were no lateral approach-related complications with direct or indirect decompression cohorts. In addition, Lim et al demonstrated a 0.0% revision rate and 4.0% rate of intraoperative dural tear among patients undergoing direct decompression. 14 With patients undergoing indirect decompression, Marchi et al demonstrated a 13.5% revision rate, involving 10 of 74 patients, including 6 patients who experienced subsidence and 4 patients who had persistent post-operative stenosis. 27 Similarly, Louie et al demonstrated a 12.0% reoperation rate among patients undergoing indirect decompression involving 3 of 25 patients. 28 All 3 patients required subsequent posterior decompression and fusion for either persistent stenosis, graft subsidence, or new instability. As the risk of reoperation for inadequate decompression or refractory stenosis appears higher with isolated indirect decompression, this risk appears to be offset by increased reoperations for epidural hematoma and infections associated with utilization of a posterior approach.
Lee et al examined patients undergoing 3-, 4-, or 5-level interbody fusion with concomitant spinal stenosis and lumbar deformity. 17 In this cohort, patients undergoing open posterior decompression had worse preoperative kyphotic deformity with pre-operative lumbar lordosis of 18.0o versus 35.0o in their percutaneous fixation group. The additional facetectomies and osteotomies performed in these patients likely contributed to worse postoperative disability and pain. Interestingly, there were no significant differences in postoperative improvements in pain and function between patients with open posterior decompression and percutaneous posterior fixation.
Attenello et al similarly compared open decompression versus percutaneous fixation after LLIF in a population of patients with adult degenerative scoliosis. 16 However, they noted lack of significant difference between groups in terms of preoperative radiographic measures of deformity (lumbar lordosis, pelvic incidence minus lumbar lordosis [PI-LL], and coronal cobb angle). Nevertheless, each decision for posterior decompression versus percutaneous instrumentation was made independently by the treating surgeon based on clinical and radiographic factors. The authors found a significantly greater improvement in overall VAS pain score in the percutaneous group compared with the open posterior decompression group. However, no significant difference was noted when analyzing VAS-leg pain, VAS-back pain, or ODI (similar to the overall findings of this systematic review).
Finally, Lim et al studied a mixed population of patients with multiple degenerative diagnoses undergoing either isolated LLIF or LLIF with posterior decompression. 14 The most frequent diagnosis among isolated LLIF patients was degenerative spondylolisthesis, while the most common diagnosis among posterior decompression patients was degenerative scoliosis. The decision to proceed with isolated lateral lumbar fusion with or without additional posterior decompression was dependent on the presence of persistent neurogenic claudication pain or radicular leg pain, despite changes in posture or sitting. Both groups demonstrated significant improvements in all patient reported outcomes by final follow up, again without significant difference in improvement between the 2 groups (consistent with the overall findings of this review). The authors therefore concluded that their clinical criteria for offering posterior decompression of persistent pain despite dynamic posture were valid in this cohort.
Significant heterogeneity in the direct and indirect decompression cohort patient populations likely contributed to the lack of clear differences in outcomes between cohorts. For example, in adult spinal deformity populations, the need for posterior facetectomies and osteotomies to achieve deformity correction may supersede the need for additional posterior neural structure decompression. Traditionally, indirect decompression with LLIF has been utilized for restoration of disc and foraminal heights as well as decompression of foraminal stenosis. Recent studies have demonstrated positive clinical and radiographic outcomes with indirect decompression, even in the setting of severe central stenosis22-24 However, none of the comparative studies in the current review directly compare patients specifically with central stenosis. Both Lee et al and Attenello et al focus on deformity populations.16,17 Lim et al report that 45.2% of indirect decompression patients (n = 19) and 37.5% of direct decompression patients (n = 3) had severe central of lateral recess stenosis. 14 Therefore, future prospective study is necessary to elucidate the value of utilizing indirect versus direct decompression techniques specifically in the setting of symptomatic central lumbar stenosis.
There are a number of limitations to the current review that should be considered a priori. First, the majority of the included studies are observational and not comparative. None of these comparative studies were randomized controlled trials. Therefore, the overall quality and strength of studies included in this review was limited. Second, heterogeneity between direct and indirect decompression cohorts is likely. For example, cases managed with additional direct decompression were more likely to be in patients with severe lumbar stenosis. Similarly, patients undergoing indirect decompression may have had isolated foraminal stenosis or milder central stenosis which obviated the need for aggressive posterior surgical intervention. In general, patient diagnosis reporting did not delineate case severity in the reviewed studies. Next, only a limited set of studies included patients undergoing lateral fusion with additional direct posterior decompression. Alternative posterior fusion techniques, such as posterolateral fusion or transforaminal interbody fusion, are more likely to be utilized in cases when posterior decompression is deemed necessary. Finally, the most commonly reported patient outcome measures that could be compared between studies were ODI and VAS pain scores. Fusion rates, other radiological measurements, and global quality of life measures were either not assessed or reported inconsistently across studies. As a result, generalizability of our findings should be considered when determining the optimal course of treatment for individual patients. Nevertheless, the results of the current systematic review are both novel and useful.
In conclusion, the current review demonstrates that indirect lateral decompression without or combined with additional posterior decompression can be effective for treatment of lumbar spinal stenosis and other degenerative pathologies. While there are trends suggesting better functional improvements with indirect decompression as well as improved restoration of lumbar lordosis with additional posterior decompression, the heterogeneity of patients in these studies makes it difficult to complete all potential comparisons between cohorts. In addition, while the complication profiles of the 2 procedures differ, the revision rates are similar. Therefore, future prospective investigation is warranted to compare these 2 techniques in a controlled patient population, such as patients with moderate to severe central stenosis, with increased power. While both direct and indirect decompression are effective in specific patient populations, without this type of prospective comparison the ideal indications for an isolated indirect lateral decompression cannot be determined.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mustfa K. Manzur, MPH, MS  https://orcid.org/0000-0002-1789-3189
https://orcid.org/0000-0002-1789-3189
Kyle W. Morse, MD  https://orcid.org/0000-0001-9136-8078
https://orcid.org/0000-0001-9136-8078
Karim A. Shafi, MD  https://orcid.org/0000-0002-6695-5593
https://orcid.org/0000-0002-6695-5593
Bridget Jivanelli Gatto, MLIS  https://orcid.org/0000-0002-8707-4859
https://orcid.org/0000-0002-8707-4859
References
- 1.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2017;26(3):671–678. [DOI] [PubMed] [Google Scholar]
- 2.Scherman DB, Rao PJ, Phan K, Mungovan SF, Faulder K, Dandie G. Outcomes of direct lateral interbody fusion (DLIF) in an Australian cohort. J Spine Surg. 2019;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin GX, Akbary K, Kotheeranurak V, et al. Clinical and radiologic outcomes of direct versus indirect decompression with lumbar interbody fusion: a matched-pair comparison analysis. World Neurosurg. 2018;119:e898–e909. [DOI] [PubMed] [Google Scholar]
- 4.Nemani VM, Aichmair A, Taher F, et al. Rate of revision surgery after stand-alone lateral lumbar interbody fusion for lumbar spinal stenosis. Spine (Phila Pa 1976). 2014;39(5):E326–E331. [DOI] [PubMed] [Google Scholar]
- 5.Campbell PG, Nunley PD, Cavanaugh D, et al. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg Focus. 2018;44(1):E6. [DOI] [PubMed] [Google Scholar]
- 6.Manzur MK, Steinhaus ME, Virk SS, et al. Fusion rate for stand-alone lateral lumbar interbody fusion: a systematic review. Spine J. 2020;20(11):1816–1825. [DOI] [PubMed] [Google Scholar]
- 7.Kotheeranurak V, Singhatanadgige W, Ratanakornphan C, Yingsakmongkol W, Hynes RA, Limthongkul W. Neutral hip position for the oblique lumbar interbody fusion (OLIF) approach increases the retroperitoneal oblique corridor. BMC Musculoskelet Disord. 2020;21(1):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzur M, Virk SS, Jivanelli B, et al. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J. 2019;19(7):1294–1301. [DOI] [PubMed] [Google Scholar]
- 9.Estefan M, Camino Willhuber GO. Laminectomy. StatPearls; 2020. [PubMed] [Google Scholar]
- 10.Ahmadian A, Verma S, Mundis GM, Oskouian RJ, Smith DA, Uribe JS. Minimally invasive lateral retroperitoneal transpsoas interbody fusion for L4-5 spondylolisthesis: clinical outcomes. J Neurosurg Spine. 2013;19(3):314–320. [DOI] [PubMed] [Google Scholar]
- 11.Castellvi AE, Nienke TW, Marulanda GA, Murtagh RD, Santoni BG. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res. 2014;472(6):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshihara H. Indirect decompression in spinal surgery. J Clin Neurosci. 2017;44:63–68. [DOI] [PubMed] [Google Scholar]
- 13.Malham GM, Parker RM, Blecher CM, Chow FY, Seex KA. Choice of approach does not affect clinical and radiologic outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patients having lateral lumbar interbody fusion at 24 months. Global Spine J. 2016;6(5):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim KZ, Daly C, Brown J, Goldschlager T. Dynamic posture-related preoperative pain as a single clinical criterion in patient selection for extreme lateral interbody fusion without direct decompression. Global Spine J. 2019;9(6):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 16.Attenello J, Chang C, Lee YP, Zlomislic V, Garfin SR, Allen RT. Comparison of lateral lumbar interbody fusion (LLIF) with open versus percutaneous screw fixation for adult degenerative scoliosis. J Orthop. 2018;15(2):486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CS, Park SJ, Chung SS, Lee JY, Yum TH, Shin SK. Mini-open anterior lumbar interbody fusion combined with lateral lumbar interbody fusion in corrective surgery for adult spinal deformity. Asian Spine J. 2016;10(6):1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veeravagu A, Li A, Swinney C, et al. Predicting complication risk in spine surgery: a prospective analysis of a novel risk assessment tool. J Neurosurg Spine. 2017;27(1):81–91. [DOI] [PubMed] [Google Scholar]
- 19.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37(1):67–76. [DOI] [PubMed] [Google Scholar]
- 20.Weiss H, Garcia RM, Hopkins B, Shlobin N, Dahdaleh NS. A systematic review of complications following minimally invasive spine surgery including transforaminal lumbar interbody fusion. Curr Rev Musculoskelet Med. 2019;12(3):328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sembrano JN, Tohmeh A, Isaacs R. SOLAS Degenerative Study Group. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine (Phila Pa 1976). 2016;41(suppl 8):S123–S132. [DOI] [PubMed] [Google Scholar]
- 22.Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Watanabe M. Comparison of radiological changes after single—position versus dual—position for lateral interbody fusion and pedicle screw fixation. BMC Musculoskelet Disord. 2019;20(1):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakashima H, Kanemura T, Satake K, et al. Indirect decompression on MRI chronologically progresses after immediate postlateral lumbar interbody fusion: the results from a minimum of 2 years follow-Up. Spine (Phila Pa 1976). 2019;44(24):E1411–E1418. [DOI] [PubMed] [Google Scholar]
- 24.Nomura H, Yamashita A, Watanabe T, Shirasawa K. Quantitative analysis of indirect decompression in extreme lateral interbody fusion and posterior spinal fusion with a percutaneous pedicle screw system for lumbar spinal stenosis. J Spine Surg. 2019;5(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira EA, Farwana M, Lam KS. Extreme lateral interbody fusion relieves symptoms of spinal stenosis and low-grade spondylolisthesis by indirect decompression in complex patients. J Clin Neurosci. 2017;35:56–61. [DOI] [PubMed] [Google Scholar]
- 26.Fujibayashi S, Hynes RA, Otsuki B, Kimura H, Takemoto M, Matsuda S. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976). 2015;40(3):E175–E182. [DOI] [PubMed] [Google Scholar]
- 27.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110–118. [DOI] [PubMed] [Google Scholar]
- 28.Louie PK, Varthi AG, Narain AS, et al. Stand-alone lateral lumbar interbody fusion for the treatment of symptomatic adjacent segment degeneration following previous lumbar fusion. Spine J. 2018;18(11):2025–2032. [DOI] [PubMed] [Google Scholar]