Abstract
Voltage-gated sodium (Nav) channels initiate action potentials in excitable tissues. Altering these channels’ function can lead to many pathophysiological conditions. Nav channels are composed of several functional and structural domains that could be targeted pharmacologically as potential therapeutic means against various neurological conditions. Mutations in Nav channels have been suggested to underlie various clinical syndromes in different tissues and in association with conditions ranging from epileptic to muscular problems. Treating those mutations that increase the excitability of Nav channels requires inhibitors that could effectively reduce channel firing. The main non-psychotropic constituent of the cannabis plant, cannabidiol (CBD), has recently gained interest as a viable compound to treat some of the conditions that are associated with Nav malfunctions. In this review, we discuss an overview of Nav channels followed by an in-depth description of the interactions of CBD and Nav channels. We conclude with some clinical implications of CBD use against Nav hyperexcitability based on a series of preclinical studies published to date, with a focus on Nav/CBD interactions.
Keywords: voltage-gated sodium channels, cannabidiol, channelopathies, Dravet syndrome, myotonia
Introduction
The cannabis plant, Cannabis sativa, contains over 120 active constituents, which are collectively called phytocannabinoids (Morales and others 2017). Cannabis originated in the Himalayas and was first cultivated in China for seed and fiber production. Early records of using cannabis medicinally can be traced to Sumerians records around 1800 B.C., which mention using this plant against a variety of diseases, including convulsions. There are more recent records of cannabis use against epilepsy in Islamic literature (ElSohly 2007; Russo and others 2008).
Over the past century, cannabis consumption became illegal in many parts of the world due to its psychotropic effects. These legal limitations also constrained cannabis research. In the 1960s, however, some research progress was made and, several years later, the mechanisms were determined by which ∆9-tetrahydrocannabinol (THC), the primary psychotropic phytocannabinoid, imparts psycho-activity. Two cannabinoid receptors, CB1 and CB2, were identified to specifically bind THC. These receptors are involved in many processes, including pain response, mood, and memory, among others (Billakota and others 2019; Pertwee 2008). THC has a high affinity for CB receptors; modulation of these receptors likely triggers the THC’s psychotropic effect. However, THC also is reported to be an analgesic, a muscle relaxant, and an anti-inflammatory.
Cannabidiol (CBD) is another key phytocannabinoid that shares a similar structure and many of the physiological effects of THC without its psycho-activity. This major difference stems from the fact that CBD has little to no affinity for the CB receptors with which THC interacts (Devane and others 1988). In fact, CBD is suggested to be a negative allosteric modulator of CB receptors (Tham and others 2019). CBD’s lack of activity at CB receptors and its apparent efficacy (both anecdotal and clinical trials) in various disorders have resulted in many proteins being proposed as CBD targets, including voltage-gated sodium (Nav) channels, voltage-gated potassium (Kv) channels, voltage-gated calcium channels, transient receptor potential (TRP) channels, G-coupled protein receptors (GPRs), and so on (De Petrocellis and others 2011; Ghovanloo and others 2018c; Kaplan and others 2017; Patel and others 2016; Ross and others 2008; Sait and others 2020).
Among these CBD targets, the Nav channel family is particularly interesting. Abnormalities in Nav function have been suggested or shown to be associated with many of the conditions in which CBD has shown efficacy, such as Dravet syndrome (DS) (Dravet 2011; Devinsky and others 2017). DS is a severe form of childhood epilepsy caused by loss of activity of Nav1.1, and Nav1.1 is the sodium channel subtype that ignites excitability in inhibitory CNS neurons. This devastating condition typically begins within the first year of life, and on onset, seizures become more frequent and unstoppable. DS affects almost every aspect of development in children who suffer from it by causing hundreds of seizures a week, the shear frequency and intensity of which prevents some of the most basic activities, including the ability to talk or walk. Unfortunately, each seizure also has the potential to be lethal (Dravet 2011; Devinsky and others 2017). The fact that CBD relieves the DS symptoms is substantially important to patients. However, from a scientific perspective, whether CBD’s mechanism of efficacy in DS involves Nav channels remains speculative.
Many cannabis constituents have also been suggested to have therapeutic effects in a range of other disorders. For instance, in patients with muscular dystrophy, cannabis could help manage pain and involuntary muscle tightness. In patients who suffer from neuropathic pain, it could significantly reduce the intensity of chronic pain and also improve sleep. Cannabis also helps with involuntary muscle tightness and reduces muscle tremors and spasticity (Baker and others 2000; Borgelt and others 2013; Iannotti and others 2019; Pertwee 2008; Ware and others 2010; Wilsey and others 2013; Woodhams and others 2015). In addition to the plant-based phytocannabinoids, much effort has gone into studying the endogenous cannabinoids, 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide (anandamide), which have important physiological implications (De Petrocellis and others 2011; Iannotti and others 2019).
Our group recently published several studies showing the full effects of CBD on the Nav channel family. We demonstrated that CBD reduces hyperexcitability associated with neuronal systems, cardiac system, and skeletal muscle fibers. We described the mechanism with which CBD acts on Nav channels, with possible implications for other pathophysiological systems (Fouda and others 2020; Ghovanloo and others 2018c; Ghovanloo and others 2021; Sait and others 2020).
In this review, we first provide a general description of Nav channels. We then describe in greater detail the interactions between CBD and Nav channels, and finally discuss potentially relevant implications of these interactions.
Voltage-Gated Sodium Channel: Structure and Function
Ion channels orchestrate an exquisite array of physiological processes, including nerve impulses, muscle contraction, and signaling in all organisms. The electric current in signaling is generated by ion flux across the cellular membrane controlled by the opening and closing of ion channels. These channels are permeable to different ions, including sodium, potassium, calcium, and chloride (Hille 2001). The direction of ion flux is determined by the membrane potential and transmembrane ionic gradients that are established by ion channels and the Na+/K+ and Ca2+ pumps, and Na+/Ca2+, Cl−/HCO3−, Na+/H−, and Na+/neurotransmitter exchangers (Purves and others 2001; Vassalle 1987). Generating a specific type of signaling, known as the action potential (AP), requires that the ion channels involved are selectively permeable to a specific ion and not to others (Hille 1975, 2001; Hodgkin and Huxley 1952). A subset of these selective ion channels is the Nav channel superfamily (Catterall 2012).
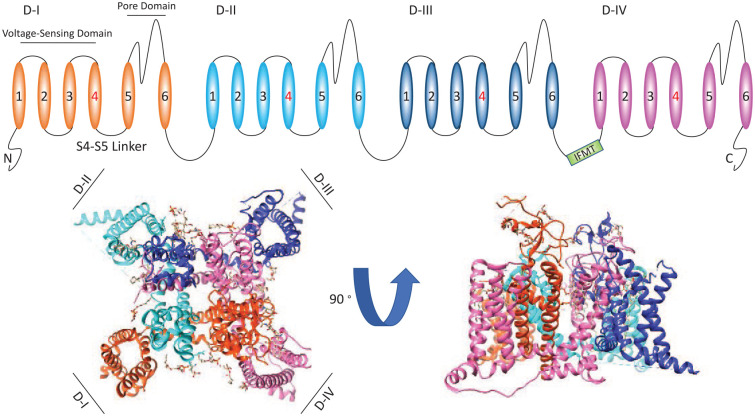
The sodium current passing through Nav channels initiates action potentials in neurons, skeletal muscles, and cardiac muscles. Nav channels are hetero-multimeric proteins composed of large ion conducting α-subunits and smaller auxiliary β-subunits (Catterall 2012; Estacion and others 2010; Ghovanloo and others 2016; Ghovanloo and Ruben 2020; Isom and others 1992; Patton and others 1994). The α-subunit is made up of a single transcript that encodes four 6-transmembrane segment domains (Catterall 2012). Each one of these four structural domains can be divided into two functional sub-domains known as the voltage-sensing domain (VSD) and the pore domain (PD) (Catterall 2012; Ghovanloo and others 2016). These two functional sub-domains are connected through the intracellular S4-S5 linker (Catterall 2012; Yarov-Yarovoy and others 2012). The VSD is formed by the first four transmembrane segments of each domain and the pore is formed by the fifth and sixth segments along with the extracellular pore loop that connects them (Catterall 2012; Ghovanloo and others 2016) (Fig. 1).
Figure 1.
An illustration of the primary structure of the α-subunit of voltage-gated sodium (Nav) channels. At the top of the figure, we show a cartoon of the Nav channel structure. At the bottom we show top and side views of the Nav1.5 (PDB ID: 6UZ3). The cartoon and structure are color-coded.
Channel opening is preceded by the outward translocation of each of the four S4 membrane spanning segments in the VSD (Cha and others 1999), driven by membrane depolarization and punctuated by electrostatic interactions between the positive charges in the S4s and negative charges in S1-S3 (Catterall 2012; DeCaen and others 2011; Yarov-Yarovoy and others 2012). S4 translocation leads to opening of the PD via the S4-S5 linker (Wisedchaisri and others 2019).
Activation is followed within milliseconds by fast inactivation. The process of fast inactivation is mediated through the interaction between the domain III-IV linker with residues on the intracellular face of the channel (Jiang and others 2020; West and others 1992). Recent cryo–electron microscopy (cryo-EM) structures of eukaryotic sodium channels revealed that fast inactivation may proceed via an allosteric mechanism. This mechanism involves the IFMT (isoleucine-phenylalanine-methionine-threonine) motif promoting pore closure by squeezing into the space between S6 and S4-S5 restriction ring. The skeletal muscle sodium channel structure shows the F residue penetrating into this space, and I and M residues to the edges around this space (Pan and others 2018; Shen and others 2017; Yan and others 2017). The allosteric mechanism of open-state fast inactivation was further elucidated using the structure of the cardiac sodium channel, where outward movement of the third VSD opens an interaction site for the fast inactivation particle. This particle in turn moves into place on the outward shift of the fourth VSD (Ghovanloo and Ruben 2020; Jiang and others 2020). This suggests that both movements are needed for open-state fast inactivation to occur. This process that happens within milliseconds of activation, blocks the channel pore, and effectively stops current conduction. This negative regulation of conductance is a way of controlling excitability.
In addition to fast inactivation, which was discovered by Hodgkin and Huxley (though they did not call it that) (Hille 2001; Hodgkin and Huxley 1952), Nav channels have a second slower inactivated state (Vilin and Ruben 2001). Repetitive or prolonged stimulation can result in slow inactivation. In a physiological setting, slow inactivation is vital to limit the frequency of firing and define the length of trains of action potentials to protect cells against excitotoxic injury (Vilin and Ruben 2001). During slow inactivation, for which outward S4 translocation is required (Silva and Goldstein 2013), two of the opposite S6 segments move toward the pore axis with the other two pointing outward. This asymmetry causes a structural collapse leading to a slowly reversible inactivation (Gamal El-Din and others 2013).
Types of Nav Currents
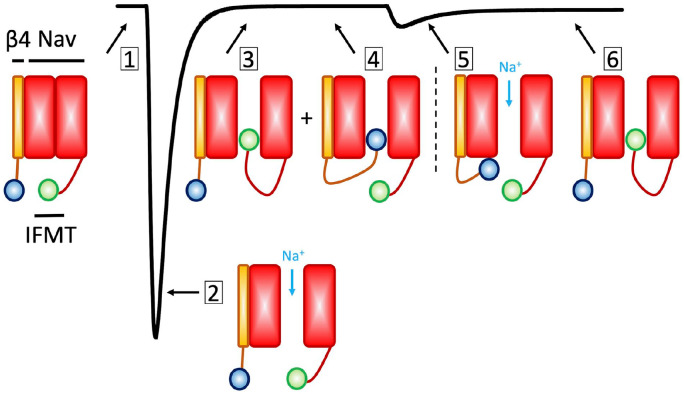
Nav channels activate on the presence of a sufficiently strong depolarizing stimulus. Once activated, a macroscopic transient sodium current is generated, which can be divided into two components: peak and late currents (Fig. 2). The late current can be further divided into persistent (typically elicited by a single square depolarizing pulse) and window currents (elicited by a ramp pulse). The peak current is known as the large-amplitude inward segment of the sodium transient. The overall sodium current tends to incompletely inactivate in both muscle and nerve cells. This incomplete inactivation results in a small, persistent current component which is only a small percentage (<5%) of the peak current amplitude (Crill 1996). The persistent current plays a vital role in excitatory cell bodies and dendrites where it increases the size of postsynaptic potentials and boosts the cell threshold during trains of action potentials (Liu and Shipley 2008). Window currents describe a range of membrane potentials over which a fraction of sodium channels is activated, but not yet inactivated. This membrane potential range is best described by the overlap of Hodgkin and Huxley activation and inactivation curves, although direct measurements are also feasible using ramp protocols. Exacerbated window currents have also been described in association with some pathological conditions (Attwell and others 1979).
Figure 2.
Types of voltage-gated sodium (Nav) currents. This cartoon illustrates the types of Nav currents described in the text. (1) The zero-sodium current condition, in which the Nav channel is in the resting state. (2) The maximal peak sodium current. (3) The fast inactivated state (Note that fast inactivation proceeds through an allosteric process, as described in the main text. The ball and chain cartoon here is only meant to illustrate the competition between the fast inactivation particle and β4, indicating two populations of channels, one bound with IFMT (isoleucine-phenylalanine-methionine-threonine) and the other with β4. (4) The β4 subunit in its bound form, thereby blocking sodium currents. (5) The unbinding of β4 causes a surge of inward sodium current. As mentioned in the text, recent findings suggest that β4 may not be necessary for resurgent current generation (White and others 2019). (6) Channels fast inactivate.
Some classes of neurons may also generate resurgent currents (Cannon and Bean 2010; Raman and Bean 1997; Raman and others 1997). These currents are a rebound of inward current that may appear following a voltage pulse or an action potential. Resurgent currents are caused by reopening of Nav channels and are in part dependent on the presence of the β4 subunit, which competes with the inactivation particle at more depolarized potentials and during repolarization. During these intermediate potentials, when the fast inactivation particle in a subset of channels is in its bound form forcing them non-conductive, another subset of channels of the population of channels may have the β4 in its bound state. Thus, the unbinding of β4 from the second population of channels causes a surge of inward sodium current that depolarizes the membrane (Fig. 2). This condition serves as a reservoir for subsequent firing. The interactions between Nav1.6 and β4 were reviewed extensively by (Cannon and Bean 2010). Recent findings suggest that the β4 subunit may not be necessary for the generation of resurgent currents. In fact, it is suggested that in the absence of β4, if Nav channels recover from fast inactivation before completion of deactivation, a resurgent-like current may be produced (White and others 2019). Overall, late and resurgent currents work together with slow inactivation to generate complex patterns of action potentials firing.
Structural Segments of the Nav Pore and Pharmacological Modulation
The Nav pore structure includes a large external vestibule, a narrow selectivity filter, a large central cavity that is lined by S6 segments that is filled with water, and an intracellular activation gate that is formed by the crossing of S6 segments at the intracellular side of the membrane (Jiang and others 2020; Pan and others 2018; Payandeh and others 2011; Yan and others 2017).
The first ion channel crystal structures described the architecture of potassium channels (Doyle and others 1998). The emergence of a multitude of crystal and cryo-EM structures revealed that the overall structure of the pore between Nav and Kv is similar. However, the structure of the ion selectivity filters, and mechanisms of ion conductance are different between the two channels. Potassium channels select potassium by direct interactions between the backbone carbonyls of residues that comprise the selectivity filter. These interactions create four ion coordination sites (Catterall 2012). In potassium channels, no amino acid charged functional groups or water molecules are involved in the selectivity process. However, in Nav channels, the selectivity filter has a high field strength on the extracellular side which is composed of amino acid side chains. This outer vestibule is followed by two ion coordination sites that are formed by backbone carbonyls (Payandeh and others 2011; Yan and others 2017). These sites allow for passage of sodium ions with four water molecules (hydration). These sites would be too large for a dry sodium ion to go through, which would be energetically unfavorable. This indicates that sodium conductance and selectivity are different to that of potassium ions.
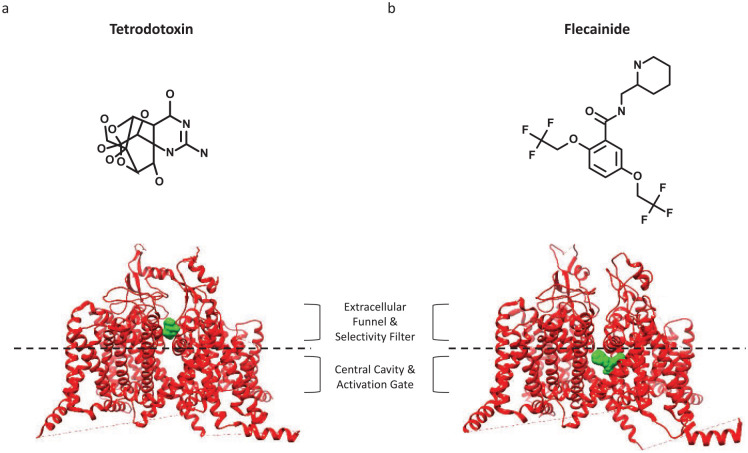
From a functional perspective, both the outer and inner segments of the PD are interaction sites for pharmacological agents. The most selective and well-known Nav blocker is tetrodotoxin (TTX), which comes from symbiotic bacteria in the pufferfish (and some other animals’) diet (Chau and others 2011; Hille 2001). The sensitivity of sodium channel subtypes to TTX has been used to divide the family into two classes: TTX-sensitive and TTX-resistant. The IC50 of TTX-sensitive (Nav1.1-4, Nav1.6-7) channels to TTX is less than 30 nM (Catterall and others 2005; Gamal El-Din and others 2013). The molecular reason underlying differential affinity for TTX in Nav has been attributed predominantly to a single homologous residue difference in the Nav pore-loop. The TTX-resistant channels have a cysteine or serine in this position, instead of a tyrosine or phenylalanine residue in TTX-sensitive channels. A recent study determined that this substitution does not alter the local conformation of the channel. However, lacking an aromatic side chain in this position may cause steric constraints that reduce TTX affinity (Ghovanloo and Ruben 2020; Jiang and others 2020) (Fig. 3).
Figure 3.
Binding of pore domain (PD) blockers on the outer and inner segments. In the middle of both structures, we show a dashed line which is meant to illustrate a division between the extracellular funnel and selectivity filter, and central cavity and activation gate. (a) The binding of tetrodotoxin (TTX) to the outer PD (more rigid part of the domain, from PDB ID: 6UZ3). (b) A view of Nav1.5 and flecainide (PDB ID: 6UZ3).
TTX is considered a state-independent Nav blocker, a function of its binding-site residing on the outer selectivity filter, which is a more rigid part of the Nav PD. In contrast, most local anesthetics (LA) are highly state-dependent Nav blockers (may also have high affinity for open state); their binding site is located below the selectivity filter, a more flexible region of the PD. This part of the channel is highly conserved among Navs (Ragsdale and others 1996). Therefore, it is unsurprising that most Nav pore blockers that interact at the LA site display little subtype selectivity. The flexibility difference between the outer and inner PD has important pharmacological implications (Fig. 3).
Because the proportion of channels populating different states is controlled by the membrane potential, the state-dependence of these compounds may be referred to as their voltage-dependence. Many such compounds also display a phenomenon known as use dependence, which occurs when the compound potency increases on higher frequency stimulations (Gamal El-Din and others 2018).
Many Nav modulating compounds are used to treat clinical conditions caused by changes to excitability. Specific examples include anticonvulsants (carbamazepine, phenytoin), local anesthetics (lidocaine), and antiarrhythmics (mexiletine) (Mantegazza and others 2010). Because these compounds largely lack selectivity across the sodium channel superfamily, they may lead to potentially undesirable side effects. All of these compounds are either neutral or weakly basic. In addition to the two general sites in the PD, recent efforts have culminated in the development of highly selective sodium channel blockers that target DIV-VSD (Ahuja and others 2015).
One recent study found that tamoxifen, an estrogen receptor modulator, and its primary and secondary metabolic products bind at the intracellular exit of the bacterial Nav channel, NavMs. This new site is distinct from the sites that other previously characterized Nav-blocking compounds interact with. This novel site could be utilized for the development of new drugs for the treatment of Nav channelopathies (Sula and others 2021).
Nav Distribution and Tissue-Specific Channelopathies
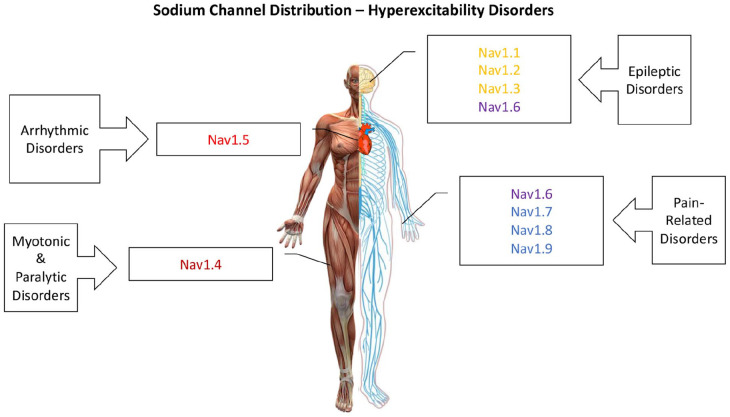
Multiple sodium channel isoforms are expressed in different tissues. Nav1.1 to Nav1.3 are primarily found in the central nervous system, although Nav1.1 and Nav1.3 can also be expressed in peripheral nervous system (Dib-Hajj and others 2010; Osteen and others 2016). Nav1.4 and Nav1.5 are expressed in skeletal and cardiac muscles, respectively. Nav1.6 is expressed in both the central and peripheral nervous systems. Nav1.7 to Nav1.9 are primarily found in the peripheral nervous system (Ghovanloo and others 2016). The expression pattern of the neuronal Nav channels depends on both the developmental stage, brain region, and cell type. Nav1.3 is expressed predominantly in neonatal brain cells; thus, it is thought to be a key contributor to brain development. In contrast, Nav1.1, Nav1.2, and Nav1.6 are highly expressed in adult brains. Furthermore, Nav1.6 displays greatest expression in unmyelinated axons (Nav1.2 and Nav1.6 are uniformly distributed along unmyelinated axons but they are clustered at the nodes of Ranvier in myelinated axons; Salzer 1997), whereas Nav1.2 is found in the cell soma (Hu and others 2009; Whitaker and others 2001) (Fig. 3). Although the different isoforms share a similar structure, their gating and response to physiological and pathophysiological modulators can vary widely.
Changes to the gating properties of sodium channels, and subsequently the current passing through them during an action potential can cause potentially fatal abnormalities in electrical signaling. Both gain-of-function (GOF) and loss-of-function (LOF) in sodium channels disrupt electrical signaling. Interestingly, several mutants display both GOF and LOF, leading to various disease phenotypes (Ghovanloo and others 2016).
In the primary sodium channel isoforms of the CNS, namely Nav1.1, 1.2, 1.3, and 1.6, both GOF and LOF elicit epilepsy syndromes (Catterall 2012; Estacion and others 2010; Veeramah and others 2012). These include relatively mild epilepsies, like benign familial neonatal-infantile seizures, or more severe forms, such as DS (Dravet 2011; Heron and others 2002; Scalmani and others 2006) and early-infantile epileptic encephalopathy-13 (O’Brien and Meisler 2013). In skeletal muscle, Nav1.4, GOF mutants elicit myotonic and paralytic syndromes (e.g., hypokalemic periodic paralysis [hypoPP]), causing an inability to relax or contract the muscle, respectively (Cannon 1996; Ghovanloo and others 2018a). Long QT-3 syndrome is due to an increase in the fraction of Nav1.5 cardiac sodium channels that fail to inactivate and, consequently, an increased persistent sodium current throughout the action potential plateau that delays repolarization (Wang and others 1995). Conversely, mutants that decrease peak Nav1.5 current cause Brugada syndrome and other diseases of conduction (Antzelevitch and others 2005). Recently, a mutation increasing window currents in Nav1.5 has been suggested in association with an atypical atrial and ventricular tachyarrhythmia (Ghovanloo and others 2020). Lastly, certain GOF mutations in Nav1.7 cause multiple pain disorders, including inherited erythromelalgia (Bankar and others 2018; Dib-Hajj and others 2007) (Fig. 4).
Figure 4.
Voltage-gated sodium (Nav) channel distribution. This is a cartoon illustrating the predominantly tissue-specific distribution of Nav channels in the human body, along with general hyperexcitability conditions that are caused by mutations in each category.
CBD Is an Inhibitor of Voltage-Dependent Sodium Currents
The disease phenotypes associated with Nav channelopathies have prompted extensive efforts to develop potentially useful novel pharmacological compounds. Specifically, reducing Nav hyperexcitability is vitally important in order to alleviate life limiting and sometimes lethal conditions. A study in 2016 first showed the Nav channels to be among CBD targets (Patel and others 2016). Over the past few years, with aid of our collaborators and colleagues across multiple disciplines, we have investigated various aspects of CBD interactions with Nav channels.
CBD is a highly hydrophobic compound with a complex profile. Many studies from different scientific disciplines suggest a wide range of molecular targets for CBD and potential therapeutic value against a variety of disorders, many of which seem unrelated to one another. For instance, in addition to the noted hyperexcitability disorders, CBD also has been suggested to possess antibiotic properties (Kosgodage and others 2019; van Klingeren and ten Ham 1976). This has created a reputation in which CBD is perceived as both a panacea and somewhat of a “snake oil.” This CBD reputation has at least two possible explanations: first, that CBD interacts with the diverse molecular targets; second, experimental assays are inadequate to investigate CBD effects, so any given proposed molecular target could be a false positive.
One way to rectify this conundrum is to investigate CBD interactions with a specific molecular target that is involved in seemingly different disorders. The Nav superfamily fits this description because Navs underlie a broad range of tissue-specific disorders.
In our first attempt to understand CBD interactions with Nav channels, we sought to determine the effects of CBD on different Navs, to find out whether CBD has any selectivity, and to discover how CBD modulates Nav gating. In a series of voltage-clamp experiments on human Nav channels (hNav1.1-7) and mouse Nav1.6, we found that CBD non-selectively inhibits Nav channels from the inactivated state (half maximal inhibitory concentration [IC50]: 1.9-3.8 µM). Interestingly, CBD’s inactivated state inhibition of Nav currents has a steep Hill slope of ~3. The steep Hill function suggests that CBD likely does not inhibit Nav channels through a 1:1 binding mechanism. Rather, there likely are multiple CBD interactions contributing to Nav inhibition from the inactivated state.
An important attribute of Nav inhibition by CBD is its ability to prevent channel opening. We found that exposures to CBD concentrations of about 3 µM (in Nav1.1, slightly above IC50), on reaching equilibrium, blocks about 90% of total macroscopic Nav conductance. However, the remaining population of channels, for which conductance is unaffected by CBD, displays an unaltered voltage-dependence of activation (Ghovanloo and others 2018c). This suggests that CBD’s presence does not modulate the threshold at which Nav channels fire, which may suggest that there is likely little impact imparted on the channel VSDs.
The elimination of VSDs as a likely site of CBD interaction on Nav channels, leaves the PD as a conceivable binding site. A hallmark of pore-blocking compounds is a stabilized inactivation. To assess whether CBD affects inactivation, we measured the voltage-dependence of steady-state inactivation following a 500-ms inactivating pulse in the same population of channels (from ~3 µM) that displayed unaltered activation. These measurements showed that CBD hyperpolarizes, and hence stabilizes inactivation (Ghovanloo and others 2018c). Indeed, we further repeated the findings in Nav1.1 in a separate study in Nav1.4 with 1 to 2 µM CBD (Ghovanloo and others 2021).
The conclusion that CBD prevents the opening of Nav channels is further affirmed by the observation of CBD’s inhibition of resurgent currents in Nav1.2 and Nav1.6 (Ghovanloo and others 2018c; Mason and Cummins 2020; Patel and others 2016). CBD also does not alter the open state fast inactivation time constants of neuronal Nav channels, suggesting that it does not interact with the sodium conduction pathway when the channel is open. Therefore, CBD’s block of conductance and resurgent currents, along with its hyperpolarization of fast inactivation, combine to make CBD an intriguing Nav inhibitor. To recapitulate, CBD, at low micromolar concentrations, blocks the majority of Nav channels and prevents them from conducting, and then makes the remaining population of unblocked channels more likely to inactivate.
CBD Is a Moderately State-Dependent Nav Inhibitor
As noted above, state-dependence is a property common to compounds that bind in the central cavity of the PD (and also VSD-binding modulators; Ahuja and others 2015; Bankar and others 2018). To determine whether CBD acts with state-dependence, we measured inhibition from −100, −90, −80, and −70 mV in Nav1.1, and −110 and −70 mV in Nav1.4. We found that CBD’s resting state inhibition has an ~10 µM IC50, which is about 10-fold less potent than the inactivated state. Interestingly, CBD’s steep Hill-curve inhibition relationship was observed at the resting state. A 10-fold difference in apparent potency between rest and inactivated states is a relatively small difference. In comparison, a well-established pore blocker like flecainide has a 60-fold difference (rest: ~600 µM and inactivated: ~10 µM) (Desaphy and others 2004). This suggests that CBD’s activity is relatively less dependent on interactions at the pore. We also found that CBD slows the Nav recovery from both fast and slow inactivated states by increasing the slow component and slow fraction of recovery from inactivation time constants. Consistent with its state-dependent properties, CBD is also a use-dependent Nav inhibitor, which could be an advantageous property against hyperexcitability disorders (Ghovanloo and others 2018c; Ghovanloo and others 2021).
These observations provide insights into CBD’s potential mechanism of Nav inhibition: (1) CBD’s state-dependence suggests interaction at the Nav central cavity of PD (the observations we made with CBD make interactions at VSDs highly unlikely), (2) CBD inhibition is a culmination of multiple interactions from both rest and inactivated states, and (3) some of CBD’s interactions could be pore-independent.
CBD Is More Potent at Lower Temperatures
We first explored CBD’s possible pore-independent pathway of Nav modulation. We began these investigations by measuring CBD inhibition of Nav channels at varying temperatures. We argued that because binding kinetics are responsive to temperature changes, if CBD’s inhibition of Nav channels follows a bimolecular scheme, then the rates of compound equilibration should increase at higher temperatures. However, we found that not only is CBD faster to inhibit Nav channels at lower temperatures, but it is also more potent when temperature is reduced (Ghovanloo and others 2018c).
CBD’s counter bimolecular temperature-dependence could be due to two possible mechanisms: (1) CBD has binding sites in areas of the Nav channels that are located in thermally volatile regions. Therefore, as temperature increases, it becomes harder for CBD to bind. (2) CBD somehow alters the biophysical properties of the membrane in which Nav channels reside. The second hypothesis seems more likely, and there is a strong precedence for amphiphilic compounds with features similar to CBD altering membrane properties and imparting manifestations on Nav channels that are also similar to CBD (Andersen and Koeppe 2007; Kapoor and others 2019).
CBD Modulates the Membrane Elasticity
Amphiphilic compounds often have a limited number of high affinity molecular targets. However, as the concentration of these compounds increase to levels in the micromolar range, they display promiscuity in targets, depending on which given molecular target is being investigated in a given experimental assay. However, this diversity of targets is suggested to be due to modulation of membrane stiffness or elasticity, rather than a direct interaction with a given target (Lundbæk and others 2004; Lundbæk and others 2010) (This effect is analogous to individuals in a swimming pool. As the properties [e.g., volume or viscosity] of the water change, so will the behaviors of the swimmers in the water). CBD and previously described amphiphiles share this property of apparent diversity of molecular protein targets. Furthermore, amphiphiles were shown to hyperpolarize the Nav channel inactivation curves without altering the voltage-dependence of activation, which is yet another commonality between CBD and amphiphiles (Ghovanloo and others 2018c; Ghovanloo and others 2021; Lundbæk and others 2004).
Investigation of amphiphilic molecules’ effects on bio-membranes have relied on gramicidin-based functional assays (Andersen and Koeppe 2007; Kapoor and others 2019). Gramicidin channels are composed of monomers that reside in each membrane leaflet. On dimerization of these monomers a continuous pore forms through the membrane. This conformational change is necessary and sufficient to conduct cationic currents. The pore diameter is ~4 Å, sufficient to allow the pore to also conduct alkali metals, protons, and water (Andersen and others 2005; Finkelstein 1974; Hladky and Haydon 1972). Gramicidin channel dimerization is directly related to the stiffness or elasticity of the membrane. Using gramicidin-based assays, it is shown that compounds that reduce the membrane stiffness or thickness (e.g., detergents) enhance the probability of gramicidin dimerization, which in turn increases the cationic gramicidin signal (Ingólfsson and others 2010; Kapoor and others 2019; Lundbæk and others 2004). Using nuclear magnetic resonance (NMR) and independently verified by MD simulations, we found that CBD tends to localize below the phosphate headgroups in bio-membrane. In contrast to what we expected, we found that CBD decreases the gramicidin signal, suggesting that it likely increases the membrane stiffness (Ghovanloo and others 2021). This contradictory behavior adds a further complication into delineating CBD’s mechanism of action. Although the complexity of the relationship between CBD’s modulation of membrane elasticity and Nav channels requires further investigations, we performed additional experiments using site-directed mutagenesis, which will be outline later in this review that will provide some insight into this relationship.
CBD Is a Pore Blocker, and the Likely Pathway Is through the Nav Fenestrations
To explore CBD’s possible interactions at the Nav pore, we tested CBD inhibition in a Nav1.1 pore-mutant construct (F1763A). The mutated F residue is conserved across mammalian Nav channels and has been indicated as a vital component of LA’s block of Nav channels. We found that destabilizing the traditional LA binding site by the F1763A mutation, only dropped the CBD inhibitory potency by ~2.5 folds from the inactivated state. The same mutation in Nav1.4 (F1586A) from the resting state also resulted in a similar ~2.5-fold drop in potency (Ghovanloo and others 2018c; Ghovanloo and others 2021). A 2.5 magnitude change in potency is considerably smaller than what has been observed with more traditional LA blockers, including tetracaine and lidocaine (for comparison, lidocaine has been shown to display ~30-fold state-dependence in cardiac Navs; Bean and others 1983). To gain further insight into CBD’s possible interacting residues in the pore, we tested its inhibition in two monomeric voltage-gated channels, Kv2.1 and the bacterial NaChBac Nav channel. We found that CBD inhibited both channels with a similar potency. These results suggested that either any potential CBD interactions inside the pore are non-crucial to its activity, or the interactions inside the pore are distinct from other traditional LA blockers, that is, less dependent on the F residue.
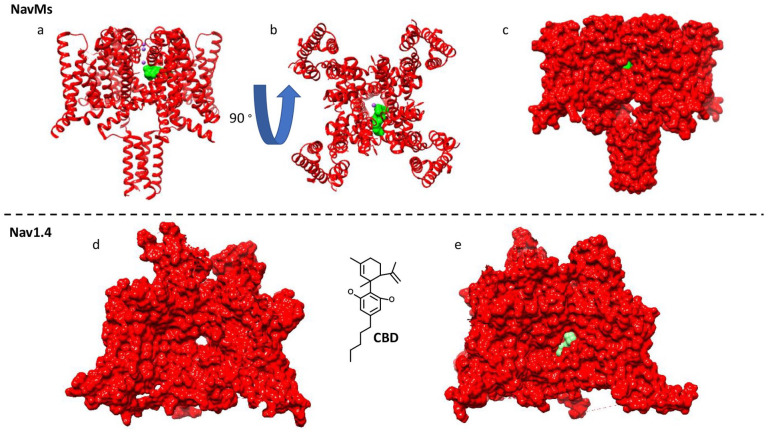
High-resolution X-ray crystallography of NavMs (another bacterial Nav channel) showed that CBD indeed interacts inside the Nav pore (the structural pose is in close agreement with suggested computational poses provided by molecular docking in mammalian Navs), at the fenestration-pore interface (Fig. 5) (Sait and others 2020). This interaction site is indeed distinct from other LAs binding site in NavMs (Bagnéris and others 2014). NavMs has a T207 in place of the conserved mammalian F residue in the pore. Interestingly, the T207A mutation in NavMs, also only slightly reduced the CBD inhibition of NavMs (Sait and others 2020), which was similar to results observed with the F to A mutations in Nav1.1 and Nav1.4 (Fig. 5a) (Ghovanloo and others 2021). These findings explain CBD’s reduced sensitivity to mutations in the traditional LA site.
Figure 5.
Cannabidiol (CBD) binding pose inside voltage-gated sodium (Nav) channels. (a-c) The crystallized binding of CBD inside the bacterial NavMs (PDB ID: 6YZ0). (d, e) The docked pose of CBD inside the human Nav1.4 structure (PDB ID: 6AGF). The poses are in close agreement.
In contrast to mammalian Nav channels, we found that CBD blocks NavMs less potently (IC50 = 18 µM) and with a shallower Hill slope (~1.5). This could be due to the smaller radius of the intramembrane fenestrations in NavMs compared to mammalian Nav orthologues (Bagnéris and others 2014). This hypothesis is predicated on the assumption that CBD’s pathway into the Nav pore involves compound penetration through the lipid phase. CBD’s preference for a pathway through the lipid phase is also congruent with its high lipophilicity (calculated log D = 6.6).
To test this hypothesis, we mutated four amino acids around the fenestrations of Nav1.4 to W residues. Performing these computations suggested that two of the fenestrations would be fully occluded with other two only partially occluded. We found the Nav1.4 WWWW construct fully abolished the steady-state CBD block of peak Nav currents, but not the more hydrophilic traditional LAs, lidocaine or flecainide (Ghovanloo and others 2021). These observations are well-explained within the confinements of the modulated receptor hypothesis (Hille 1977), which suggests that the central cavity of the PD is accessible to compounds from both the cytosolic activation gate and the lipid phase. The extend through which a given compound transverses through either pathway depends on its physicochemical properties. Indeed, molecular dynamics simulations support the CBD pathway through the fenestrations and into the pore (Ghovanloo and others 2021).
One intriguing finding from the Nav1.4 WWWW construct was that even though occluding fenestrations abolishes CBD’s peak current block, it does not impact CBD’s stabilization of inactivation (Ghovanloo and others 2021). As CBD’s effect on membrane elasticity is opposite to other amphiphiles, it is not possible to be certain that CBD’s effect on inactivation is due to modulation of the membrane; however, there may be an association between the two effects.
Proposed Blocking Scheme for CBD
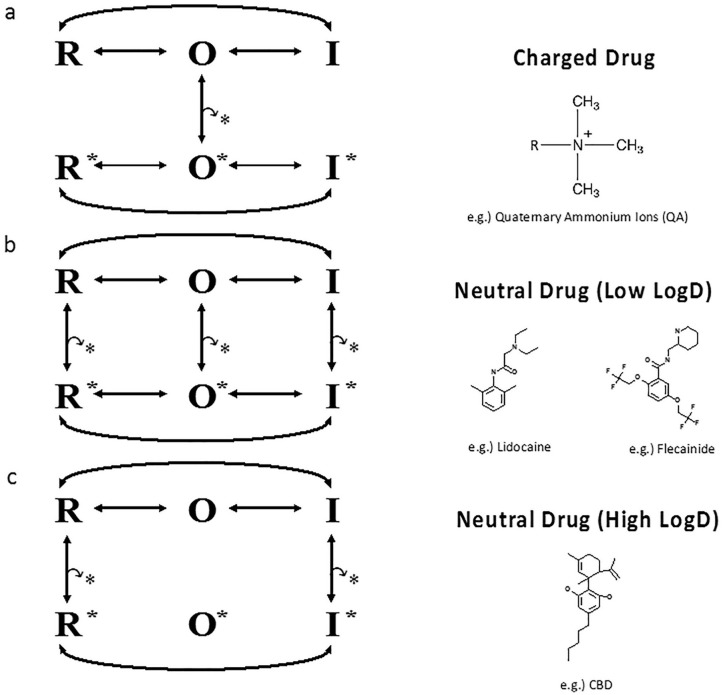
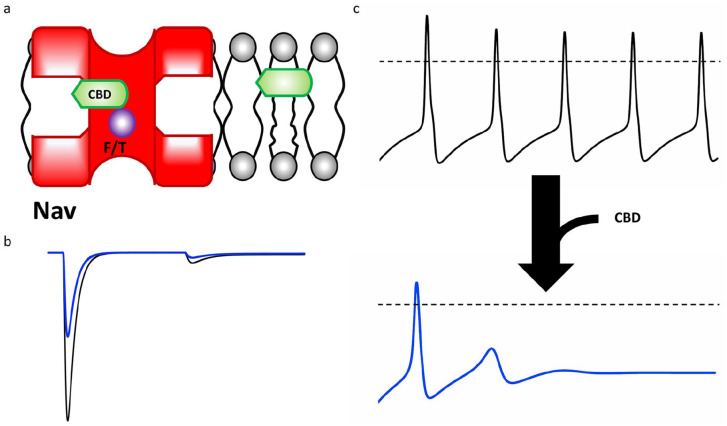
From a pharmacological perspective, the CBD results described are the first (to our knowledge) detailed mechanistic description of how an ultra-hydrophobic compound inhibits Nav channels. These results prompt us to propose that CBD does not interact with the open state of the channel. This prediction is consistent with the overall trend that has been proposed for LAs that are charged or neutral with low log Ds (Ghovanloo and others 2018c; Ghovanloo and others 2021; Hille 1977, 2001) and suggests that, as the drug becomes more hydrophobic, it tends to interact more with resting and inactivated states (Fig. 6). One caveat to the scheme proposed in Figure 6 is that it is based on a single ultra-hydrophobic compound. To determine whether this scheme holds true for other compounds with similar physicochemical properties to CBD, other compounds must be tested (Fig. 7a shows a cartoon representation of CBD pathway from the lipid phase through Nav fenestration and into the pore).
Figure 6.
Proposed channel blocking scheme for an ultra-hydrophobic compound. Hypothesis for block by local anesthetics (LAs) (based on Hille 1977, 2001; Hondeghem and Katzung 1977). (a, b) The first two models are based on previous studies. (a) Voltage-gated sodium (Nav) states and transitions with charged drug molecules. Charged (hydrophilic) drug may come and go only while the gate is open. (b) Neutral (hydrophobic) drug can bind and unbind even if when the gate is closed. Therefore, two pathways exist for drug to reach its receptor in the pore. The hydrophilic pathway is closed when the gate is closed. (c) The third model is based on our results from our studies on CBD and Nav channels. The star indicates drug. We propose as the drug becomes more hydrophobic, its interaction with the channel transitions from only the open-state (O) to only interactions with rest (R) and inactivated (I) states.
Clinical Implications: Neuronal, Cardiac, and Skeletal Muscle Systems
The landmark event that initiated the surge of molecular research into CBD effects and potential clinical implications was the clinical success and subsequent Food and Drug Administration approval of Epidiolex (commercial name of therapeutic CBD) for use against Dravet and Lennox-Gastaut syndromes (Devinsky and others 2017). CBD’s potential preference for blocking persistent and resurgent currents over peak currents was proposed as a possible mechanism for clinical efficacy (Mason and Cummins 2020; Patel and others 2016). Because resurgent currents are primarily associated with Nav1.2 and Nav1.6 (the Nav1.2 isoform gets partially replaced by Nav1.6 within the first year of life; Spratt and others 2019), which are presumed to be the predominant Nav isoforms in excitatory neurons, then CBD’s preference for inhibiting resurgent currents could give it functional selectivity. This indicates that because DS is a LOF in Nav1.1 (the predominant Nav channel subtype in inhibitory neurons), the inhibition of Nav1.6 resurgent currents would reduce the overall hyperexcitability associated with epilepsy (Patel and others 2016). Therefore, CBD restores the balance between Nav1.1 and Nav1.6, maintaining proper nerve conduction. In other words, reducing the activity of Nav1.6 excitability would implicate a reduction in Nav1.1 LOF.
An important consideration pertaining to functional selectivity is tissue/cell-specific excitability properties. For instance, although CBD has a slight preference in blocking persistent over peak currents (as measured in Nav1.6, peak IC50 = ~10 µM and persistent IC50 = ~6.4 µM) (Ghovanloo and others 2018c), this preference is entirely voltage protocol dependent. Typically, eliciting persistent currents requires holding the membrane potential very negative followed by a step pulse to a depolarizing potential. The IC50 numbers provided above were taken using a protocol in which the membrane was held at −120 mV, followed by a 100 ms pulse to −20 mV. As expected, 10 µM CBD blocks about 50% of the channels that were at the resting state, while blocking about ~60% of the persistent current. However, as we have seen, CBD blocks ~50% of the peak sodium current at ~2 to 3 µM from the inactivated state. This indicates that CBD’s slight preference for blocking persistent current may only be functionally relevant if the cell type has a resting membrane potential that is sufficiently negative. If the resting membrane potential is about −75 to −65 mV (this is roughly the average range in most neurons; Buchanan 1993; Ghovanloo and others 2021; Williams and others 2002), then the Nav channels are also about half inactivated, which would mean that CBD’s persistent to peak preference is unlikely to be a part of its efficacy mechanism. This principle may hold in some cardiac cells, in which both the resting membrane potential and Nav1.5 voltage-dependence of inactivation are about −80 to −90 mV (O’Hara and others 2011). In contrast, in skeletal muscle fibers, where the resting membrane potential is close to −90 mV, with Nav1.4 being half inactivated at ~−65 mV, CBD’s persistent to peak preference could theoretically play a role against disorders such as myotonia (Cannon and others 1993).
Recently, several studies have undertaken efforts to identify potential clinical applications for CBD in various systems. These studies include measuring CBD effects on the excitability of human induced pluripotent stem cell (iPSC)–derived neurons (Ghovanloo and others 2018c) and rabbit cardiomyocytes (Orvos and others 2020), among others. In every case, CBD has been shown to block sodium and potassium currents (including hERG, IC50 = 6.5 µM; Orvos and others 2020). One simple conclusion would be to claim that CBD could be a viable therapeutic to reduce any hyperexcitability condition, wherever it appears, from the brain to the heart (Fig. 7b and c). Indeed, future research and clinical trials might succumb to this conclusion; however, it is worth pointing out that many of the clinical claims are based on in vitro, in silico, or ex vivo assays. While such studies are vitally important to gain insight into mechanisms of action and identification of possible novel applications, the translation of findings from the lab bench to the clinic is a long and complex process. Additionally, there are other considerations that need to be accounted for, including compound bioavailability and tissue distribution, both of which depend on mode of administration for CBD (Lim and others 2020; Millar and others 2018). Furthermore, if every report of CBD activity on all/any given molecular target was physiologically relevant (most of which in the low micromolar range), then CBD may be viewed as a toxin as much as a therapeutic.
Figure 7.
Cannabidiol (CBD) pathway and effect on excitability. (a) The pathway of CBD from the lipid phase through the voltage-gated sodium (Nav) fenestration and into the pore, where it interacts to some extent by the local anesthetic (LA) site F or T residues, depending on the Nav channel. (b) CBD blocking generic peak, persistent, and resurgent sodium currents. (c) The general effect CBD on a generic action potential morphology.
CBD Protects against Glucose-Induced Oxidative Stress and Cardio-Cytotoxicity, In Vitro/In Silico
Diabetes-induced cardiovascular complications are a major cause of death. In an in vitro study using transiently transfected cells, we found that high glucose concentrations impart various gating changes onto Nav1.5 (Fouda and others 2020). These changes include depolarizing shifts to activation and inactivation curves, slowing of recovery from inactivation, and exacerbation of persistent currents. Reflecting the hyperglycemic gating changes onto a ventricular action potential model culminated in a computationally prolonged action potential duration (LQT-3).
Exposures of 5 µM CBD alleviated the high glucose mediated gating defects. Interestingly, the results from cell viability and fluorescence assays suggested that CBD works not only at the level of Nav1.5 in the cell membrane, but it also directly reduces production of reactive oxygen species that accompanies hyperglycemia. Both activities contribute to alleviating the Nav1.5-relaetd cytotoxicity (Fouda and others 2020).
CBD Alleviates Myotonic Phenotype in an In Vitro/In Silico Assay of Skeletal Muscle Hyperexcitability
Skeletal muscle hyperexcitability can impose serious limitations on a patient’s quality of life, and cannabinoids have been suggested to possess therapeutic potential against many of these conditions, in various assays (Borgelt and others 2013; Ghovanloo and others 2021; Iannotti and others 2019). Mutations that cause hyperexcitability in Nav1.4 are plentiful, and their biophysical consequences are complex. Although a given mutation may alter only a single component of the channel gating, many clinically relevant mutations tend to alter multiple aspects of channel biophysics (Ghovanloo and others 2016). For instance, a single missense mutation may right-shift activation (LOF) and also exacerbate persistent currents (GOF). The overall channotype (channel sequence variation profile) is a mixture of both defects (Klassen and others 2011).
We recently identified a naturally occurring mutation, P1158S (causes myotonia and periodic paralysis in the same patient (Sugiura and others 2003; Webb and Cannon 2008)), that increases the pH-sensitivity of Nav1.4 (Ghovanloo and others 2018a; Ghovanloo, and others 2018b; Peters and others 2018). The most intriguing aspect of P1158S is that its channotype culminates in multiple degrees of hyperexcitability. We found that lowering the pH causes depolarizing shifts to both activation and inactivation curves, and that it has exacerbated persistent currents relative to WT Nav1.4. Incorporating the gating differences on to the Cannon action potential model (Cannon and others 1993) suggested that at higher pH, P1158S displays an action potential morphology that is characteristic of periodic paralysis, and as pH is lowered, the action potential adopts a more myotonic morphology. We used this relationship between P1158S and pH to develop an in vitro/in silico assay to test CBD effects. We found that adding CBD to P1158S at a lowered pH alleviated the myotonic behaviour on action potential simulations. Additionally, CBD similarly affected the simulated periodic paralysis phenotype at an elevated pH (Ghovanloo and others 2021).
Concluding Remarks
Structural homology between Nav channel subtypes presents a problematic barrier for subtype-specific drug development. Highly conserved residues in the pore region are those to which many small molecules bind, which underlies the difficulty in developing drug therapies that are specific to individual Nav subtypes. Nevertheless, small molecules are widely used and therapeutically efficacious. Although CBD has similar effects and dose-dependence across the Nav family, a strong case for its therapeutic potential for treating a variety of Nav-related disorders can be made on the basis of its clinical efficacy in treating DS. It seems reasonable to speculate that the propensity for CBD to alter membrane properties results in modulating multiple targets rather than only affecting specific sodium channels. Rigorous clinical trials are necessary to confirm the therapeutic potential of CBD to treat other disorders, including those associated with skeletal muscle.
In conclusion, the work presented in this article reviews CBD’s effects and mechanism of action on Nav and membrane and suggests that CBD has therapeutic potential against several conditions. Finally, this work could be a first stepping-stone toward determining whether CBD or related compounds could develop or contribute to the development of other promising therapeutics.
Acknowledgments
Much of the work presented and reviewed here was conducted by our colleagues and collaborators. In particular, we thank all of the members of the research groups led by Drs. Samuel Goodchild, Lucie Delemotte, Olaf Andersen, Bonnie Wallace, Jenifer Thewalt, Charles Cohen, and JP Johnson.
Footnotes
Authors’ Note: A version of some sections of this manuscript has been incorporated in M.-R. Ghovanloo’s doctoral thesis (Ghovanloo 2020).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Natural Science and Engineering Research Council of Canada and the Rare Disease Foundation to PCR and M-RG (CGS-D: 535333-2019 and MSFSS: 546467-2019), a MITACS Accelerate fellowship in partnership with Xenon Pharma, Inc. to M-RG (IT10714).
ORCID iDs: Mohammad-Reza Ghovanloo  https://orcid.org/0000-0002-2171-0744
https://orcid.org/0000-0002-2171-0744
Peter C. Ruben  https://orcid.org/0000-0002-7877-5178
https://orcid.org/0000-0002-7877-5178
References
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, and others. 2015. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 350:aac5464. http://www.sciencemag.org/cgi/doi/10.1126/science.aac5464 [DOI] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE. 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36:107–30. http://www.annualreviews.org/doi/10.1146/annurev.biophys.36.040306.132643 [DOI] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE, Roux B. 2005. Gramicidin channels. IEEE Trans Nanobioscience 4:10–9. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, and others. 2005. Brugada syndrome: Report of the Second Consensus Conference Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111:659–70. [DOI] [PubMed] [Google Scholar]
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. 1979. The steady state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflügers Arch Eur J Physiol 379:137–42. [DOI] [PubMed] [Google Scholar]
- Bagnéris C, DeCaen PG, Naylor CE, Pryde DC, Nobeli I, Clapham DE, and others. 2014. Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc Natl Acad Sci U S A 111:8428–33. https://www.pnas.org/cgi/doi/10.1073/pnas.1406855111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, and others. 2000. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404:84–7. [DOI] [PubMed] [Google Scholar]
- Bankar G, Goodchild SJ, Howard S, Nelkenbrecher K, Waldbrook M, Dourado M, and others. 2018. Selective NaV1.7 antagonists with long residence time show improved efficacy against inflammatory and neuropathic pain. Cell Rep 24:3133–45. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. 1983. Lidocaine block of cardiac sodium channels. J Gen Physiol 81:613–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billakota S, Devinsky O, Marsh E. 2019. Cannabinoid therapy in epilepsy. Curr Opin Neurol 32:220–6. [DOI] [PubMed] [Google Scholar]
- Borgelt LM, Franson KL, Nussbaum AM, Wang GS. 2013. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 33:195–209. http://doi.wiley.com/10.1002/phar.1187 [DOI] [PubMed] [Google Scholar]
- Buchanan JT. 1993. Electrophysiological properties of identified classes of Lamprey spinal neurons. 70:2313–25. [DOI] [PubMed] [Google Scholar]
- Cannon SC. 1996. Sodium channel defects in myotonia and periodic paralysis. Annu Rev Neurosci 19:141–64. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Bean BP. 2010. Sodium channels gone wild: resurgent current from neuronal and muscle channelopathies. J Clin Invest 120:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC, Brown RH, Corey DP. 1993. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J 65:270–88. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1225722&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. 2012. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590:2577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. 2005. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57:397–409. [DOI] [PubMed] [Google Scholar]
- Cha A, Ruben PC, George AL, Fujimoto E, Bezanilla F. 1999. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron 22:73–87. [DOI] [PubMed] [Google Scholar]
- Chau R, Kalaitzis JA, Neilan BA. 2011. On the origins and biosynthesis of tetrodotoxin. Aquat Toxicol 104:61–72. [DOI] [PubMed] [Google Scholar]
- Crill WE. 1996. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58:349–62. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, and others. 2011. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163:1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Yarov-Yarovoy V, Scheuer T, Catterall WA. 2011. Gating charge interactions with the S1 segment during activation of a Na+ channel voltage sensor. Proc Natl Acad Sci U S A 108:18825–30. https://www.pnas.org/content/108/46/18825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J-F, De Luca A, Didonna MP, George AL, Camerino Conte D, Luca ADE. 2004. Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block. J Physiol 554:321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–13. [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, and others. 2017. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 376:2011–20. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. 2007. From genes to pain: Nav1.7 and human pain disorders. Trends Neurosci 30:555–63. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. 2010. Sodium channels in normal and pathological pain. Annu Rev Neurosci 33:325–47. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, and others. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Dravet C. 2011. The core Dravet syndrome phenotype. Epilepsia 52:3–9. [DOI] [PubMed] [Google Scholar]
- ElSohly MA. 2007. Marijuana and the cannabinoids. Springer. [Google Scholar]
- Estacion M, Gasser A, Dib-Hajj SD, Waxman SG. 2010. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp Neurol 224:362–8. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. 1974. Aqueous pores created in thin lipid membranes by the antibiotics nystatin, amphotericin B and gramicidin A. Implications for pores in plasma membranes. In: Callingham BA, editor. Drugs and transport processes. Macmillan. p. 241–50. [Google Scholar]
- Fouda MA, Ghovanloo MR, Ruben PC. 2020. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br J Pharmacol 177:2932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamal El-Din TM, Lenaeus MJ, Zheng N, Catterall WA. 2018. Fenestrations control resting-state block of a voltage-gated sodium channel. Proc Natl Acad Sci 115:13111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamal El-Din TM, Martinez GQ, Payandeh J, Scheuer T, Catterall WA. 2013. A gating charge interaction required for late slow inactivation of the bacterial sodium channel NavAb. J Gen Physiol 142:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghovanloo M-R. 2020. Inhibition of voltage-dependent sodium currents by cannabidiol. https://ir.lib.sfu.ca/item/20992 [DOI] [PMC free article] [PubMed]
- Ghovanloo M-R, Abdelsayed M, Peters CH, Ruben PC. 2018. a. A mixed periodic paralysis & myotonia mutant, P1158S, imparts pH-sensitivity in skeletal muscle voltage-gated sodium channels. Sci Rep 8:6304. http://europepmc.org/abstract/med/29674667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghovanloo M-R, Aimar K, Ghadiry-Tavi R, Yu A, Ruben PC. 2016. Physiology and pathophysiology of sodium channel inactivation. Curr Top Membr 78:479–509. [DOI] [PubMed] [Google Scholar]
- Ghovanloo M-R, Atallah J, Escudero CA, Ruben PC. 2020. Biophysical characterization of a novel SCN5A mutation associated with an atypical phenotype of atrial and ventricular arrhythmias and sudden death. Front Physiol 11:610436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghovanloo M-R, Choudhury K, Bandaru TS, Fouda MA, Rayani K, Rusinova R, and others. 2021. Cannabidiol inhibits the skeletal muscle Nav1.4 by blocking its pore and by altering membrane elasticity. J Gen Physiol 153:e202012701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghovanloo M-R, Peters CH, Ruben PC. 2018. b. Effects of acidosis on neuronal voltage-gated sodium channels: Nav1.1 and Nav1.3. Channels 12:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghovanloo M-R, Ruben PC. 2020. Say cheese: structure of the cardiac electrical engine is captured. Trends Biochem Sci 45:369–71. [DOI] [PubMed] [Google Scholar]
- Ghovanloo M-R, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. 2018. c. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 293:16546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SE, Crossland KM, Andermann E, Phillips HA, Hall AJ, Bleasel A, and others. 2002. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet 360:851–2. [DOI] [PubMed] [Google Scholar]
- Hille B. 1975. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol 66:535–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 1977. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 2001. Ion channels of excitable membranes. Sinauer. [Google Scholar]
- Hladky SB, Haydon DA. 1972. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta 274:294–312. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. 1952. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116:449–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. 1977. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta 472:373–98. [DOI] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. 2009. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat Neurosci 12:996–1002. [DOI] [PubMed] [Google Scholar]
- Iannotti FA, Pagano E, Moriello AS, Alvino FG, Sorrentino NC, D’Orsi L, and others. 2019. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Br J Pharmacol 176:1568–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson HI, Lea Sanford R, Kapoor R, Andersen OS. 2010. Gramicidin-based fluorescence assay; for determining small molecules potential for modifying lipid bilayer properties. J Vis Exp 44:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BFX, Offord J, Charbonneau H, and others. 1992. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science 256:839–42. [DOI] [PubMed] [Google Scholar]
- Jiang D, Shi H, Tonggu L, Gamal El-Din TM, Lenaeus MJ, Zhao Y, and others. 2020. Structure of the cardiac sodium channel. Cell 180:122–134.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Stella N, Catterall WA, Westenbroek RE. 2017. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A 114:11229–34. http://www.pnas.org/lookup/doi/10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Peyear TA, Koeppe RE, Andersen OS. 2019. Antidepressants are modifiers of lipid bilayer properties. J Gen Physiol 151:342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, and others. 2011. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 145:1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosgodage US, Matewele P, Awamaria B, Kraev I, Warde P, Mastroianni G, and others. 2019. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front Cell Infect Microbiol 9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Sharan S, Woo S. 2020. Model-based analysis of cannabidiol dose-exposure relationship and bioavailability. Pharmacother J Hum Pharmacol Drug Ther 40:291–300. [DOI] [PubMed] [Google Scholar]
- Liu S, Shipley MT. 2008. Intrinsic conductances actively shape excitatory and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci 28:10311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Birn P, Hansen AJ, Søgaard R, Nielsen C, Girshman J, and others. 2004. Regulation of sodium channel function by bilayer elasticity. J Gen Physiol 123:599–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. 2010. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface 7:373–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. 2010. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol 9:413–24. [DOI] [PubMed] [Google Scholar]
- Mason ER, Cummins TR. 2020. Differential inhibition of human Nav1.2 resurgent and persistent sodium currents by cannabidiol and GS967. Int J Mol Sci 21:2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Yates AS, O’Sullivan SE. 2018. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol 9:1365. https://www.frontiersin.org/article/10.3389/fphar.2018.01365/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, Hurst DP, Reggio PH. 2017. Molecular targets of the phytocannabinoids: a complex picture. Prog Chem Org Nat Prod 103:103–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JE, Meisler MH. 2013. Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front Genet 4:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara T, Virág L, Varró A, Rudy Y. 2011. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol 7:e1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvos P, Pászti B, Topal L, Gazdag P, Prorok J, Polyák A, and others. 2020. The electrophysiological effect of cannabidiol on hERG current and in guinea-pig and rabbit cardiac preparations. Sci Rep 10:16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, and others. 2016. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 534:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Li Z, Zhou Q, Shen H, Wu K, Huang X, and others. 2018. Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science 362:eaau2486. [DOI] [PubMed] [Google Scholar]
- Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N, Cummins TR. 2016. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain 139:2164–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DE, Isom LL, Catterall WA, Goldin AL. 1994. The adult rat brain β1 subunit modifies activation and inactivation gating of multiple sodium channel α subunits. J Biol Chem 269:17649–55. [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. 2011. The crystal structure of a voltage-gated sodium channel. Nature 475:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CH, Ghovanloo M-R, Gershome C, Ruben PC. 2018. pH modulation of voltage-gated sodium channels. Handb Exp Pharmacol 246:147–60 [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, and others. 2001. Neuroscience. 2nd ed. Active transporters create and maintain ion gradients. Sinauer. [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. 1996. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A 93:9270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. 1997. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17:4517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. 1997. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19:881–91. [DOI] [PubMed] [Google Scholar]
- Ross HR, Napier I, Connor M. 2008. Inhibition of recombinant human T-type calcium channels by Δ9-tetrahydrocannabinol and cannabidiol. J Biol Chem 283:16124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Jiang HE, Li X, Sutton A, Carboni A, Del Bianco F, and others. 2008. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J Exp Bot 59:4171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sait LG, Sula A, Ghovanloo MR, Hollingworth D, Ruben PC, Wallace BA. 2020. Cannabidiol interactions with voltage-gated sodium channels. Elife 9:e58593. https://elifesciences.org/articles/58593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. 1997. Clustering sodium channels at the node of Ranvier: close encounters of the axon-glia kind. Neuron 18:843–6. [DOI] [PubMed] [Google Scholar]
- Scalmani P, Rusconi R, Armatura E, Zara F, Avanzini G, Franceschetti S, and others. 2006. Effects in neocortical neurons of mutations of the Nav1.2 Na+ channel causing benign familial neonatal-infantile seizures. J Neurosci 26:10100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhou Q, Pan X, Li Z, Wu J, Yan N. 2017. Structure of a eukaryotic voltage-gated sodium channel at near atomic resolution. Science 355:eaal4326. [DOI] [PubMed] [Google Scholar]
- Silva JR, Goldstein SAN. 2013. Voltage-sensor movements describe slow inactivation of voltage-gated sodium channels II: a periodic paralysis mutation in Na V 1.4 (L689I). J Gen Physiol 141:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt PWE, Ben-Shalom R, Keeshen CM, Burke KJ, Clarkson RL, Sanders SJ, and others. 2019. The autism-associated Gene Scn2a contributes to dendritic excitability and synaptic function in the prefrontal cortex. Neuron 103:673–685.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Makita N, Li L, Noble PJ, Kimura J, Kumagai Y, and others. 2003. Cold induces shifts of voltage dependence in mutant SCN4A, causing hypokalemic periodic paralysis. Neurology 61:914–8. [DOI] [PubMed] [Google Scholar]
- Sula A, Hollingworth D, Ng LCT, Larmore M, DeCaen PG, Wallace BA. 2021. A tamoxifen receptor within a voltage-gated sodium channel. Mol Cell 81:1160–1169.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. 2019. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol 176:1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M. 1987. Contribution of the Na+/K+-pump to the membrane potential. Experientia 43:1135–40. [DOI] [PubMed] [Google Scholar]
- van Klingeren B, ten Ham M. 1976. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 42:9–12. [DOI] [PubMed] [Google Scholar]
- Veeramah KR, O’Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, and others. 2012. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet 90:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilin YY, Ruben PC. 2001. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys 35:171–90. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, and others. 1995. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80:805–11. [DOI] [PubMed] [Google Scholar]
- Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, and others. 2010. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 182:E694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J, Cannon SC. 2008. Cold-induced defects of sodium channel gating in atypical periodic paralysis plus myotonia. Neurology 70:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. 1992. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci U S A 89:10910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. 2001. Comparative distribution of voltage-gated sodium channel proteins in human brain. Mol Brain Res 88:37–53. [DOI] [PubMed] [Google Scholar]
- White H V, Brown ST, Bozza TC, Raman IM. 2019. Effects of FGF14 and Na V β4 deletion on transient and resurgent Na current in cerebellar Purkinje neurons. J Gen Physiol 151:1300-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Häusser M. 2002. Membrane potential bistability is controlled by the hyperpolarization-activated current/H in rat cerebellar Purkinje neurons in vitro. J Physiol 539:469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. 2013. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 14:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisedchaisri G, Tonggu L, McCord E, Gamal El-Din TM, Wang L, Zheng N, and others. 2019. Resting-state structure and gating mechanism of a voltage-gated sodium channel. Cell 178:993–1003.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Sagar DR, Burston JJ, Chapman V. 2015. The role of the endocannabinoid system in pain. Handb Exp Pharmacol 227:119–43. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhou Q, Wang L, Wu J, Zhao Y, Huang G, and others. 2017. Structure of the Nav1.4-β1 complex from electric eel. Cell 170:470–482.e11. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan C-Y, Scheuer T, Baker D, and others. 2012. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci 109:E93–102. http://www.pnas.org/cgi/doi/10.1073/pnas.1118434109 [DOI] [PMC free article] [PubMed] [Google Scholar]