Abstract
The unicellular cyanobacterium Synechocystis sp. strain PCC 6803 has two putative pathways for ammonium assimilation: the glutamine synthetase-glutamate synthase cycle, which is the main one and is finely regulated by the nitrogen source; and a high NADP-dependent glutamate dehydrogenase activity (NADP-GDH) whose contribution to glutamate synthesis is uncertain. To investigate the role of the latter, we used two engineered mutants, one lacking and another overproducing NADP-GDH. No major disturbances in the regulation of nitrogen-assimilating enzymes or in amino acids pools were detected in the null mutant, but phycobiline content, a sensitive indicator of the nutritional state of cyanobacterial cells, was significantly reduced, indicating that NADP-GDH plays an auxiliary role in ammonium assimilation. This effect was already prominent in the initial phase of growth, although differences in growth rate between the wild type and the mutants were observed at this stage only at low light intensities. However, the null mutant was unable to sustain growth at the late stage of the culture at the point when the wild type showed the maximum NADP-GDH activity, and died faster in ammonium-containing medium. Overexpression of NADP-GDH improved culture proliferation under moderate ammonium concentrations. Competition experiments between the wild type and the null mutant confirmed that the presence of NADP-GDH confers a selective advantage to Synechocystis sp. strain PCC 6803 in late stages of growth.
Glutamate can be synthesized through two alternative ammonium assimilation pathways: one carried out sequentially by glutamine synthetase (GS) and glutamate synthase (GOGAT), and another catalyzed by glutamate dehydrogenase (GDH). The GS-GOGAT cycle is the one that plants and bacteria use the most (26), while GDH is the main one in many fungi (19). In enterobacteria, GDH plays an auxiliary role, being able to operate as the only pathway in media containing high concentrations of ammonium (2). In addition, results of competition experiments have suggested that enterobacteria use GDH for glutamate synthesis when energy supply is limiting (13).
The GS-GOGAT cycle is the main ammonium assimilation pathway in cyanobacteria (23), although NADP-GDH is present in a number of cyanobacterial strains (28). In these organisms, GS is finely regulated by the nitrogen source and exhibits a lower activity when cells are cultured in ammonium-containing media (11, 29). Synechocystis sp. strain PCC 6803 is a unicellular cyanobacterium, able to grow in phototrophic or myxotrophic conditions, that shows a high NADP-dependent GDH activity (10). A minor NAD-dependent activity has also been detected (5), but no putative gene coding for an independent NAD-GDH has been identified subsequent to sequencing of the whole genome of Synechocystis sp. strain PCC 6803 (16). Therefore, this activity could be ascribed either to a secondary activity of another enzyme or to a member of a new family of GDHs.
GS is rapidly inactivated in Synechocystis sp. strain PCC 6803 by the addition of ammonium to the culture medium (24). In addition, GS synthesis is significantly diminished in ammonium-containing media (25). The correlation between these properties of Synechocystis GS and the occurrence of NADP-GDH has favored the hypothesis of an ammonium assimilatory role for GDH in ammonium-rich medium, similar to what has been described for enterobacteria (2). This hypothesis is compatible with the contribution of NADP-GDH to ammonium tolerance by cyanobacteria, shown by Lightfoot et al. (18).
The gdhA gene of Synechocystis sp. strain PCC 6803, coding for NADP-GDH, has been cloned by complementation of an Escherichia coli gdhA mutant (6). In that genetic background, it is able to operate as the only ammonium-assimilating enzyme (6). The gdhA gene is transcriptionally regulated by the growth phase in Synechocystis, with maximum activity at the late stage of growth (6).
To clarify the role of NADP-GDH in Synechocystis sp. strain PCC 6803 and the biological significance of the occurrence of two putative ammonium assimilation pathways in cyanobacteria, we have made use of two mutant strains. One, SCh11, lacks NADP-GDH; the other, SCh12, overproduces the enzyme. Our results show that although nonessential for viability, NADP-GDH is necessary for proliferation during the late stage of growth in both nitrate- and ammonium-containing media. Nitrogen metabolism is affected in the null mutant, as indicated by the low phycocyanin content, although no major alterations in the regulation of nitrogen assimilation enzymes were detected. We propose that NADP-GDH plays an auxiliary role in nitrogen assimilation in cyanobacteria that is critical at late stages of growth.
MATERIALS AND METHODS
Strains and growth conditions.
Synechocystis sp. PCC 6803 strain SFCΩ5 (4) was used as the wild type. The gdhA-null mutant of Synechocystis used was strain SCh11 (6). To overproduce NADP-GDH, we transformed SFCΩ5 with plasmid pSCh11 as described by Chauvat et al. (3). Plasmid pSCh11 was constructed by ligating the 1.8-kb PvuII-EcoRI DNA fragment of Synechocystis containing the entire gdhA gene with plasmid pFF11T (8) previously restricted with EcoRI. After treatment of the ligation mixture with S1 nuclease to make blunt the nonligated EcoRI end, the DNA was ligated again. All strains were grown photoautotrophically at 35°C on BG11 medium (33), which contains 18 mM nitrate as the nitrogen source, under continuous illumination (125 μE · m−2 · s−1; white light). The cultures were bubbled with 1.5% (vol/vol) CO2 in air. When ammonium was used as the nitrogen source, BG11 medium lacking nitrate was supplemented with 10 mM NH4Cl and the medium was buffered with 20 mM N-tris(hydroxymethyl)-methyl-2-aminoethanesulfonic acid (TES) buffer.
Growth curves.
To determine growth, cells were inoculated, at a final concentration of 1 μg of chlorophyll per ml, into 250-ml Erlenmeyer flasks containing 50 ml of BG11 medium buffered with 150 mM HEPES-NaOH (pH 7.5). Ammonium cultures contained NH4Cl at the indicated concentration. To balance ionic strength, the cultures were supplemented with KCl until the highest NH4Cl concentration of each experiment was reached. Cultures were shaken at 150 rpm under white light (65 μE · m−2 · s−1) at 30°C. Samples were drawn at various times, and growth was estimated by chlorophyll determination.
Competition experiments.
In the competition experiments, Synechocystis sp. PCC 6803 strains SFCΩ5 and SCh11 were grown separately and used to inoculate 250-ml Erlenmeyer flasks, containing 50 ml of BG11 medium, to a final concentration of 0.3 μg of chlorophyll per ml. The cells were previously counted under a microscope to ensure a 1:1 ratio between the two strains. Cultures were grown with constant shaking under white light as specified above. A set of cultures was reinoculated with 1/10 of the culture every 4 days, while reinoculation of the another set was done with 1/5 of the culture every 15 days. The proportion between the two populations was calculated after plating on BG11 medium with and without chloramphenicol to discriminate between total cells and null-mutant cells, which are chloramphenicol resistant.
Determination of enzymatic activities.
NADP-dependent GDH aminating activity was assayed in vitro as previously described (6). GS was determined in situ by using the Mn2+-dependent γ-glutamyltransferase assay of cells permeabilized with mixed alkyltrimethylammonium bromide (24). Nitrate and nitrite reductases were determined in situ with dithionite-reduced methyl viologen as the reductant (14, 15). NAD-GDH, isocitrate dehydrogenase, and GOGAT activities were determined as described elsewhere (5, 9, 22).
Absorption spectra.
To obtain the absorption spectra, whole cells were washed by centrifugation, resuspended in 10 mM NaCl, and scanned in the turbid sample compartment of an SLM-Aminco DW2000 spectrophotometer.
Analytical methods.
To determine the intracellular pools of amino acids, cells lysates were obtained by the addition of 0.9 ml of a culture in exponential phase to 0.1 ml of 2 N HCl, followed by vigorous shaking and centrifugation at 12,000 × g for 5 min at 4°C. The amino acid concentrations in the supernatants were determined by high-pressure liquid chromatography as previously described (22). Protein in whole cells was determined by the method of Lowry et al. (19a) as modified by Markwell et al. (21), using ovalbumin as the standard. Chlorophyll was determined as described by MacKinney (20).
Determination of C/N ratios.
Cells were harvested by centrifugation, washed twice with 20 mM NaCl, and dried at 80°C for 2 days. Samples of about 1 mg (dry weight) were used to determine the C and N content of the cells, utilizing a Carlo Erba Instrumentazione 1106/R elemental analyzer.
Northern blot analysis.
RNA blotting experiments were carried out as previously described (6), using a 347-bp BstEII-BstEII gdhA labeled fragment as a probe. As a control, the filter was later hybridized with a probe for the RNA subunit of Synechocystis sp. strain PCC 6803 RNase P (rnpB).
RESULTS
Mutant strains of Synechocystis sp. PCC 6803 affected in NADP-GDH activity.
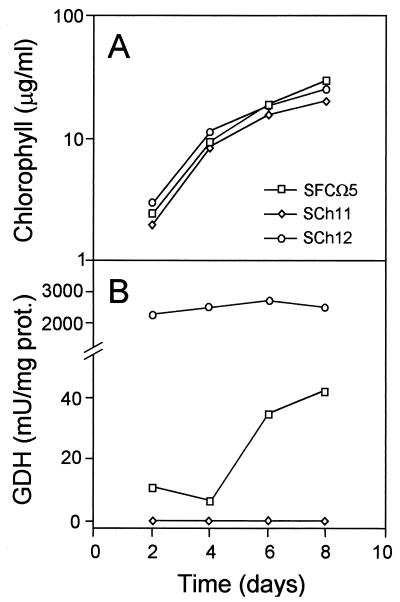
To clarify the function of the NADP-GDH in Synechocystis sp. strain PCC 6803, we used two mutant strains: SCh11, a previously described NADP-GDH null mutant obtained by disrupting the gdhA gene (6); and SCh12, an NADP-GDH overproducer constructed by inserting the gdhA gene in plasmid pFF11T (8), immediately downstream of the very strong tac promoter (see Materials and Methods for details). The recombinant plasmid was used to transform the wild-type strain SFCΩ5 (4). SCh12 showed an NADP-GDH activity about 100-fold higher than the wild-type level (Fig. 1B). This activity showed no significant variation over time, in contrast to the NADP-GDH activity of the wild type, which increased at the latest stages of growth. The null strain SCh11 never exhibited NADP-GDH activity (Fig. 1B).
FIG. 1.
Evolution of NADP-GDH activity during growth of the Synechocystis sp. PCC 6803 strains used in this work. Cells were grown on ammonium as described in Materials and Methods. The initial chlorophyll concentration was 0.1 μg/ml. prot., protein.
Both mutant strains were viable and retained the main nutritional behavior of the wild type with regard to the use of nitrogen sources, since they grew when plated on medium containing either nitrate or ammonia, in the presence or in the absence of glucose. They were also able to utilize arginine as the only nitrogen source and could not grow in the presence of glutamine (data not shown), thus behaving like the wild type with respect to amino acids (17).
From a regulatory standpoint, the short-term ammonium effects on GS activity and on the glutamate and glutamine pools were the same in all three strains, although the steady-state pool of glutamine was somewhat higher in the null mutant, indicating that NADP-GDH influences the amino acid fluxes to a certain extent (data not shown). The levels and changes with age of the culture of GS and other enzymes involved in nitrogen metabolism, such as nitrate and nitrite reductases, GOGAT, NAD-GDH, or isocitrate dehydrogenase, were the same in the mutants and the wild type. The same was true with the clear nitrate-versus-ammonium pattern observed in GS and nitrate or nitrite reductase activities, being high in nitrate-containing medium and low in the presence of ammonium (data not shown).
Effect of NADP-GDH levels in the initial phase of growth.
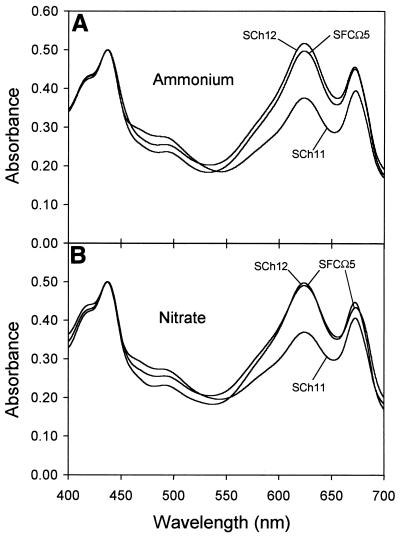
As cultures on solid media do not provide information on growth rate, we investigated the time course of liquid cultures. Figure 1A shows that the mutant and wild-type strains grew equally well during the initial phase, despite their differences in NADP-GDH activity levels (Fig. 1B). However, the SCh11 culture presented a greenish aspect that was due to its lower light absorption in the phycocyanin-absorbing region of the spectrum (Fig. 2). The 437-to-624-nm absorption ratio was 35% higher in SCh11 than in the NADP-GDH-containing strains. The same result was obtained with either nitrate or ammonium as the nitrogen source (Fig. 2).
FIG. 2.
Absorption spectra of the Synechocystis sp. PCC 6803 strains used in this work. Cells were grown as described in Materials and Methods to a chlorophyll concentration of 1 μg/ml. The nitrogen source used was ammonium (A) or nitrate (B). Spectra were recorded as described in Materials and Methods and normalized to an absorbance value of 0.5 at 437 nm.
To check if there was a heavy unbalance in the overall nitrogen assimilation in the initial stage of growth, we determined the C/N ratios of the cultures. Table 1 shows that there were no significant differences in C/N ratio among the three strains when growing in ammonium. The differences in nitrate-grown cultures, although statistically significant at α = 0.01, were rather small, and the C/N ratio was lower in the NADP-GDH-containing strains.
TABLE 1.
C/N ratios of Synechocystis sp. PCC 6803 strains at various growth stages
| Strain | C/N ratioa
|
|||
|---|---|---|---|---|
| Cultures in nitrate
|
Cultures in ammonium
|
|||
| Initial stage | Late stage | Initial stage | Late stage | |
| SFCΩ5 | 4.065 ± 0.029 | 4.480 ± 0.039 | 4.041 ± 0.076 | 4.605 ± 0.128 |
| SCh11 | 4.105 ± 0.012b | 4.583 ± 0.040b | 4.045 ± 0.087 | 4.574 ± 0.096 |
| SCh12 | 4.024 ± 0.017b | 4.495 ± 0.137 | 4.055 ± 0.068 | 4.663 ± 0.156 |
Chlorophyll contents were about 1 and 10 μg/ml for initial and late stages of growth, respectively. Values are the averages ± standard deviations of eight determinations.
Statistically different from the wild-type value (SFCΩ5) at α = 0.01.
From the results shown above, it follows that NADP-GDH activity has an observable, although limited, effect in the initial phase of growth.
Effect of NADP-GDH levels in the late stage of growth.
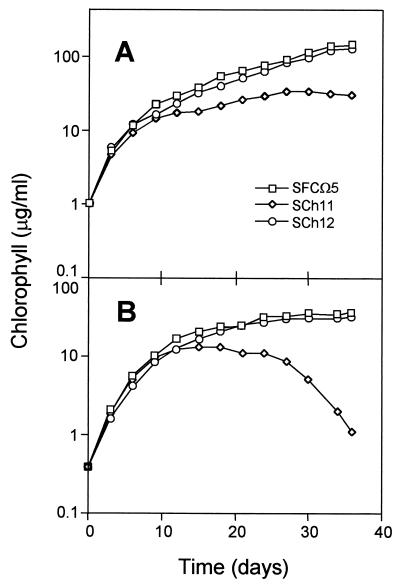
After the initial growth, the cultures reached a phase in which light was limiting and the growth rate decreased. Under those conditions, the mutant lacking NADP-GDH grew at a drastically reduced rate at a time when wild-type cells and those overexpressing the enzyme were still growing vigorously (Fig. 3A). The differences were even more evident when high levels of ammonium were present in the culture (Fig. 3B). In that case SCh11 cells not only stopped growing but also died, very likely due to a toxic effect of ammonium. However, the strain overexpressing NADP-GDH did not become more ammonium tolerant, and only at a moderate concentration of ammonium (15 mM) did SCh12 grow faster than the wild type (data not shown).
FIG. 3.
Growth curves of the Synechocystis sp. PCC 6803 strains used in this work. Cells were grown in nitrate-containing media as described in Materials and Methods in the absence (A) or presence (B) of 100 mM NH4Cl.
The effects of NADP-GDH levels on the absorption spectra, although still present, were less marked in the late stages of growth than in cells rapidly growing on nitrate medium. The wild-type strain and the NADP-GDH overproducer exhibited equal light absorption in the 624-nm region, which was higher than that of the null mutant (data not shown). In late-stage cultures of ammonium-growing cells, a similar result was observed, although in this case the 624-nm absorption of the wild-type culture was midway between those of the overproducer and the null mutant (data not shown).
The C/N ratios determined in cultures in the late stages of growth were similar in all three strains when they were growing in ammonium (Table 1). Only minor differences were found between nitrate cultures of the null mutant and those of NADP-GDH-containing strains, being higher in the null mutant (Table 1).
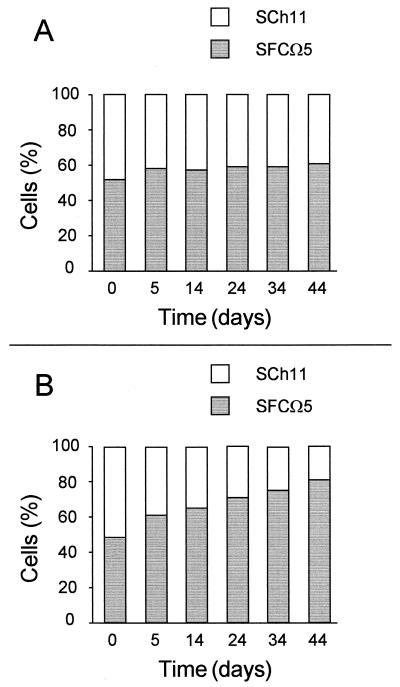
The inability of SCh11 to grow optimally in the late stages suggests that NADP-GDH, despite being nonessential for Synechocystis, confers a selective advantage to this microorganism. To test this hypothesis, we determined the ability of the mutant to compete with the wild type in a mixed culture. When the mixed population was cultured for more than 1 month at the exponential rate, that is, using a low inoculum every 4 days, the frequency of mutant cells in the population diminished only slightly (10%) and mainly in the first days of the experiment (Fig. 4A). The difference was much more apparent when the cultures were reinoculated every 15 days, that is, when they were allowed to reach growth phases close to stationary prior to reinoculation. In this case, after 44 days of culture and two reinoculations, less than 20% of the total population were mutant cells (Fig. 4B). From the results shown above, we conclude that the presence of NADP-GDH activity is a considerable selective advantage for Synechocystis sp. strain PCC 6803 at the late stages of growth.
FIG. 4.
Competition between Synechocystis sp. PCC 6803 wild-type strain SFCΩ5 and the gdhA null mutant SCh11 in mixed cultures. Cultures were diluted 10-fold every 4 days (A) or 5-fold every 15 days (B). The percentage of each strain was determined at the indicated times as described in Materials and Methods.
Light availability and role of NADP-GDH.
To check if light limitation was the cause of the loss in competing ability of the null mutant versus the wild-type strain observed at late stages, we cultured both strains at low light intensity (5 μE·m−2·s−1) for 18 days. Under those conditions, both strains grew linearly from an initial chlorophyll concentration of 0.5 μg·ml−1 to 11.5 ± 0.55 and 13.4 ± 0.97 μg·ml−1 for the null mutant and the wild type, respectively.
The transfer to low light intensity of a mid-log-phase culture of the wild-type strain growing at normal light intensity did not induce an increase in either NADP-GDH activity or gdhA mRNA (data not shown). Moreover, a chloramphenicol acetyltransferase fusion of the regulated gdhA promoter (6) showed no positive regulation of gdhA in response to a decrease in light intensity. Taken together, these data suggest that light availability is not the only physiological parameter determining the functionality of NADP-GDH in Synechocystis.
DISCUSSION
There is overwhelming evidence that in Synechocystis sp. strain PCC 6803, ammonium assimilation takes place through the GS-GOGAT cycle (24, 27, 31), which raises the question of the physiological role of an NADP-GDH with clear assimilatory features (10). Until now the coexistence of these two pathways had been discussed in terms of the different Km values for ammonium—low for GS and high for NADP-GDH—and of their different relative levels of activity in nitrate- and ammonium-containing media (9, 10). This viewpoint was a consequence of directly adapting the model developed for E. coli to Synechocystis sp. strain PCC 6803. In enterobacteria, NADP-GDH is proposed to carry out the assimilation of inorganic nitrogen when high levels of ammonium are present in the medium (30). Given the results presented above, we believe that this point of view does not apply to the situation in Synechocystis. Rather, we think that the alternatives are not ammonium versus nitrate or high versus low ammonium concentration but rather initial versus late stages of growth.
The fact that the absence of NADP-GDH produces a deleterious phenotype only during the late stages of growth is in good agreement with the regulation of this activity throughout the time course of the culture. The level of NADP-GDH increases after the initial growth phase (Fig. 1B), with only minor deviations observed between nitrate- and ammonium-grown cells (data not shown). This time course of activity is driven by transcriptional regulation of the gdhA gene (6), correlating the levels of gdhA expression with the functional importance of NADP-GDH in each stage of growth.
If NADP-GDH plays a role in nitrogen assimilation in Synechocystis, its absence should produce detectable changes in the parameters related to it. The phycobiliprotein content, significantly lower in the null mutant (Fig. 2), confirms this hypothesis. However, other indicators of nitrogen metabolism, such as the pools of Glu, Gln, and Asp or the levels of the main enzymes related to nitrogen assimilation, i.e., nitrate and nitrite reductases or GS, did not show major variations between the mutants and the wild-type strain, nor did we observe any major changes in the C/N ratio of the cells (Table 1). Furthermore, the fine regulation by nitrogen source that most of these enzymes exhibit (12) is not disturbed in the mutant lacking NADP-GDH or in the strain that overexpresses this enzyme. Therefore, the contribution of NADP-GDH to nitrogen assimilation by Synechocystis, though present, is not essential. This idea is reinforced by the inability to isolate GOGAT-null mutants of Synechocystis sp. strain PCC 6803 (27), indicating that NADP-GDH cannot replace GOGAT’s role in glutamate synthesis.
The lower tolerance to high ammonium concentrations of the null mutant (Fig. 3B) also supports a biosynthetic role for NADP-GDH, reducing the toxic, intracellular levels of that ion. In fact, the expression of E. coli NADP-GDH in Synechococcus sp. strain PCC 6301, a cyanobacterium naturally lacking this enzyme, protected against ammonium toxicity (18). Our results confirm this phenomenon, although ammonium detoxification is not the only role of NADP-GDH in cyanobacteria, since the lack of this enzyme also produces a clear effect in cells grown on nitrate medium.
Mutants lacking NADP-GDH without showing apparent phenotypes have been described for several enterobacteria and for Corynebacterium glutamicum (1). In all of these cases, no major disturbance of nitrogen metabolism was observed. In E. coli, Salmonella typhimurium, and Klebsiella pneumoniae, a second mutation, affecting GOGAT activity, is required to make NADP-GDH essential (30). Therefore, we wonder whether a phenotype similar to the one described in this work also occurs in those species.
Competition experiments between wild-type and gdhA null-mutant strains of E. coli indicate a selective advantage of the wild type under conditions of carbon starvation (13). Such conditions could be considered analogous to what takes place in Synechocystis during the late stages of growth, when the energy and reduced ferredoxin supply starts to be limiting due to reduced photosynthesis. In that case, the presence of an ammonium-assimilating enzyme such as NADP-GDH could be a valuable supplement to the GS-GOGAT pathway, since it does not need ATP and uses NADPH, a reductant readily obtainable from reserves, instead of the reduced ferredoxin required by GOGAT. In fact, in Synechococcus cells cultured at low light intensity, Lightfoot et al. (18) observed a positive effect on growth of the presence of an NADP-GDH. Nevertheless, light availability is not the only parameter governing the metabolic role of NADP-GDH in Synechocystis, as can be concluded from the following facts: (i) the lack of NADP-GDH causes a pigment phenotype at the initial stage, when light is not limiting; (ii) low light intensity slightly reduces but does not prevent growth of the null mutant; and (iii) light limitation does not induce NADP-GDH activity or the accumulation of gdhA mRNA in a mid-log-phase culture. Therefore, other metabolic factors in addition to light intensity must influence the regulation and functionality of NADP-GDH in the late stages of growth.
In short, we envisage two different scenarios: an initial stage of growth in which the low cell density of the culture allows an optimal photosynthetic metabolism, and a second period of high cell density and lower growth rate. In both, the NADP-GDH behaves identically but its lack or abundance is reflected differently.
In the initial stage, the activity of NADP-GDH, in addition to the operation of the GS-GOGAT cycle, produces a slight nitrogen surplus that is stored as phycocyanin at a time when a high amount of this pigment is not necessary to capture light (9). Therefore, as we have previously shown, the elimination of NADP-GDH activity in Synechocystis sp. strain PCC 6803 by disruption of the gdhA gene has no lethal effect (6) and is revealed only as a lower level of phycocyanin. In fact, this shows that ammonium assimilation is somewhat impaired in the null mutant, since phycobiliproteins are very sensitive indicators of the nutritional state of cyanobacterial cells (7).
As the culture grows and light becomes limiting, more nitrogen ought to be invested in the synthesis of auxiliary pigments. At the same time, GS activity declines (32) and NADP-GDH activity rises (Fig. 1B). In this situation, the relative contribution of NADP-GDH to ammonium assimilation increases, and its absence is a clear disadvantage with respect to the wild type, which not only has a higher nitrogen assimilation rate but also exhibits a higher initial pigment content. Consequently, cells without NADP-GDH grow slower than the wild type and are outnumbered in the competence studies (Fig. 4).
Exponential growth rates are probably an exception in natural conditions, where organisms are usually limited by trophic parameters. In the ecosystems where cyanobacteria live, light is often not optimal and much less abundant than under laboratory conditions. The fact that NADP-GDH is necessary during limited-growth conditions is therefore not a trivial circumstance but very likely crucial for the survival of Synechocystis sp. strain PCC 6803 in nature.
ACKNOWLEDGMENTS
This work was supported by grants from DGICYT (PB91-0127 and PB94-1444) and by Junta de Andalucía (CVI 0112), Spain. S. Chávez and J. C. Reyes were recipients of a predoctoral fellowship from M.E.C. (Spain).
The strain SFCΩ5 of Synechocystis PCC 6803 and plasmid pFF11T were kindly provided by F. Chauvat.
REFERENCES
- 1.Börmann-El Kholy E R, Eikmanns B J, Gutmann M, Sahm H. Glutamate dehydrogenase is not essential for glutamate formation by Corynebacterium glutamicum. Appl Environ Microbiol. 1993;59:2329–2331. doi: 10.1128/aem.59.7.2329-2331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley J E, Magasanik B. Mutants of Klebsiella aerogenes lacking glutamate dehydrogenase. J Bacteriol. 1974;114:544–550. doi: 10.1128/jb.117.2.544-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauvat F, De Vries L, Van der Ende A, Van Arkel G A. A host-vector system for gene cloning in the cyanobacterium Synechocystis PCC 6803. Mol Gen Genet. 1986;204:185–191. [Google Scholar]
- 4.Chauvat F, Labarre J, Ferino F. Development of genes transfer system for the cyanobacterium Synechocystis PCC 6803. Plant Physiol Biochem. 1988;26:629–637. [Google Scholar]
- 5.Chávez S, Candau P. An NAD-specific glutamate dehydrogenase from cyanobacteria. Identification and properties. FEBS Lett. 1991;285:35–38. doi: 10.1016/0014-5793(91)80719-j. [DOI] [PubMed] [Google Scholar]
- 6.Chávez S, Reyes J C, Chauvat F, Florencio F J, Candau P. The NADP-glutamate dehydrogenase of the cyanobacterium Synechocystis 6803: cloning, transcriptional analysis and disruption of the gdhA gene. Plant Mol Biol. 1995;28:173–188. doi: 10.1007/BF00042048. [DOI] [PubMed] [Google Scholar]
- 7.Collier J L, Grossman A R. A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J. 1994;13:1039–1047. doi: 10.1002/j.1460-2075.1994.tb06352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferino F, Chauvat F. A promoter-probe vector-host system for the cyanobacterium, Synechocystis PCC6803. Gene. 1989;84:257–266. doi: 10.1016/0378-1119(89)90499-x. [DOI] [PubMed] [Google Scholar]
- 9.Florencio F J, Chávez S, Muro-Pastor M I, Reyes J C, Marqués S, Mérida A, Candau P. Interaction between photosynthesis and nitrogen assimilating enzymes in cyanobacteria. In: Barber J, Guerrero M G, Medrano H, editors. Trends in photosynthesis research. 1992. Andover, England: Intercept Ltd.; 1992. pp. 231–240. [Google Scholar]
- 10.Florencio F J, Marqués S, Candau P. Identification and characterization of a glutamate dehydrogenase in the unicellular cyanobacterium Synechocystis PCC 6803. FEBS Lett. 1987;223:37–41. [Google Scholar]
- 11.Florencio F J, Ramos J L. Purification and characterization of glutamine synthetase from the unicellular cyanobacterium Anacystis nidulans. Biochim Biophys Acta. 1985;838:39–48. [Google Scholar]
- 12.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria—1994. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 487–517. [Google Scholar]
- 13.Helling R B. Why does Escherichia coli have two primary pathways for synthesis of glutamate? J Bacteriol. 1994;176:4664–4668. doi: 10.1128/jb.176.15.4664-4668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero A, Flores E, Guerrero M G. Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119, and Nostoc sp. strain 6719. J Bacteriol. 1981;145:175–180. doi: 10.1128/jb.145.1.175-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero A, Guerrero M G. Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J Gen Microbiol. 1986;132:2463–2468. [Google Scholar]
- 16.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 17.Labarre J, Chauvat F, Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989;171:3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lightfoot D A, Baron A J, Wootton J C. Expression of the Escherichia coli glutamate dehydrogenase gene in the cyanobacterium Synechococcus PCC6301 causes ammonium tolerance. Plant Mol Biol. 1988;11:335–344. doi: 10.1007/BF00027390. [DOI] [PubMed] [Google Scholar]
- 19.Lomnitz A, Calderón J, Hernández G, Mora J. Functional analysis of ammonium assimilation enzymes in Neurospora crassa. J Gen Microbiol. 1987;133:2333–2340. [Google Scholar]
- 19a.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.MacKinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- 21.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 22.Marqués S, Florencio F J, Candau P. Ammonia assimilating enzymes from cyanobacteria: in situ and in vivo assay using high-performance liquid chromatography. Anal Biochem. 1989;180:152–157. doi: 10.1016/0003-2697(89)90104-8. [DOI] [PubMed] [Google Scholar]
- 23.Meeks J C, Wolk C P, Lockau W, Schilling N, Shaffer P W, Chien W-S. Pathways of assimilation of [13N]N2 and 13NH4 by cyanobacteria with and without heterocysts. J Bacteriol. 1978;134:125–130. doi: 10.1128/jb.134.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mérida A, Candau P, Florencio F J. Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 by the nitrogen source: effect of ammonium. J Bacteriol. 1991;173:4095–4100. doi: 10.1128/jb.173.13.4095-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mérida A, Leurentop L, Candau P, Florencio F J. Purification and properties of glutamine synthetases from the cyanobacteria Synechocystis sp. strain PCC 6803 and Calothrix sp. strain PCC 7601. J Bacteriol. 1990;172:4732–4735. doi: 10.1128/jb.172.8.4732-4735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miflin B J, Lea P J. Ammonia assimilation. In: Miflin B J, editor. The biochemistry of plants. 1980. New York, N.Y: Academic Press; 1980. pp. 169–202. [Google Scholar]
- 27.Navarro F, Chávez S, Candau P, Florencio F J. Existence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis sp. PCC 6803. Isolation and insertional inactivation of gltB and gltS genes. Plant Mol Biol. 1995;27:753–767. doi: 10.1007/BF00020228. [DOI] [PubMed] [Google Scholar]
- 28.Neilson A H, Doudoroff M. Ammonia assimilation in blue-green algae. Arch Mikrobiol. 1973;89:15–22. doi: 10.1007/BF00409395. [DOI] [PubMed] [Google Scholar]
- 29.Orr J, Haselkorn R. Regulation of glutamine synthetase activity and synthesis in free-living and symbiotic Anabaena spp. J Bacteriol. 1982;152:626–635. doi: 10.1128/jb.152.2.626-635.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitzer L J, Magasanik B. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine and d-alanine. In: Neidhart F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 302–320. [Google Scholar]
- 31.Reyes J C, Florencio F J. A new type of glutamine synthetase in cyanobacteria: the protein encoded by the glnN gene supports nitrogen assimilation in Synechocystis sp. strain PCC 6803. J Bacteriol. 1994;176:1260–1267. doi: 10.1128/jb.176.5.1260-1267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes, J. C., and F. J. Florencio. Unpublished data.
- 33.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]