Abstract
Polypharmacy in people living with HIV/AIDS (PLWHA) is a rising morbidity that exacts hefty economic burden on health budgets in addition to other adverse clinical outcomes. Despite recent advances, uncertainty remains around its exact definition in PLWHA. In this systematic review and Meta-analysis, we explored relevant databases (PUBMED, EMBASE, CROI) for studies evaluating polypharmacy in PLWHA from January 2000 to August 2021 to ascertain the exact numerical threshold that defines this morbidity. Two independent reviewers extracted and reviewed relevant variables for analyses. The review included a total of 31 studies involving n = 53,347 participants with a mean age of 49.5 (SD ± 17.0) years. There was a total of 36 definitions, with 93.5% defining polypharmacy as the concomitant use of 5 or more medications. We found significant variation in the numerical definition of polypharmacy, with studies reporting it as “minor” (N = 3); “major” (N = 29); “severe” (N = 2); “excessive” (N = 1); and “higher” (N = 1). Most studies did not incorporate a duration (84%) in their definition and excluded ART medications (67.7%). A plurality of studies in PLWHA have established that polypharmacy in this cohort of patients is the intake of ≥ 5 medications (including both ART and non-ART). To standardize the approach to addressing this rising morbidity, we recommend incorporation of this definition into national and international PLWHA treatment guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12981-022-00461-4.
Keywords: PLWHA, Polypharmacy, Drug interactions, HIV, AIDS, Antiretroviral
Introduction
One of the evolving challenges with regards to therapeutics in people living with HIV/AIDS (PLWHA) is the increasing number of daily medications patients often must take [1–3]. This is invariably a consequence of the rising multimorbidity associated with increasing survival seen in these patient cohorts as well as those of the general population [4]. The latter although attributable to multiple factors, the role of antiretroviral therapy (ART) drugs is by far the most important on reducing mortality of PLWHA [3]. Unfortunately, since the incorporation of these drugs into national and international treatment guidelines, the total number of HIV and non-HIV medications used by PLWHA daily has exponentially increased. This has conferred enormous burden on this cohort of patients [5], including the costly consequences of polypharmacy such as drug–drug [6, 7], drug–food [8], and pharmacogenetic interactions [9].
In the general population, polypharmacy has often been defined as the daily ingestion of five or more medications [10]. Whilst there appears to be consensus regarding the definition of polypharmacy in the general population [10], uncertainty still exists as to what exactly constitutes polypharmacy in PLWHA [11]. This uncertainty revolves around both the numerical threshold (< 5, ≥ 5, or ≥ 10 medications etc.) [11], as well as whether HIV medications [12] were part of the numerical count of polypharmacy or not. Whilst several reports have attempted to explore patterns, determinants, and consequences of polypharmacy across various populations in both inpatient and outpatient settings around the world [8, 13–15], an enduring consensus around its exact definition in PLWHA remains unexplored. The most recent attempt at a numerical characterization of polypharmacy in this cohort of patients was a narrative review by Back et al. [1]. A recent meta-analysis by Danjuma et al. explored for the first time the prevalence as well as global trends of polypharmacy across different demographic populations of PLWHA (in press). It reported a period prevalent rate of polypharmacy amongst PLWHA of around 33% across the world and rising. Notably most of the studies included in this review synthesis had different medication thresholds for what constitutes polypharmacy.
In this study, we aimed to carry out a comprehensive synthesis of all studies that have investigated polypharmacy in PLWHA with the view to ascertaining what exactly constitutes polypharmacy in this cohort of patients; and in so doing engender potentially useful prescriptive consensus around this rising morbidity.
Method
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist and the Cochrane Handbook guidelines [16]. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO)—CRD42020170071.
Eligibility criteria
The following databases were searched from 1 January 2000 to 30 August 2021: PubMed; EMBASE, Conference on Retroviruses and Opportunistic Infections (CROI), Cochrane Database of Systematic Reviews; Science Citation Index and Database of Abstracts of Reviews of Effects (DARE). Reference lists of included studies were also manually searched to identify relevant articles that were not yielded from the database search. Databases were searched using the Boolean operator ‘AND’ to combine terms from different categories, while ‘OR’ was utilized for terms under one category. The following Medical Subject Headings (MeSH) terms and keywords were used: (HIV [tiab] OR “people living with HIV” [MeSH] AND polypharmacy[tiab]). Studies were considered for inclusion if they were published in English language between 1st January 2000 and 30th august 2021; and focused on PLWHA with age greater than 18 years old. All studies that incorporated at least one definition (numerical, descriptive or both) of polypharmacy amongst PLWHA on ART medicines were eligible for inclusion irrespective of design. We excluded studies that failed to clearly define what constitutes polypharmacy either numerically or descriptively in their methodology. Additional file 1: Table S2 shows results of literature search.
Study selection
Following completion of literature search from relevant databases, duplicates were removed utilizing EndNote 20® (2021 Clarivate). Screening of titles, abstracts (using Rayyan QCRI software), and full papers (using Microsoft excel) was conducted independently by two reviewers (SK and FA) according to the inclusion and exclusion criteria. Discrepancies were resolved through consensus or by adjudication by a third reviewer (MID).
Risk of bias assessment
Two reviewers (MID, LMN) carried out a comprehensive risk of bias assessment of the reviewed studies using the Cochrane checklist for the assessment of risk of bias [17]. Where disagreement arose, this was resolved with consensus or adjudication by the third reviewer (AE). This is to ascertain the methodological quality of the reviewed studies. Details of this checklist has exhaustively been explained elsewhere, but in brief it is comprised of six standard criteria: including concealment of allocation, adequate sequence generation, blinding of participants and personnel, from selective reporting, blinded assessment of primary outcome(s), freedom from other risks of bias, and adequately addressed incomplete outcome data. A risk of bias table showing the grading of the studies is shown in Additional file 1: Table S1.
Data extraction
A data extraction form was designed by two reviewers (MID and SK) and piloted on five included studies. We extracted the following variables from each study: first author, year of publication, center where the study was carried out, study design, duration of HIV/AIDS, HIV viral load (where available), number of PLWHA, polypharmacy definition, number of patients satisfying criteria for polypharmacy, socio-demographic parameters. Where studies explored different definitions of polypharmacy, we included all definitions they explored. Statistical analyses were conducted in Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC. 2021.
Results
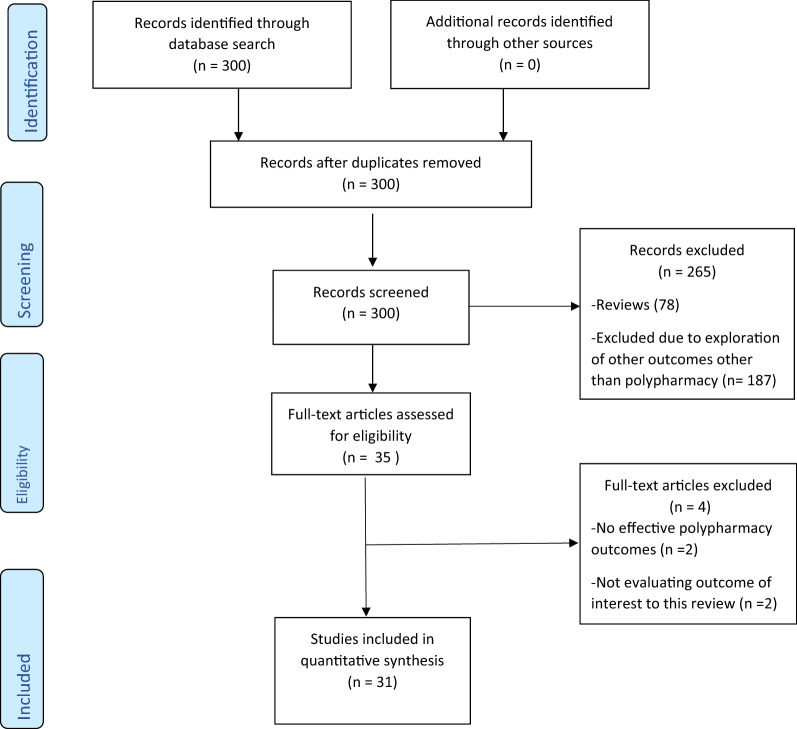
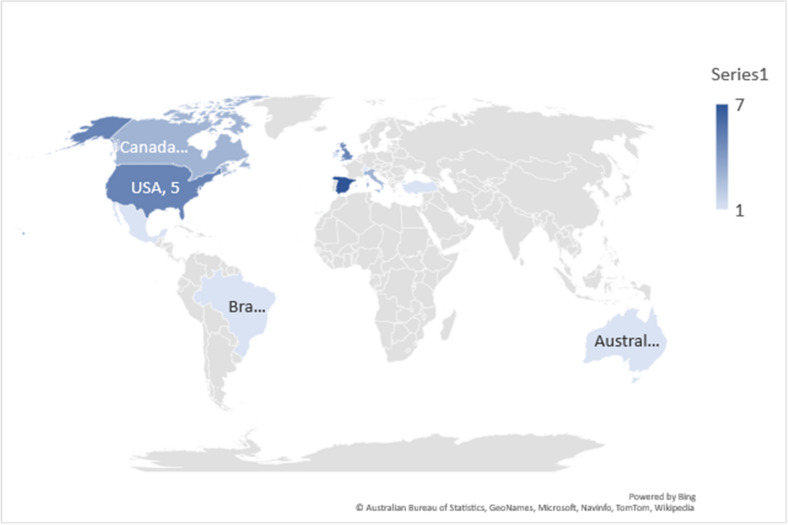
Figure 1 gives a PRISMA chart of result of our search strategy and studies included in this synthesis. We identified a total of N = 31 studies [2, 6, 8, 11–13, 15, 18–41] across 4 continents (Fig. 2) that fulfilled the criteria for inclusion in the systematic review.
Fig. 1.
PRISMA flow chart for study selection
Fig. 2.
Map showing the distribution of studies included in the review. (Spain = 7; Italy = 3; Ireland = 1; USA = 5; Turkey = 1; Canada = 3: UK = 5; Mexico = 1; Australia = 1; Brazil = 1; Switzerland = 1)
The total number of patients with polypharmacy as defined by the included studies was (N = 53347). The mean age of the patient cohort was 49.5 (SD ± 17.0) years, a significant proportion of which were males (67%). There was a total of 36 definitions of polypharmacy, with studies further divided based on the magnitude of polypharmacy (Table 1); minor polypharmacy (N = 3); major polypharmacy (N = 29); “severe” polypharmacy (N = 2); “excessive” polypharmacy (N = 1); “higher” polypharmacy (N = 1) (Table 1).
Table 1.
Baseline and socio-demographic characteristics of the reviewed studies
| Author | Study location | Study design | Duration of HIV (years) | HIV viral load (copies/ml) | Sample size (N) | PLWH | Age mean | Proportion of male population | Number of medications | Numerical definition | Duration of polypharmacy | Descriptive (HIV, non-HIV, or both) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cantudo-Cuenca 2014 | Spain | Prospective observational | UD (70.4%) | 594 | 118 | 47 | 0.801 | ≥ 5 | Yes | Non-HIV | ||
| Gimeno-Gracia 2015 | Spain | Retrospective | 13.6 | UD (88%) | 118 | 27 | 54.8 | 77.1 | ≥ 5 | Yes | > 1 day, > 90 days, > 180 days | Both |
| Gimeno-Gracia 2016 | Spain | Retrospective (descriptive) | UD (91.9%) | 199 | 53 | 52 | 73.4 | ≥ 5 | Yes | > 1 day, > 90 days, > 180 days | Non-HIV | |
| Guaraldi 2016 | Italy | Cross sectional | 19 | 2944 | 992 | 48.37 | 66.3 | ≥ 5 | Yes | 4 months | Non-HIV | |
| Guaraldi 2018 | Italy | Cross sectional | 1573 | 1258 | 71.47 | 82.7 | ≥ 5 | Yes | Non-HIV | |||
| Halloran 2019 | Ireland | Prospective observational | 1072 | 158 | 56 | ≥ 5 | Yes | Both | ||||
| Holtzman 2013 | USA | Cross sectional | 3810 | 3810 | 44 | 79 | ≥ 5 | Yes | both | |||
| Justice 2018 | USA | Prospective observational | 49,285 | 9473 | 97.5 | < 5 and ≥ 2 | Yes | 90 | Non-HIV | |||
| Kara 2019 | Turkey | Cross sectional | 145 (80.1%) | 181 | 37 | 40.4 mean | 79.6 | ≥ 5 | Yes | Both | ||
| Krentz 2016 | Canada | Prospective observational | 1329 | 1329 | 75.8 | ≥ 5 | Yes | Both | ||||
| Lopes 2019 | UK | Cross sectional | 2680 | 2680 | 46 mean | 86 | ≥ 5 | Yes | Non-HIV | |||
| Lopez-Centeno 2019 | Spain | Cross sectional | 6636451 | 22945 | 48 (PLWH), 41 (control) | 78.28. (PLWH), 48.02 (Controls) | ≥ 5 | Yes | Non-HIV | |||
| Mata-Marín 2019 | Mexico | Case control | 8 | 41–48 (63–80%) | 125 | 125 | ≥ 5 | Yes | 0 | Non-HIV | ||
| Mazzitelli 2019 | UK | Prospective observational | UD | 790 | 790 | 55.8 | 92.8 | ≥ 10, ≥ 5 | Yes | 0 | Non-HIV | |
| Morillo-Verdugo 2019 | Spain | Cross sectional | 184 (84.4) | 223 | 223 | 53 | 86.5 | ≥ 6, ≥ 11, ≥ 21 | Yes | Both | ||
| Nozza 2017 | Italy | Cross sectional | 17 | UD (92%) | 1222 | 1222 | 70 | 83.7 | ≥ 5 | Yes | 121 days | Non-HIV |
| Okoli 2020 | 25 countries | Prospective (multicentre) | 2112 | 2112 | 70.4 | ≥ 5 | Yes | – | Both | |||
| Patel 2015 | UK | Cross sectional | 299 | 16 | 58 mean | 94.6 | ≥ 5 | Yes | Both | |||
| Siefried 2017 | Australia | Prospective observational | 522 | 522 | 50.8 | 94.6 | ≥ 5 | Yes | Non-HIV | |||
| Ssonko 2018 | Uganda | Cross sectional | 411 | 239 | ≥ 4 | Yes | Non-HIV | |||||
| Titon 2021 | USA | Case control | 9.2 | UD | 156 | 52 | 60 | 38.5 | ≥ 5 | Yes | Non-HIV | |
| Vinuesa-Hernando 2021 | Brazil | Prospective observational | 30 | 30 | 71 | 73 | ≥ 5, ≥ 10 | Yes | Both | |||
| Arant 2021 | Spain | Prospective cohort | 348 | 106 | 77.2 (Cohort 1) and 72.1 (Cohort 2) | ≥ 5 | Yes | Both | ||||
| Ramos 2021 | USA | Prospective observational | 39 | 24 | 54.5 | 87.2 | ≥ 11 | Yes | Both | |||
| Calcagno 2021 | Cross sectional | 2432 | 1158 |
PLWH: 51.6 Controls: 47.7 |
69.8 (PLWH), 68.8 (Control) | ≥ 10 | Yes | Non-HIV | ||||
| Loste 2020 | Italy | Cross sectional | 10 | UD | 91 | 91 | 71.2 | 81.3 | ≥ 5 | Yes | Non-HIV | |
| Livio 2020 | Spain | Retrospective | 18 | 159 (91%) | 175 | 175 | 78 | 71 | ≥ 5 | Yes | Non-HIV | |
| Kuznetsov 2021 | UK | Prospective observational | 11.3 | 150 | 150 | 38.3 | 66 | ≥ 5 | Yes | Daily | Both | |
| Allemann 2017 | Canada | Prospective observational | 2 | 2 | 48 | 50 | ≥ 3 | Yes | 4 months | |||
| Ware 2019 | Switzerland | Prospective observational | 12.5 | UD (57.7%) | 3160 | 1715 | 53 | 100 | ≥ 5 | Yes | Non-HIV | |
| Ware 2016 | USA | Prospective observational | 12 | UD (48.2%) | 3160 | 1715 | 46 | 100 | ≥ 5 | Yes | Non-HIV |
UD undetected HIV viral load
Numerical definition of polypharmacy in PLWHA
There was marked variation in the numerical definition of polypharmacy, with studies reporting it as “minor”, “major”, “excessive”, “severe”, and “higher”. Of the studies included in our review, 93.5% (N = 29) defined polypharmacy as the concomitant use of greater than or equal to 5 medications. Several studies had “outlying” definitions for polypharmacy that were different from those mentioned in other studies. These definitions included ≥ 2 [12]; ≥ 3 [12]; ≥ 4 [13]; ≥ 6 [11, 26]; ≥ 10 [27, 40]; ≥ 11 [11, 26]; ≥ 21 [11].
Duration of polypharmacy
Amongst the included studies, 16% (N = 5) incorporated the duration of treatment to the definition of polypharmacy, while a significant proportion 84% (N = 26) only provided the numerical definition of polypharmacy with no additional information on its duration. Gimeno-Gracia et al. further stratified drug exposure based on the following duration: greater than 1 day; greater than 90 days; and greater than 180 days [14, 37]. Three studies included a duration of more than or equal to 4 consecutive months [18, 21, 31]. In their definition of polypharmacy, Justice et al. included the duration of more than or equal to 90 consecutive days [12].
ART or non-ART medication polypharmacy
With regards to constituents of medication regimen included in the definitions, 67.7% of studies (N = 21) specified that the polypharmacy definition included only non-ART medications, while 27% (N = 9) studies included both ART and non-ART medications in their definitions. One study did not specify whether ART and non-ART medications were used as part of the adjudication process of polypharmacy (3.2%) [24].
Discussion
To our knowledge, this review represents the first comprehensive systematic synthesis of studies that have explored polypharmacy in PLWHA to define what the term polypharmacy entails in this context. We have identified and evaluated 31 studies that defined polypharmacy in PLWHA forming a pooled sample size of 53,347 from eleven countries. We found wide variability in the way polypharmacy was defined amongst PLWHA; with an iteration of a total of 36 definitions from the reviewed studies. We found a significant proportion (93.5%) of studies included in our report defined polypharmacy as ≥ 5 concomitant medications. Additionally, up to 67.7% have explicitly specified these concomitant medications as non-ART medications. Following a systematic synthesis of the results from all studies, we found that a definition of polypharmacy including ≥ 5 non-ART medications over any period is representative of what most studies put forth as their definition.
Several recent studies have identified HIV polypharmacy as a growing problem that needs to be addressed. However, the lack of consensus around a unified definition hindered attempts at exactly estimating the burden as well as robust appraisal of its consequences and interventions to mitigate its effect on therapeutics. Without adequately defining what constitutes polypharmacy, researchers, administrators, and clinicians are all liable to grossly misidentifying patients who are at risk for polypharmacy. This leaves patients susceptible to the dangerous (but avoidable) harms associated with polypharmacy including problematic interactions (drug–drug, drug–food, and pharmacogenetic), adverse effects, rising therapeutic costs, medication non-concordance, increased hospitalizations, and sometimes avoidable mortality [2]. The situation is especially serious in PLWHA since they are a high-risk therapeutic population ab initio with a high pill burden due to their rigid ART medication regimes. What has exacerbated this in recent years amongst these patient cohorts, is their increasing survival often associated with a tandem rise in prevalence of associated comorbidities [12, 22, 37, 42, 43]. Taken together, this provides the enabling milieu for potentially harmful polypharmacy to ensue often with far reaching implication for a range of morbidities as highlighted above.
Having a unified definition for polypharmacy ensures that at hospital, community, and administrative levels therapeutic decisions regarding the pill burden of PLWHA are more nuanced and uniform. Additionally, a well-established definition can also assist in strengthening and clarifying communication between different stakeholders to for example help encourage mindfulness and attention while prescribing medications, and minimize downstream adverse outcomes in these cohorts of patients. Finally, having a standardized definition for polypharmacy will allow a more robust comparison of parameters and outcomes related to pill burden in PLWHA and hence draw more precise conclusions.
Although most studies (93.5%) defined polypharmacy as the intake of 5 or more medications, there were several “outlier” studies that explored alternative definitions. The “outlier” definitions ranged from as low as ‘ ≥ 2 medications’ to as high as ‘ > 21 medications’, and it wasn’t immediately apparent from reviewing these studies how these thresholds were arrived at. From the average pill count of PLWHA starting at 3 medications, it is unlikely that adoption of polypharmacy thresholds < 5 in these patients is likely to be of any determinative value as literally all population of PLWHA will thus be classified as “cases”. Conversely, definitions at the upper part of the extreme (such as ≥ 21 medications) are likely misclassify a high proportion of PLWHA with high pill burden but which has not reached the high threshold of 21 medications. Yet other studies employed descriptive terms such as “excessive” [11] or “severe” (≥ 10 Medications) [24, 27], and “higher” [40] polypharmacy to convey the magnitude of pill burden, but these subcategories were not consistently mentioned across all studies.
Although, incorporating duration of drug exposure into the definition of polypharmacy allows for a better understanding of the magnitude of the problem, the time threshold it prescribes has the potential to preclude some patient cohorts from beneficial interventions simply because they fail to satisfy this arbitrary exposure thresholds. In our review, two studies included the duration of at least 4 months in their definition of polypharmacy (Nozza et al., Guaraldi et al.) [18, 21] while most other studies did not specify a duration. A patient taking several medications for 3 months may not be classified as a polypharmacy case according to these two studies but may be considered a polypharmacy case according to studies that did not include a duration of drug exposure in their definition. This has serious implications when it comes to deciding which patients will benefit from interventions aimed at reducing the harms caused by polypharmacy. If one definition of polypharmacy required a duration of more than 4 months, patient A who took 6 medications for 5 months would be offered interventions while patient B who took 9 medications for 2 months would not be a candidate for the same interventions (although both patients may be exposed to the same adverse outcomes of polypharmacy); including drug-drug, drug-food, and pharmacogenetic interactions amongst others. If a patient met the numerical criteria of polypharmacy but did not meet the criteria in terms of duration, they should not be excluded from interventions that address the effects of polypharmacy. The decision about whether a patient will benefit from interventions should be solely based on the adverse effects they are experiencing secondary to polypharmacy.
When incorporating duration as part of the polypharmacy definition, and when this same definition is used as criteria to guide interventions, it is important to consider both the number of medications and the duration of use simultaneously. If a patient has been using medications for a long time, fewer number of medications should be acceptable to meet the criteria for polypharmacy. Alternatively, if a patient has been using an excessively large number of medications but for a short period of time, interpretation of the duration of medication exposure should be more nuanced. In the general population for instance, before Masnoon et al.’s consensus review [10], Nishtala et al. [44] defined polypharmacy as the use of five to nine medications for 90 days or more, while Veehof et al. [45] described it as the ingestion of two or more medications for more than 240 days in a year. Although the duration was longer in the definition proposed by Veehof et al., the threshold for number of medications was lower in Nishtala et al.
Studies may agree with regards to the duration of medication exposure, but still diverge with regards to the numerical definition of polypharmacy. In patients with PLWHA, it is our observation that consistency amongst studies in only one component of polypharmacy definition (e.g. duration) is not enough. For instance, Nozza et al. [21], Guaraldi et al. [18], and Alleman et al. [31] all included a duration of 4 months in their definitions, different medication thresholds for what constitutes polypharmacy. More specifically, Alleman et al. considers ≥ 3 medications as polypharmacy while Nozza et al. and Guaraldi et al. considered ≥ 5 medications. According to the polypharmacy definition proposed by Alleman et al., a patient taking 4 medications for a duration of 5 months would fall under the definition of polypharmacy and may be eligible for interventions, but the same patient may not be offered interventions according to Nozza et al. or Guaraldi et al. This highlights the importance of having consistency in terms of both number of medications as well as duration of treatment.
While the majority (67.7%) of studies included in our review only comprised non-ART medications in their definitions of polypharmacy, a significant proportion (27%) embraced both ART and non-ART medications. Kara et al. [19] included both categories in their definition of polypharmacy, while Halloran et al. [6] only involved non-ART; although both studies had the same numerical definition for polypharmacy (5 or more medications). A patient taking three ART and three non-ART medications will meet the criteria for polypharmacy according to Kara et al. but not Halloran et al. Consistency with regards to the classes of medications included in the definition of polypharmacy is essential. The argument for inclusion of both ART and non-ART medications in the numerical count of polypharmacy is not difficult to discern. Inevitably ART drugs are increasingly associated with secondary therapeutic morbidities such as dyslipidemias, various kidney morbidity phenotypes (amongst others) and do often require additional and increasing number of medications (such as statins) to manage them. Therefore, although the latter class of drugs may not in the strictest sense of word be ART’s, but they constitute a package of inevitable therapeutics associated with PLWHA. Additionally, bidirectional and pharmacogenetic interactions highlighted earlier between ART and non-ART drugs have become an integral and sometimes unavoidable part of ART therapeutics (especially of they are not treatment-limiting). And in risk stratification of therapeutic burden such as polypharmacy in PLWHA, any attempt at excluding non-ART drugs from the definition has the potential to underestimate this risks/burden. Indeed, Justice et al.’s report did showed that more than two non-ART polypharmacy was associated with about 68% increased risk of mortality amongst PLWHA (hazard ratio 1.68, 95% CI 1.50–1.89). The novelty of Justice et al. ‘s study has been the first report to adjust for severity of illness to ascertain the exact contribution of both ART and non-ART related polypharmacy to morbidity and mortality outcomes in PLWHA population. In the light of these, and other factors exhaustively “ventilated” elsewhere, with regards to PLWHA, we recommend that both ART and non-ART medications be included in the definition of polypharmacy.
Despite the stated observations regarding the increasing risk of polypharmacy with rising survival of PLWHA, recent advances in drug delivery systems in these patients cohorts may likely alter our projection of polypharmacy risks. Cabotegravir and Rilpivirine (long-acting antiretroviral combination) both of which have received market authorization from European Medicines Agency (EMEA), and Food and Drug Administration (FDA) amongst other drug regulatory agencies look set to revolutionize the pill count per PLWHA [46, 47]. For example, a hitherto sustained pill burden of about 365 days may ultimately be reduced to just about 6 intramuscular injections per year. Further advances (such as the novel Islatravir (a first-in-class nucleoside reverse transcriptase translocation inhibitor) currently formulated as an implant with a dosing interval of about 1 year is speculated to significantly change this polypharmacy numerical dynamic going forward [48, 49]. Numerically the effect of this in the pill count per PLWHA will be considerably low, but what effect this will have on other downstream consequences of polypharmacy (bidirectional interactions) remains unknown and will need to be explored by future studies.
Strengths and limitations
The key strength of this study lies in its novelty as the first attempt at unifying the various interactions of polypharmacy definitions in PLWHA currently in use in existing literature; thus providing therapeutic policy makers, researchers, and clinicians with an important tool to classify PLWHA in the context of polypharmacy. Furthermore, our methodology anchored on a robust systematic synthesis of current evidence from a plurality of studies contrast significantly from previous attempts that expounded a narrative inference to resolving this uncertainty. Finally, due to its loose inclusion criteria (both observational cohort studies and randomized controlled trials), this has allowed us to analyze a large, pooled sample size. This lends external validity to the study and ensures that the results are generalizable to a greater and more diverse patient population.
As has been observed from previous exploration of these data schemes, our study was limited by the same constraints; including missing data that sometimes could not be retrieved from some of the authors of the included studies; large variability and imprecise data points that may skew our results. Additionally, we have only included studies that were published in English language, it is however unlikely that the range of uncaptured data from studies in other languages are likely to alter the final point estimate of polypharmacy definition in any meaningful way. Future reviews will improve our understanding of the burden of polypharmacy in PLWHA by including the type of medications predominantly accounting for the excess numerical count as well as their duration of exposure. Despite this, the findings of this review are likely to engender a more robust and reproducible consensus around this rising morbidity.
Conclusion
A plurality of studies in PLWHA have established that polypharmacy in this cohort of patients is the intake of ≥ 5 medications (both ART and non-ART). We recommend the incorporation of this definition into national and international PLWHA treatment guidelines to standardize the approach to addressing this rising morbidity.
Supplementary Information
Additional file 1: Table S1. Results of rias of bias assessment of the included studies. Studies. Table S2. Review search strategy.
Author contributions
MID was involved in review concept development, regulatory/PROSPERO approval, independent reviews, and data analyses, writing initial and final manuscript draft; SK and FW were involved in independent reviews and data collection; LMN was involved in collecting and analyzing data; all authors (MID, SK, FW, LMN, AE) were involved in writing and reviewing the manuscript. All authors read and approved the final manuscript.
Funding
The study had no structural funding support.
Availability of data and materials
All data relating to this work is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
None of the authors have any competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Back D, Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J Int AIDS Soc. 2020;23(2):e25449. doi: 10.1002/jia2.25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guaraldi G, Malagoli A, Calcagno A, Mussi C, Celesia BM, Carli F, Piconi S, De Socio GV, Cattelan AM, Orofino G. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr. 2018;18(1):99. doi: 10.1186/s12877-018-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PS, Satcher Johnson A, Pembleton ES, Stephenson R, Justice AC, Althoff KN, Bradley H, Castel AD, Oster AM, Rosenberg ES, Mayer KH, Beyrer C. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021;397(10279):1095–1106. doi: 10.1016/S0140-6736(21)00395-0. [DOI] [PubMed] [Google Scholar]
- 4.Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–1196. doi: 10.1080/14740338.2018.1546841. [DOI] [PubMed] [Google Scholar]
- 5.McIsaac DI, Wong CA, Bryson GL, van Walraven C. Association of polypharmacy with survival, complications, and healthcare resource use after elective noncardiac surgery: a population-based cohort study. Anesthesiol J Am Soc Anesthesiol. 2018;128(6):1140–1150. doi: 10.1097/ALN.0000000000002124. [DOI] [PubMed] [Google Scholar]
- 6.Halloran MO, Boyle C, Kehoe B, Bagkeris E, Mallon P, Post FA, Vera J, Williams I, Anderson J, Winston A. Polypharmacy and drug–drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther. 2019 doi: 10.3851/IMP3293. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS ONE. 2014;9(11):e112133. doi: 10.1371/journal.pone.0112133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siefried KJ, Mao L, Cysique LA, Rule J, Giles ML, Smith DE, McMahon J, Read TR, Ooi C, Tee BK. Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS. 2018;32(1):35. doi: 10.1097/QAD.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danjuma MI, Egan D, Abubeker IY, Post F, Khoo S. Polymorphisms of tenofovir disoproxil fumarate transporters and risk of kidney tubular dysfunction in HIV-positive patients: genetics of tenofovir transporters. Int J STD AIDS. 2018 doi: 10.1177/0956462418786562. [DOI] [PubMed] [Google Scholar]
- 10.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatrics. 2017 doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillo-Verdugo R, de Robustillo-Cortés MA, Martín LA-K, de SotomayorPaz MÁ, de Naranjo FLL, González CVA. Determination of a cutoff value for medication regimen complexity index to predict polypharmacy in HIV+older patient. Rev Esp Quimioter. 2019;32(5):458. [PMC free article] [PubMed] [Google Scholar]
- 12.Justice AC, Gordon KS, Skanderson M, Edelman EJ, Akgün KM, Gibert CL, ReLo V, Rimland D , Womack JA, Wyatt CM. Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS. 2018;32(6):739. doi: 10.1097/QAD.0000000000001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ssonko M, Stanaway F, Mayanja HK, Namuleme T, Cumming R, Kyalimpa JL, Karamagi Y, Mukasa B, Naganathan V. Polypharmacy among HIV positive older adults on anti-retroviral therapy attending an urban clinic in Uganda. BMC Geriatr. 2018;18(1):125. doi: 10.1186/s12877-018-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimeno-Gracia M, Crusells-Canales MJ, Javier Armesto-Gomez F, Rabanaque-Hernández MJ. Prevalence of concomitant medications in older HIV+patients and comparison with general population. HIV Clin Trials. 2015;16(3):117–124. doi: 10.1179/1528433614Z.0000000012. [DOI] [PubMed] [Google Scholar]
- 15.Patel R, Moore T, Cooper V, McArdle C, Perry N, Cheek E, Gainsborough N, Fisher M. An observational study of comorbidity and healthcare utilisation among HIV-positive patients aged 50 years and over. Int J STD AIDS. 2016;27(8):628–637. doi: 10.1177/0956462415589524. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 63. Hoboken: John Wiley & Sons; 2022. [Google Scholar]
- 18.Guaraldi G, Menozzi M, Zona S, Calcagno A, Silva AR, Santoro A, Malagoli A, Dolci G, Mussi C, Mussini C. Impact of polypharmacy on antiretroviral prescription in people living with HIV. J Antimicrob Chemother. 2017;72(2):511–514. doi: 10.1093/jac/dkw437. [DOI] [PubMed] [Google Scholar]
- 19.Kara E, İnkaya aç, hakli da, demirkan sk, ünal S. Polypharmacy and drug-related problems among people living with HIV/AIDS: a single-center experience. Turkish J Med Sci. 2019;49(1):222–9. doi: 10.3906/sag-1807-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS. 2016;30(1):11–17. doi: 10.1089/apc.2015.0199. [DOI] [PubMed] [Google Scholar]
- 21.Nozza S, Malagoli A, Maia L, Calcagno A, Focà E, De Socio G, Piconi S, Orofino G, Cattelan AM, Celesia BM. Antiretroviral therapy in geriatric HIV patients: the GEPPO cohort study. J Antimicrob Chemother. 2017;72(10):2879–2886. doi: 10.1093/jac/dkx169. [DOI] [PubMed] [Google Scholar]
- 22.Okoli C, de Los RP, Eremin A, Brough G, Young B, Short D. Relationship between polypharmacy and quality of life among people in 24 countries living with HIV. Prev Chronic Dis. 2020;5(17):E22–E22. doi: 10.5888/pcd17.190359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titon JP, Titon OJ, Júnior VS, Wendt GW, Follador FAC, Vieira AP, Ferreto LED. Sociodemographic, behavioral, and geriatric characteristics in older adults with and without HIV: a case-control study. Medicine (Baltimore) 2021;100(30):e26734. doi: 10.1097/MD.0000000000026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinuesa-Hernando JM, Gimeno-Gracia M, Malo S, Sanjoaquin-Conde I, Crusells-Canales MJ, Letona-Carbajo S, Gracia-Piquer R. Potentially inappropriate prescriptions and therapeutic complexity in older HIV patients with comorbidities. Int J Clin Pharm. 2021;43(5):1245–1250. doi: 10.1007/s11096-021-01242-1. [DOI] [PubMed] [Google Scholar]
- 25.Arant EC, Harding C, Geba M, Targonski PV, McManus KA. Human Immunodeficiency Virus (HIV) and aging: multimorbidity in older people with HIV in one nonurban Southeastern Ryan white HIV/AIDS program clinic. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofaa584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GuzmánRamos MI, ManzanoGarcia M, las de RobustilloCortés MA, GutiérrezPizarraya A, MorilloVerdugo R. Influence of CMO pharmaceutical care model-based intervention on readmission rate in high risk HIV patients the INFARDAR study. Rev Española Quimioter. 2021 doi: 10.37201/req/025.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calcagno A, de Nicolò A, Pizzi C, Trunfio M, Tettoni C, Ferrara M, Alcantarini C, Trentini L, D’Avolio A, Di Perri G, Bonora S. Medication burden and clustering in people living with HIV undergoing therapeutic drug monitoring. Br J Clin Pharmacol. 2021 doi: 10.1111/bcp.14869. [DOI] [PubMed] [Google Scholar]
- 28.Loste C, Moltó J, Pérez-Álvarez N, Puig J, Echeverría P, Bonjoch A, Fumaz CR, Lemos B, Estany C, Clotet B, Negredo E. Potential prescribing issues among older HIV-infected subjects in a mediterranean cohort: does the current prevalence give cause for concern? Br J Clin Pharmacol. 2021;87(3):1310–1317. doi: 10.1111/bcp.14513. [DOI] [PubMed] [Google Scholar]
- 29.Livio F, Deutschmann E, Moffa G, Rrustemi F, Stader F, Elzi L, Braun DL, Calmy A, Yerly S, et al. Analysis of inappropriate prescribing in elderly patients of the Swiss HIV cohort study reveals gender inequity. J Antimicrob Chemother. 2021;76(3):758–764. doi: 10.1093/jac/dkaa505. [DOI] [PubMed] [Google Scholar]
- 30.Kuznetsov S, Eremin A, Zaytseva E, Young B, Basova A, Paice A, Marin O, de los Rios P, Okoli C. Treatment challenges and health conditions among people living with HIV with or without substance use disorder in the Russian federation. AIDS Care - Psychol Socio-Med Asp AIDS/HIV. 2021 doi: 10.1080/09540121.2021.1960945. [DOI] [PubMed] [Google Scholar]
- 31.Allemann SS, Dürsteler KM, Strasser J, Vogel M, Stoeckle M, Hersberger KE, Arnet I. Novel remote electronic medication supply model for opioid-dependent outpatients with polypharmacy-first long-term case study. Harm Reduct J. 2017;14(1):10–17. doi: 10.1186/s12954-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware D, Palella FJ, Jr, Chew KW, Friedman MR, D’Souza G, Ho K, Plankey M. Prevalence and trends of polypharmacy among HIV-positive and -negative men in the multicenter AIDS cohort study from 2004 to 2016. PLoS ONE. 2018;13(9):e0203890–e0203890. doi: 10.1371/journal.pone.0203890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware D, Palella FJ, Chew KW, Friedman MR, D’Souza G, Ho K, Plankey M. Examination of polypharmacy trajectories among HIV-positive and HIV-negative men in an ongoing longitudinal cohort from 2004 to 2016. AIDS Patient Care STDS. 2019;33(8):354–365. doi: 10.1089/apc.2019.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, Wood K, Brooks JT, Investigators H. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med. 2013;28(10):1302–1310. doi: 10.1007/s11606-013-2449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantudo-Cuenca MR, Jiménez-Galán R, Almeida-González CV, Morillo-Verdugo R. Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. J Manag Care Pharm. 2014;20(8):844–850. doi: 10.18553/jmcp.2014.20.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gimeno-Gracia M, Sánchez-Rubio-Ferrández J, de LA Robustillo-Cortés M, Morillo-Verdugo R. Prevalence of polypharmacy and pharmacotherapy complexity in elderly people living with HIV in Spain. POINT study. Farm Hosp organo of Expr Cient la Soc Esp Farm Hosp. 2020;44(4):127–34. doi: 10.7399/fh.11367. [DOI] [PubMed] [Google Scholar]
- 37.Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, Compaired-Turlán V, Rabanaque-Hernández MJ. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging. 2016;11:1149. doi: 10.2147/CIA.S108072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Centeno B, Badenes-Olmedo C, Mataix-Sanjuan Á, McAllister K, Bellón JM, Gibbons S, Balsalobre P, Pérez-Latorre L, Benedí J, Marzolini C, Aranguren-Oyarzábal A, Khoo S, Calvo-Alcántara MJ, Berenguer J. Polypharmacy and drug-drug interactions in HIV-infected subjects in the region of Madrid, Spain: a population-based study. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2019 doi: 10.1093/cid/ciz811. [DOI] [PubMed] [Google Scholar]
- 39.Mata-Marín JA, Martínez-Osio MH, Arroyo-Anduiza CI, de Berrospe-Silva MÁ, Chaparro-Sánchez A, Cruz-Grajales I, Cruz-Herrera JE, Uribe-Noguez LA, Gaytán-Martínez JE, Jerónimo-Morales M. Comorbidities and polypharmacy among HIV-positive patients aged 50 years and over: a case–control study. BMC Res Notes. 2019;12(1):1–6. doi: 10.1186/s13104-019-4576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzitelli M, Milinkovic A, Pereira B, Palmer J, Tong T, Asboe D, Boffito M. Polypharmacy and evaluation of anticholinergic risk in a cohort of elderly people living with HIV. AIDS. 2019;33(15):2439–2441. doi: 10.1097/QAD.0000000000002403. [DOI] [PubMed] [Google Scholar]
- 41.Lopes S, O’Day K, Meyer K, Van Stiphout J, Punekar Y, Radford M, Haas JS. Comedication prescription patterns and potential for drug-drug interactions with antiretroviral therapy in people living with human immunodeficiency virus type 1 infection in Germany. Pharmacoepidemiol Drug Saf. 2020;29(3):270–278. doi: 10.1002/pds.4928. [DOI] [PubMed] [Google Scholar]
- 42.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi: 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014;62(3):447–453. doi: 10.1111/jgs.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishtala PS, Salahudeen MS. Temporal trends in polypharmacy and hyperpolypharmacy in older New Zealanders over a 9-Year period: 2005–2013. Gerontology. 2015;61(3):195–202. doi: 10.1159/000368191. [DOI] [PubMed] [Google Scholar]
- 45.Veehof L, Stewart R, Haaijer-Ruskamp F, Jong BM. The development of polypharmacy. A longitudinal study Fam Pract. 2000;17(3):261–267. doi: 10.1093/fampra/17.3.261. [DOI] [PubMed] [Google Scholar]
- 46.Boyd MA, Cooper DA. Long-acting injectable ART: next revolution in HIV? Lancet. 2017;390:1468–1470. doi: 10.1016/S0140-6736(17)31962-1. [DOI] [PubMed] [Google Scholar]
- 47.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: Phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 48.Matthews RP, Zang X, Barrett S et al. Next-generation islatravir implants projected to provide yearly HIV prophylaxis. Conference on Retroviruses and Opportunistic Infections (CROI). 2021. Abstract 88
- 49.Flexner C, Thomas DL, Swindells S. Creating demand for long-acting formulations for the treatment and prevention of HIV, tuberculosis, and viral hepatitis. Curr Opin HIV AIDS. 2019;14:13–20. doi: 10.1097/COH.0000000000000510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Results of rias of bias assessment of the included studies. Studies. Table S2. Review search strategy.
Data Availability Statement
All data relating to this work is available from the corresponding author on reasonable request.