Abstract
The COVID-19 pandemic led to increased distress in many children, particularly in girls. Socio-emotional vulnerability, as well as psychiatric symptomatology prior to or during the initial stages of the pandemic, have been identified as important predictors of this distress. Still, it is unclear whether the pandemic also had physiological repercussions in children. If so, it remains to be determined whether these same predictors could provide insight into inter-individual variability. This longitudinal study aimed to investigate the effects of socio-emotional vulnerability, as well as pre-pandemic internalizing and externalizing symptoms, on hair cortisol concentrations (HCC) in response to the COVID-19 pandemic in healthy youth. In June 2020 (T1), 69 healthy children (M = 11.57 y/o) who visited the laboratory between 2017 and 2019 (T0) provided a 6 cm hair sample. This technique allowed us to quantify cortisol secretion during the three months preceding the COVID-19 pandemic (Segment A) and during the first three months of the first wave of the pandemic in Quebec, Canada (Segment B). At T0, participants completed the Dominic Interactive to assess pre-pandemic internalizing and externalizing symptoms. A socio-emotional composite score (SECS) was derived using a weighted z-score with the following constructs: anxiety sensitivity (Childhood Anxiety Sensitivity Index) measured at T0, trait anxiety (Trait subscale of the State-Trait Anxiety Inventory for Children (STAI-C)), intolerance of uncertainty (Intolerance of Uncertainty Scale for Children), and trait rumination (Children’s Response Style Scale) measured at T1. A linear regression was conducted using the percent change in HCC across Segment A and B as the dependent variable, where SECS, pre-pandemic internalizing and externalizing symptoms, and sex were used as predictors. We found a main effect of sex, with girls presenting increased HCC reactivity compared to boys. We also found that SECS and internalizing symptoms negatively predicted HCC, whereas the opposite relationship was found between externalizing symptoms and HCC reactivity. For healthy children, our results suggest that previous psychiatric symptoms and socio-emotional vulnerability may be risk factors for the presentation of diverging cortisol response patterns in response to an adverse life event (such as the COVID-19 pandemic).
Keywords: Hair cortisol, COVID-19, Youth, Socio-emotional vulnerability, Internalizing symptoms, Externalizing symptoms, Sex differences
1. Introduction
Due to the coronavirus disease 2019 (COVID-19), Canada was among one of the many countries that implemented strict confinement measures to limit the spread of the virus. Though effective at taming the propagation of the virus (Hassan et al., 2021), these measures drastically disrupted the daily lives of children. Such disruptions were observed in school settings, as well as in the daily routines and social lives of children (Courtney et al., 2020). Thus, it is not surprising that studies have reported high levels of stress-related psychological distress in children (Brown et al., 2020, Cost et al., 2021, Courtney et al., 2020, Fitzpatrick et al., 2020, Marques de Miranda et al., 2020). Important sex differences have also been reported. For instance, compared to younger children and boys, adolescent girls have an increased risk of presenting elevated depression and anxiety symptoms (for a meta-analysis, see Ma et al., 2021). A longitudinal study conducted by our research group showed that youth presenting elevated pre-pandemic socio-emotional vulnerability displayed an increase and maintenance of anxiety and post-traumatic symptoms when faced with the pandemic (Raymond et al., 2022). Yet, it remains unclear whether the adverse life event of the pandemic was able to “get under the skin” and affect the biological stress system of these children.
When the brain detects a threat in the environment, a physiological cascade is triggered in the body. For instance, the hypothalamic-pituitary-adrenal (HPA) axis is activated, for which the main end product is cortisol (Sapolsky et al., 2000). The latter is commonly measured in saliva, which indicates an acute concentration of basal (i.e. diurnal rhythm) or reactive (i.e. stress-induced) cortisol (Gray et al., 2018, Stalder et al., 2017). In the last decade, a growing number of studies have focused on using hair samples as a method of quantifying cortisol. Unlike saliva, this technique allows researchers to examine cumulative cortisol secretion. Given that hair strands grow at a rate of about one centimetre per month, hair samples allow for a retrospective measure of biological stress (Stalder et al., 2017). Although short-term activation of the HPA axis is adaptive, chronic cortisol secretion may have deleterious effects on mental health, given the presence of cortisol receptors in brain regions required for various cognitive and emotional processes, such as emotional regulation (Jentsch et al., 2019, Raymond et al., 2018). In addition, the developing brains of children are at an even greater risk of suffering from the potentially harmful effects of chronic cortisol secretion (Lupien et al., 2009). Therefore, it is crucial to identify youth who are at risk of displaying a strong reaction to a major stressful life event, such as the pandemic. Some predisposing factors have previously been identified as modulators of the physiological stress system activity in children.
To begin, stable and consistent internal characteristics have been established as predisposing factors. Four personality traits have received notable scientific attention as per their effect on the HPA axis in children. The first personality trait is anxiety sensitivity, which is defined as the belief that somatic, cognitive, and social anxiety symptoms will have harmful consequences such as increasing anxiety (Naragon-Gainey, 2010). The second is trait anxiety, which refers to persistent anticipation about one or many situations to which a person might be exposed (Hishinuma et al., 2001). The third is intolerance of uncertainty, where an individual may experience anxiety due to an intolerance to unknown elements or situations (Boswell et al., 2013). The final personality trait is a tendency to ruminate which is characterized by intrusive and recurrent thoughts related to negative events (Sorg et al., 2012). Each of these four individual socio-emotional predictors was found to be associated with basal (Adam, 2006, Byrne et al., 2020, Oskis et al., 2011, van der Vegt et al., 2009) and/or reactive (Lanni et al., 2012, Rodgers et al., 2019) salivary cortisol levels in both healthy and clinical youth populations. However, the directionality (positive vs. negative) of these associations remains unclear. Furthermore, although these factors tend to correlate amongst each other, studies tend to assess their individual predictive value on HPA axis activity rather than their combined contribution (Boelen et al., 2010, Cox et al., 2001, Hensley and Varela, 2008, Muris et al., 2001). Recently, our research group demonstrated the combined contribution of these factors in the prediction of anxiety and post-traumatic symptoms in reaction to COVID-19 in youth (Raymond et al., 2022). The integration of these predictors could provide a more global portrait of the contribution that socio-emotional vulnerability plays on HPA functioning in children.
Another potential predisposing factor is the presence of psychiatric symptoms. The latter can be divided into two broad categories: internalizing (e.g., depression, anxiety) and externalizing (e.g., disruptive behaviour, attention problems, hyperactivity) symptoms. Indeed, numerous cross-sectional studies have found that altered HCC was associated with internalizing (Ford et al., 2019, Gray et al., 2018, Lu et al., 2018, Rietschel et al., 2016, Sandstrom et al., 2021, Stalder et al., 2017) and externalizing (Grotzinger et al., 2018, Kao et al., 2018, Pauli-Pott et al., 2017, Pauli-Pott et al., 2019, Schloß et al., 2018, White et al., 2017) symptoms in youth. Nevertheless, findings regarding the directionality of these effects (positive vs. negative) are inconclusive. In addition, as these studies were mainly conducted in clinical populations, it becomes difficult to pinpoint the temporality of the association between altered HPA axis activity, socio-emotional factors, and psychiatric symptomatology. In other words, do these associations precede the pathology or are they a consequence of it? Further, this raises the question of whether the presence of symptoms in children prior to exposure to an adverse event may modulate their physiological stress response to the event.
To summarize, previous studies have shown that both socio-emotional vulnerability factors (anxiety sensitivity, trait anxiety, intolerance of uncertainty, and rumination) and psychiatric symptoms (internalizing and externalizing) are correlated with HPA axis activity in children. However, the lack of longitudinal studies and heterogeneity of the populations studied made it difficult to decipher the directionality and temporality of these associations. Given the major life changes that COVID-19 brought for many children, the pandemic offered an opportunity to test these research questions. Along these lines, one study found that elevated HCC in mothers and their children was associated with increased COVID-related family stress (Perry et al., 2022). Though to date, the individual predictors of cortisol variations in response to the pandemic remain undocumented.
The current study utilized a convenience sample of individuals that had participated in a previous pre-COVID-19 study. Within the context of this pre-pandemic study, participants completed personality and symptomatology questionnaires. Thus, the objective of the current study was to have a better understanding of 1) socio-emotional vulnerability (assessed via personality traits measured before the pandemic or in its early stages) and 2) pre-pandemic internalizing and externalizing symptoms of HCC in response to the COVID-19 pandemic in healthy youth (participants aged 8–12). In light of the important sexual differences that are present in several psychopathologies associated with the stress system, the second objective of this study was to test the moderating role of biological sex on these associations.
2. Material and methods
2.1. Participants
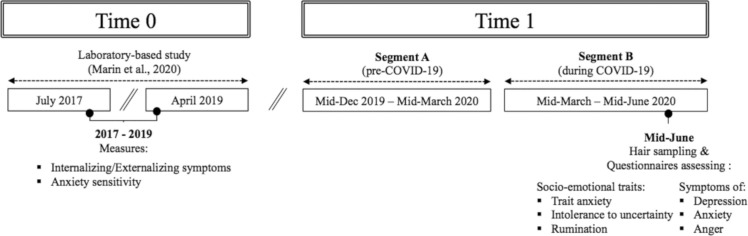
Participants were recruited for this study subsequent to their participation in one of our laboratory-based experiments that occurred between 2017 and 2019 (T0). This previous research aimed to study observational fear learning within families (for further details on the purpose of the study, methods used, and obtained results, see Marin et al., 2020 and Bilodeau-Houle et al., 2020). For the said study, parent-child dyads were recruited through advertisements on social media and posters in the surroundings of our research centre. Among the parents of the 92 children that were contacted, 84 (91.3%) children (42 girls and 42 boys) agreed to participate in this follow-up (T1) which intended to 1) assess distress longitudinally between June 2020 and March 2021 (Raymond et al., 2022) and; 2) assess HCC in reaction to the COVID-19 pandemic (see Fig. 1 for timeline overview). Three boys were excluded from the analyses due to insufficient hair length, resulting in a sample size of 81 children.
Fig. 1.
Timeline overview. Timeline of hair collection as a function of the implementation of COVID-19 health restriction measures in Canada.
In the current study, participants were aged between 9 and 14 years old (M = 11.57, SD = 1.49). At T0, children were free of chronic physical illnesses, psychopathologies, and were not taking medication. At T1, two children were taking medication that may have influenced glucocorticoids (melatonin and combined oral contraceptives). Two other children were exposed to a potentially traumatic event (alongside exposure to the COVID-19 pandemic) between T0 and T1, though neither child received a psychiatric diagnosis thereafter. To verify whether this would impact our results, we ran our analyses twice (once including these four subjects, once without). As the inclusion of these participants did not significantly impact our findings, all participants were included in the final set of analyses.
2.2. Computerized task assessing internalizing and externalizing symptoms
The Dominic Interactive (T0; Valla et al., 2000) is a 15-minute standardized computerized questionnaire that is used to assess seven common psychopathologies in youth. Dominic is the main character presented throughout the task. The character is shown in 91 different situations which represent the criteria for certain psychopathologies in the DSM-5. These situations illustrate Dominic in certain situations of his daily life (e.g., an image of Dominic crying). A simple sentence is added to the image, allowing the child to better understand the situation (e.g., Do you often feel sad or depressed, like Dominic?). In response to the different items, the child must answer yes or no. The major strengths of the Dominic Interactive are that it decreases social desirability effects, as well as its use of multimodal stimuli to increase the child’s comprehension of the situations and to ensure that the results are not biased by the child’s reading abilities. Another important strength of this task is that the character with whom the child must identify can be programmed to be either male (Dominic) or female (Dominique) and from different nationalities. This is advantageous as it increases the task’s ecological validity. The questionnaire assesses the child’s tendency to exhibit internalizing (specific phobias, separation anxiety, generalized anxiety, and depression) and externalizing symptoms (opposition, conduct problems, inattention, hyperactivity, and impulsivity). Three scores can be attributed to the child’s responses to each situation: “There is no problem”, “There might be a problem”, and “There is a problem”. Thereafter, scores can be converted into two continuous subscales for internalizing and externalizing symptoms. Both scales have good internal consistencies, ranging between 0.72 and 0.89 (Dugré et al., 2001, Valla et al., 2000). Similar internal consistencies were found in our sample, with a Cronbach’s α of.771 and.885 for the “internalizing” and “externalizing” subscales, respectively.
2.3. Questionnaires assessing socio-emotional vulnerability
2.3.1. Childhood Anxiety Sensitivity Index (CASI; T0)
To assess anxiety sensitivity, children completed the French version of the CASI (Stassart and Etienne, 2014). This validated questionnaire for children (ages 8–17) includes 18 items that can be answered on a 3-point scale. The total scores range from 18 to 54. The validated French version of the CASI has an internal consistency of 0.82 (Stassart and Etienne, 2014). In our sample, we found an internal consistency of α = 0.828 for the CASI.
2.3.2. State-Trait Anxiety Inventory for Children (STAIC; T1)
To assess anxiety, the French version of the STAIC (Turgeon and Chartrand, 2003) was used. Based on the adult form of the instrument (STAI; Spielberger, 1983), the STAIC consists of two scales that have been validated for children aged 7–17. Each scale contains 20 items: a State scale (STAIC-S) which measures transient anxiety reactions to certain situations and a Trait scale (STAIC-T) which measures a stable predisposition to react anxiously to any situation. Each item is answered on a 3-point scale. The total scores on each scale range from 20 to 60. The STAIC-S scale allowed us to assess each child’s current anxiety-related symptoms and the STAIC-T allowed for the assessment of each child’s trait anxiety (this measure was used to compute socio-emotional vulnerability). The validated French version of the STAIC shows good internal consistency (0.78 and 0.82 for the state and trait scale, respectively; Turgeon and Chartrand, 2003). In our sample, we found an internal consistency of.881 and.901 for the trait and state scale, respectively.
2.3.3. Intolerance of uncertainty scale for children (IUSC; T1)
The IUSC (Comer et al., 2009) was used to assess intolerance of uncertainty. The IUSC is a validated scale for children (ages 7–17) which assesses the tendency to react negatively to uncertain situations and events on an emotional, cognitive, and behavioural level. For each of the 27 statements, participants are asked to indicate how well the items describe them on a scale of 1–5. The overall scores range from 27 to 135. The psychometric indices for the original version demonstrate good validity (internal consistency of 0.92). Members of our team translated the original IUSC from English to French using a double-blind back-translation method. In our sample, we found an internal consistency of α = 0.916 for the IUSC.
2.3.4. Children’s response styles scale (CRSS; T1)
The CRSS (Ziegert and Kistner, 2002) is a 20-item self-report questionnaire validated for children (ages 8–17) that measures a child’s tendency to ruminate, as well as the tendency to seek distraction in response to feelings of sadness. For our study, the rumination subscale was used. The rumination subscale (10 items) represents thoughts and behaviours that maintain a focus on emotions. Items are rated on a five-point Likert scale. Scores range from 10 to 50. The validated French version shows excellent internal consistency for each of the factors, ranging from 0.78 to 0.85 (Le Van et al., 2021). In our sample, we found an internal consistency of α = 0.823 for the CRSS.
2.4. Questionnaires assessing current symptomatology (covariates)
Given that the presence of psychiatric symptoms in the early stages of the pandemic may modulate HCC, we also assessed current feelings of anger, as well as depression and anxiety symptoms in children at T1. Thus, to control for the effects of these potentially confounding variables in the main analyses, participants completed the following questionnaires at T1.
2.4.1. Anger expression scale for children (AESC; T1)
The AESC (Steele et al., 2009) is a 26-item self-report inventory assessing the expression of anger in children ages 7–17. Each item is answered on a 4-point Likert scale. The questionnaire is based on a list of potential items generated to reflect trait anger as well as multiple facets of anger expression and control and is divided into four subscales: 1) Trait anger; 2) Anger expression; 3) Internalizing anger and 4) Anger control. For our study, the Anger expression subscale was used. The AESC has an internal consistency ranging from 0.84 to 0.91 depending on the subscale (Steele et al., 2009). Members of our team translated the original AESC from English to French using a double-blind back-translation method.
2.4.2. Children’s depression inventory (CDI; T1)
The French version of the CDI (Saint-Laurent, 1990) is a 27-item questionnaire measuring depression symptomatology in children ages 7–17. Each item can be answered with either a 0 (no symptomatology), 1 (mild symptomatology), or 2 (severe symptomatology). The total scores range from 0 to 54. According to the clinical cut-off scores for the CDI, a score of 15, 20, and 25 are indicative of mild, moderate, and severe depression, respectively. The validated French version of the CDI has an internal consistency of 0.92 (Saint-Laurent, 1990).
2.4.3. State-trait anxiety inventory for children (STAIC; T1)
The state subscale of the French version of the STAIC (Turgeon and Chartrand, 2003) was used to assess state anxiety (see Section 2.3 above for psychometric details).
2.5. Questionnaire completion
At T0, participants completed the aforementioned questionnaires in the laboratory. At T1, questionnaires were completed via Qualtrics, an online and highly secure platform. To access the questionnaires on Qualtrics, a personalized URL link was sent to each participant via email.
2.6. Hair sample collection
In June 2020 (T1), children were instructed to collect hair samples at home with the help of a parent. As demonstrated by Enge et al. (2020), self-collection of hair samples is a valid method to measure HCC. For our study, participants needed to provide a sample of 6 cm in length (to allow for the analysis of two 3 cm segments within the same sample). As hair grows 1 cm per month on average (Stalder and Kirschbaum, 2012), each segment (A and B) should represent a period of approximately three months: Segment A represents hair growth from approximately mid-December 2019 to mid-March 2020 (before the start of the first wave of COVID-19 in Canada) and Segment B represents hair growth from mid-March 2020 to mid-June 2020 (corresponding to the first wave of the pandemic in Canada; see Fig. 1).
As validated by Ouellet-Morin et al. (2016), we provided participants with hair sampling kits, as well as an explanatory guide that detailed the hair sampling process. Participants were instructed to collect their samples from the occipital region of the scalp. To do so, participants had to comb their hair, separate a strand of at least 1 cm wide, and place the hair clamps 1 cm from the scalp to hold the hair in place. To ensure that the sample was sufficient, participants were told to lay their sample on a piece of cardboard provided by our research team. The cardboard featured several lines that were separated by 1 cm (similar to those on a ruler) to help the participant determine whether more/less hair needed to be added to their sample. Then, they had to cut the section of the hair as close to the scalp as possible with a pair of scissors. The sample was then laid on the piece of aforementioned cardboard, secured with adhesive tape, and inserted in a plastic bag and envelope provided by our team. Participants sent their hair samples to the laboratory in a postage-paid envelope. After the hair collection, parents completed an online home-based questionnaire assessing the characteristics of their child’s hair (e.g., washing frequency, hair products (type and frequency of use), and health (physical and mental health problems, medication).
2.7. Hair analyses
Hair analyses were conducted at the Centre for Studies on Human Stress in Canada (http://humanstress.ca/saliva-lab/general-information/). The protocol validated by Davenport et al. (2006) was used for hair washing and steroid extraction. Each segment of hair was placed in a Falcon tube. Next, 2.5 mL of isopropanol was added and the tube was mixed on an overhead rotator for 3 min. After decanting, the wash cycle was repeated twice and the hair samples were thereafter allowed to dry for 12 h. For each participant, two 3 cm segments of hair (25 mg each) were analyzed separately to quantify cortisol concentrations in the corresponding periods (Segments A and B). Samples were assayed in duplicate using a luminescence immunoassay (detection range: 0.005–4 µg/dl; intra-assay coefficient of variation = 5.24%; inter-assay coefficient of variation = 8.78%).
2.8. General protocol
At T0, children completed the CASI (anxiety sensitivity), as well as the Dominic Interactive (internalizing and externalizing symptoms). At T1, participants completed questionnaires to assess socio-emotional vulnerability (STAIC-T, IUSC, CRSS), as well as current psychiatric symptoms (STAIC-S, AESC, CDI). They also provided hair samples at T1 (for a timeline overview, see Fig. 1).
2.9. Statistical analyses
2.9.1. Initial treatment of the data and preliminary analyses
Socio-emotional composite score: To create a socio-emotional composite score, z-scores were generated for each of the following questionnaires: CASI, STAIC-T, IUSC, and CRSS. These scores were then averaged for each participant to provide a composite score. This score was referred to as the socio-emotional composite score (SECS; see Raymond et al. (2022)) for further details).
Current anxio-depressive symptoms (assessed at T1): To create a composite score for anxio-depressive symptoms at T1, z-scores were generated for both the CDI and STAIC-S questionnaires. Thereafter, scores were averaged for each participant to provide a weighted score (referred to as internalizing symptoms at T1). This variable was included in the analyses to ensure that our results were not better explained by internalizing distress experienced during the pandemic.
Control variables: Given that the CASI (included in the SECS) and CDI were completed between 2017 and 2019 (T0), the time elapsed between T0 and T1 was included as a covariate in the main analyses. Furthermore, sex and age at T1 were included in the models given that age impacts HCC (for a meta-analysis, see Gray et al., 2018). Finally, to ensure that our results were not attributable to current internalizing and externalizing distress, we also controlled for anxio-depressive symptoms (composite score) and anger at T1. To determine whether additional covariates should be included in our statistical models, we conducted analyses on covariates previously identified by Stalder et al. (2017). For continuous variables (hair washing frequency, physical activity, body mass index), bivariate correlation analyses were conducted with HCC. T-tests were used for categorical variables (hair washing in the last 24 h, medication use). Finally, we verified the intercorrelations between the main predictors.
2.9.2. Main analyses
Analyses were run using IBM SPSS Statistics version 26. After examining the data, scores were standardized (z-scores). Scores falling below − 3.29 or above + 3.29 were considered outliers and winsorized to 3.29 (thresholds based on Tabachnick and Fidell, 2007). One participant exhibited extreme (elevated) scores for Segment A of HCCs and two presented elevated scores for Segment B. We ran the analyses twice: once including the winsorized values and once excluding them. As no difference was found between the two sets of analyses, winsorized data were included in the final analyses. Before conducting the statistical analyses, the distribution of our variables was also assessed for skewness and kurtosis. Using indices for acceptable limits of ± 2 (Aguinis et al., 2013), the data was found to be normally distributed. Therefore, no transformations were applied to the raw values.
First, to assess the change in HCCs between Segment A (pre-pandemic) and Segment B (during the pandemic), data were analyzed via a repeated measures ANOVA with time as the within-subjects factor and sex as the between-subjects factor.
Second, according to guidelines provided by Cohen et al. (2003), a linear regression was used to examine the main effects of SECS. Pre-pandemic internalizing and externalizing symptoms, as well as their interaction with sex, were used as predictors of change in HCC. Sex and the three predictors (SECS, pre-pandemic internalizing and externalizing symptoms at T0) were included in the first model and the interaction terms (SECS*sex, pre-pandemic internalizing symptoms*sex, pre-pandemic externalizing symptoms*sex) were included in the second model. When significant interactions were found, the effect of the predictor was plotted as a function of the other predictor. To decompose this interaction, this step was followed by simple slope tests (Aiken et al., 1991). Standardized predictors were used to compute the interaction terms and in the analyses.
3. Results
3.1. Preliminary analyses
As presented in Table 1A and Table 1B, none of the potential covariates reached statistical significance. Therefore, no covariates were included in the main analyses.
Table 1.
Preliminary analyses amongst the potential covariates. H: hours.
| Descriptive statistics |
Segment A | Segment B | |||
|---|---|---|---|---|---|
| A. | M (SD) | r | p | r | p |
| Bivariate correlations | |||||
| Hair washing frequency /week | 3.57 (0.79) | -0.196 | .134 | -0.162 | .205 |
| Physical activity (h/week) |
3.42 (4.19) | -0.167 | .206 | .107 | .407 |
| Body mass index | 19.79 (4.46) | .192 | .146 | .013 | .424 |
| B. | N | t | p | t | p |
| T-tests | |||||
| Hair washing (last 24 h) | 28 | .004 | .947 | .063 | .802 |
| Use of medication | 11 | .116 | .735 | .327 | .57 |
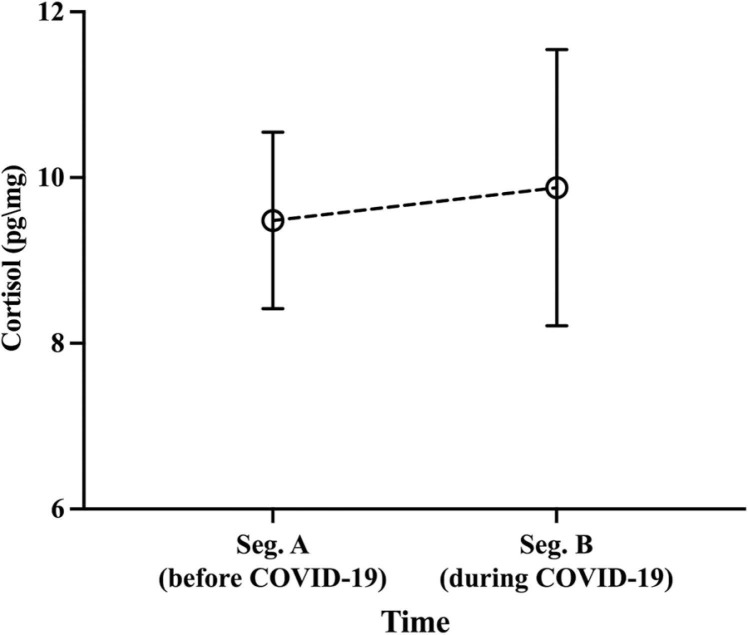
The participants had mean HCC levels of 9.48 pg/mg (SD = 1.07) in Segment A and 9.88 pg/mg (SD = 1.67) in Segment B, with both segments being highly correlated [r = 0.855; p < .001]. The repeated measures ANOVA revealed no main effect of Time [F(1,65) = 0.297 p = .588], sex [F(1,65) = 0.657 p = .421], or Time*Sex interaction [F(1,65) = 0.973 p = .327] (see Fig. 2). Subsequently, we used the percentage change between the two segments to reduce iterations and increase our statistical power. The percent change was calculated as follows: ((Segment B-Segment A)/Segment A)* 100. This figure was used to facilitate the interpretation of the results. A multivariate ANOVA revealed sex differences, with girls presenting higher scores on the SECS and pre-pandemic internalizing symptoms compared to boys. No sex difference was found for pre-pandemic externalizing symptoms (see Table 2).
Fig. 2.
Mean hair cortisol concentrations as a function of time (Segment A pre-COVID-19 vs. Segment B during COVID-19). Means are adjusted for age and sex.
Table 2.
A. Correlation matrix of the main predictors. The table describes the correlations among the different predictors included in the analyses. r (p values). B. Sex differences for the main predictors, the socio-emotional traits, as well as the distress scores. Mean (SE) * indicates statistical significance set at p < .05. SECS: socio-emotional composite score.
| A. | SECS | Internalizing symptoms | Externalizing symptoms | |
|---|---|---|---|---|
| SECS | – | – | – | |
| Internalizing symptoms | .266 (0.019)* | – | – | |
| Externalizing symptoms | .109 (0.344) | .703 (<0.001)* | – | |
| B. | Sex differences | pvalues | ||
| Girls | Boys | |||
| Main predictors | ||||
| SECS | .19 (0.11) | -0.14 (0.11) | .036 * | |
| Internalizing symptoms | 15.74 (1.24) | 11.13 (1.26) | .015 * | |
| Externalizing symptoms | 9.44 (0.96) | 9.74 (0.97) | .826 | |
| Socio-emotional traits | ||||
| Trait anxiety | 33.44 (1.21) | 28.95 (0.87) | .004 * | |
| Intolerance to uncertainty | 52.02 (2.65) | 53.36 (1.93) | .687 | |
| Anxiety sensitivity | 30.44 (0.98) | 26.96 (0.79) | .007 * | |
| Rumination | 31.13 (1.11) | 30.76 (0.94) | .798 | |
| Distress at T1 | ||||
| State anxiety | 29.83 (0.76) | 29.03 (0.70) | .440 | |
| Depression | 8.2 (0.86) | 7.24 (0.80) | .411 | |
| Anger | 36.38 (0.82) | 36.44 (0.84) | .959 | |
3.2. Main and interaction effects
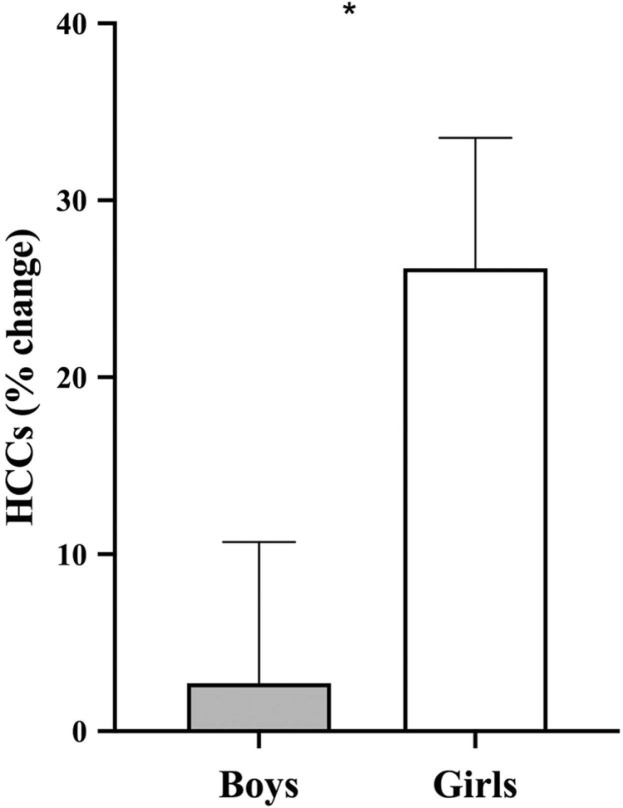
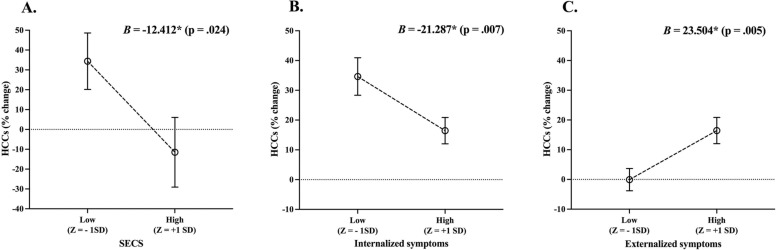
With the intention to account for the effect of the covariates, the first step of the model was non-significant [F(5,61) = 1.192 p = .324] (see Table 3A). The second step of the model was used to analyze the main effects of SECS as well as pre-pandemic internalizing and externalizing symptoms at T0 [F(8,61) = 3.353, p = .004]. We found a main effect of sex, with girls presenting higher HCC % changes as opposed to boys (see Table 3B and Fig. 3). We also found a main effect of SECS, where greater SECS predicted lower HCC % changes (see Table 3B and Fig. 4A). A main effect of pre-pandemic internalizing symptoms was also found, where symptoms at T0 predicted higher HCC % changes. Further, we found a significant main effect of pre-pandemic externalizing symptoms, where greater symptoms at T0 predicted higher HCC % changes at T1 (see Table 3B, Fig. 4B and C).
Table 3.
Main and interaction effects of sex, SECS, internalizing and externalizing symptoms on hair cortisol concentrations % change. * indicates statistical significance set at p < .05. SECS: socio-emotional composite score.
| HCCs (% change) |
|||||
|---|---|---|---|---|---|
|
B |
SE |
β |
t |
p |
|
| A. Control variables (model 1) | |||||
| Time elapsed (Time 1 - Time 0) | -0.595 | 0.831 | -0.096 | -0.717 | .477 |
| Sex | 23.792 * | 10.223 | .303 | 2.326 | .024 |
| Age at T1 | -0.649 | 3.597 | -0.024 | -0.180 | .857 |
| Anxio-depressive symptoms at T1 | -1.937 | 6.640 | -0.038 | -0.292 | .772 |
| Anger at T1 | 0.224 | 0.998 | .029 | 0.224 | .823 |
| Adjusted R2= 0.016% | |||||
| B. Main effects (model 2) | |||||
| Time elapsed | -0.659 | 0.737 | -0.106 | -0.893 | 376 |
| Sex | 43.275 * | 10.216 | .552 | 4.236 | > 0.001 |
| Age at T1 | -3.122 | 3.391 | -0.116 | -0.921 | .361 |
| Anxio-depressive symptoms at T1 | -2.155 | 6.557 | -0.042 | -0.329 | .744 |
| Anger at T1 | 0.491 | 0.910 | .065 | 0.540 | .591 |
| SECS | -12.412 * | 5.332 | -0.296 | -2.328 | .024 |
| Internalizing symptoms at T0 | -21.287 * | 7.589 | -0.581 | -2.805 | .007 |
| Externalizing symptoms at T0 | 23.504 * | 8.046 | .579 | 2.921 | .005 |
| Adjusted R2= 23.9% | |||||
Fig. 3.
Sex differences in hair cortisol % changes. Estimated marginal means considering age and time elapsed (Time 1 - Time 0). HCCs: hair cortisol concentrations. * indicates statistical difference set at p < .05.
Fig. 4.
Effects of SECS (A), pre-pandemic internalizing (B) and pre-pandemic externalizing (C) symptoms on hair cortisol concentration % changes. Means are adjusted for sex, age, internalizing symptoms, and anger at T1. 1 SD below and above the mean for SECS (A), pre-pandemic internalizing (B) and pre-pandemic externalizing (C) symptoms. SECS: socio-emotional composite score; HCCs; hair cortisol concentrations. * indicates statistical significance set at p < .05.
4. Discussion
This longitudinal study aimed to have a better understanding of the effect of the COVID-19 pandemic on HCC in healthy youth. Further, we aimed to elucidate the predictive value of certain predisposing factors known to alter HPA axis activity in children. Specifically, we examined the impact of sex, pre-pandemic socio-emotional vulnerability, as well as internalizing and externalizing symptoms on capillary cortisol levels corresponding to the three months preceding and succeeding the announcement of the first general lockdown in Quebec, Canada.
Across our sample, we found no significant difference between the pre-pandemic hair segment and the segment corresponding to the first three months of the lockdown in Quebec. In other words, the pandemic did not seem to affect the cortisol response for our sample as a whole. From a biological standpoint, this result raises the importance of understanding who responded to the pandemic. By examining the percent change in hair cortisol between both hair segments (before and during the pandemic), we found that girls showed a greater HCC reactivity in response to the pandemic compared to boys. To our knowledge, this is the first study to demonstrate sex differences in HCC reactivity to a potentially long-lasting stressor in children. However, some studies in youth have investigated sex differences in cortisol reactivity to an experimental stressor (Gunnar et al., 2009, Klimes–Dougan et al., 2001, Kudielka and Kirschbaum, 2005, Sumter et al., 2010). While some reported no sex differences in salivary cortisol responses (Kudielka and Kirschbaum, 2005, Sumter et al., 2010), others have observed differences. For example, a higher cortisol response to the Trier Social Stress Test for Children (an experimental psychosocial stressor; (Seddon et al., 2020)) was found in adolescent girls as opposed to younger boys and girls (Gunnar et al., 2009, Klimes–Dougan et al., 2001).
From this, it remains unclear why girls had a greater hair cortisol response to the pandemic as opposed to boys. The nature of the stressor may provide a potential explanation for this finding. Indeed, the first three months of the COVID-19-related confinement measures resulted in prolonged periods of isolation, where children were home-schooled and prohibited from engaging with others outside of their immediate family. Of note, social isolation has been shown to affect the HPA axis activity of females more strongly than males. This finding has been observed in both animal (Pisu et al., 2016, Weintraub et al., 2010) and human (for a review, see Cacioppo et al., 2015) models. Thus, our study suggests that the nature of the stressor is an important factor to consider when exploring sex differences for the physiological stress response.
In addition, we found that socio-emotional vulnerability (represented by the composite score SECS) negatively predicted HCC percent change in youth. In a first publication based on the same sample (Raymond et al., 2022), we demonstrated that the socio-emotional vulnerability score predicted both anxiety and post-traumatic stress symptoms in youth during the year following the first wave of the pandemic. As these personality traits are known to favour the development of clinical anxiety (Aktar et al., 2017, Alkozei et al., 2014, Boswell et al., 2013, Brandes and Bienvenu, 2006; H. M. Brown et al., 2016; Naragon-Gainey, 2010), our current results add to the existing literature by showing that these traits predict a lower reactivity of the physiological stress system in response to a long-lasting stressor. However, three of the four predictors included in the composite score of socio-emotional vulnerability (trait anxiety, intolerance of uncertainty, and rumination) were measured when the pandemic was already present in the province of Quebec (T1). Therefore, one may wonder whether our results were solely due to a pre-pandemic socio-emotional vulnerability or whether the vulnerability score was amplified by the pandemic. Fortunately, we measured internalizing and externalizing symptoms (two predictors known to be closely associated with the stress response) before the pandemic.
Indeed, we found that HCC percent change was associated with internalizing and externalizing symptoms measured 1–3 years prior to the pandemic. First, similar to SECS, we found that internalizing symptoms predicted a decreased HCC reactivity to the pandemic. The literature on the association between HCC and internalizing symptoms is mixed. Certain studies have reported a positive association (Lu et al., 2018, Rietschel et al., 2016), negative association (Vives et al., 2015), and others have found no association between HCC and internalizing symptoms (Gray et al., 2018, Sandstrom et al., 2021, White et al., 2017). In addition, our results revealed that children who presented increased pre-pandemic externalizing symptoms showed higher HCC in reaction to the pandemic. The literature investigating this association is quite divergent as well. For example, a negative association between HCC and externalizing symptoms was found in a sample of teenagers who had experienced numerous instances of childhood maltreatment (White et al., 2017). In contrast to the studies described above, we analyzed the percentage change in hair cortisol when faced with a potentially long-term stressor rather than a single cortisol concentration at a specific point in time. Using a methodology similar to the one in this study (two segments of 3 cm of hair), Kornelsen et al. (2019) found a positive association between externalizing disorders and HCC change over the course of six months in a sample of children suffering from a chronic physical illness.
Several methodological factors may explain the heterogeneity of the results in the literature. The first potential factor is sample type. Indeed, the inclusion of populations with psychiatric diagnoses, participants at different stages of puberty, as well as the lack of consideration for sex differences may considerably influence the results of a study. For example, to maximize cortisol detection in hair, some authors tend to only recruit girls as their hair tends to be longer (Sandstrom et al., 2021, Schuler et al., 2017). Several significant methodological strengths of this study include the recruitment of both sexes and the use of a longitudinal design. Our results showed a negative association between pre-pandemic internalizing symptoms and cortisol reactivity to a major life event such as the pandemic. Nevertheless, further research is needed to elucidate the directionality of the association between HCC and internalized/externalized symptoms and to unveil the mechanisms underlying this association.
This association could be explained by neurobiological mechanisms. Both forms of symptomatology have been associated with neurobiological differences in brain regions necessary for the regulation of the HPA axis. Indeed, numerous studies have reported structural and functional dysfunction of the amygdala (an important region for threat detection and for triggering of the HPA axis; Bishop et al., 2004) and the prefrontal cortex (important for emotion regulation; Sullivan and Gratton, 2002) in children suffering from various externalizing (for a review, see Noordermeer et al., 2016) and internalizing disorders (for a review, see Martin et al., 2009). It would be interesting for future studies to verify the mediating role of the volume/activity of these brain regions on the association between internalizing and externalizing symptoms on HCC secreted in response to a prolonged stressor. Indeed, studies using a multimodal approach (hormonal, neural, and self-report data) will undoubtedly contribute to a better understanding of the neurobiological mechanism underlying the development of anxiety in youth.
Given that stress hormones can have deleterious effects on long-term cognitive functioning and mental health (Lupien et al., 2009), it might be tempting to interpret the negative association between internalizing symptoms and HCC as promising. However, it remains unclear whether blunted HPA reactivity is adaptive when faced with a prolonged stressor. Indeed, animal (Cohen et al., 2006) and human (Marin et al., 2019) studies have suggested that blunted HPA activity may be predictive of poor mental health outcomes in the aftermath of trauma/stress exposure. For example, it has been shown that a high cortisol awakening response in the aftermath of exposure to workplace violence moderated the association between acute stress disorder symptoms and post-traumatic stress symptoms two months following exposure to the trauma (Marin et al., 2019). It remains to be determined whether an exacerbated or reduced cortisol response predicts poor adaptation during exposure to a long-term stressor (such as COVID-19). Consequently, members of our research team are exploring this important research question.
This study contains several limitations. First, cortisol washout could have occurred across the two hair segments, given that we compared newer (Segment B) to older (Segment A) hair (Stalder and Kirschbaum, 2012). This methodological issue could have contributed to the obtained results. Although we could not eliminate the impact of this potential effect on our results, previous studies have demonstrated that cortisol remains stable in hair for up to 6 months (Dettenborn et al., 2010; Rajcani et al., 2021)). In support of this, we found a strong correlation between the HCC values found in the two hair segments. Second, our limited sample size did not allow for the verification of whether sex and age moderated the association between the predisposing factors and HCC. It would be interesting for future studies with a larger sample to verify whether the observed associations are stronger in a particular sex or age group. For example, given that adolescents and girls were found to be more distressed by the pandemic (for a meta-analysis, see Ma et al., 2021), future studies should explore whether these findings are observable on a physiological level. As an increased vulnerability to experience clinical anxiety emerges at puberty (Beesdo et al., 2009), another limitation of the current study is that we did not identify the puberty stage of each child. Therefore, future research should investigate whether puberty status influences the nature of the associations between SECS, internalizing and externalizing symptoms, as well as HCC reactivity to prolonged stress. Due to limited sample size, another limitation of our study was the failure to account for various environmental factors (such as parental stress and socioeconomic status). Also, we did not account for gender identity and therefore, cannot verify whether all children in our sample were cisgender. Given the significant role of gender on stress reactivity (Dedovic et al., 2009) and psychiatric symptoms (Vlassoff, 2007), future studies should consider this important variable in their research. Another important limitation of this study was the lack of family (e.g., family structure) and relationship measures (e.g., parental attachment, nature of family interactions) in our analyses. In addition, we did not account for the emotional, financial, or functional impact that the COVID-19 pandemic may have had on the familial level. These factors may have influenced our results. Moreover, we did not measure how children perceived the COVID-19 pandemic. In other words, although we defined the pandemic as a “long-term stressful event”, we are unaware to which extent the children in our sample perceived this life event as stressful and how other stressful situations might have contributed to our results. With that said, the majority of studies investigating individual differences in HPA axis activity are cross-sectional. Though informative, these studies cannot inform us about the long-term predictive value of predisposing factors on a future stressor. Therefore, the longitudinal nature of the present study represents an important strength.
As reported by a wealth of studies, the first wave of the COVID-19 pandemic and the associated confinement measures represented an adverse life event for many children around the globe. Using a neuroendocrine marker of long-term stress (hair cortisol concentrations), we showed that the pandemic affected the biological stress system of certain children. In other words, the pandemic was able to “get under the skin” of some children. This study provides insight into the long-term effects of socio-emotional vulnerability, as well as internalizing and externalizing symptoms on HPA axis activity in healthy youth. Due to the potentially deleterious effects of altered stress hormone secretion during periods of brain development, it is crucial to identify the vulnerability factors that alter biological stress system reactivity when faced with adversity. Further studies are needed to better understand the long-term effects of HCC alterations on mental health outcomes in youth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Dr. Marin holds a salary award from the Fonds de recherche du Québec-Santé (FRQS) and the study was supported by a start-up grant from FRQS to Dr. Marin. Dr. Raymond holds a post-doctoral grant from the FRQS.
References
- Adam E.K. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Aguinis H., Gottfredson R.K., Joo H. Best-practice recommendations for defining, identifying, and handling outliers. Organ. Res. Methods. 2013;16(2):270–301. doi: 10.1177/1094428112470848. [DOI] [Google Scholar]
- Aiken L.S., West S.G., Reno R.R. SAGE; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Aktar E., Nikolić M., Bögels S.M. Environmental transmission of generalized anxiety disorder from parents to children: Worries, experiential avoidance, and intolerance of uncertainty. Dialog-. Clin. Neurosci. 2017;19(2):137–147. doi: 10.31887/DCNS.2017.19.2/eaktar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkozei A., Cooper P.J., Creswell C. Emotional reasoning and anxiety sensitivity: Associations with social anxiety disorder in childhood. J. Affect. Disord. 2014;152–154:219–228. doi: 10.1016/j.jad.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K., Knappe S., Pine D.S. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr. Clin. North Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. https://doi.org/10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau-Houle A., Bouchard V., Morand-Beaulieu S., Herringa R.J., Milad M.R., Marin M.-F. Anxiety sensitivity moderates the association between father-child relationship security and fear transmission. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.579514. https://www.frontiersin.org/article/10.3389/fpsyg.2020.579514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Lawrence A.D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen P.A., Vrinssen I., van Tulder F. Intolerance of uncertainty in adolescents: correlations with worry, social anxiety, and depression. J. Nerv. Ment. Dis. 2010;198(3):194–200. doi: 10.1097/NMD.0b013e3181d143de. [DOI] [PubMed] [Google Scholar]
- Boswell J.F., Thompson-Hollands J., Farchione T.J., Barlow D.H. Intolerance of uncertainty: a common factor in the treatment of emotional disorders. J. Clin. Psychol. 2013;69(6) doi: 10.1002/jclp.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M., Bienvenu O.J. Personality and anxiety disorders. Curr. Psychiatry Rep. 2006;8(4):263–269. doi: 10.1007/s11920-006-0061-8. [DOI] [PubMed] [Google Scholar]
- Brown H.M., Meiser-Stedman R., Woods H., Lester K.J. Cognitive vulnerabilities for depression and anxiety in childhood: specificity of anxiety sensitivity and rumination. Behav. Cogn. Psychother. 2016;44(1):30–42. doi: 10.1017/S1352465814000472. [DOI] [PubMed] [Google Scholar]
- Brown S.M., Doom J.R., Lechuga-Peña S., Watamura S.E., Koppels T. Stress and parenting during the global COVID-19 pandemic. Child Abus. Negl. 2020;110 doi: 10.1016/j.chiabu.2020.104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne K.A., Peters C., Willis H.C., Phan D., Cornwall A., Worthy D.A. Acute stress enhances tolerance of uncertainty during decision-making. Cognition. 2020;205 doi: 10.1016/j.cognition.2020.104448. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Cacioppo S., Capitanio J.P., Cole S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015;66(1):733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Zohar J., Gidron Y., Matar M.A., Belkind D., Loewenthal U., Kozlovsky N., Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry. 2006;59(12):1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Comer J.S., Roy A.K., Furr J.M., Gotimer K., Beidas R.S., Dugas M.J., Kendall P.C. The intolerance of uncertainty scale for children: a psychometric evaluation. Psychol. Assess. 2009;21(3):402–411. doi: 10.1037/a0016719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost K.T., Crosbie J., Anagnostou E., Birken C.S., Charach A., Monga S., Kelley E., Nicolson R., Maguire J.L., Burton C.L., Schachar R.J., Arnold P.D., Korczak D.J. Mostly worse, occasionally better: impact of COVID-19 pandemic on the mental health of Canadian children and adolescents. Eur. Child Adolesc. Psychiatry. 2021 doi: 10.1007/s00787-021-01744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney D., Watson P., Battaglia M., Mulsant B.H., Szatmari P. COVID-19 impacts on child and youth anxiety and depression: challenges and opportunities. Can. J. Psychiatry. 2020;65(10):688–691. doi: 10.1177/0706743720935646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.J., Enns M.W., Taylor S. The effect of rumination as a mediator of elevated anxiety sensitivity in major depression. Cogn. Ther. Res. 2001;25(5):525–534. doi: 10.1023/A:1005580518671. [DOI] [Google Scholar]
- Davenport M.D., Tiefenbacher S., Lutz C.K., Novak M.A., Meyer J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147(3):255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Wadiwalla M., Engert V., Pruessner J.C. The role of sex and gender socialization in stress reactivity. Dev. Psychol. 2009;45(1):45–55. doi: 10.1037/a0014433. [DOI] [PubMed] [Google Scholar]
- Dettenborn L., Tietze A., Bruckner F., Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Dugré S., Trudel M., Valla J.-P. Considérations individuelles et culturelles en santé mentale des enfants: Le Dominique à l′épreuve. [Individual and cultural considerations concerning the mental health of children: Use of the Dominic–R.] Rev. Can. De. Psycho-Educ. 2001;30(1):119–138. [Google Scholar]
- Enge S., Fleischhauer M., Hadj-Abo A., Butt F., Kirschbaum C., Schmidt K., Miller R. Comparison of hair cortisol concentrations between self- and professionally-collected hair samples and the role of five-factor personality traits as potential moderators. Psychoneuroendocrinology. 2020;122 doi: 10.1016/j.psyneuen.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick K.M., Harris C., Drawve G. Fear of COVID-19 and the mental health consequences in America. Psychol. Trauma. 2020;12(S1):S17–S21. doi: 10.1037/tra0000924. [DOI] [PubMed] [Google Scholar]
- Ford J.L., Boch S.J., Browning C.R. Hair cortisol and depressive symptoms in youth: an investigation of curvilinear relationships. Psychoneuroendocrinology. 2019;109 doi: 10.1016/j.psyneuen.2019.104376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N.A., Dhana A., Van Der Vyver L., Van Wyk J., Khumalo N.P., Stein D.J. Determinants of hair cortisol concentration in children: a systematic review. Psychoneuroendocrinology. 2018;87:204–214. doi: 10.1016/j.psyneuen.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Grotzinger A.D., Mann F.D., Patterson M.W., Tackett J.L., Tucker-Drob E.M., Harden K.P. Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psychol. Sci. 2018;29(5):688–699. doi: 10.1177/0956797617742981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Wewerka S., Frenn K., Long J.D., Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev. Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M., Haque M.E., Tozal M.E. Efficacy the of confinement policies on the COVID-19 spread dynamics in the early period of the pandemic. ArXiv. 2021;2111:03020. [Physics]. http://arxiv.org/abs/2111.03020. [Google Scholar]
- Hensley L., Varela R.E. PTSD symptoms and somatic complaints following hurricane katrina: the roles of trait anxiety and anxiety sensitivity. J. Clin. Child Adolesc. Psychol. 2008;37(3):542–552. doi: 10.1080/15374410802148186. [DOI] [PubMed] [Google Scholar]
- Vives A., De Angel V., Papadopoulos A., Strawbridge R., Wise T., Young A.H., Arnone D., Cleare A.J. The relationship between cortisol, stress and psychiatric illness: new insights using hair analysis. J. Psychiatr. Res. 2015;70:38–49. doi: 10.1016/j.jpsychires.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Hishinuma E.S., Miyamoto R.H., Nishimura S.T., Goebert D.A., Yuen N.Y., Makini G.K., Andrade N.N., Johnson R.C., Carlton B.S. Prediction of anxiety disorders using the state-trait anxiety inventory for multiethnic adolescents. J. Anxiety Disord. 2001;15(6):511–533. doi: 10.1016/s0887-6185(01)00079-2. [DOI] [PubMed] [Google Scholar]
- Jentsch V.L., Merz C.J., Wolf O.T. Restoring emotional stability: cortisol effects on the neural network of cognitive emotion regulation. Behav. Brain Res. 2019;374 doi: 10.1016/j.bbr.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Kao K., Doan S.N., St., John A.M., Meyer J.S., Tarullo A.R. Salivary cortisol reactivity in preschoolers is associated with hair cortisol and behavioral problems. Stress. 2018;21(1):28–35. doi: 10.1080/10253890.2017.1391210. [DOI] [PubMed] [Google Scholar]
- Klimes–Dougan B., Hastings P.D., Granger D.A., Usher B.A., Zahn–Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev. Psychopathol. 2001;13(3):695–719. doi: 10.1017/S0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kornelsen E., Buchan M.C., Gonzalez A., Ferro M.A. Hair cortisol concentration and mental disorder in children with chronic physical illness. Chronic Stress. 2019;3 doi: 10.1177/2470547019875116. 2470547019875116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lanni K.E., Schupp C.W., Simon D., Corbett B.A. Verbal ability, social stress, and anxiety in children with Autistic Disorder. Autism.: Int. J. Res. Pract. 2012;16(2):123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van F., Zebdi R., Burgy S., Souillot R., Julien-Sweerts S., Fouques D. Le processus de rumination mentale chez les enfants de 8 à 11 ans: Adaptation et validation française de la Children’s Response Styles Scale/échelle des styles de réponses chez les enfants. Neuropsychiatr. De. l′Enfance Et. De. l′Adolescence. 2021;69(2):105–111. doi: 10.1016/j.neurenf.2020.10.002. [DOI] [Google Scholar]
- Lu Q., Pan F., Ren L., Xiao J., Tao F. Sex differences in the association between internalizing symptoms and hair cortisol level among 10-12 year-old adolescents in China. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0192901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Ma L., Mazidi M., Li K., Li Y., Chen S., Kirwan R., Zhou H., Yan N., Rahman A., Wang W., Wang Y. Prevalence of mental health problems among children and adolescents during the COVID-19 pandemic: a systematic review and meta-analysis. J. Affect. Disord. 2021;293:78–89. doi: 10.1016/j.jad.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M.-F., Geoffrion S., Juster R.-P., Giguère C.-E., Marchand A., Lupien S.J., Guay S. High cortisol awakening response in the aftermath of workplace violence exposure moderates the association between acute stress disorder symptoms and PTSD symptoms. Psychoneuroendocrinology. 2019;104:238–242. doi: 10.1016/j.psyneuen.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Marin M.-F., Bilodeau-Houle A., Morand-Beaulieu S., Brouillard A., Herringa R.J., Milad M.R. Vicarious conditioned fear acquisition and extinction in child–parent dyads. Sci. Rep. 2020;10(1):17130. doi: 10.1038/s41598-020-74170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques de Miranda D., da Silva Athanasio B., Sena Oliveira A.C., Simoes-e-Silva A.C. How is COVID-19 pandemic impacting mental health of children and adolescents. Int. J. Disaster Risk Reduct. 2020;51 doi: 10.1016/j.ijdrr.2020.101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.I., Ressler K.J., Binder E., Nemeroff C.B. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr. Clin. North Am. 2009;32(3):549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Schmidt H., Merckelbach H., Schouten E. Anxiety sensitivity in adolescents: Factor structure and relationships to trait anxiety and symptoms of anxiety disorders and depression. Behav. Res. Ther. 2001;39(1):89–100. doi: 10.1016/S0005-7967(99)00179-5. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol. Bull. 2010;136(1):128–150. doi: 10.1037/a0018055. [DOI] [PubMed] [Google Scholar]
- Noordermeer S.D.S., Luman M., Oosterlaan J. A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol. Rev. 2016;26(1):44–72. doi: 10.1007/s11065-015-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A., Loveday C., Hucklebridge F., Thorn L., Clow A. Anxious attachment style and salivary cortisol dysregulation in healthy female children and adolescents. J. Child Psychol. Psychiatry. 2011;52(2):111–118. doi: 10.1111/j.1469-7610.2010.02296.x. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U., Schloß S., Ruhl I., Skoluda N., Nater U.M., Becker K. Hair cortisol concentration in preschoolers with attention-deficit/hyperactivity symptoms—Roles of gender and family adversity. Psychoneuroendocrinology. 2017;86:25–33. doi: 10.1016/j.psyneuen.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U., Schloß S., Skoluda N., Nater U.M., Becker K. Low hair cortisol concentration predicts the development of attention deficit hyperactivity disorder. Psychoneuroendocrinology. 2019;110 doi: 10.1016/j.psyneuen.2019.104442. [DOI] [PubMed] [Google Scholar]
- Perry N.B., Donzella B., Troy M.F., Barnes A.J. Mother and child hair cortisol during the COVID-19 pandemic: associations among physiological stress, pandemic-related behaviors, and child emotional-behavioral health. Psychoneuroendocrinology. 2022;137 doi: 10.1016/j.psyneuen.2021.105656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu M.G., Garau A., Boero G., Biggio F., Pibiri V., Dore R., Locci V., Paci E., Porcu P., Serra M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience. 2016;320:172–182. doi: 10.1016/j.neuroscience.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Rajcani J., Vytykacova S., Solarikova P., Brezina I. Stress and hair cortisol concentrations in nurses during the first wave of the COVID-19 pandemic. Psychoneuroendocrinology. 2021;129 doi: 10.1016/j.psyneuen.2021.105245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C., Marin M.-F., Majeur D., Lupien S. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018 doi: 10.1016/j.pnpbp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Raymond C., Provencher J., Bilodeau-Houle A., Leclerc J., Marin M.-F. A longitudinal investigation of psychological distress in children during COVID-19: the role of socio-emotional vulnerability. Eur. J. Psychotraumatology. 2022;13(1):2021048. doi: 10.1080/20008198.2021.2021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel L., Streit F., Zhu G., McAloney K., Kirschbaum C., Frank J., Hansell N.K., Wright M.J., McGrath J.J., Witt S.H., Rietschel M., Martin N.G. Hair cortisol and its association with psychological risk factors for psychiatric disorders: a pilot study in adolescent twins. Twin Res. Hum. Genet.: Off. J. Int. Soc. Twin Stud. 2016;19(5):438–446. doi: 10.1017/thg.2016.50. [DOI] [PubMed] [Google Scholar]
- Rodgers J., Goodwin J., Parr J.R., Grahame V., Wright C., Padget J., Garland D., Osborne M., Labus M., Kernohan A., Freeston M. Coping with Uncertainty in Everyday Situations (CUES©) to address intolerance of uncertainty in autistic children: study protocol for an intervention feasibility trial. Trials. 2019;20(1):385. doi: 10.1186/s13063-019-3479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Laurent L. Étude psychométrique de l′Inventaire de dépression pour enfants de Kovacs auprès d′un échantillon francophone. Can. J. Behav. Sci. / Rev. Can. Des. Sci. Du Comport. 1990;22(4):377–384. doi: 10.1037/h0078990. [DOI] [Google Scholar]
- Sandstrom A., Daoust A.R., Russell E., Koren G., Hayden E.P. Hair cortisol concentrations predict change in girls’ depressive symptoms. Eur. J. Dev. Psychol. 2021;18(2):184–198. doi: 10.1080/17405629.2020.1774359. [DOI] [Google Scholar]
- Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schloß S., Ruhl I., Müller V., Becker K., Skoluda N., Nater U.M., Pauli-Pott U. Low hair cortisol concentration and emerging attention-deficit/hyperactivity symptoms in preschool age. Dev. Psychobiol. 2018;60(6):722–729. doi: 10.1002/dev.21627. [DOI] [PubMed] [Google Scholar]
- Schuler K.L., Ruggero C.J., Goldstein B.L., Perlman G., Klein D.N., Kotov R. Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. J. Adolesc. Health.: Off. Publ. Soc. Adolesc. Med. 2017;61(6):767–772. doi: 10.1016/j.jadohealth.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J.A., Rodriguez V.J., Provencher Y., Raftery-Helmer J., Hersh J., Labelle P.R., Thomassin K. Meta-analysis of the effectiveness of the Trier Social Stress Test in eliciting physiological stress responses in children and adolescents. Psychoneuroendocrinology. 2020;116 doi: 10.1016/j.psyneuen.2020.104582. [DOI] [PubMed] [Google Scholar]
- Sorg S., Vögele C., Furka N., Meyer A.H. Perseverative Thinking in Depression and Anxiety. Front. Psychol. 2012:3. doi: 10.3389/fpsyg.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C.D. (1983). State-Trait Anxiety Inventory for Adults. https://doi.org/10.1037/t06496–000.
- Stalder T., Kirschbaum C. Analysis of cortisol in hair—State of the art and future directions. Brain, Behav., Immun. 2012;26(7):1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Stalder T., Steudte-Schmiedgen S., Alexander N., Klucken T., Vater A., Wichmann S., Kirschbaum C., Miller R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Stassart C., Etienne A. A French Translation of the Childhood Anxiety Sensitivity Index (CASI): Factor Structure, Reliability and Validity of This Scale in a Nonclinical Sample of Children. Psychol. Belg. 2014;54(2):222–241. doi: 10.5334/pb.an. [DOI] [Google Scholar]
- Steele R.G., Legerski J.-P., Nelson T.D., Phipps S. The anger expression scale for children: initial validation among healthy children and children with cancer. J. Pediatr. Psychol. 2009;34(1):51–62. doi: 10.1093/jpepsy/jsn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1–2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Sumter S.R., Bokhorst C.L., Miers A.C., Van Pelt J., Westenberg P.M. Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35(10):1510–1516. doi: 10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. xxvii. Allyn & Bacon/Pearson Education; 2007. (Using multivariate statistics, 5th ed). [Google Scholar]
- Turgeon L., Chartrand É. Psychometric properties of the french canadian version of the state-trait anxiety inventory for children. Educ. Psychol. Meas. 2003;63(1):174–185. doi: 10.1177/0013164402239324. [DOI] [Google Scholar]
- Valla J.P., Bergeron L., Smolla N. The Dominic-R: a pictorial interview for 6- to 11-year-old children. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):85–93. doi: 10.1097/00004583-200001000-00020. [DOI] [PubMed] [Google Scholar]
- van der Vegt E.J.M., van der Ende J., Kirschbaum C., Verhulst F.C., Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults: a study of international adoptees. Psychoneuroendocrinology. 2009;34(5):660–669. doi: 10.1016/j.psyneuen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Vlassoff C. Gender differences in determinants and consequences of health and illness. J. Health, Popul., Nutr. 2007;25(1):47–61. [PMC free article] [PubMed] [Google Scholar]
- Weintraub A., Singaravelu J., Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- White L.O., Ising M., von Klitzing K., Sierau S., Michel A., Klein A.M., Andreas A., Keil J., Quintero L., Müller-Myhsok B., Uhr M., Gausche R., Manly J.T., Crowley M.J., Kirschbaum C., Stalder T. Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. J. Child Psychol. Psychiatry. 2017;58(9):998–1007. doi: 10.1111/jcpp.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegert D.I., Kistner J.A. Response Styles Theory: Downward Extension to Children. J. Clin. Child Adolesc. Psychol. 2002;31(3):325–334. doi: 10.1207/S15374424JCCP3103_04. [DOI] [PubMed] [Google Scholar]