Abstract
FtsZ is an ancestral homologue of tubulin that polymerizes in a GTP-dependent manner. In this study, we used 90° angle light scattering to investigate FtsZ polymerization. The critical concentration for polymerization obtained by this method is similar to that obtained by centrifugation, confirming that the light scattering is proportional to polymer mass. Furthermore, the dynamics of FtsZ polymerization could be readily monitored by light scattering. Polymerization was very rapid, reaching steady state within 30 s. The length of the steady-state phase was proportional to the GTP concentration and was followed by a rapid decrease in light scattering. This decrease indicated net depolymerization that always occurred as the GTP in the reaction was consumed. FtsZ polymerization was observed over the pH range 6.5 to 7.9. Importantly, Mg2+ was not required for polymerization although it was required for the dynamic behavior of the polymers. It was reported that 7 to 25 mM Ca2+ mediated dynamic assembly of FtsZ (X.-C. Yu and W. Margolin, EMBO J. 16:5455–5463, 1997). However, we found that Ca2+ was not required for FtsZ assembly and that this concentration of Ca2+ reduced the dynamic behavior of FtsZ assembly.
FtsZ is a highly conserved protein that appears to be present in all prokaryotes, where it has an essential role in cell division (16). It assembles at midcell into a cytoskeletal structure designated the FtsZ ring (Z ring) that directs the process of septation (2, 3). It was suggested that the Z ring formed through the polymerization of FtsZ, and all subsequent work supports this hypothesis (15). One activity of this ring is to recruit other division proteins to the division site, where they participate in formation of the septum (16).
FtsZ has limited sequence identity to eukaryotic tubulins, suggesting that it is an ancestral homologue (19). The recent solution of the structures of FtsZ and tubulin support this conclusion, as the structures are remarkably similar (13, 22). In addition to these sequence and structural similarities there is functional similarity, as both tubulin and FtsZ are GTPases that can polymerize into higher-order structures. Like tubulin’s GTPase, FtsZ’s GTPase activity increases dramatically with the protein concentration (5, 14, 28, 29). Both have a low basal rate at protein concentrations too low to support polymerization and a significantly increased rate at concentrations above the critical concentration needed for assembly. Tubulin assembles into microtubules that are composed of protofilaments, head-to-tail polymers of the αβ dimer (7). FtsZ also assembles into protofilaments (8, 19), although it is not known how these protofilaments are arranged in the Z ring.
FtsZ readily polymerizes in the presence of DEAE-dextran, a polycation that also promotes tubulin polymerization (8, 19). In the presence of DEAE-dextran, the FtsZ protofilaments tend to bundle or form sheets. Assembly of FtsZ into bundles of protofilaments at neutral pH was also observed by Bramhill and Thompson (4) but was later reported to be due to an inadvertent drop in the pH below 6 (9). More recently, assembly of FtsZ into bundles of protofilaments was observed in the presence of 7 to 25 mM Ca2+, a concentration 1,000-fold higher than the physiological concentration (30). Although these various conditions have been used to promote FtsZ assembly, FtsZ can readily assemble in their absence (20). Under such conditions, FtsZ assembles into a mixture of single protofilaments and polymers that contain two to three protofilaments. Importantly, under these more physiological conditions the FtsZ polymers, like microtubules, are dynamic, depolymerizing as the GTP is exhausted (20). Thus, GTP hydrolysis plays a role in regulating FtsZ polymer dynamics similar to its role in dictating microtubule dynamics.
In our previous study, we used electron microscopy and centrifugation to characterize FtsZ polymerization (20). Here we report that FtsZ polymerization can be monitored by 90° angle light scattering, a technique that we used to examine FtsZ assembly under a variety of conditions, including exposure to various divalent cations.
MATERIALS AND METHODS
Purification and polymerization of FtsZ.
Escherichia coli FtsZ was purified as described earlier (20). However, in that description the ultracentrifugation step was inadvertently omitted. High-speed centrifugation of the 10,000 rpm supernatant was done at 38,000 rpm for 1 h in a Beckman 50.2 Ti rotor to remove the membrane fraction. The supernatant was subjected to 30% ammonium sulfate fractionation. All steps before and after this step in the purification procedure are the same as described previously (20, 21).
For the standard polymerization assay, FtsZ was incubated at 30°C in 50 mM morpholine ethanesulfonic acid (MES)-NaOH (pH 6.5)–10 mM MgCl2–50 mM KCl (polymerization buffer) for various time periods. Polymerization was initiated by adding 1 mM GTP to the prewarmed reaction mixture. In some experiments the pH, the concentration of GTP, or some other component of the polymerization reaction was altered; such changes are indicated where relevant. Piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-NaOH (50 mM) was used for polymerization at pH 6.9, and HEPES-NaOH (50 mM) was used for polymerization at pH 7.5 and 7.9. For electron microscopic analysis, 1 mM GTP was added to FtsZ at 200 μg/ml (5 μM) in the appropriate buffer and incubated for 10 min at 30°C. Samples were then prepared for visualization as described previously (20). For assay by centrifugation, polymerization of FtsZ (200 μg/ml) was initiated in the appropriate buffer by adding 1 mM GTP, and the reaction was immediately centrifuged at 25°C in a Beckman TLA 100.2 rotor at 80,000 rpm for 15 min. Processing the pellet for protein estimation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described earlier (17, 20).
Light scattering assay of FtsZ polymerization.
FtsZ polymerization was measured by 90° angle light scattering in a Hitachi fluorometer (model F-3010) with both the excitation and emission wavelengths set at 350 nm and a slit width of 1.5 nm. FtsZ was added to a final concentration of 500 μg/ml (12.5 μM) or as specified in the appropriate buffer to a fluorometer cuvette with a 1-cm path length. The cuvette was then placed in a cuvette chamber that was maintained at 30°C by a circulating water bath, and data were collected for 8 min to establish a baseline. Then the cuvette was removed, and 1 mM GTP or any other nucleotide was added to achieve a final reaction volume of 300 μl. The reaction mixture was gently stirred with a pipette tip, and the cuvette was returned to the cuvette chamber for data collection for a specified period of time. The reading at time zero is the first reading taken after the cuvette was returned to the chamber following nucleotide addition. The elapsed time for the nucleotide addition step was 20 to 30 s. The net change in light scattering following nucleotide addition was plotted as a function of time. Although data were collected every 2 s, for purposes of plotting we used only data obtained every 30 s or 1 min and in some instances 2 min.
FtsZ GTPase activity.
FtsZ was incubated in reaction mixtures with a final volume of 50 μl at 30°C. The components of the reaction mixtures are described where appropriate in the text. The reaction was initiated by adding 1 mM [γ-32P]GTP (250 to 400 cpm/pmol). At indicated times, 5-μl aliquots were withdrawn and assayed for the liberation of 32P exactly as described previously (18, 20).
RESULTS
FtsZ polymers can be measured by 90° angle light scattering.
We have recently shown that GTP-dependent polymerization of FtsZ can be assayed by electron microscopy and centrifugation (20). The FtsZ polymers that are formed are 7 to 20 nm wide, depending on whether they exist as a single protofilament or a bundle of two to three protofilaments. Actin filaments and microtubules are 6 and 25 nm wide, respectively, and their assembly can be assayed by electron microscopy or centrifugation as well as by a more convenient light scattering assay offering real-time kinetics (11, 12). We therefore explored whether FtsZ polymerization could be assayed by light scattering since development of such an assay would be useful to further characterize FtsZ polymerization.
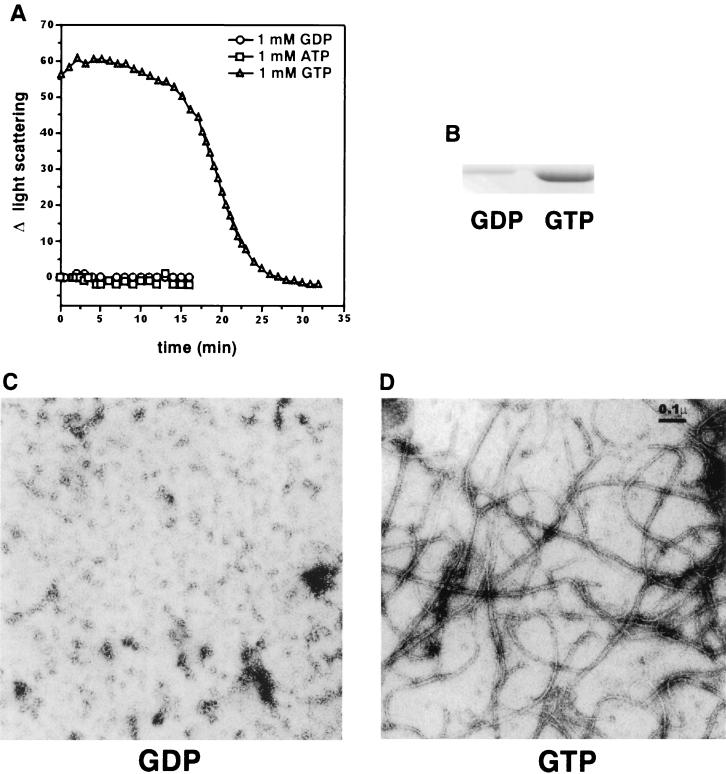
To assess FtsZ polymerization by light scattering, we used conditions where FtsZ forms dynamic polymers that hydrolyze GTP and undergo net disassembly upon exhaustion of GTP (20). No significant light scattering was detected by a spectrophotometer with a wavelength setting of 350 nm even at FtsZ concentrations of up to 1 mg/ml. We therefore monitored polymerization by 90° angle light scattering in a fluorometer since this is a more sensitive method than measuring turbidity in a spectrophotometer (25). As described in Materials and Methods, FtsZ at 500 μg/ml (12.5 μM) was incubated in polymerization buffer (50 mM MES-NaOH [pH 6.5], 10 mM MgCl2, 50 mM KCl) for 8 min to establish the baseline light scattering signal, and then 1 mM GTP was added to initiate polymerization. As seen in Fig. 1A, at time zero, which is the time of the first reading obtained after nucleotide addition, the increase in light scattering was about 60 U, a level which remained constant for about 15 min. Thereafter the light scattering signal started to decrease rapidly and nearly reached 0 by 25 min. In contrast, there was no change in the light scattering when FtsZ was incubated with either 1 mM GDP or ATP (Fig. 1A). The change in light scattering was observed specifically with GTP and not with other nucleotides, which indicated that we were detecting FtsZ polymer formation and subsequent disassembly.
FIG. 1.
Assay of GTP-dependent polymerization of FtsZ by 90° angle light scattering, centrifugation, and electron microscopy. (A) Polymerization of FtsZ was assayed by 90° angle light scattering in a fluorometer. FtsZ at a concentration of 500 μg/ml (12.5 μM) in 294 μl of polymerization buffer (50 mM MES-NaOH [pH 6.5], 10 mM MgCl2, 50 mM KCl) in a fluorometer cuvette was incubated at 30°C for 8 min to establish a baseline. The reaction was initiated with the addition of nucleotide to 1 mM as indicated, and light scattering was monitored. The net change in light scattering (Δ light scattering) following nucleotide addition was plotted against time. (B) For the assay of FtsZ polymerization by centrifugation, 1 mM GDP or GTP was added to FtsZ (200 μg/ml [5 μM]) in 100 μl of polymerization buffer. The reaction mixtures were centrifuged for 15 min, and the FtsZ pellets obtained were analyzed by SDS-PAGE and Coomassie blue staining. (C and D) For analysis of FtsZ polymerization by electron microscopy, 1 mM GDP (C) or GTP (D) was added to FtsZ (200 μg/ml) in polymerization buffer at 30°C and incubated for 10 min. Samples were then prepared for visualization by electron microscopy.
In this and subsequent experiments, we observed that maximum light scattering was reached by the time the first reading was taken. This rapid increase in light scattering (occurring within the 20 to 30 s required for GTP addition and mixing) was also observed when the experiment was done at 15°C, although the net increase in light scattering was slightly reduced (data not shown). This result indicates that FtsZ undergoes rapid polymerization under these conditions and reaches a steady state where polymer mass is constant, as indicated by the plateau in the light scattering signal. During the steady-state phase the polymers are presumably dynamic, undergoing rapid assembly and disassembly. The rapid decline in light scattering that follows the steady state coincides with the time of GTP exhaustion due to hydrolysis (see below). Thus, the rapid decline in the light scattering indicates the end of the steady-state phase when polymerization is prevented by the absence of GTP and only depolymerization is occurring. This issue is examined in more detail later.
To support our conclusions that the increase in light scattering was due to formation of FtsZ polymers, we also monitored polymerization by electron microscopy and centrifugation. FtsZ at 200 μg/ml (5 μM) was incubated in polymerization buffer, and the reaction was initiated with the addition of either 1 mM GDP or GTP. For centrifugation analysis, the reaction mixture was immediately centrifuged for 15 min, and the pellets were analyzed by SDS-PAGE and quantitated by protein determination. For electron microscopy analysis, samples were processed after incubation for 10 min at 30°C. Figure 1B shows the amount of FtsZ in the pellet. Quantitation revealed that 50% of the FtsZ was in the pellet following GTP addition but less than 5% was in the pellet following GDP addition. These values are similar to those reported previously (20). Electron microscopy revealed (Fig. 1C and D) no polymer formation with the addition of GDP, whereas there was a network of FtsZ polymers, 7 to 20 nm wide, following GTP addition. These results confirm that FtsZ polymers are present under the conditions of the light scattering assay, and we therefore conclude that the GTP-dependent increase in light scattering is due to the formation of FtsZ polymers.
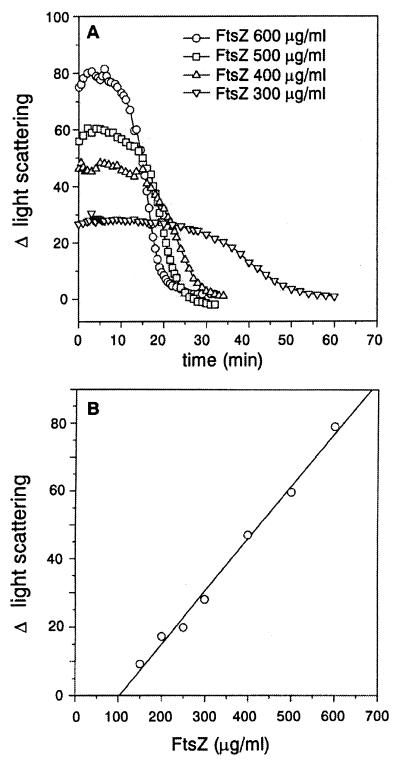
The change in light scattering due to the formation of FtsZ polymers might correlate with the mass of the polymers formed since the polymers are quite long. It is known that light scattering is proportional to polymer mass and is not affected by the length of polymers as long as L/λ > 3.5, where L is the length of the polymer and λ is the wavelength of the incident light (11). We tested this by varying the FtsZ concentration from 300 to 600 μg/ml in the polymerization assay (Fig. 2A). The increase in light scattering, averaged over the steady-state phase, was 27 U with FtsZ at 300 μg/ml; this level increased with increasing concentrations of FtsZ to 80 U at 600 μg/ml. Plotting the increase in light scattering against the FtsZ concentration allowed the critical concentration for polymerization to be determined. The intercept on the x axis for such a plot yields a critical concentration of 100 μg/ml (2.5 μM) (Fig. 2B), close to the critical concentration (1.5 μM) determined by centrifugation (20). We therefore conclude that the change in light scattering is a measure of polymer mass.
FIG. 2.
Light scattering is proportional to FtsZ concentration. Light scattering measurements were done as described in Materials and Methods and in the legend to Fig. 1. (A) FtsZ at different concentrations, as indicated, was incubated in polymerization buffer for 8 min at 30°C, and polymerization initiated by the addition of 1 mM GTP. (B) To obtain the critical concentration for FtsZ polymerization, the average values of the net change in light scattering (Δ light scattering) during the steady state were plotted against FtsZ concentration. The intercept on the x axis is the critical concentration.
We previously demonstrated by electron microscopy that FtsZ polymers persist as long as GTP is present and disappears when GTP is exhausted by hydrolysis (20). As seen in Fig. 2A, the length of the steady-state period, where the polymer mass remains constant, varied inversely with the FtsZ concentration. The length of the period correlates with the expected persistence of GTP at these different FtsZ concentrations (20). At all concentrations of FtsZ, this plateau is followed by a decrease in the light scattering, finally reaching values manifested by unpolymerized FtsZ. This finding was further examined in the assays described below.
The dynamic nature of FtsZ polymers can be monitored by 90° angle light scattering.
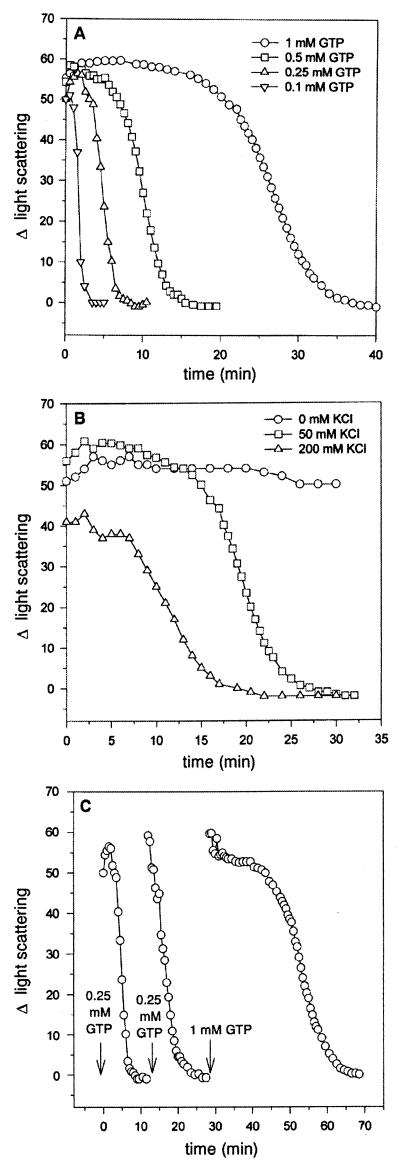
To verify the above interpretation of the light scattering data, we manipulated reaction conditions to vary the length of time for which GTP would persist in the polymerization reaction. First, we varied the GTP concentration. Polymerization was initiated by adding GTP at different concentrations to reactions containing FtsZ at 500 μg/ml (12.5 μM) in polymerization buffer (Fig. 3A). The initial change in light scattering was not noticeably affected by GTP concentration, but it was apparent, that with increasing concentrations of GTP, the steady state persisted longer. With 0.1 mM GTP there was a decline in light scattering within 1 min, reaching zero in 3 min, whereas with 1 mM GTP the light scattering was constant for about 20 min before it started to decline, reaching zero in 35 min. This result demonstrates that the length of the steady state is proportional to the GTP concentration and is consistent with the interpretation of the above results for assays in which the FtsZ concentration was varied.
FIG. 3.
Assessment of the dynamic nature of FtsZ polymers by light scattering. Light scattering measurements were done as described in Materials and Methods and in the legend to Fig. 1. (A) FtsZ at a concentration of 500 μg/ml (12.5 μM) was incubated in polymerization buffer for 8 min at 30°C, and poly- merization was initiated by adding GTP to various final concentrations as indicated. (B) FtsZ at a concentration of 500 μg/ml (12.5 μM) was incubated for 8 min at 30°C in polymerization buffer containing different concentrations of KCl as indicated, and polymerization was initiated by adding 1 mM GTP. (C) FtsZ at a concentration of 500 μg/ml (12.5 μM) in polymerization buffer was incubated for 8 min at 30°C, and polymerization was initiated by adding 0.25 mM GTP. After depolymerization, additional rounds of FtsZ polymerization were induced by the addition of GTP as indicated.
In another approach, the KCl concentration in the polymerization buffer was varied since it is known that the rate of GTP hydrolysis by FtsZ is affected by the KCl concentration (18). Previous results have shown that in 50 mM MES-NaOH (pH 6.5) and 10 mM MgCl2, the rate of GTP hydrolysis increases with increasing amounts of KCl between 0 and 200 mM. The rate is 3.5 times faster with 200 mM than with 50 mM KCl and nearly 8.5 times faster than in the absence of KCl (20). Polymerization was initiated by adding 1 mM GTP to FtsZ (500 μg/ml) in polymerization reaction mixtures containing 0, 50, and 200 mM KCl, and the light scattering was monitored. As seen in Fig. 3B, in the absence of KCl when the GTPase activity is low, the change in light scattering is constant for over 30 min. In contrast, with 50 and 200 mM KCl, the light scattering starts to decline after 15 and 7.5 min and reaches zero after about 15 and 25 min, respectively. This experiment therefore demonstrates that changing the KCl concentration, which affects the rate of FtsZ’s GTPase activity, results in predictable changes in the length of the steady state. Therefore, by varying FtsZ, GTP, and KCl concentrations (Fig. 2A, 3A, and 3B), conditions which affect how long GTP will persist in the polymerization reaction, we see that the persistence of the polymers (the steady-state period) correlates with the persistence of the GTP.
We then tested whether FtsZ would recycle if additional GTP was added. As seen in Fig. 3C, a cycle of polymerization was initiated with 0.25 mM GTP, and by about 8 min the FtsZ was depolymerized. When 0.25 mM GTP was again added, there was again an increase in the light scattering, indicative of polymerization followed shortly by depolymerization. Another cycle of polymerization was initiated with the addition of 1 mM GTP, which due to the higher GTP concentration resulted in a more prolonged response. The recycling of FtsZ polymers as monitored by light scattering is similar to that observed by electron microscopy (20), confirming that light scattering can indeed be an alternative assay for FtsZ polymerization.
FtsZ polymerizes over a broad pH range.
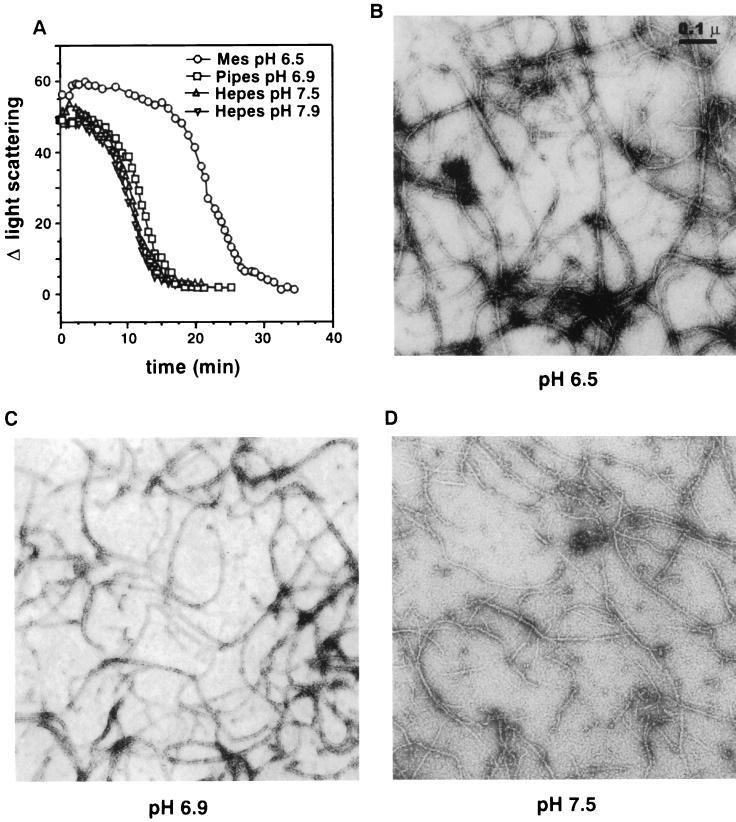
Our previous studies on FtsZ polymerization (20) and the experiments described here were done mostly at pH 6.5. We did report previously that polymer formation as assayed by electron microscopy occurred at pH 7.2 but did not appear as abundant as that at pH 6.5 (20). We therefore examined the effect of pH on FtsZ polymerization more thoroughly by light scattering, centrifugation, and electron microscopy. As seen in Fig. 4A, GTP-dependent polymerization was examined at pH 6.5, 6.9, 7.5, and 7.9. At each pH an increase in light scattering occurred, indicating polymers were formed. However, the steady-state period was shorter at the higher pHs than at pH 6.5. Upon measuring the GTPase activity at the higher pHs, we found that in each case the rate was 25% higher than at pH 6.5 (data not shown). This difference contributes to the decreased length of the steady state at these higher pHs, as the GTP would be consumed earlier. Also at pH 6.5, the initial change in light scattering was consistently 20 to 30% greater than at other pH values.
FIG. 4.
Effect of pH on FtsZ polymer formation. For polymerization at pH 6.9, 50 mM PIPES-NaOH was used; for pH 7.5 and 7.9, 50 mM HEPES-NaOH was used. The MgCl2 and KCl concentrations in these buffers were 10 and 50 mM, respectively. The standard polymerization buffer was used for the assay at pH 6.5. (A) Light scattering measurements were done as described in Materials and Methods and in the legend to Fig. 1. FtsZ at a concentration of 500 μg/ml (12.5 μM) was incubated at 30°C for 8 min in buffers at different pH values, and polymerization was initiated by adding 1 mM GTP. (B to D) For electron microscopy analyses of FtsZ polymers formed at different pHs, polymerization was initiated by adding 1 mM GTP to FtsZ at 200 μg/ml (5 μM) in buffers at pH 6.5 (B), pH 6.9 (C), and 7.5 (D). The reaction mixtures were incubated at 30°C for 10 min, and then samples were prepared for visualization by electron microscopy.
Quantitative determination of FtsZ polymerization by centrifugation in various buffers (all containing 10 mM MgCl2 and 50 mM KCl) with different pHs revealed that the amounts of FtsZ recovered in the pellets were 50% at pH 6.5, 35% at pH 6.9 and 7.5 and 25% at pH 7.9 (Table 1). The presence of polymers was also verified by electron microscopy (Fig. 4B to D). At pH 6.5, the polymers are long, form a dense network, and vary from 7 to 20 nm in width (Fig. 4B). At pH 6.9, the polymers are similar to those formed at pH 6.5 except that shorter polymers are more noticeable (Fig. 4C). At pH 7.5, the polymers are less bundled and so the 7-nm-wide polymers are more prevalent (Fig. 4D). Shorter polymers are also present. At pH 7.9, the polymers formed are similar to those formed at pH 7.5 (data not shown). The lower amounts of FtsZ pelleted at higher pHs may be due to less polymer formation; also, the shorter polymers may not sediment as well. Nonetheless, it is amply clear that FtsZ can polymerize over a broad range of pH values.
TABLE 1.
Effects of pH and Mg2+ on the recovery of FtsZ in the pellet
| Variable | % FtsZ in pelleta |
|---|---|
| pH | |
| 6.5 | 50 |
| 6.9 | 35 |
| 7.5 | 35 |
| 7.9 | 25 |
| [Mg2+] (mM) | |
| 0 | 32 |
| 2.5 | 27 |
| 5.0 | 43 |
| 10.0 | 50 |
Percentage of the total amount of FtsZ (200 μg/ml) in the reaction mixture.
Effect of Mg2+ on polymer formation.
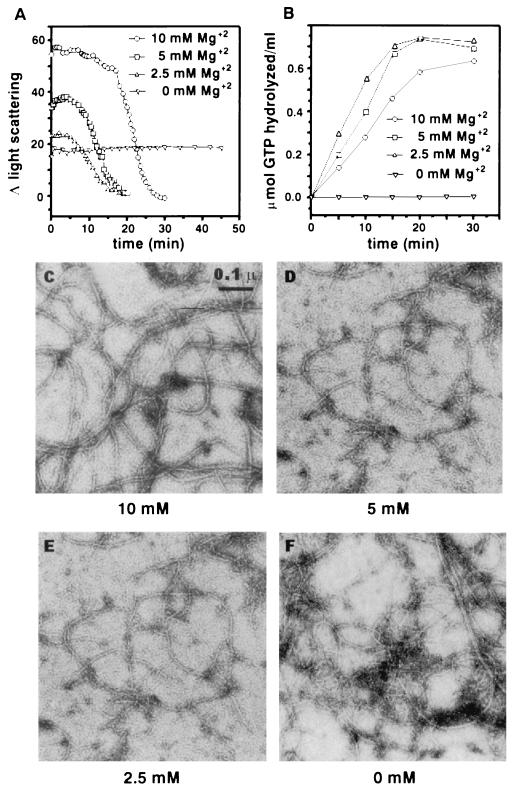
FtsZ requires Mg2+ for GTP hydrolysis (6, 18, 26) but not for GTP binding (18, 26). In the absence of Mg2+, FtsZ forms polymers as seen by electron microscopy and as assayed by centrifugation (20). The concentration of Mg2+ that we used for polymerization was 10 mM, and therefore it was relevant to carefully examine the effects of the Mg2+ concentration on polymerization. At all concentrations of Mg2+ examined, as well as in the absence of Mg2+, a significant increase in light scattering was detected (Fig. 5A). The initial change in light scattering was directly correlated with the Mg2+ concentration, indicating that polymer mass decreased with decreasing Mg2+ concentration. The initial increases in light scattering with 0, 2.5, and 5 mM Mg2+ were 36, 46, and 76% of the increase observed with 10 mM Mg2+ (Table 1). Importantly, in the absence of Mg2+ the change in light scattering remained constant for as long as 45 min, suggesting that polymers formed but did not turn over. A similar observation was made when polymer formation was assayed by centrifugation (20).
FIG. 5.
Effect of Mg2+ on FtsZ polymerization and GTPase activity. Light scattering measurements were done as described in Materials and Methods and in the legend to Fig. 1. (A) FtsZ at a concentration of 500 μg/ml (12.5 μM) was incubated at 30°C for 8 min in polymerization buffer with different MgCl2 concentrations as indicated. Polymerization was initiated by adding 1 mM GTP. (B) The FtsZ GTPase reaction was initiated by adding 1 mM [γ-32P]GTP to a reaction mixture at 30°C containing FtsZ at a concentration of 500 μg/ml in polymerization buffer with different MgCl2 concentrations as indicated. At indicated times, samples were assayed for 32P formation from [γ-32P]GTP as described earlier (18). For electron microscopy analyses of FtsZ polymers formed with different concentrations of MgCl2, polymerization was initiated by adding 1 mM GTP to FtsZ at 200 μg/ml (5 μM) in polymerization buffer containing 10 mM (C), 5 mM (D), 2.5 mM (E), and 0 mM MgCl2 (F). The reaction mixtures were incubated at 30°C for 10 min, and then samples were prepared for visualization by electron microscopy.
The inclusion of 2.5 to 10 mM Mg2+ in the reaction resulted in dynamic behavior of the polymers, as indicated by the rapid decrease in the light scattering occurring at 10 to 30 min after the reaction was initiated. There was, however, a noticeable difference in the response among the different Mg2+ concentrations examined. The steady state was shorter at lower Mg2+ concentrations than at 10 mM Mg2+. One possible explanation is that the rate of GTP hydrolysis increases at lower Mg2+ concentrations, leading to faster consumption of GTP. We therefore examined the GTPase activity of FtsZ at various Mg2+ concentrations (Fig. 5B). Indeed, with 1 (data not shown) and 2.5 mM Mg2+, the rate of GTP hydrolysis is about twice as fast as at 10 mM Mg2+, and with 5 mM it is about 1.5 times as fast. Thus, at these lower concentrations of Mg2+, the polymers are more dynamic than at 10 mM Mg2+.
When the FtsZ polymers formed in the presence of 0, 2.5, and 5.0 mM Mg2+ were measured by centrifugation, the amounts pelleted were 64, 54, and 86%, respectively of that pelleted with 10 mM Mg2+ (Table 1). These values are in accord with what we observed by light scattering except that we have consistently seen slightly more FtsZ in the pellet without Mg2+ than with 2.5 mM Mg2+. We also examined the morphology of the polymers at these various Mg2+ concentrations by electron microscopy. At 10 mM Mg2+, the polymers are 7 to 20 nm wide, as reported previously (20), but at lower (5 and 2.5 mM) Mg2+ concentrations and especially in the absence of Mg2+, there is a predominance of polymers that are 7 nm wide (Fig. 5C to E). Thus, it appears that Mg2+ promotes the lateral association of FtsZ polymers. We also observed that shorter polymers were more visible at lower Mg2+ concentrations, especially 1 mM (data not shown). This finding argues that Mg2+ also affects the stability of protofilaments, presumably affecting the rate of disassembly.
FtsZ polymerization does not require Ca2+.
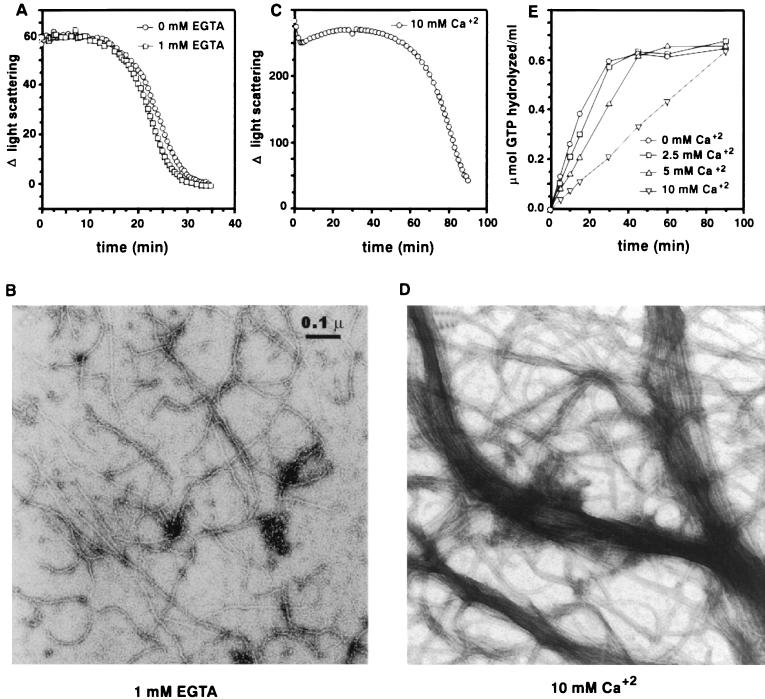
In our previous polymerization studies (20) and in all experiments described here, Ca2+ was not added to the buffers because it was considered not required for polymerization of FtsZ. However, Yu and Margolin (30) reported that a relatively high concentration of Ca2+ (>7 mM) was required for polymerization. Therefore, we examined the effect of Ca2+ on polymerization by light scattering and electron microscopy. To rule out the possibility that low levels of Ca2+ contaminating our buffers influenced polymerization, we examined the effect of EGTA on the polymerization reaction. With 1 mM EGTA, the kinetics and quantity of polymer formation were virtually identical to those for the control without EGTA (Fig. 6A). Essentially the same results were obtained with 5 mM EGTA, although the initial change in light scattering was slightly less (data not shown). The polymers formed in the presence of EGTA (Fig. 6B) appear similar to those for the control (Fig. 5C). Thus, Ca2+ is not required for polymerization of FtsZ.
FIG. 6.
Effect of Ca2+ on FtsZ polymerization and FtsZ’s GTPase activity. (A and C) FtsZ at 500 μg/ml (12.5 μM) was incubated at 30°C for 8 min in polymerization buffer with or without 1 mM EGTA (A) or with 10 mM Ca2+ (C). Polymerization was initiated with the addition of 1 mM GTP, and light scattering measurements were done as described in Materials and Methods and in the legend to Fig. 1. (B and D) For electron microscopic analyses of FtsZ polymers, polymerization was initiated by adding 1 mM GTP to FtsZ at 200 μg/ml (5 μM) in polymerization buffer containing 1 mM EGTA (B) or 10 mM CaCl2 (D). The reaction mixtures were incubated at 30°C for 10 min, and then samples were prepared for visualization by electron microscopy. (E) The FtsZ GTPase reaction was initiated by adding 1 mM [γ-32P]GTP to a reaction mixture at 30°C containing FtsZ at a concentration of 500 μg/ml in polymerization buffer with different concentrations of CaCl2 as indicated. At indicated times, samples were assayed for 32P formation as described earlier (18, 20).
Although Ca2+ is not required for polymerization of FtsZ, we examined the effects of the high concentrations of Ca2+ used by Yu and Margolin (30). Monitoring polymerization of FtsZ by light scattering indicated that polymerization occurred in the presence of 10 mM Ca2+; however, there were several differences (Fig. 6C). First, the amount of light scattering was markedly enhanced by Ca2+. Electron microscopic examination of polymers formed in the presence of 10 mM Ca2+ revealed dramatic bundling (Fig. 6D), presumably responsible for the increased light scattering. Second, the polymers were much less dynamic, as the length of the steady-state phase was markedly increased (Fig. 6C). This result suggested that millimolar concentrations of Ca2+ reduced the dynamic aspect of FtsZ polymers. To examine this possibility further, we measured the effect of 10 mM Ca2+ on the GTPase activity of FtsZ. We found that the GTPase activity was inhibited fourfold by 10 mM Ca2+ (Fig. 6E), similar to the result reported by Yu and Margolin (30). Little effect was observed at 2.5 mM. Thus, it appears that the major effects of 10 mM Ca2+ are to induce bundling of the protofilaments and inhibit the GTPase activity, which reduces the dynamic behavior of FtsZ polymers.
DISCUSSION
In this study we have shown that 90° angle light scattering can be used to investigate FtsZ polymerization. We found that Mg2+ is not required for assembly but is required for the dynamic behavior of the polymers. We found that Ca2+ is not required for FtsZ polymerization and observed that at high concentrations, it induced bundling of protofilaments and reduced the dynamic aspect of FtsZ assembly. We also found that FtsZ assembly occurred throughout the pH range from 6.5 to 7.9.
Since light scattering had not been previously applied to FtsZ polymerization, we felt it necessary to analyze this in some detail. The theoretical analysis of light scattering by very long rod particles indicates that the amount of scattered light is dependent only on the total weight concentration of the rods provided that the length of rods is much greater than the wavelength of the incident light (11). That this is indeed the case for FtsZ polymers was confirmed by using the data obtained by light scattering to estimate the critical concentration. The value of 2.5 μM that was obtained is similar to that obtained previously by centrifugation (20).
The light scattering assay confirmed the dynamic nature of the FtsZ polymers. Under the conditions that we used assembly occurred quickly, reaching a steady state by the time we began measurements. However, we could readily observe the disappearance of polymers which occurred as the GTP was consumed. This correlation between disappearance of the polymers and exhaustion of GTP occurred under a variety of conditions. By altering the GTP, FtsZ, Mg2+, Ca2+, or KCl concentration we could manipulate the time at which GTP was consumed, and this always coincided with the time at which the polymers disappeared. In addition, conditions that inhibited GTPase activity but not assembly, e.g., the removal of Mg2+ or the addition of 10 mM Ca2+, inhibited or reduced the dynamic nature of FtsZ polymers.
Our study revealed that FtsZ polymers form in the absence of Mg2+, indicating that GTP hydrolysis is not required for assembly. Such polymers were very stable, however, indicating that GTP hydrolysis is required for the polymers to depolymerize. Furthermore, in examining the effect of the Mg2+ concentration on FtsZ assembly, we found that the steady state persisted longer at 10 mM Mg2+ than at lower concentrations of Mg2+. This difference could be explained by the effect of Mg2+ on the GTPase activity. The activity was enhanced at lower Mg2+ concentrations (1 to 5 mM), resulting in a more rapid consumption of GTP and the more rapid disappearance of the polymers. At lower Mg2+ concentrations the protofilaments were also shorter, indicating that disassembly was more rapid.
Under our experimental conditions, the basic unit of FtsZ assembly is a protofilament. At higher Mg2+ concentrations, there is a tendency for the protofilaments to align along the long axis to give polymers with two to three protofilaments. It is likely that at 10 mM Mg2+, where this bundling is more noticeable, the increased bundling results in a slower turnover of FtsZ polymers that also reduces the rate of GTP hydrolysis. Since this bundling appears to be induced by the elevated Mg2+ concentration, the physiological significance is unclear. This bundling is also affected by pH. Our study revealed that FtsZ polymerization occurs over a considerable pH range (6.5 to 7.9), although within this pH range assembly is most efficient at pH 6.5, similar to results with tubulin (23). However, the protofilaments also displayed increased bundling at acidic pH, where the GTP hydrolysis is reduced. Again, these results are consistent with the increased bundling reducing the rate of GTP hydrolysis. Lu et al. (14) also suggested that increased bundling was associated with decreased GTPase activity.
We also examined the role of Ca2+ since Yu and Margolin (30) reported that 7 to 25 mM Ca2+ induced dynamic assembly of FtsZ. In our initial studies we did not add Ca2+, assuming it was not required for assembly or the dynamic behavior of FtsZ polymers. The possibility of contaminating Ca2+ influencing the polymerization was ruled out by demonstrating that EGTA had no effect on assembly. Addition of Ca2+ in the millimolar range reduced the dynamic aspect of FtsZ assembly and inhibited the GTPase activity. Interestingly, millimolar concentrations of Ca2+ do not affect the critical concentration, as Yu and Margolin (30) estimated a critical concentration in the range of 2.5 to 3 μM, similar to the critical concentration that we found in the absence of Ca2+. The high concentration of Ca2+ notably increased the amount of light scattered. Inspection of the polymers formed in the presence of 10 mM Ca2+ revealed that Ca2+ dramatically increased bundling of protofilaments, leading to very large bundles. At these high concentrations Ca2+ must promote lateral associations between protofilaments, causing bundling much like DEAE-dextran-induced assembly of FtsZ (19). The bundling of FtsZ protofilaments by polycations is just one example of the bundling of charged biopolymers by polycations (27).
We suspect that the effects of Ca2+ are unlikely to be physiologically relevant because of the high concentration (>2.5 mM) required for Ca2+ to effect FtsZ polymerization compared to the estimated free Ca2+ concentration of 0.1 to 1.0 μM in vivo (10). Therefore, in vivo Ca2+ is unlikely to influence FtsZ assembly directly, although we cannot rule out some indirect role, for example, through an effect on another protein that regulates FtsZ assembly. The failure of Yu and Margolin (30) to observe polymerization in vitro with less than 7 mM Ca2+ is likely due to the assay that they employed; the fluorescence microscopic assay using FtsZ-GFP probably requires large structures that are relatively stable for visualization. As we have shown here, this is precisely the effects on FtsZ polymerization caused by 10 mM Ca2+.
The very dynamic nature of FtsZ polymers observed in vitro raises questions about how they are stabilized in vivo. For example, Z rings once formed exist for some time before they are used in division (1). Also, Z rings appear to persist in cell division mutants that are blocked after Z-ring assembly (1) or in cells treated with an inhibitor of septal peptidoglycan biosynthesis (24), suggesting that the polymers that make up the ring are stable under these conditions. As we have shown here, the assembly of protofilaments into bundles, however they are induced, decreases the dynamics of FtsZ polymers. Thus, any mechanism that causes association of protofilaments in vivo would contribute to their stability. In analogy with other polymerizing systems, FtsZ polymers likely have stabilizing factors such as cross-linking, nucleating, and capping factors.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant GM29764 from the National Institutes of Health.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addinall S G, Lutkenhaus J. FtsZ spirals and arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–238. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- 3.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 4.Bramhill D, Thompson C M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci USA. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis A, Sage C R, Wilson L, Farrell K W. Purification and biochemical characterization of tubulin from the budding yeast Saccharomyces cerevisiae. Biochemistry. 1993;82:8823–8835. doi: 10.1021/bi00085a013. [DOI] [PubMed] [Google Scholar]
- 6.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 7.Desai A, Mitchison T J. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Erickson H P, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to alpha/beta and gamma tubulin. J Cell Biol. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson H P, Taylor D W, Taylor K A, Bramhill D. Bacterial cell division protein FtsZ assembles into protofilament sheet and minirings, structural homologs of tubulin polymers. Proc Natl Acad Sci USA. 1996;93:519–523. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangola P, Rosen B P. The maintenance of intracellular calcium in Escherichia coli. J Biol Chem. 1987;262:12570–12574. [PubMed] [Google Scholar]
- 11.Gaskin F, Cantor C R, Shelanski M L. Turbidimetric measurements of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974;89:737–758. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- 12.Korn E D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982;62:672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- 13.Lowe J, Amos L. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 14.Lu C, Stricker J, Erickson H P. FtsZ from Escherichia coli, Azotobacter vinelandii, and Thermotoga maritima-quantitation, GTP hydrolysis and assembly. Cell Motil Cytoskel. 1998;40:71–86. doi: 10.1002/(SICI)1097-0169(1998)40:1<71::AID-CM7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Lutkenhaus J. FtsZ ring in bacterial cytokinesis. Mol Microbiol. 1993;9:404–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 16.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee A, Cao C, Lutkenhaus J. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in E. coli. Proc Natl Acad Sci USA. 1998;95:2885–2890. doi: 10.1073/pnas.95.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee A, Dai K, Lutkenhaus J. E. coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee A, Lutkenhaus J. Purification, assembly, and localization of FtsZ. Methods Enzymol. 1998;298:296–305. doi: 10.1016/s0076-6879(98)98026-0. [DOI] [PubMed] [Google Scholar]
- 22.Nogales E, Wolf S G, Downing K H. Structure of the alphabeta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 23.Olmstead J B, Borissy G G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975;14:2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- 24.Pogliano J, Pogliano K, Weiss D S, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rai S S, Wolff J. Vinblastine-induced formation of tubulin polymers is electrostatically regulated and nucleated. Eur J Biochem. 1997;250:425–431. doi: 10.1111/j.1432-1033.1997.0425a.x. [DOI] [PubMed] [Google Scholar]
- 26.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 27.Tang J X, Janmey P A. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J Biol Chem. 1996;271:8556–8563. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Lutkenhaus J. FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Lutkenhaus J. FtsZ ring: the eubacterial division apparatus conserved in archaebacteria. Mol Microbiol. 1996;21:313–320. doi: 10.1046/j.1365-2958.1996.6421360.x. [DOI] [PubMed] [Google Scholar]
- 30.Yu X-C, Margolin W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997;16:5455–5463. doi: 10.1093/emboj/16.17.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]