Abstract
Introduction
Postoperative delirium (POD) is a common and distressing complication after thoracic surgery. S-ketamine has neuroprotective properties as a dissociative anaesthetic. Emerging literature has indicated that S-ketamine can reduce cognitive impairment in patients with depression. However, the role of S-ketamine in preventing POD remains unknown. Therefore, this study aims to evaluate the effect of intraoperative prophylactic S-ketamine compared with that of dexmedetomidine on the incidence of POD in elderly patients undergoing non-cardiac thoracic surgery.
Methods and analysis
This will be a randomised, double-blinded, placebo-controlled, positive-controlled, non-inferiority trial that enrolled patients aged 60–90 years undergoing thoracic surgery. The patients will be randomly allocated in a ratio of 1:1:1 to S-ketamine, dexmedetomidine or normal saline placebo groups using computer-generated randomisation with a block size of six. The primary outcome will be the incidence of POD within 4 days after surgery and this will be assessed using a 3-Minute Diagnostic Confusion Assessment Method two times per day. The severity and duration of POD, the incidence of emergence delirium, postoperative pain, quality of sleep, cognitive function, and the plasma concentrations of acetylcholine, brain-derived neurotrophic factor, tumour necrosis factor-α and incidence of adverse events will be evaluated as secondary outcomes.
Ethics and dissemination
Ethical approval has been obtained from the Institutional Review Board of the Cancer Hospital and the Institute of Guangzhou Medical University (ZN202119). At the end of the trial, we commit to making a public disclosure available, regardless of the outcome. The public disclosure will include a publication in an appropriate journal and an oral presentation at academic meetings.
Trial registration number
ChiCTR2100052750 (NCT05242692).
Keywords: delirium & cognitive disorders, geriatric medicine, thoracic surgery
Strengths and limitations of this study.
In this randomised controlled trial, we will evaluate, for the first time, the prophylactic effect of S-ketamine on postoperative delirium in elderly patients undergoing non-cardiac thoracic surgery.
Methodology strengths of this non-inferiority study involve placebo and positive comparators, concealed assignment, blinded assessment and representative sample size.
It is a pragmatic trial that will occur in a real-world setting with standardised anaesthetic management. Moreover, the study team is equipped with a rich experience in postoperative neurocognitive function assessment.
This is a single-centre study that exclusively involves thoracic surgery; therefore, the generalisability may not be extrapolated.
An anticipated non-inferiority margin ratio of 1.5 in our trial may be too large, and consequently, the sample size may be underestimated.
Introduction
Postoperative delirium (POD) is a neuropsychiatric disorder in elderly patients, manifested as an acute onset of altered and fluctuating consciousness, inattention and disorganised thinking. POD occurs in hospital up to 1 week postoperatively or until discharge (whichever occurs first), and typically the highest incidence is observed during the first 72 hours.1 The incidence of POD varies between 4% and 60%, depending on the age and surgical type, although its incidence is underestimated since the hypoactive subtype is not well appreciated.2–7 POD is associated with prolonged hospital stay, long-term cognitive and social dysfunction, and even death.8–10 The 1-year survival probability is reduced by approximately 10% for each additional day of POD.11 The pathophysiological mechanisms of delirium have not been well elucidated, and neuroinflammation remains a topic of mainstream research interest. Furthermore, its development results from the complicated interaction of multifactorial risks, such as pain, opioids, sleep deprivation and inflammation, which poses a challenge for the prevention and treatment of POD.12 Although various techniques, including multicomponent non-pharmacological interventions, are suggested to reduce the risks, there are limited pharmacological methods to reduce the incidence of delirium.13
Dexmedetomidine is a highly selective α-2 adrenergic receptor agonist that is associated with sedative, sympatholytic and anti-inflammatory effects, and has the highest-ranking possibility of preventing POD in a recent network meta-analysis.10 Furthermore, the plausibility of dexmedetomidine’s positive effects on POD is enhanced by evidence of less anticholinergic activity and opioid-sparing properties.14 Postoperative prophylactic low-dose dexmedetomidine could remarkably reduce the incidence of delirium during 7 days after non-cardiac surgery15; moreover, perioperative infusion of dexmedetomidine halved the incidence of delirium in the elderly after major cardiac and non-cardiac surgery without the increase in adverse effects.16 17 A randomised controlled trial (RCT) found that intraoperative dexmedetomidine did not decrease POD or affect cognitive function in the elderly undergoing major non-cardiac surgery.18 A meta-analysis including 11 RCTs revealed that perioperative dexmedetomidine reduced the incidence of POD in elderly patients after non-cardiac surgery, but this came at the cost of an increased incidence of hypotension and bradycardia.19 A meta-analysis of 1301 patients undergoing cardiac surgery revealed that dexmedetomidine decreased POD.20 Nevertheless, this meta-analysis should be interpreted with caution because several of the included studies did not consider delirium as the primary outcome, the methodology of delirium assessment varied and dexmedetomidine administration was also inconsistent, with differing doses and duration. Furthermore, the finding that dexmedetomidine prevents POD is also controversial. In the DECADE trial, continuous infusions of dexmedetomidine, started at induction and maintained for 24 hours, failed to reduce delirium in patients recovering from cardiac surgery. Notably, dexmedetomidine non-significantly aggravated delirium, probably mediated by hypotension.21 However, the plausibility that dexmedetomidine prevents POD should be discussed separately, because physiopathology and incidence of delirium are quite different between non-cardiac surgery and cardiac surgery (frequent cerebral embolism). The heterogeneous ways that dexmedetomidine is administrated (preoperative or postoperative or both, bolus, continuous, etc) also complicated the analysis even more. As with all pharmacological treatment options, the side effects of dexmedetomidine are bradycardia and hypotension in a dose-dependent manner, and more strikingly in the elderly; hence, close haemodynamic monitoring is warranted.
Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, is pharmacologically rationalised as an effective medication for reducing POD, probably due to its neuroprotective properties. Under surgical conditions, the enhanced AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/NMDA signalling, caused by the activation of cytokine receptors, and high mobility group box 1 facilitate an increased influx of glutamate in hippocampal neurons, which ultimately promotes glutamate toxicity.22 Ketamine can mitigate neuronal apoptosis by inhibiting the activation of NMDA receptors and the transduction of excitatory signals.23 The assumption of ketamine’s beneficial effects on delirium is also strengthened by evidence of its opioid-sparing and antidepressant effects. Depression and delirium, induced by similar pathophysiological mechanisms, are thought to overlap.24 25 A small sample size of an RCT indicated that a low-dose single bolus of ketamine at induction significantly attenuated delirium after cardiac surgery. However, the PODCAST Study showed that low-dose ketamine failed to decrease POD, pain and opioid consumption, and generated a dose-dependent increase in the occurrence of negative experiences.26 The PRIDe Study offered no possibility for ketamine to prevent postoperative cognitive decline, including delirium.27 Ketamine remains an off-label treatment for POD with factors that limit widespread use including its dissociative effects and abuse potential.
S-ketamine is the S(+) enantiomer of ketamine, which has a higher affinity with aspartate receptor and µ-opioid receptor. The anaesthetic potency of S-ketamine is twofold higher than that of racemic ketamine, and it has higher in vivo clearance rate characterised by lower incidence of adverse reactions.28 Animal experiments showed that S-ketamine, rather than racemic ketamine, could alleviate the injury of hippocampal neurons exposed to glutamate in rodents; a subanaesthetic dose of S-ketamine could remarkably mitigate neuroinflammation by inhibiting microglia proliferation and TLR4/NF-ĸB signalling pathway activation, which consequently improved neurocognitive function.29 30 Additionally, S-ketamine could promote the plasticity of hippocampal neurons and improve the function of neurons in the prefrontal and hippocampal neural circuits.31 A study on healthy volunteers showed that S-ketamine exhibited pro-neuroplastic effects on hippocampal structure, which may improve cognitive function after surgery.32 Moreover, a recent study on human metabolome revealed that S-ketamine decreases the levels of circulating branched chain amino acids which inhibit the synthesis and release of serotonin and norepinephrine in the brain. Thus, S-ketamine could, in theory, increase the effects of serotonin and norepinephrine in the brain, and contribute to the improvement of depression and cognitive impairment.33 Furthermore, we hypothesise that the sympathomimetic and analgesic properties of S-ketamine might partially explain its non-inferior property for delirium prevention compared with dexmedetomidine. Though S-ketamine has stronger potency and lower incidence of adverse reactions, the evidence that it reduces the incidence of POD is fairly insufficient.
Since the effects of S-ketamine on POD lack good-quality evidence, we designed the current prospective, randomised, double-blinded, placebo-controlled and positive-controlled, non-inferiority trial to investigate the effect of intraoperative prophylactic S-ketamine on POD in elderly patients undergoing thoracic surgery compared with dexmedetomidine.
Methods
Study setting and design
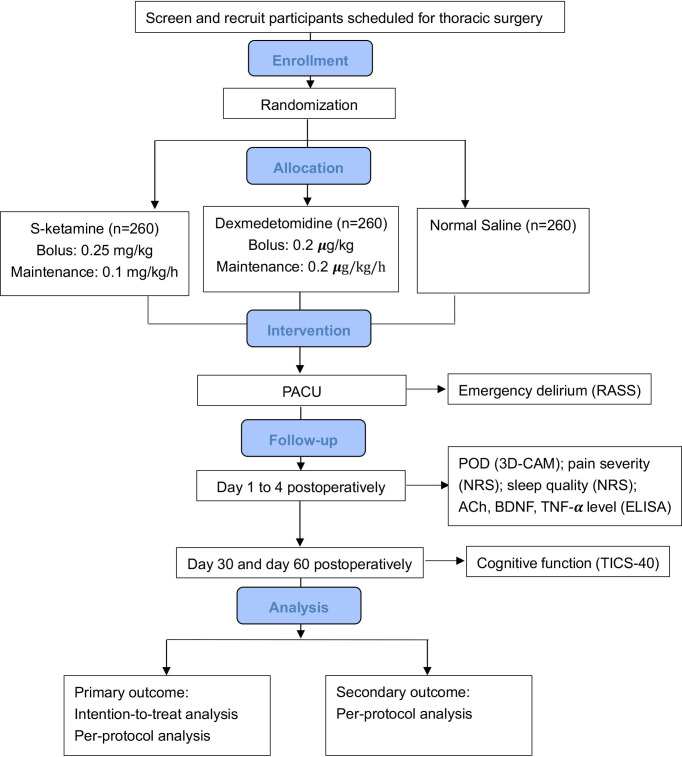
This study will be conducted at the Cancer Hospital and Institute of Guangzhou Medical University (Guangzhou, Guangdong, China, with principal investigator (PI) YY). The study activities are expected to commence in March 2022 and be completed in December 2023. The study design is in accordance with the standard protocol items for randomised trials guidelines. The overall schedule is illustrated in table 1, and the Consolidated Standards of Reporting Trials flow diagram is shown in figure 1. The current study protocol is the fifth version.
Table 1.
Schedule of enrolment, interventions and assessments for the trial
| Enrolment | Allocation | Post-allocation | Close-out | ||||||||
| Preoperative assessment | Allocation | Before induction | Recovery | 4 hours after surgery | 24 hours after surgery | 48 hours after surgery | 72 hours after surgery | 96 hours after surgery | 30 days after surgery | 60 days after surgery | |
| Time point | −T1 | T0 | T1 | T2 | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
| Enrolment | |||||||||||
| Eligibility screen | X | ||||||||||
| Informed consent | X | ||||||||||
| Allocation | X | ||||||||||
| Interventions | |||||||||||
| S-ketamine |

|
||||||||||
| Dexmedetomidine |

|
||||||||||
| Normal saline |

|
||||||||||
| Assessments | |||||||||||
| Postoperative delirium (3D-CAM) | X | X | X | X | X | ||||||
| Pain severity (NRS) | X | X | X | ||||||||
| Sleep quality (NRS) | X | X | X | X | |||||||
| Cognitive function (TICS-40) | X | X | |||||||||
| Haemodynamic variables |

|
||||||||||
| Emergence delirium (RASS) | X | ||||||||||
| Plasma biomarkers (ACh, BDNF, TNF-α) | X | X | X | ||||||||
ACh, acetylcholine; BDNF, brain-derived neurotrophic factor; 3D-CAM, 3-Minute Diagnostic Confusion Assessment Method; NRS, Numerical Rating Scale; RASS, Richmond Agitation-Sedation Scale; TICS-40, Telephone Interview for Cognitive Status-40; TNF-α, tumour necrosis factor-α.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. 3D-CAM, 3-Minute Diagnostic Confusion Assessment Method; ACh, acetylcholine; BDNF, brain-derived neurotrophic factor; NRS, Numerical Rating Scale; PACU, post-anaesthesia care unit; POD, postoperative delirium; RASS, Richmond Agitation-Sedation Scale; TICS-40, Telephone Interview for Cognitive Status-40; TNF-α, tumour necrosis factor-α.
Participant recruitment
Inclusion criteria
Aged 60–90 years old.
Both sexes.
American Society of Anesthesiologists (ASA) physical status classification I–III.
Diagnosed with pulmonary, oesophageal or mediastinal disorders.
Undergoing open or video-assisted thoracic surgery, including lobectomy, segmentectomy, pneumonectomy, oesophagectomy or resection of the mediastinal tumour.
General anaesthesia with one-lung ventilation (OLV) or bronchial blocker.
Expected operation duration of 2 hours or more.
Voluntary participation in the trial and signed informed consent.
Exclusion criteria
History of severe psychiatric disease.
History of glaucoma or hyperthyroidism.
History of severe hepatic (Child-Pugh grade C) or renal (requirement for renal replacement therapy) disorders.
Body mass index (BMI) ˃35 kg/m2.
Dementia history or baseline Mini-Mental State Examination (MMSE) score of <23.
Severe audio-visual impairments, or inability to speak Mandarin or Cantonese precluding communication.
Sinus bradycardia (heart rate (HR) <50 beats per minute (bpm)), sick sinus or Wolff-Parkinson-White syndrome, or second-degree atrioventricular block and over.
Uncontrolled hypertension (baseline value >200/110 mm Hg).
Allergic to dexmedetomidine or S-ketamine.
Taking sedatives, antidepressants or glucocorticoids.
Alcohol or illicit drug misuse disorder.
Life expectancy of less than 2 months due to extensive tumour metastasis.
Participants’ consent
All patients scheduled for thoracic surgery will be screened 1 day before the operation for eligibility at the preoperative evaluation clinic (or on Friday for those who will undergo surgery the following Monday). Eligible patients will be informed by the study team coordinator. For the sake of voluntary participation, all patients will be informed about the aims, procedures, benefits, possible risks of study and how to react if risks occur. If interested in enrolment, the patients or their next of kin will sign the written consent form in triplicate.
Randomisation and blindness
A randomisation code will be generated in a block size of six on the website of http://www.Randomization.com and kept in a sealed opaque envelope by an anaesthetist nurse. The patients will be randomly allocated by a ratio of 1:1:1 to the S-ketamine group (S group), dexmedetomidine group (D group) or control group (C group) by an anaesthetist nurse. Dispensing and labelling of the study drugs will be performed by a pharmacist. Both the anaesthetist nurse and the pharmacist will not be involved in the following research or follow-up. The randomisation protocol will be kept secure by the anaesthetist nurse. The primary investigator, and the clinicians collecting the data, are allowed to unmask the randomisation protocol only when both recruitment and the database are closed.
The labelled ‘study medication’ syringes (50 mL), identical in appearance, and the infusion regimen formulated by the pharmacist based on the randomisation, will be distributed to the attending anaesthesiologists responsible for anaesthetic management as soon as the research team informs the central pharmacy about the patient heading for surgery. In order to avoid anaesthesiologists’ speculation about the randomised assignment, the study drugs will be infused at the same rate (see table 2). The anaesthesiologists, patients, investigators responsible for follow-up and statisticians will be all blinded to the randomised allocations until the final statistical analyses are completed. The blindness will be unmasked by the primary investigator in a medical emergency, including deterioration of the patient’s condition intraoperatively or adverse events (AEs) postoperatively.
Table 2.
Study drugs and administrative protocol (take a 60 kg patient as an example)
| Group | Concentration | Loading dose | Maintenance dose |
| S-ketamine | 1 mg/mL | 0.25 mg/kg | 0.1 mg/kg/hour |
| ie, the administrative protocol of a 60 kg patient will be a loading dose of 15 mL and a maintenance dose of 6 mL/hour | |||
| Dexmedetomidine | 2 µg/mL | 0.2 µg/kg | 0.2 µg/kg/hour |
| ie, the administrative protocol of a 60 kg patient will be a loading dose of 15 mL and a maintenance dose of 6 mL/hour | |||
| Control | Normal saline | — | — |
| ie, the administrative protocol of a 60 kg patient will be a loading dose of 15 mL and a maintenance dose of 6 mL/hour | |||
Standard anaesthetic management
On the day of the operation, the patients will be admitted to the operating room after random assignment. Vital signs will be routinely monitored, including HR, blood pressure (BP), oxyhaemoglobin saturation by pulse oximetry (SpO2), end-tidal carbon dioxide partial pressure (EtCO2), nasopharyngeal temperature and urine output throughout surgery. Pre-oxygenation with 100% oxygen for 15 min before the induction of anaesthesia will be delivered to the patient using a face mask. Atropine will be administered intravenously in avoidance of excessive secretions.
After arterial line and central venous line are cannulated under ultrasound guidance, anaesthesia induction will be performed by administration of midazolam (0.05 mg/kg), propofol (1–2 mg/kg) or etomidate (0.2 mg/kg), and sufentanil (0.2–0.4 µg/kg). After the patient becomes unconscious, rocuronium (0.6 mg/kg) will be injected intravenously. Bronchial intubation will be performed smoothly with a video laryngoscope after 3-minute positive pressure ventilation. The tip of double lumen tubes (DLTs) will be inserted into the glottis under direct vision and advanced until a mild resistance is perceived. After the fibreoptic bronchoscope is fully lubricated, it will be advanced into the tracheal lumen of the DLTs until the carina is identified. Afterwards, the ideal position of the bronchial lumen (the blue bronchial cuff should be invisible for left DLTs, the opening in the upper lobe of the right lung should be visible for right DLTs) will be verified. Dual-controlled ventilator modes (ie, pressure-controlled ventilation with volume guaranteed or pressure-regulated volume control) will be applied. One-lung protective ventilation regimen will be conducted by a combination of tidal volumes of 6 mL/kg or lower, by predicted body weight, with a positive end-expiratory pressure of 6 cmH2O or beyond based on guidelines and expert opinion for optimal practice during OLV.34 High inspiratory fractions of oxygen (>70%) will be administered to maintain SpO2 higher than 94%. In addition, continuous positive airway pressure regimen will be considered when necessary. The respiratory rate will be adjusted to maintain EtCO2 at 35–45 mm Hg. Sedative maintenance will be performed with a TCI (target-controlled infusion) of propofol according to the Schnider model at a plasma concentration (Cp) of 2–3 µg/mL to maintain the bispectral index (BIS) value between 40 and 60. Analgesic maintenance will be achieved with a TCI of remifentanil according to the Minto model at a Cp of 1–6 ng/mL to fluctuate the HR and BP within the baseline value ±20%. An intermittent bolus of rocuronium will be administered to maintain Train of four (TOF) <1 intraoperatively. Forced-air warm blankets will be used to ensure an intraoperative body temperature of 36°C–37°C. The surgeon will implement an intercostal nerve block (T3–7) with 20 mL of 0.5% ropivacaine under direct thoracoscopic view before placing a chest tube. The sign of a successful block is the presence of pleural displacement. All participants will be given hydromorphone (0.015 mg/kg) when a chest tube is placed for the sake of prophylaxis of hyperalgesia.
A patient-controlled analgesia (PCA) device, with hydromorphone (0.15 mg/kg) and ondansetron (12 mg) in a total volume of 100 mL, will be connected to the intravenous cannula at the end of surgery. The device is programmed to administer a background dose of 2 mL/hour, as well as a bolus dose of 0.5 mL with a lockout interval of 15 min for 48 hours. Hydromorphone (0.008 mg/kg) will be administered if the Numerical Rating Scale (NRS) score is >5 despite the PCA regimen. Residual neuromuscular blockade will be routinely reversed with neostigmine (40 µg/kg) and atropine (20 µg/kg), and the endotracheal tube will be removed when the patients are able to follow verbal commands.
Study drugs’ administration
S-ketamine (50 mg, 2 mL) is diluted to 50 mL (1 mg/mL) with 48 mL normal saline; dexmedetomidine (200 µg, 2 mL) is diluted to 100 mL (2 µg/mL) with 98 mL normal saline; the control group only receives 50 mL normal saline in light of blindness. All drugs are identical in appearance, packaged in identical 50 mL syringes labelled with ‘study medications’. The loading dose of study drugs will be infused within 10 min before induction, and the maintenance dose will be infused at a constant rate continuously until skin closure. In the preliminary trial, we found that a loading dose of 0.4 µg/kg dexmedetomidine led to obvious bradycardia and transient hypertension events. Therefore, we modified the loading dose of dexmedetomidine to 0.2 µg/kg. In addition, in order to ensure blindness, the infusion speed of dexmedetomidine is consistent with that of S-ketamine, which also reduces the side effects of dexmedetomidine. The detailed administrative protocol of study drugs is shown in table 2.
Data collection
The following data will be collected through patient interviews and abstractions from the electronic medical record system:
Preoperative data collection
Patient demographic data including age (years), sex, height (cm), weight (kg), BMI (kg/m2) and education level (years).
ASA classification, Charlson Comorbidity Index, baseline MMSE and type of surgery.
Plasma biomarker concentrations including acetylcholine (ACh), brain-derived neurotrophic factor (BDNF) and tumour necrosis factor-α (TNF-α) before the administration of study drugs (T1).
Intraoperative data collection
Haemodynamic parameters including HR (bpm), mean arterial pressure (mm Hg), SpO2 and BIS value at 15-minute intervals.
Hypotension and bradycardia episodes (see table 3).
Hypertension and tachycardia episodes (see table 3).
Duration of desaturation (SpO2 <94%, min).
The cumulative dosage of norepinephrine (µg) and atropine (mg).
The consumption of propofol (mg) and opioids (converted to morphine milligram equivalent (MME) by Global RPH).
Surgery, anaesthesia and OLV duration (min).
Time to extubation (min, duration from discontinuation of propofol to removal of the tracheal tube).
Emergence agitation (Richmond Agitation-Sedation Scale (RASS) score ≥1).
Plasma biomarker concentrations at the end of operation (T2).
Table 3.
The definitions of adverse events and corresponding medication rescue
| Adverse events | Severity | Definition | Treatment |
| Hypotension (SBP <90 mm Hg or DBP <50 mm Hg or MAP <80% baseline) | Mild Moderate Severe Life-threatening |
SBP 80–89 mm Hg SBP 70–79 mm Hg >2 min SBP 60–69 mm Hg >1 min SBP 60–69 mm Hg and unresponsive to norepinephrine or SBP <60 mm Hg |
Close monitoring Norepinephrine 4 µg* Norepinephrine 8 µg† Intensive intervention and suspension of the study |
| Hypertension (SBP >140 mm Hg or DBP >90 mm Hg or MAP >120% baseline) | Mild Moderate Severe Life-threatening |
SBP 141–160 mm Hg or DBP 91–100 mm Hg SBP 160–170 mm Hg or DBP 101–110 mm Hg >3 min SBP 171–180 mm Hg or DBP 111–120 mm Hg >2 min SBP >180 mm Hg or DBP >120 mm Hg and unresponsive to NG |
Close monitoring Urapidil 12.5 mg Urapidil 25 mg or NG 50 µg Intensive intervention and suspension of the study |
| Bradycardia (HR <60 bpm) | Mild Moderate Severe Life-threatening |
HR 55–60 bpm HR 50–54 bpm >3 min HR 40–50 bpm >2 min HR <40 bpm and unresponsive to atropine |
Close monitoring Atropine 0.5 mg Atropine 1.0 mg Intensive intervention and suspension of the study |
| Tachycardia (HR <60 bpm) | Mild Moderate Severe Life-threatening |
HR 90–100 bpm HR 101–110 bpm >3 min HR 111–130 bpm >2 min HR >130 bpm and unresponsive to esmolol |
Close monitoring Esmolol 20 mg Esmolol 40 mg Intensive intervention and suspension of the study |
| Hypoxaemia (SpO2 <94%) | Mild Moderate Severe Life-threatening |
SpO2 90%–94% SpO2 80%–90% >3 min SpO2 70%–79% >2 min SpO2 <70% and unresponsive to two-lung ventilation |
Close monitoring CPAP Two-lung ventilation Intensive intervention and suspension of the study |
| Emergence delirium | Mild Severe |
RASS 1–2 RASS 3–4 |
Limb restraint Propofol 30 mg |
| Hallucination/nystagmus | NA | 3D-CAM | Haloperidol 10 mg |
*Followed by continuous infusion with 0.01–0.1 µg/kg/min when necessary.
†Followed by continuous infusion with 0.1–0.2 µg/kg/min when necessary.
bpm, beats per minute; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; 3D-CAM, 3-Minute Diagnostic Confusion Assessment Method; HR, heart rate; MAP, mean arterial pressure; NA, not applicable; NG, nitroglycerine; RASS, Richmond Agitation-Sedation Scale; SBP, systolic blood pressure; SpO2, oxyhaemoglobin saturation by pulse oximetry.
Postoperative data collection
Incident POD between 4 hours after surgery and the 4th postoperative day, and two times per day from postoperative day 1 to postoperative day 4 (08:00–10:00) with an interval of at least 6 hours.
Severity and duration of delirium.
Postoperative pain at 4 hours, 1 and 2 days after surgery.
Consumption of hydromorphone (mg).
Quality of sleep within 4 days after surgery.
Cognitive function at 30 and 60 days after surgery.
Plasma biomarker concentrations at the fourth day after surgery (T3).
The Data Safety and Monitoring Board (DSMB) is consist of three senior anaesthesiologists and one surgeon who are blinded to the study. The DSMB will provide independent oversight of the SKED trial and will review the study data for the participant safety as well as case report form (CRF) storage. The data will be entered into the EpiData V.4.6 database protected by password only accessible to the DSMB. Then, the data will be exported from EpiData database to a statistical package for analysis by biostatisticians independent of the study.
Outcomes
Primary outcomes
The primary outcome will be the incidence of POD as defined by any positive assessment between 4 hours after surgery and the 4th postoperative day.
Secondary outcomes
The main secondary outcome will be the subtype, severity and duration of POD. Other prespecified secondary outcomes will be the incidence of emergence delirium; pain severity at 4 hours, 1 and 2 days after surgery; quality of sleep within 4 days after surgery; cognitive function at 30 and 60 days after surgery; plasma biomarker (ACh, BDNF, TNF-α) concentrations at T1–3; and incidence of AEs.
Measurement of outcomes
Measurement of delirium
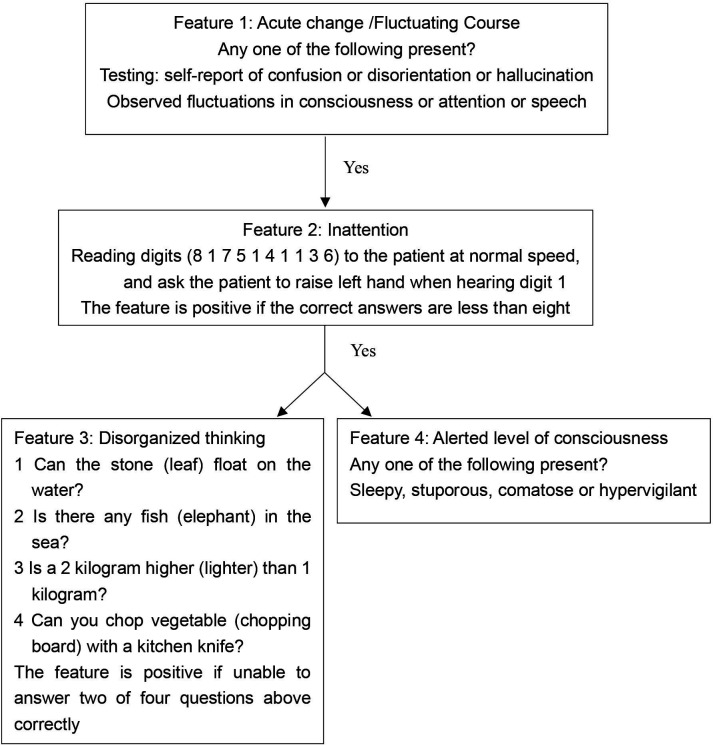
Delirium will be assessed using a validated 3-Minute Diagnostic Confusion Assessment Method (3D-CAM Chinese version, with a sensitivity of 84%–99% and specificity of 90%–97%)35 36 or Confusion Assessment Method for the Intensive Care Unit (CAM-ICU), for patients who have a tracheal tube or underwent tracheostomy.37 The 3D-CAM resolves the four diagnostic features of delirium: (1) acute onset and fluctuating course, (2) inattention, (3) disorganised thinking and (4) altered level of consciousness. A patient who displays both features 1 and 2, with either feature 3 or 4, will be diagnosed with delirium (see figure 2).35 Delirium assessments will be performed only when patients can be aroused sufficiently with a RASS score of −3 to 4 (online supplemental table 1). Patients with POD will be classified into three subtypes. Hyperactive delirium will be defined when the RASS score ranges from 1 to 4; hypoactive delirium will be defined when the RASS score ranges from −1 to −3; and mixed delirium will be defined when the RASS score ranges from 1 to 4 and −1 to −3 alternatively. The severity of POD will be rated using the CAM-Severity Short-Form Scale (CAM-S) (online supplemental table 2). Mild-to-moderate delirium will be defined as a CAM-S score of 3–5, while severe delirium will be defined as a CAM-S score of 6–7.38
Figure 2.
Overview of 3-Minute Diagnostic Confusion Assessment Method assessment.
bmjopen-2022-061535supp001.pdf (52.2KB, pdf)
Four investigators who are not involved in perioperative care will be responsible for POD assessments and will be trained by a psychiatrist with regard to symptoms, diagnosis and treatment of delirium. Furthermore, the psychiatrist will explain the protocols of 3D-CAM and CAM-ICU in detail, and will perform the simulation training of delirium assessment until a κ value over 0.8 is achieved between investigators and psychiatrists. The training process will be repeated every 4–6 months throughout the study. In addition, the chart-based delirium identification instrument with the information primarily derived from electronic medical records system and recalling descriptions of caregivers will be employed to detect any cases of delirium in patients that may occur outside of in-person delirium assessments (online supplemental table 3).39
Pain and sleep quality measurement
Postoperative pain at rest and during a cough will be evaluated using an 11-point NRS (0=no pain, 0<NRS<4 (mild pain), 4≤NRS<7 (moderate pain), 7≤NRS<10 (severe pain), NRS=10 (worst pain imaginable)). Postoperative sleep quality will also be evaluated using the NRS (0=best-quality sleep, 10=worst-quality sleep).
Cognitive function measurement
Postoperative cognitive function will be assessed using the Chinese version of the Telephone Interview for Cognitive Status-40 (TICS-40). The TICS-40 scale used in this study consists of nine items with a maximum score of 40 points, including the following variables and corresponding points: address (3 points), current date (5 points), counting backwards (2 points), word-list recalling (10 points), subtractions (5 points), object naming (2 points), repetition (1 point), the president’s and prime minister’s names (2 points), and delayed recall of the word list (10 points). A score below 21 will be defined as mild cognitive impairment (online supplemental table 4).40
Biomarker concentration measurement
Venous blood (approximately 6 mL) will be sampled and stored on ice in vacutainer tubes containing EDTA. Within 30 min, the samples will be centrifuged at 4°C for 20 min at 2000× g to obtain plasma and then stored at −80°C. We will measure ACh, BDNF and TNF-α levels by ELISA method (in accordance to manufacturer’s instructions). The biomarker assay will be performed by a specialist who is blinded to the randomisation (online supplemental text for the rationale of biomarkers selected).
bmjopen-2022-061535supp002.pdf (19.9KB, pdf)
Adverse events
An AE can be any unfavourable and unintended symptom or side effect temporally associated with the use of study medications. The potential AEs that may be considered in this trial are bradycardia, hypotension, tachycardia, hypertension, arrhythmia, nystagmus, hypersalivation, euphoria, emergence agitation, hallucinations and nightmares. It is possible, but very unlikely, that low-dose S-ketamine (50 mg in total) administered intraoperatively will cause these psychiatric effects. Potential AEs and medical rescue are shown in table 3.41
Serious AEs are rare, life-threatening events that may be associated with the study drugs or perioperative incidents, such as death or serious cardiocerebral vascular events.
Sample size calculation
The sample size was calculated for the main outcome, the incidence of POD, using PASS software V.11.0. Based on previous studies and our recently completed data, we estimated that the incidence of POD in elderly patients undergoing non-cardiac thoracic surgery was 40%.12 42–46 Assuming that dexmedetomidine is associated with a 40% relative reduction in the incidence of POD, the non-inferiority margin rate ratio (RR) of S-ketamine versus dexmedetomidine will be set at 1.5.15 27 47 48 To achieve a two-sided type I error of 5% and 80% power, 729 participants (243 patients per arm) will be recruited. To accommodate a 5% dropout rate, the final sample size will be 780 (260 patients per arm).
Statistical methods
Kolmogorov-Smirnov test will be used to evaluate the normal distribution of continuous variables. Normally distributed data will be presented as means±SD, and non-normally distributed data will be presented as medians with IQRs. Categorical data will be summarised as counts (proportions).
The absolute standardised difference (ASD) will be used for the comparison of baseline data among the three groups, that is, the absolute difference in means, mean ranks or proportions divided by the combined SD. Baseline variables with ASD >0.013 (ie, 1.96 ) are considered to be imbalanced and will be adjusted for in all analyses when necessary.
For the primary outcome, the incidence of POD, the intention-to-treat approach and per-protocol (PP) approach will be performed. Pearson’s Χ2 test will be applied to compare proportions with the primary outcome among groups. The difference among groups will be expressed as RR and 95% CI, while non-inferiority will be identified if the upper limit of 95% CI of RR is <1.5. For the secondary outcomes, only the PP approach will be used. Normally distributed data will be analysed with one-way analysis of variance (ANOVA); non-normally distributed data will be analysed with Kruskal-Wallis test. The median difference will be calculated using the Hodges-Lehmann estimation on the basis of the Kruskal-Wallis test. AEs that are presented as incidences will be compared by calculating the 95% CI of the incidence difference: incidence (S group)–incidence (D group), and non-inferiority will be achieved if the upper limit of 95% CI is <5%. The superiority for outcomes will be assessed when non-inferiority is verified.
To account for correlation among repeated measurements, such as NRS scores for pain and sleep quality, plasma biomarker concentrations and cognitive function, will be compared using generalised estimating equation analysis among groups. The time to delirium will be calculated with the Kaplan-Meier estimator, and the differences among groups will be assessed by the log-rank test. The number needed to treat will be estimated for the primary outcome.
Missing values will be adjusted using random forest imputation in the missForest package. However, missing values, due to fatigue in the assessment or the patient’s inability to cooperate, will be imputed with positive results or means in the corresponding treatment group and time point. If the patient did not have a delirium assessment at all (eg, dropout or death), no values will be imputed. The last assessment is used to replace the missing value to estimate the incidence of POD in patients who are discharged or die within 4 days, while the missing value of assessment per day does not need to be replaced.
The p values and CIs reported from one-way ANOVA and Kruskal-Wallis test will be considered to illustrate statistical significance if they are less than 0.017% and 98.3%, respectively, accounting for three pairwise comparisons. The family-wise significance and CI levels among the three groups will be set at 0.05% and 95%, respectively. For the pain intensity score, a 1.1 decrease will be considered the minimal clinically important difference.49
Analyses will be conducted using IBM SPSS V.25.0, R statistics V.4.1.2 (R Project for Statistical Computing) and GraphPad Prism V.8.0 (GraphPad Software, San Diego, California, USA).
Ethics and confidentiality
Ethical approval was obtained from the Institutional Review Board (IRB) of the Cancer Hospital and the Institute of Guangzhou Medical University (ZN202119). The study has also been registered at Chictr.org.cn with the identifier ChiCTR2100052750. The personal information of the participants will not be disclosed unless authorisation is approved. In addition, each participant will be provided with a unique identity code, the information of which will be properly secured. The CRF and EpiData database will be retained for a minimum of 10 years.
Patient and public involvement
No patients or public representatives were involved in the design of this trial.
Dissemination
At the end of the trial, we commit to making public disclosure available despite the outcome. Public disclosure will include publication in an appropriate journal or oral presentation at an academic meeting. The PI will be considered the first or corresponding author. The investigators who contribute a minimum of 4 months to the trial will be coauthors; otherwise, they will be acknowledged in the publication.
Discussion
Lung cancer ranks first among all malignancies in China, and anatomical pulmonary resection is a major component of multimodal therapy according to the lung cancer guidelines.12 However, more than 40% of patients undergoing lung cancer surgery are inflicted by severe depression-related psychological suffering postoperatively.50 Depression is an independent predictor of POD in patients who undergo orthopaedic and cancer surgeries.24 Based on its pharmacological mechanisms and antidepressant effects, we speculate that S-ketamine would be non-inferior to dexmedetomidine in reducing POD to some extent in the elderly, with fewer episodes of hypotension or less opioid consumption. Hypotension is pertinent to delirium, and minimisation of intraoperative hypotension episodes is recommended to reduce POD.51 Additionally, the administration of opioids (long-acting opioids in particular) is closely related to POD in a dose-dependent manner. Hence, it is critical to abate opioid consumption in order to curtail delirium.8
Although previous studies have demonstrated that ketamine failed to reduce the incidence of POD in patients undergoing major cardiac or non-cardiac surgery, we will deploy a different administrative protocol to evaluate the effect of an isomer of ketamine on POD accompanied by dexmedetomidine as a positive comparator and by an optimal sample size. Dexmedetomidine is a highly recommended agent in the prevention and treatment of POD; however, it is commonly accompanied by hypotension and bradycardia in the elderly. As the prevention of POD is more practical and effective than the treatment itself, creating a means of prevention for delirium is extraordinarily indispensable. We believe that the possible result will be one of the following: (1) S-ketamine will be non-inferior to dexmedetomidine in the prevention of POD; meanwhile, more stable haemodynamics, lower postoperative pain severity or other beneficial secondary outcomes will be observed with S-ketamine intervention. Side effects will be compared between groups, all of which will be our desirables. This suggests that S-ketamine will be an optimal choice for limiting delirium emergence in the elderly, and further studies should be performed to evaluate its effect on long-term cognitive function. (2) S-ketamine will be non-inferior to dexmedetomidine in POD prevention with comparable secondary outcomes; however, it will be accompanied by frequent side effects. This indicates that S-ketamine will be clinically valueless for delirium prevention, which is also possible in view of the results from previous studies on ketamine (PODCAST and PRIDe Study). (3) S-ketamine will be inferior to dexmedetomidine in the prevention of POD, which is probably because dexmedetomidine is recognised as the most effective medication for delirium, and fewer studies have compared the two drugs.
The SKED protocol has many limitations. First, the current trial is launched at special time when inclusion may be constrained by local SARS-CoV-2 pandemic. As such, the research period may take longer than anticipated. Second, this is a single-centre study that exclusively involves thoracic surgery; therefore, the generalisability may not be extrapolated. Third, an anticipated non-inferiority margin ratio of 1.5 in our trial may be too large, and consequently, the sample size may be underestimated. Fourth, a dropout rate of 5% seems a bit low as AEs due to dexmedetomidine may be higher than that, if so, we would enlarge the sample size upon approval from the IRB.
Supplementary Material
Acknowledgments
The authors would like to express sincere thanks for the support of the Department of Anaesthesia and the Division of Thoracic Surgery at the Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, Guangdong, China.
Footnotes
WW and AZ contributed equally.
Correction notice: This article has been corrected since it first published. Author 'Yu Gu' has been added as the corresponding author.
Contributors: WW, AZ, XZ, MZ and YY participated in the study design. WW, AZ and XZ co-drafted the protocol manuscript. YG and YY contributed to sample size calculation and statistical consultation. WW and XZ developed the case report forms and the EpiData database. WW, LL and CT conducted the preliminary trial. YY served as the primary investigator and provided the funding. All authors completed 3D-CAM and CAM-ICU training courses and obtained good clinical practice certificates.
Funding: This study is funded by the Cancer Hospital and Institute of Guangzhou Medical University 5555 project and Guangdong Provincial Medical Science and Technology Foundation (grant no. B2019035).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018;121:1005–12. 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin 2015;33:505–16. 10.1016/j.anclin.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015;220:136–48. 10.1016/j.jamcollsurg.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 4.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202–11. 10.1213/ANE.0b013e3182147f6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin CA, O'Gorman T, Stern E, et al. Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg 2019;154:328–34. 10.1001/jamasurg.2018.5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Lei L, Ji M, et al. Predicting postoperative delirium after microvascular decompression surgery with machine learning. J Clin Anesth 2020;66:109896. 10.1016/j.jclinane.2020.109896 [DOI] [PubMed] [Google Scholar]
- 7.Migirov A, Chahar P, Maheshwari K. Postoperative delirium and neurocognitive disorders. Curr Opin Crit Care 2021;27:686–93. 10.1097/MCC.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindroth H, Bratzke L, Purvis S, et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open 2018;8:e019223. 10.1136/bmjopen-2017-019223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S-K, Lim T, Cho H, et al. Comparative effectiveness of pharmacological interventions to prevent postoperative delirium: a network meta-analysis. Sci Rep 2021;11:11922. 10.1038/s41598-021-91314-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisani MA, Kong SYJ, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009;180:1092–7. 10.1164/rccm.200904-0537OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei W, Zheng X, Gu Y, et al. Effect of general anesthesia with thoracic paravertebral block on postoperative delirium in elderly patients undergoing thoracoscopic lobectomy: a randomized-controlled trial. BMC Anesthesiol 2022;22:1–10. 10.1186/s12871-021-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth 2020;125:492–504. 10.1016/j.bja.2020.06.063 [DOI] [PubMed] [Google Scholar]
- 14.Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology 2016;124:362–8. 10.1097/ALN.0000000000000951 [DOI] [PubMed] [Google Scholar]
- 15.Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 16.Li C-J, Wang B-J, Mu D-L, et al. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg 2020;107:e123–32. 10.1002/bjs.11354 [DOI] [PubMed] [Google Scholar]
- 17.van Norden J, Spies CD, Borchers F, et al. The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: a randomised, double-blind placebo-controlled trial. Anaesthesia 2021;76:1342–51. 10.1111/anae.15469 [DOI] [PubMed] [Google Scholar]
- 18.Deiner S, Luo X, Lin H-M, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg 2017;152:e171505. 10.1001/jamasurg.2017.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan H, Liu C, Ma X, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth 2019;66:1489–500. 10.1007/s12630-019-01440-6 [DOI] [PubMed] [Google Scholar]
- 20.Duan X, Coburn M, Rossaint R, et al. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth 2018;121:384–97. 10.1016/j.bja.2018.04.046 [DOI] [PubMed] [Google Scholar]
- 21.Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet 2020;396:177–85. 10.1016/S0140-6736(20)30631-0 [DOI] [PubMed] [Google Scholar]
- 22.Safavynia SA, Goldstein PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatry 2018;9:752. 10.3389/fpsyt.2018.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth 2010;24:131–42. 10.1053/j.jvca.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 24.Elsamadicy AA, Adogwa O, Lydon E, et al. Depression as an independent predictor of postoperative delirium in spine deformity patients undergoing elective spine surgery. J Neurosurg Spine 2017;27:209–14. 10.3171/2017.4.SPINE161012 [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan R, Inouye SK, Meagher D. Delirium and depression: inter-relationship and clinical overlap in elderly people. Lancet Psychiatry 2014;1:303–11. 10.1016/S2215-0366(14)70281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267–75. 10.1016/S0140-6736(17)31467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollinger A, Rüst CA, Riegger H, et al. Ketamine vs. haloperidol for prevention of cognitive dysfunction and postoperative delirium: a phase IV multicentre randomised placebo-controlled double-blind clinical trial. J Clin Anesth 2021;68:110099. 10.1016/j.jclinane.2020.110099 [DOI] [PubMed] [Google Scholar]
- 28.Krystal JH, Charney DS, Duman RS. A new rapid-acting antidepressant. Cell 2020;181:7. 10.1016/j.cell.2020.02.033 [DOI] [PubMed] [Google Scholar]
- 29.Himmelseher S, Pfenninger E, Kochs E, et al. S(+)-ketamine up-regulates neuronal regeneration associated proteins following glutamate injury in cultured rat hippocampal neurons. J Neurosurg Anesthesiol 2000;12:84–94. 10.1097/00008506-200004000-00003 [DOI] [PubMed] [Google Scholar]
- 30.Himmelseher S, Pfenninger E, Georgieff M. The effects of ketamine-isomers on neuronal injury and regeneration in rat hippocampal neurons. Anesth Analg 1996;83:505–12. 10.1097/00000539-199609000-00011 [DOI] [PubMed] [Google Scholar]
- 31.Treccani G, Ardalan M, Chen F, et al. S-ketamine reverses hippocampal dendritic spine deficits in flinders sensitive line rats within 1 h of administration. Mol Neurobiol 2019;56:7368–79. 10.1007/s12035-019-1613-3 [DOI] [PubMed] [Google Scholar]
- 32.Höflich A, Kraus C, Pfeiffer RM, et al. Translating the immediate effects of S-ketamine using hippocampal subfield analysis in healthy subjects-results of a randomized controlled trial. Transl Psychiatry 2021;11:200. 10.1038/s41398-021-01318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nummela AJ, Laaksonen LT, Laitio TT, et al. Effects of dexmedetomidine, propofol, sevoflurane and S-ketamine on the human metabolome: a randomised trial using nuclear magnetic resonance spectroscopy. Eur J Anaesthesiol 2022;39:1097. 10.1097/EJA.0000000000001591 [DOI] [PubMed] [Google Scholar]
- 34.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) Society and the European Society of thoracic surgeons (ESTs). Eur J Cardiothorac Surg 2019;55:91–115. 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 35.Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014;161:554–61. 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu D-L, Ding P-P, Zhou S-Z, et al. Cross-cultural adaptation and validation of the 3D-CAM Chinese version in surgical ICU patients. BMC Psychiatry 2020;20:133. 10.1186/s12888-020-02544-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10. 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 38.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014;160:526–33. 10.7326/M13-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 2005;53:312–8. 10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- 40.Wang J-H, Huang J, Guo F-Q, et al. Circulating neurofilament light predicts cognitive decline in patients with post-stroke subjective cognitive impairment. Front Aging Neurosci 2021;13:665981. 10.3389/fnagi.2021.665981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 2008;26:985–1028. 10.1016/j.ajem.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 42.Humeidan ML, Reyes J-PC, Mavarez-Martinez A, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the Neurobics randomized clinical trial. JAMA Surg 2021;156:148–56. 10.1001/jamasurg.2020.4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung DM, Ahn HJ, Yang M, et al. Hydroxyethyl starch is associated with early postoperative delirium in patients undergoing esophagectomy. J Thorac Cardiovasc Surg 2018;155:1333–43. 10.1016/j.jtcvs.2017.10.077 [DOI] [PubMed] [Google Scholar]
- 44.Fuchita M, Khan SH, Perkins AJ, et al. Perioperative risk factors for postoperative delirium in patients undergoing esophagectomy. Ann Thorac Surg 2019;108:190–5. 10.1016/j.athoracsur.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 45.Smith PJ, Rivelli SK, Waters AM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care 2015;30:126–9. 10.1016/j.jcrc.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249:173–8. 10.1097/SLA.0b013e31818e4776 [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Zhu M, Gao Z, et al. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: a double-blind, randomised clinical trial. Eur J Anaesthesiol 2021;38:S9–17. 10.1097/EJA.0000000000001382 [DOI] [PubMed] [Google Scholar]
- 48.Perbet S, Verdonk F, Godet T, et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med 2018;37:589–95. 10.1016/j.accpm.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 49.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 2001;18:205–7. 10.1136/emj.18.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S, Kang CH, Hwang Y, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer. Eur J Cardiothorac Surg 2016;49:e16–21. 10.1093/ejcts/ezv336 [DOI] [PubMed] [Google Scholar]
- 51.Wesselink EM, Kappen TH, van Klei WA, et al. Intraoperative hypotension and delirium after on-pump cardiac surgery. Br J Anaesth 2015;115:427–33. 10.1093/bja/aev256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061535supp001.pdf (52.2KB, pdf)
bmjopen-2022-061535supp002.pdf (19.9KB, pdf)