Abstract
Background:
Meningiomas correspond to one-third of all primary central nervous system tumors. Approximately 9% of them are spheno-orbital meningiomas (SOMs), presenting significant clinical symptoms as visual impairment and orbital esthetics. This article aims to evaluate exophthalmos’ improvement in a surgical series without orbital reconstruction.
Methods:
We consecutively included all patients diagnosed with SOM, admitted to a single institution for 10 years. Surgical resection was the standard of care, associated or not with adjuvant radiation therapy. The radiological investigation included preoperative and postoperative head CT or MRI. We quantified proptosis through imaging.
Results:
Forty patients composed this series, 87.5% were female. Proptosis was the most common presentation (90%), followed by decreased visual acuity (65%), motility deficit (20%), and headache (20%). Gross total resection was achieved in 65% of the procedures. In late outcomes, 78% of the patients maintained or improved visual acuity and 85% maintained or improved headache. Proptosis significantly improved after surgery and along with the follow-up (P < 0.001). Ten patients were submitted to adjuvant RT, six of them after a subtotal resection. All patients of this subgroup had proptosis. It was observed a higher frequency of worse in visual acuity in patients submitted to RT (71% vs. 28%, P = 0.038).
Conclusion:
Resection of SOM was sufficient to stop the evolution of visual deficit and allowed the improvement of proptosis. Orbital reconstruction does not seem to be an essential step in reducing enophthalmos.
Keywords: Central nervous system tumor, Exophthalmos, Gross total resection, Meningioma, Orbit reconstruction, Spheno-orbital meningiomas

INTRODUCTION
Meningiomas correspond to one-third of all primary central nervous system (CNS) tumors. Approximately 9% of them are spheno-orbital meningiomas (SOMs).[23,24] Most found in female around 50 years old, they are classified as en-plaque meningiomas, and they usually exhibit slow growth pattern, with a thin dural layer, bone invasion, and hyperostosis of the sphenoid wing.[6,7,21,29]
SOM management is challenging, since tumor grows into the cavernous sinus, with compression of the cranial nerves II, III, IV, V, and VI. The most related symptoms are decreased visual acuity, trigeminal neuralgia, diplopia, ptosis, and proptosis[26,32] (prevalence around 90%).[28] Subtotal resection (STR) occurs in 10–80% of the procedures, with a recurrence rate varying from 8% to 56%.[3,27,29,32] Adjuvant radiation therapy, often indicated for patients with macroscopic residual tumor, is not a consensus. Due to their indolent nature, SOM requires a long postoperative follow-up. However, most of the studies about SOM are short case series, with no sufficient postoperative time to describe outcomes. In their meta-analysis, Fisher et al. found a follow-up range between 3 and 135 months.[8] The same meta-analysis related improvement of 96% of the patients concerning diplopia and motility deficit after surgical resection, with hypesthesia of CNV as the most common postoperative complication.[8]
This article aims to evaluate exophthalmos’ improvement in a surgical series without orbital reconstruction and complications analysis. We discuss the preoperative presentation, surgical strategy, adjuvant treatment, and long-term outcomes.
MATERIALS AND METHODS
Setting
This retrospective case series consecutively included all patients older than 18 years, diagnosed with SOM who underwent surgical resection in a Brazilian single institution between 2008 and 2018. The exclusion criteria were patients who lost the follow-up. Informed consent for use of the radiological images obtained from each patient at the time of surgery, in accordance with the ethical guidelines approved by our university (research protocol 200/05). The patients consented to the procedures. Medical records provided us with demographic information, tumor characteristics, treatment details, and tumor progression, which were recorded under the research protocol. A total of 40 patients were included and 11 patients (27.5%) underwent the first resection in other services and were reoperated in this series.
Surgical management
We retrospectively reviewed perioperative clinical data records from a large brain tumor databank. The radiological investigation included pre- and postoperative head CT and MRI of the skull and orbit.
The standard of care was a pretemporal craniotomy, associated with superolateral orbitotomy for tumor resection [Figure 1], graded according to the Simpson scale.[30] Gross total resection (GTR) was defined as Simpson Grade I, II, and III resections, whereas STR included Simpson Grade IV cases. The definition of extent of resection (EOR) is not uniform among studies, in part due to the difficulties to adapt the Simpson scale, originally applied for convexity meningiomas,[30] to SOM resection: some authors consider only Simpson Grade I as a GTR,[28] others believe Simpson Grades I and II,[9,11,17] and finally, some series define GTR as Simpson I, II, and III.[3,13] The lack of consensus about basic concepts hinders comparisons among them.
Figure 1:
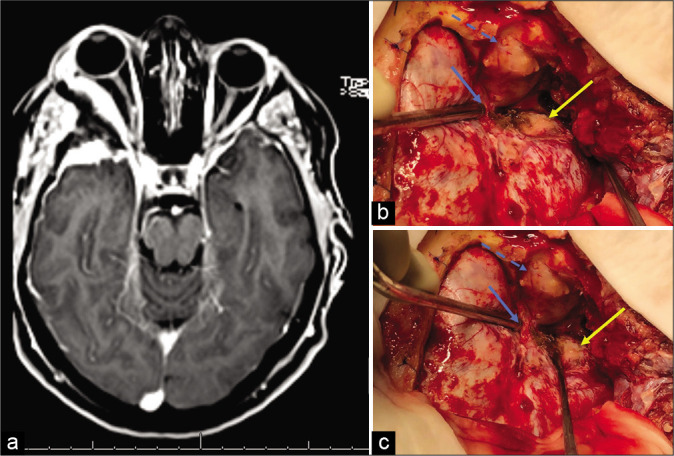
(a) MRI-T1 gadolinium: right spheno-orbital meningioma. (b and c) Standard surgical strategy. We performed a pretemporal craniotomy. Blue arrow: superior orbital fissure exposed; blue dashed arrow: periorbita visualization after orbitotomy and intraorbital tumor resection; yellow arrow: tumor implantation. The greater sphenoid wing was drilled and removed.
In our service, we do not perform reconstruction of orbital walls after orbital decompression. The association of adjuvant fractionated stereotactic radiotherapy (FSRT) was made under the attending team’s discretion, considering the following factors: age, recurrence, and postoperative visual acuity. The FSRT protocol applied 50 Gy to the tumor bed in 28 sessions with 1.8 Gy/fraction.
Histopathological analysis categorized tumors into five different subtypes (meningothelial, transitional, fibrous, fibroblastic, and microcystic), graded according to the WHO Classification of Tumors of the CNS at the time of diagnosis.[15,16] Clinical follow-up was made 15 days after surgery, 2 months, 6 months, and then one visit every year. The variables of interest were demographics, preoperative clinical presentation, histology, the EOR, and adjuvant FSRT.
Outcomes
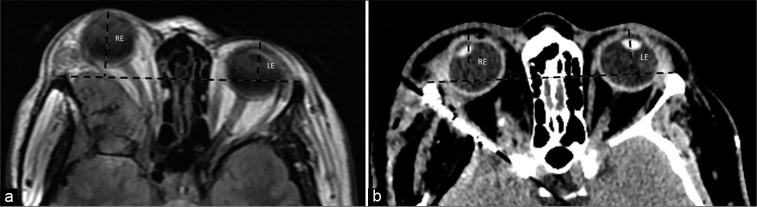
The primary outcome analyzed was proptosis improvement after the surgery. Proptosis assessment is essential in orbital reconstruction in craniofacial anomalies. We use an axial head computed tomography with a thin slice. Preoperative proptosis was measured using the exophthalmos index (EI) as presented by Scarone et al.[28]
We selected axial images in the orbital plane of the CT scan and MRI on the optic nerve level bilaterally. A single examiner analyzed all CTs/MRIs, placing a transverse tangent on the anterior lateral orbital rim of both sides and calculating the distance from the corneal center to the tangent line at a 90° angle. Then, we compared the difference in length (mm) between the pre- and postoperative scans to evaluate the exophthalmos’ improvement [Figure 2].
Figure 2:
A 53 y/o patient presenting right exophthalmos and decreased visual acuity. (a) Preoperative MRI showing a spheno-orbital meningioma, with a quantifiable proptosis of 10.8 mm. (b) Early CT scan after a Simpson II resection, with the absence of deviation. RE: right eye, LE: left eye. Black dashed lines representing exophthalmos index as presented by Scarone et al.
As secondary outcomes, we verified visual acuity, ocular motility, and campimetry exclusively through physical examination. All the 40 patients were examined by a neurosurgeon and an ophthalmologist. In addition, the incidence of headache and perioperative complications was recorded.
Statistical analysis
We described categorical variables with absolute and relative frequencies. Continuous variables were described with mean and standard deviations (for variables with normal distribution) and median with interquartile range (for nonnormal data). We assessed normal distribution through the methods of asymmetry and kurtosis. To compare the baseline characteristics of the stratified subgroups by recurrence and adjuvant radiotherapy, the Fisher’s exact test was used for categorical variables and the Mann– Whitney U-test for independent samples or for continuous variables as applicable. We analyzed variation of proptosis along the study using ANOVA for repeated measures. The significance level was <0.05. All analyses were performed using the software SPSS (IBM SPSS Statistics for Windows, version 24.0. Armonk, NY: IBM Corp.).
RESULTS
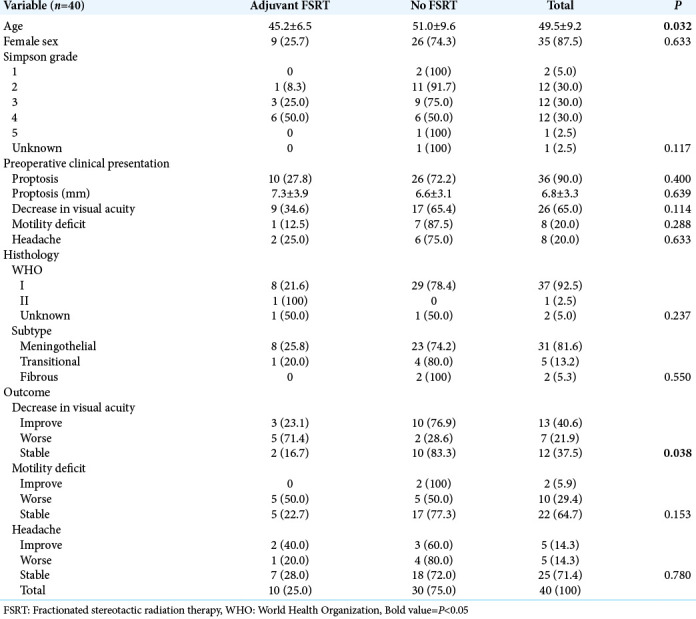
A total of 40 patients composed this case series; 87.5% were female. The mean age at surgery was 49.5 ± 9.2 years. All patients presented symptoms related to the tumor at the preoperative clinical evaluation. Twenty-nine patients had newly diagnosed meningiomas, while 11 patients presented recurrences of previously resected tumors. Proptosis was the most common presentation (90%), followed by decreased visual acuity (65%), motility deficit (20%), and headache [20%, Table 1]. Six patients had severe vision loss, two with only luminous perception, and four patients with amaurosis. A decrease of the visual campus was present in three patients with temporal hemianopsia. Finally, two patients presented seizures.
Regarding surgical resection, GTR was achieved in 65% of the procedures. We used periosteum grafts for dural reconstruction. Thirty-nine cases were diagnosed with the WHO Grade I meningiomas and only one patient with the WHO Grade II (atypical) meningioma. The most prevalent histology subtype was the meningothelial (81%). Perioperative complications included three surgical site infections, three postoperative seizures, two strokes, one pulmonary thromboembolism, and one patient with insipidus diabetes. One patient presented a cerebral spinal fluid fistula, requiring a second approach and reconstruction with a fascia lata graft. In late outcomes, 78% of the patients maintained or improved visual acuity; regarding headache, associated pharmacological therapy allowed the improvement or the stability of 85% of patients [Table 1].
Table 1:
General sample characterization.
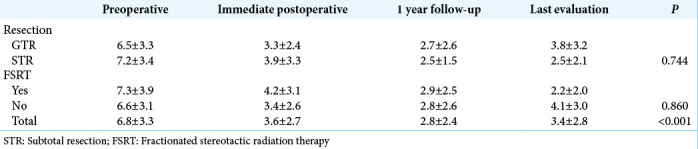
The radiological evaluation had a median follow-up of 39 months. Proptosis significantly improved after surgery and along the follow-up [P < 0.001, Table 2].
Table 2:
Average of proptosis (mm) along the study.
Ten patients presented recurrence/regrowing and were submitted to adjuvant FSRT, six of them after an STR. All patients of this subgroup had proptosis. It was observed a higher frequency of worse in visual acuity in patients submitted to FSRT [71% vs. 28%, P = 0.038, Table 1]. It was observed proptosis improvement (quantified in imaging) in the FSRT group compared with other patients [2.2 ± 2.0 mm vs. 4.1 ± 3.0 mm, P = 0.147]. Until now, these patients are still with controlled disease.
DISCUSSION
Like other meningiomas, SOM is mostly benign tumors which exhibit a remarkable bone invasion, associated with a dural en-plaque growth. This morphology, related to the proximity of delicate structures, hinders the total resection of these lesions and increases recurrence/regrowth.[6,7]
Due to the 11 patients (27.5%) who underwent the first resection in other services and were reoperated in this series, we preferred to evaluate clinical follow-up and quantify proptosis rather than stratify individuals according to tumor recurrence. We observed around one-third of STR in our service. Although it was expected a relatively worse outcome in the STR group, it had a slightly lower quantifiable proptosis in the last evaluation than the GTR group (2.5 ± 2.1 mm vs. 3.8 ± 3.2 mm). This finding suggests even an incomplete removal of the tumor can provide good late outcomes.
Ten patients underwent FSRT and EOR influenced adjuvant treatment since six of these patients had an STR. In addition, this group presented a higher prevalence of visual acuity worsening in the last follow-up. This finding is associated with a worse preoperative setting in the FSRT group, since nine of the 10 patients submitted to FSRT presented preoperative decrease visual acuity, against 56% of the patients without FSRT. Nonetheless, patients submitted to STR associated with adjuvant FSRT had lower proptosis in the late outcome.
There was a high prevalence of proptosis (90%) in our population, similar to the previous case series.[28,29] Patients presented a significant improvement after surgery, sustained during the follow-up (6.8 ± 3.3 mm preoperatively vs. 3.4 ± 2.8 mm in the last evaluation), demonstrating that surgical resection was effective in most cases.
The surgical technique involves the pretemporal craniotomy, to remove all the bone and tumor from the temporal pole until the foramen rotundum, and associate the superolateral orbitotomy, allowing orbital decompression and the removal of the intraorbital tumor. There are many approaches for this tumor. As discussed in a recent meta-analysis,[8] the most used surgical technique was the pterional craniotomy associated or not orbital wall, anterior clinoid, and optic canal removal.[2,18] Since 2008, orbitotomy through supraciliary or trans-eyelid approach started to be used. Recently, the use of endoscope for lateral orbitotomy and tumor resection is increasing allowing minimally invasive approaches for specific cases, in which there is an invasion of the lateral portion of the orbit.[20]
Reconstruction of orbital walls and the lesser or greater sphenoid wings, especially with autologous bone graft, has been endorsed by some authors. The main advantages reported are the esthetic improvement and prevention of pulsating exophthalmos, oculomotor muscle fibrosis, meningoceles, and enophthalmos.[3-5,10-12,14,22,29] Although we do not perform reconstruction in our service, only one patient had enophthalmos in the late follow-up, and no patient presented pulsating exophthalmos or meningoceles. In a case series of 60 patients, Mariniello et al.[17] did not observe any of these complications. They reported good/acceptable esthetic results in more than 90% of their cases.[17] Previously, Maroon et al., studying 200 patients, also did not found the incidence of pulsating exophthalmos or poor cosmetic outcomes.[19] As supported by other authors,[1,25,28,31] we believe that orbital reconstruction is not mandatory in most cases. This allows a surgery with greater cost-effectiveness, reducing costs for the health system.
There are some limitations needed to be addressed. We assessed clinical data retrospectively. In addition, only 10 patients underwent radiotherapy (25%), hindering the analysis of radiotherapy effect on SOM recurrence. Finally, there was a high rate of follow-up withdrawal, compromising data about late outcomes. This is a case series and just shows a tendency for exophthalmos improvement without enophthalmos in patients who did not underwent orbital reconstruction. More studies are necessary to give more significance for these findings, including randomized clinical trials.
CONCLUSION
Although a demanding procedure, surgical resection of SOM was effective in stopping the evolution of visual deficit and improvement of proptosis. Adjuvant RT was reserved for patients with STR and worst visual acuity. The nonreconstruction of the orbit does not seem to correlate with a higher prevalence of enophthalmos. Most of the perioperative complications were solved during the hospital stay and did not affect late clinical outcome.
Footnotes
How to cite this article: dos Santos AG, Paiva WS, da Roz LM, do Espirito Santo MP, Teixeira MJ, Figueiredo EG, et al. Spheno-orbital meningiomas: Is orbit reconstruction mandatory? Long-term outcomes and exophthalmos improvement. Surg Neurol Int 2022;13:318.
Contributor Information
Alexandra Gomes dos Santos, Email: alexandra.gomes@fm.usp.br.
Wellingson Silva Paiva, Email: wellingsonpaiva@yahoo.com.br.
Leila Maria da Roz, Email: leilaroz@hotmail.com.
Marcelo Prudente do Espirito Santo, Email: prudanet@gmail.com.
Manoel Jacobsen Teixeira, Email: manoeljacobsen@gmail.com.
Eberval G. Figueiredo, Email: ebgadelha@yahoo.com.
Vinicius Trindade Gomes da Silva, Email: viniciustrindade@hotmail.com.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A, Hashemi SM. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int. 2015;6:79. doi: 10.4103/2152-7806.157074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baucher G, Troude L, Roux A, Loundou A, Boucekine M, Meling T, et al. Predictors of visual function after resection of skull base meningiomas with extradural anterior clinoidectomy. Neurosurg Rev. 2022;45:2133–49. doi: 10.1007/s10143-021-01716-w. [DOI] [PubMed] [Google Scholar]
- 3.Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: Clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013;27:84–90. doi: 10.3109/02688697.2012.709557. [DOI] [PubMed] [Google Scholar]
- 4.Brusati R, Biglioli F, Mortini P, Raffaini M, Goisis M. Reconstruction of the orbital walls in surgery of the skull base for benign neoplasms. Int J Oral Maxillofac Surg. 2000;29:325–30. [PubMed] [Google Scholar]
- 5.Chambless LB, Mawn LA, Forbes JA, Thompson RC. Porous polyethylene implant reconstruction of the orbit after resection of spheno-orbital meningiomas: A novel technique. J Craniomaxillofac Surg. 2012;40:e28–32. doi: 10.1016/j.jcms.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Cushing H. The cranial hyperostosis produced by meningeal endotheliomas. Arch Neurol Psychiatry. 1922;8:139–54. [Google Scholar]
- 7.Cushing H, Eisenhardt L. Vol. Vol. 27. Toronto: Springer Charles C Thomas; 1938. The Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical end Results; p. 185. [Google Scholar]
- 8.Fisher FL, Zamanipoor Najafabadi AH, Schoones JW, Genders SW, van Furth WR. Surgery as a safe and effective treatment option for spheno-orbital meningioma: A systematic review and meta-analysis of surgical techniques and outcomes. Acta Ophthalmol. 2021;99:26–36. doi: 10.1111/aos.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güdük M, Özduman K, Pamir MN. Sphenoid wing meningiomas: Surgical outcomes in a series of 141 cases and proposal of a scoring system predicting extent of resection. World Neurosurg. 2019;125:e48–59. doi: 10.1016/j.wneu.2018.12.175. [DOI] [PubMed] [Google Scholar]
- 10.Heufelder MJ, Sterker I, Trantakis C, Schneider JP, Meixensberger J, Hemprich A, et al. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthalmic Plast Reconstr Surg. 2009;25:223–6. doi: 10.1097/IOP.0b013e3181a1f345. [DOI] [PubMed] [Google Scholar]
- 11.Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J. Spheno-orbital meningiomas: Outcome after microsurgical treatment: A clinical review of 30 cases. Neurol Res. 2010;32:314–25. doi: 10.1179/016164109X12464612122614. [DOI] [PubMed] [Google Scholar]
- 12.Idowu OO, Ashraf DC, Magill ST, Kersten RC, McDermott MW, Vagefi MR. Multidisciplinary frontotemporal orbitozygomatic craniotomy for spheno-orbital meningiomas: Ophthalmic and orbital outcomes. Ophthalmic Plast Reconstr Surg. 2021;37:18–26. doi: 10.1097/IOP.0000000000001662. [DOI] [PubMed] [Google Scholar]
- 13.Ko CC, Lim SW, Chen TY, Chen JH, Li CF, Shiue YL. Prediction of progression in skull base meningiomas: Additional benefits of apparent diffusion coefficient value. J Neurooncol. 2018;138:63–71. doi: 10.1007/s11060-018-2769-9. [DOI] [PubMed] [Google Scholar]
- 14.Leroy HA, Leroy-Ciocanea CI, Baroncini M, Bourgeois P, Pellerin P, Labreuche J, et al. Internal and external spheno-orbital meningioma varieties: Different outcomes and prognoses. Acta Neurochir (Wien) 2016;158:1587–96. doi: 10.1007/s00701-016-2850-0. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 17.Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F, et al. Spheno-orbital meningiomas: Surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir. 2008;69:175–81. doi: 10.1055/s-2008-1077077. [DOI] [PubMed] [Google Scholar]
- 18.Mariniello G, de Divitiis O, Bonavolontà G, Maiuri F. Surgical unroofing of the optic canal and visual outcome in basal meningiomas. Acta Neurochir (Wien) 2013;155:77–84. doi: 10.1007/s00701-012-1485-z. [DOI] [PubMed] [Google Scholar]
- 19.Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80:202–8. doi: 10.3171/jns.1994.80.2.0202. [DOI] [PubMed] [Google Scholar]
- 20.Maugeri R, Graziano F, Basile L, Gulì C, Giugno A, Giammalva GR, et al. Trends in Reconstructive Neurosurgery. In: Visocchi M, Mehdorn HM, Katayama Y, von Wild KR, editors. Cham. Springer International Publishing; 2017. [Google Scholar]
- 21.Mourits MP, van der Sprenkel JW. Orbital meningioma, the Utrecht experience. Orbit. 2001;20:25–33. doi: 10.1076/orbi.20.1.25.2640. [DOI] [PubMed] [Google Scholar]
- 22.Nagahama A, Goto T, Nagm A, Tanoue Y, Watanabe Y, Arima H, et al. Spheno-orbital meningioma: Surgical outcomes and management of recurrence. World Neurosurg. 2019;126:e679–87. doi: 10.1016/j.wneu.2019.02.123. [DOI] [PubMed] [Google Scholar]
- 23.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1–100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pompili A, Derome PJ, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge clinical features, surgical therapy, and long-term observations: Review of 49 cases. Surg Neurol. 1982;17:411–6. doi: 10.1016/s0090-3019(82)80006-2. [DOI] [PubMed] [Google Scholar]
- 25.Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60:214–21. doi: 10.1227/01.NEU.0000255415.47937.1A. [DOI] [PubMed] [Google Scholar]
- 26.Saeed P, van Furth WR, Tanck M, Kooremans F, Freling N, Streekstra GI, et al. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien) 2011;153:395–402. doi: 10.1007/s00701-010-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33:260–6. doi: 10.1016/j.jcms.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Scarone P, Leclerq D, Héran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009;111:1069–77. doi: 10.3171/2009.1.JNS081263. [DOI] [PubMed] [Google Scholar]
- 29.Shrivastava RK, Sen C, Costantino PD, Della Rocca R. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103:491–7. doi: 10.3171/jns.2005.103.3.0491. [DOI] [PubMed] [Google Scholar]
- 30.Simpson D. The recurrence of intracranlal meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talacchi A, De Carlo A, D’Agostino A, Nocini P. Surgical management of ocular symptoms in spheno-orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg Rev 2014 ; 37:301–9. doi: 10.1007/s10143-014-0517-y. [DOI] [PubMed] [Google Scholar]
- 32.Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL, et al. Spheno-orbital meningiomas surgery: Multicenter management study for complex extensive tumors. World Neurosurg. 2018;112:e145–56. doi: 10.1016/j.wneu.2017.12.182. [DOI] [PubMed] [Google Scholar]