Abstract
Background:
The third segment of the vertebral artery (V3) is vulnerable during far lateral and retrosigmoid approaches. Although the suboccipital triangle (SOT) is a useful anatomical landmark, the relationship between V3 and the muscles forming the triangle is not well-described. We aimed to demonstrate the relationship between the V3, surrounding muscles, and SOT in clinical cases.
Methods:
Operative videos of patients with the vertebral artery (VA) and posterior inferior cerebellar artery (PICA) aneurysms treated with occipital artery-PICA bypass through the far lateral approach were examined. Videos from January 2015 to October 2021 were retrospectively reviewed to determine anatomy of the V3 and the SOT.
Results:
Fourteen patients were included in this study. The ipsilateral V3 was identified without injury in all patients using the bipolar cutting technique. The lateral 68.2% of the horizontal V3 segment, including the V3 bulge, was covered by the inferomedial part of the superior oblique muscle (SO). The medial 23.9% was covered by the inferolateral part of the rectus capitis posterior major muscle. The inferomedial part of the horizontal V3 segment is located within the SOT.
Conclusion:
Most of the V3, including the V3 bulge, were located beneath the SO and the inferomedial part of V3 located within the SOT. Elevation of the SO should be performed carefully using the bipolar cutting technique to avoid injury to the V3. To the best of our knowledge, this is the first description of the V3 relative to the SOT in the clinical setting.
Keywords: Far lateral approach, Suboccipital triangle, Superior oblique muscle, V3 segment, Vertebral artery

INTRODUCTION
The suboccipital segment or the third segment of the vertebral artery (VA) (V3) extends from the transverse foramen of C1 to the foramen magnum. This segment of the VA runs horizontally in the groove of the posterior arch of C1 (J-groove or C1 sulcus arteriosus).[4,8,16,27] The V3 runs a tortuous course and is prone to accidental iatrogenic injury during retrosigmoid and far lateral approaches.[2,17,18] The suboccipital triangle (SOT) is formed by the superior oblique (SO), inferior oblique (IO), and rectus capitis posterior major muscles (RCPM) and is usually used as an important anatomical landmark for V3. The V3 and C1 nerve roots lying in the groove of C1 are localized deep within the triangle.[2,6,10,12,19,21,28] Identification and skeletonization of V3 are a key step in craniovertebral junction surgery.[4,10,16] Castillo et al. divided the V3 into five subsegments: infraforaminal, intraforminal, supraforaminal, horizontal, and intramembranous.[4] Meybodi et al. simply classified the V3 into three subsegments: the C1 transverse foramen segment, the sulcus arteriosus (horizontal subsegment) segment, and the segment just before the dural entrance.[16]
Dorsolateral and far lateral approaches (retrocondylar, transcondylar, and transcondylar fossa variants) are indicated when accessing the lower clivus, anterolateral foramen magnum, VA, or posterior inferior cerebellar artery (PICA). The V3 is usually identified, skeletonized, and preserved while utilizing these approaches.[3,9,13,14,15,22,28] This study aimed to identify the course of the V3 relative to the SOT and the three muscles forming this triangle in the clinical setting and propose an alternative method for identifying and skeletonizing the V3.
MATERIALS AND METHODS
Patient selection
The operative videos of patients with VA and PICA aneurysms and dissections treated by occipital artery-PICA (OA-PICA) bypass through the far lateral approach between January 2015 and October 2021 were retrospectively reviewed. The V3 anatomy relative to the surrounding muscles and SOT was examined.
Operative technique
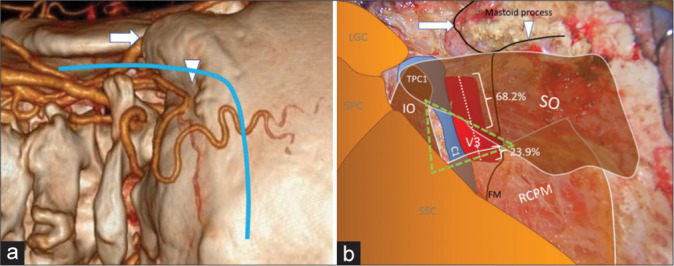
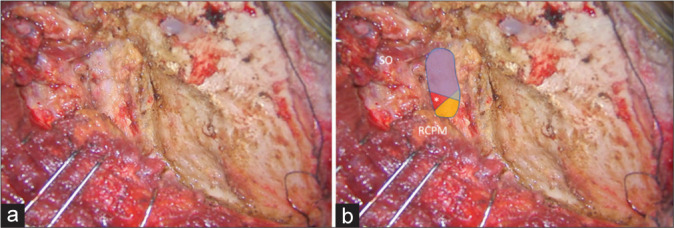
With the patient in the semi-prone park bench position [Figure 1a], a reverse “L” shape incision [Figure 1b] was made.[23,29] The sternocleidomastoid, splenius capitis, longissimus capitis, and semispinalis capitis muscles and the posterior belly of the digastric muscle were elevated in layers, and the transverse process of C1 and the SOT, which is formed by the SO, IO, and RCPM, was identified [Figures 1c and d].[23] The SO, which originated from the upper surface of the C1 transverse process, was attached to the inferior nuchal line of the occipital bone. The IO extended from the spinous process of C2 to the C1 transverse process. The RCPM was attached to the inferior nuchal line and inserted on the spinous process of C2. After resection of the fibrofatty tissue covering the SOT using the bipolar cutting technique,[25] the SO was elevated from the suboccipital bone inferolaterally toward the C1 transverse process using monopolar cautery. The RCPM, which was located beneath the SO, was preserved. When the inferolateral margin of the RCPM was reached, the bipolar cutting technique was used to further elevate the SO from the underlying V3 toward the C1 transverse process [Figures 2a and b and Video 1]. The vertebral venous plexus surrounding the VA was meticulously coagulated. The inferior border of the V3 was dissected from the IO and C1 groove using the bipolar cutting technique [Figures 3a and b]. The RCPM was inferomedially elevated from the suboccipital bone toward the posterior arch of C1 using monopolar cautery. The inferolateral rim of RCPM adhering to the V3 was separated from the VA using the bipolar cutting technique [Figures 3c and d, Video 2]. A craniotomy and transcondylar approach was performed [Figures 3e and f].
Figure 1:
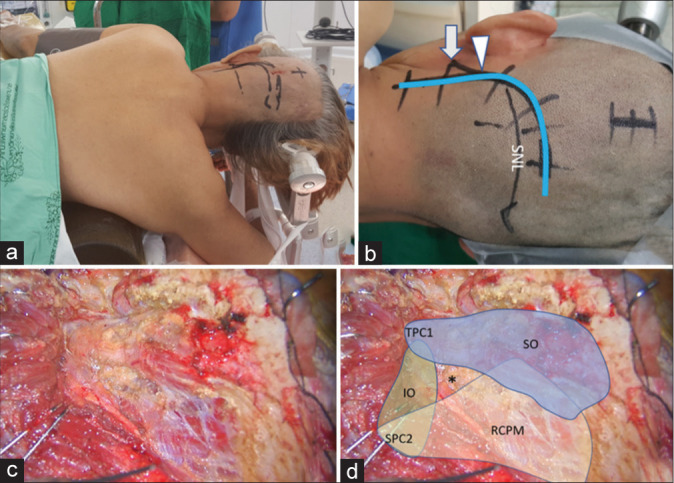
(a) Semi-prone park bench position. (b) Reverse “L” shape incision relative to the mastoid tip (arrow), mastoid groove (arrowhead), and superior nuchal line (SNL). (c and d) After the sternocleidomastoid, splenius capitis, longissimus capitis, and semispinalis capitis muscles and the posterior belly of digastric muscle were elevated in layers, the transverse process of C1 (TPC1) and the spinous process of C2 (SPC2) were palpated and the suboccipital triangle (asterisk) formed by the superior oblique (SO in blue color), inferior oblique (IO in green color), and rectus capitis posterior major (RCPM in yellow color) muscles was identified.
Figure 2:
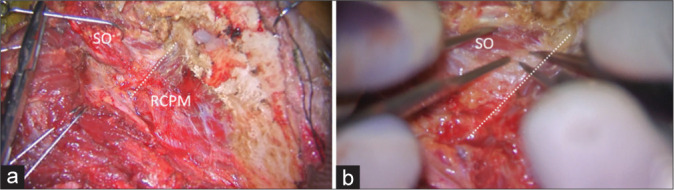
Intraoperative photograph demonstrating left suboccipital muscle dissection. (a) After inferolateral elevation of the superior oblique muscle (SO) from the rectus capitis posterior major muscle (RCPM), the inferolateral border (dashed line) of the RCPM was identified as the landmark of the third segment of the vertebral artery (V3) and the starting point for the bipolar cutting technique to separate the SO from the V3. (b) Magnified photograph of Panel A demonstrated the inferolateral border (dashed line) of the RCPM and the partially elevated SO.
Figure 3:
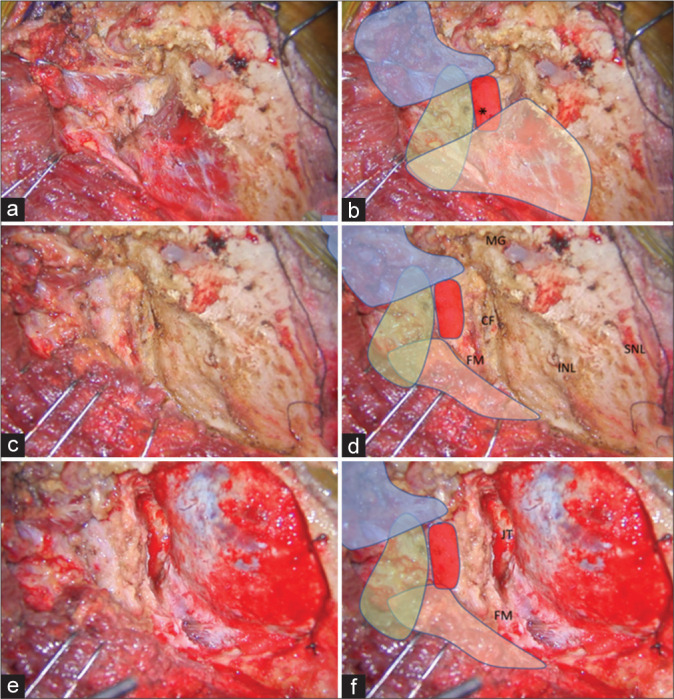
(a and b) After the SO (in blue color) was inferolaterally elevated, the V3 (red color) was identified beside the RCPM (yellow color) and IO (green color). The location of the center of the suboccipital triangle is marked with an asterisk. (c and d) After the RCPM (yellow color) was inferomedially elevated from the inferior nuchal line, the foramen magnum (FM) was exposed. The condylar fossa (CF), SNL, and mastoid groove (MG) were also identified. (e and f) After retrosigmoid craniotomy, removal of the posterolateral part of the FM, and drilling of the CF, the posterior part of jugular tubercle (JT), and the medial part of the occipital condyle, the transcondylar exposure was completed.
Further dissection of the suboccipital muscle to expose the V3 segment of the vertebral artery.
Quantitative measurements
After full exposure of the V3 was completed, the long axis of the V3 was marked [Figure 4]. The lengths of V3 covered by the SO and RCPM and the whole length of the V3 were measured as “a,” “b,” and “c,” respectively, as shown in Figure 4. The percentages of the V3 covered by the SO and RCPM were calculated as a/c × 100 and b/c × 100, respectively. The part of V3 segment located in the center of the SOT and the location of the V3 bulge related to the center of the SOT were also noted.
Figure 4:
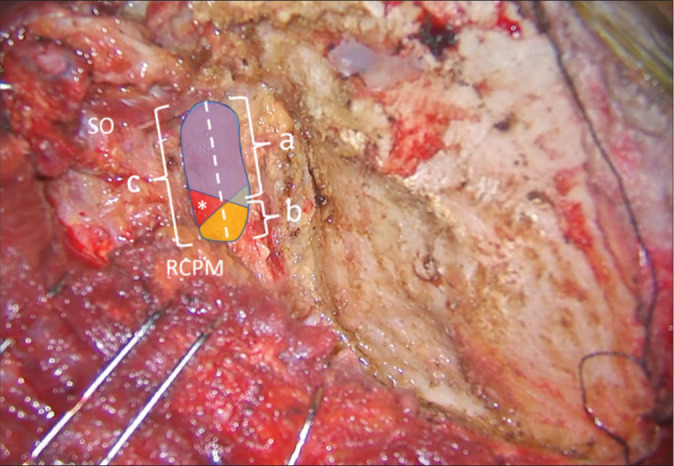
Method of quantitative measurement of the V3 length on the left side. The superior oblique (SO) and rectus capitis posterior major (RCPM) muscles were identified. The whole length of the V3 was marked as a dashed line and measured as “c.” The length of the V3 covered by SO (blue color) and RCPM (yellow color) were measured as “a” and “b,” respectively. The center of the suboccipital triangle above the V3 is indicated with an asterisk.
RESULTS
Patient characteristics
The far lateral transcondylar or transcondylar fossa approaches and OA-PICA bypass was performed in 14 patients with VA and proximal PICA aneurysms and dissections. The ipsilateral V3 was identified in all patients. The average patient age was 54 years. All patients suffered from subarachnoid hemorrhage (SAH) at initial presentation. VA dissections were the most common type of lesion (64.3%), followed by saccular aneurysms of the VA-PICA junction (28.6%). Surgeries were performed on the left side in 10 patients (71.4%) and on the right side in four patients (28.6%) [Table 1].
Table 1:
Patient characteristics and operative findings.
Operative findings
After elevation of the SO, the V3 covered by the venous plexus was observed just inferolateral to the inferolateral border of the RCPM. After full elevation of the SO and RCPM, the lateral 60–72% of the V3 was covered by the inferomedial part of the SO. The medial 23–25% of the V3 was covered by the inferolateral part of RCPM. On average, the SO and RCPM covered the lateral 68.2% and medial 23.9% of the V3, respectively [Figure 5]. The part of V3 segment located within the center of the SOT was the inferomedial part. The V3 bulge was related to the lateral part of the SOT (lateral to the center of the SOT) and covered by the SO. The RCPM and rectus capitis posterior minor covered the foramen magnum. No intraoperative VA injury occurred. No arcuate foramen (an aperture formed by the presence of a complete bony bridge on the posterior arch of the atlas) or flat sulcus arteriosus were detected [Table 1].
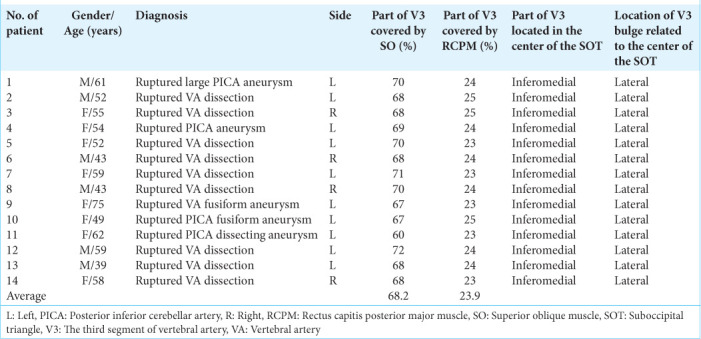
Figure 5:
(a) Three-dimensional reconstructed computed tomography angiography of the left side of the craniocervical junction demonstrating the skin incision (blue line), mastoid tip (arrow), and mastoid groove (arrowhead). (b) An intraoperative photograph, similar to Panel A, demonstrating the mastoid tip (arrow), mastoid groove (arrowhead), the third segment of the vertebral artery (V3 and red color), the suboccipital triangle (green triangle), and the related suboccipital muscles. The lateral 68.2% and medial 23.9% of the V3 are covered by the superior oblique muscle (SO) and rectus capitis posterior major muscle (RCPM), respectively. The inferomedial part of the V3 is located within the suboccipital triangle (green triangle). (C1: Posterior arch of C1, FM: Foramen magnum, IO: Inferior oblique muscle, LGC: Longissimus capitis muscle, RCPM: Rectus capitis posterior major muscle, SO: Superior oblique muscle, SPC: Splenius capitis muscle, SSC: Semispinalis capitis muscle, TPC1: Transverse process of C1).
ILLUSTRATIVE CASES
Case 1 (Patient 1)
A 61-year-old male presented with sudden alteration of consciousness (AOC). The World Federation of Neurosurgical Societies (WFNS) grade was 5 at the presenting hospital. Computed tomography (CT) showed a diffuse SAH. A large and wide neck saccular aneurysm at the first and second segment of the left PICA was detected by computed tomography angiography (CTA). A left far lateral transcondylar approach [Figures 1-3] for OA-PICA bypass with aneurysm trapping was performed. The left V3 was exposed without injury. The lateral 70% and the medial 24% of the V3 were covered by the SO and RCPM, respectively. The inferomedial part of the V3 was located within the SOT [Figure 6]. Complete obliteration of the aneurysm with patent OA-PICA bypass was confirmed through immediate postoperative CTA. No further neurological deficits were detected. A Glasgow outcome scale (GOS) score of 5 was achieved 3 months after surgery.
Figure 6:
Illustrative Case 1 (Patient 1) (a and b) Intraoperative finding of the left suboccipital muscle dissection and exposure for the far lateral transcondylar approach. (b) The superior oblique (SO) and rectus capitis posterior major muscle (RCPM) were elevated. The third segment of the vertebral artery (blue, red, and yellow colors) was exposed. The blue color was covered by the SO, which included the lateral 70% of the third segment of the vertebral artery. The yellow color was covered by the RCPM, which included the medial 24% of the third segment of the vertebral artery. The red color was located within the center of the suboccipital triangle (asterisk).
Case 2 (Patient 13)
A 39-year-old male presented with sudden AOC with an initial WFNS grade of 4. CT showed diffuse SAH predominantly at the premedullary cistern. A left vertebral artery dissection (VAD) with PICA involvement was detected through CTA. A left far lateral transcondylar fossa approach was performed [Figure 7]. OA-PICA bypass and blind-alley formation (occlusion of the proximal VA and PICA origin) were performed. The left V3 was exposed without injury. The lateral 68% and medial 24% of the V3 were covered by the SO and RCPM, respectively. The inferomedial part of the V3 was located within the SOT [Figure 8]. Complete obliteration of the aneurysm with good patency of the OA-PICA bypass was confirmed through immediate postoperative CTA. Postoperative dysphagia and hoarse voice were detected. The patient recovered and achieved a GOS score of 4, 3 months after surgery.
Figure 7:
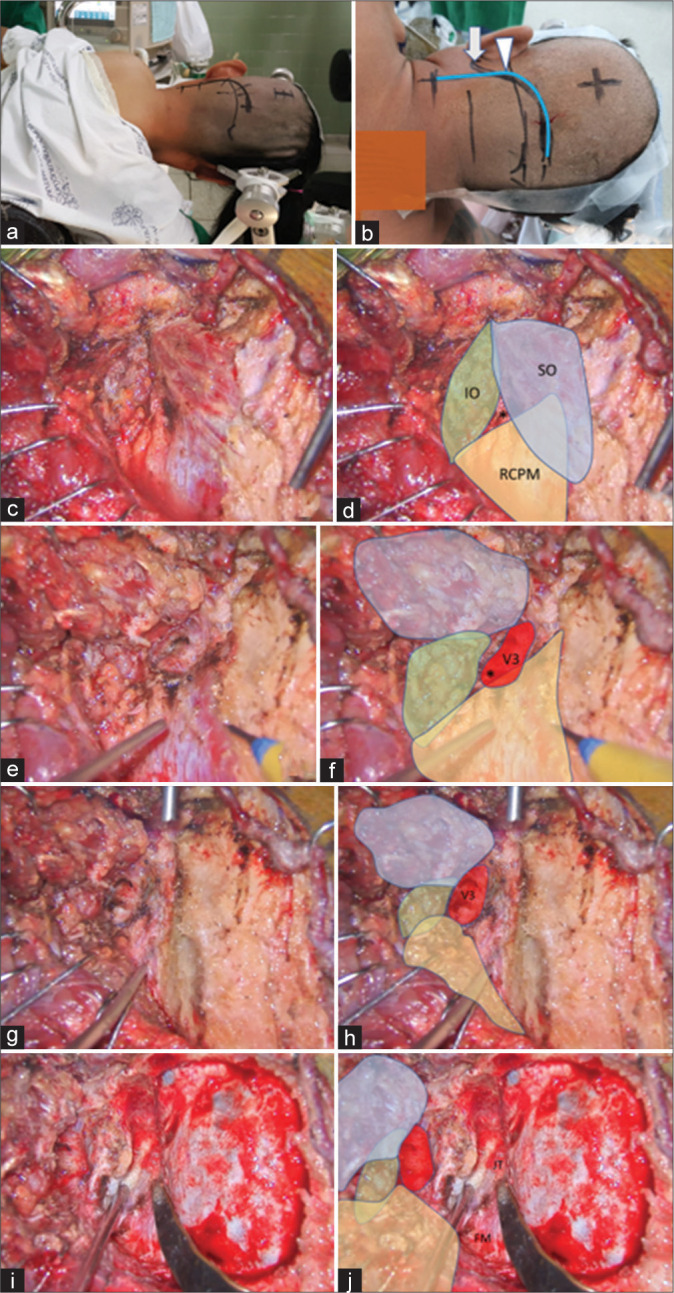
Illustrative Case 2 (Patient 13). (a) Right semi-prone park bench position. (b) Skin incision (blue line) relative to the mastoid tip (arrow) and mastoid groove (arrowhead). (c-h) The steps to expose the third segment of the left vertebral artery are demonstrated, similar to Figure 1-3. The superior oblique (SO, blue color), inferior oblique (IO, green color), and rectus capitis posterior major (RCPM, yellow color) muscles; the third segment of the vertebral artery (V3, red color); and the center of the suboccipital triangle (asterisk) are shown. (I and j) The left far lateral transcondylar fossa approach.
Figure 8:
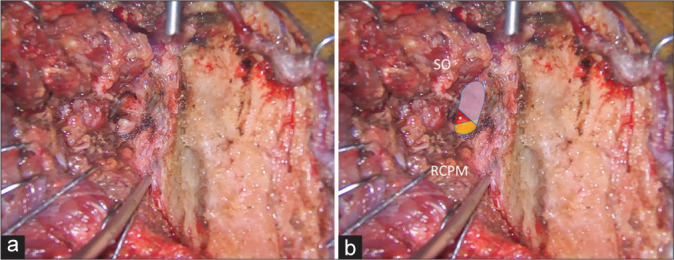
Illustrative Case 2 (Patient 13). (a and b) Intraoperative findings during the left suboccipital muscle dissection and exposure for the far lateral transcondylar approach. (b) The superior oblique (SO) and rectus capitis posterior major (RCPM) muscles were elevated. The third segment of the vertebral artery (blue, red, and yellow colors) was exposed. The blue color was covered by the SO, which included the lateral 68% of the third segment of the vertebral artery. The yellow color was covered by RCPM, which included the medial 24% of the third segment of the vertebral artery. The red color was located within the center of the suboccipital triangle (asterisk).
Case 3 (Patient 14)
A 58-year-old female presented with sudden AOC. The WFNS grade was 4 at the first hospital. CT showed a diffuse SAH. A right VAD and right PICA originating from the dissecting segment were detected through CTA. OA-PICA bypass and blind-alley formation were performed through a right far lateral transcondylar approach [Figure 9]. The right V3 was exposed without injury. The lateral 68% and medial 23% of the V3 were covered by the SO and RCPM, respectively. The inferomedial part of V3 was located within the SOT [Figure 10]. Complete obliteration of the VAD with patent OA-PICA bypass was confirmed through immediate postoperative CTA. No further neurological deficits were detected. The patient recovered and achieved a GOS score of 5, 3 months after the surgery.
Figure 9:
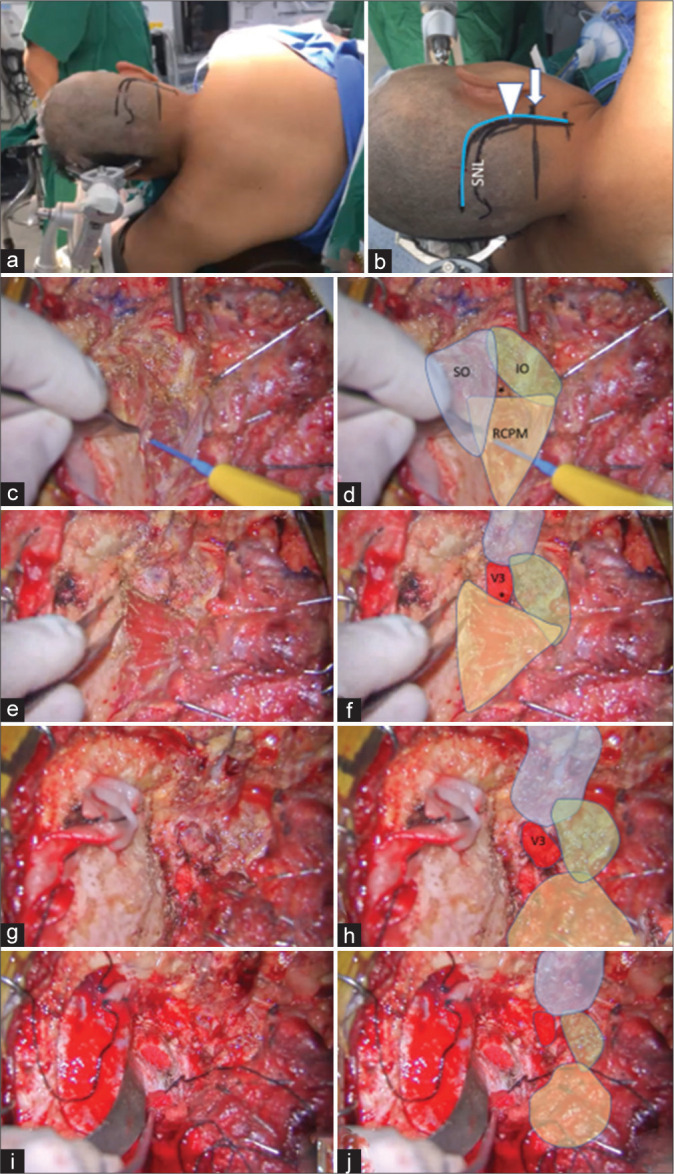
Illustrative Case 3 (Patient 14). (a) Left semi-prone park bench position. (b) Skin incision (blue line) relative to the mastoid tip (arrow), mastoid groove (arrowhead), and superior nuchal line (SNL). (c-h). The steps to expose the third segment of the right vertebral artery are demonstrated, similar to Figures 1-3. The superior oblique (SO, blue color), inferior oblique (IO, green color), and rectus capitis posterior major (RCPM, yellow color) muscles; the third segment of the vertebral artery (V3, red color); and the center of the suboccipital triangle (asterisk) are shown. (i and j) Right far lateral transcondylar approach.
Figure 10:
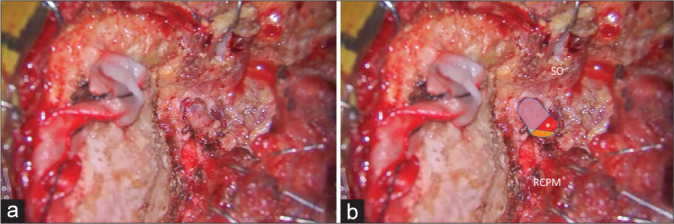
Illustrative Case 3 (Patient 14). (a and b) Intraoperative findings during right suboccipital muscle dissection and exposure for the far lateral transcondylar approach. (b) The superior oblique (SO) and rectus capitis posterior major (RCPM) muscles were elevated. The third segment of the vertebral artery (blue, red, and yellow colors) was exposed. The blue color was covered by the SO, which included the lateral 68% of the third segment of the vertebral artery. The yellow color was covered by the RCPM, which included the medial 23% of the third segment of the vertebral artery. The red color was located within the center of the suboccipital triangle (asterisk).
DISCUSSION
Surgical anatomy of the V3
The relationship between the V3 and the surrounding bones was well-described in cadaveric studies by Lang and Kessler[11] and Ulm et al.;[26] however, the relationship between the V3 and the suboccipital muscles was not described. Ulm et al. reported that the V3 has variable normal anatomy, which impacts common surgical procedures involving the craniovertebral junction. They identified two V3 sites that are vulnerable to injury during lateral suboccipital approaches: the medial border of the V3 and the superior surface of the midpoint of the V3, which often bulges posteriorly into the suboccipital muscular triangle. The first site was vulnerable to injury while exposing the C1 arch, and the second site was particularly prone to injury when extending the lateral suboccipital exposure down to the level of the foramen magnum.[26]
The relationship between the V3 and the surrounding muscles was described by Meybodi et al.[17] and Castillo et al.[4] in cadaveric studies. Castillo et al. described the exposure of the V3 (at its exit from the C1 transverse foramen) after retraction of the SO.[4] Meybodi et al.[17] described the close relationship between the V3 bulge in the SOT and the medial edge of the SO. The V3 bulge was found within 5.2–18.4 mm (mean, 10.2 mm) of the medial superficial edge of the SO. The most posterior bulging V3 section was located at the lateral part of the SOT, not the center of the triangle. They also found that the plane of the skin incision and suboccipital muscle dissection was directed toward the SO while performing retrosigmoid and paramedian suboccipital craniotomies using straight or lazy-S incisions. Hence, the SO was a good landmark for locating the V3. The authors concluded that if the SO was accidentally cut, the V3 would be injured. These cadaveric findings were similar to the operative findings of our clinical study. Most of the V3, including the V3 bulge, was usually covered by the SO.
Operative techniques for V3 exposure
Several techniques for V3 exposure were described previously. Youssef et al. suggested an interfascial dissection technique that followed the natural plane between the deep suboccipital muscle fascia, the posterior atlantooccipital membrane, and the periosteum of the C1 and C2 laminae and the lateral masses.[30] Wanibuchi et al. suggested four anatomical landmarks to identify the V3: the inferior sigmoid point, the occipital midline dural point, the posterior tubercle of C1, and the sulcus arteriosus. This technique should be used carefully because the sulcus arteriosus varies (flat variation and formation of arcuate foramen). Furthermore, the use of cadavers and the neutral position of the cadaveric head limit application of this study.[27]
Meybodi et al. suggested that the V3 could be localized using the distance from the atlanto-mastoid line. The V3 location is 40% of the distance from the mastoid process. However, the posterior tubercle of C1 is difficult to palpate because of the bulky muscles of the suboccipital region. Furthermore, this study was limited because of the use of cadavers and the position of the cadaveric head.[17] Meybodi et al. also performed a cadaveric study and suggested the use of the inferior nuchal line to localize the V3 during the retrosigmoid approach. The mean depths of the V3 relative to the medial and lateral ends of the vertical arm of the inferior nuchal line were 24.9 and 8.3 mm, respectively. Monopolar cautery should be avoided when exposing the inferior part of the inferior nuchal line, which is close to the V3.[24]
George and Laurian also described techniques for V3 exposure, as follows. With the anterolateral approach, the tip of the C1 transverse process was identified first. The insertion of the SO was detached from the C1 transverse process and dissected superomedially above the V3. The C1 groove was identified and the VA was secured within the groove.[7] With the posterolateral approach, the midline of the posterior arch of the C1 and spinous process of C2 were identified first. The subperiosteal plane of C1 was exposed laterally from the midline toward the transverse process to identify the C1 groove and the V3 was secured in the groove.[4,7]
Ota et al. described a transcondylar fossa approach for bilateral V3 exposure to treat complex VA aneurysms. They used a multiple-layer technique for suboccipital muscle dissection and identified the posterior arch of C1, SO, and RCPM as the landmarks for V3.[20] Balik and Takizawa described a surgical technique for V3 exposure using multiple-layer muscle dissection and bipolar coagulation. In this approach, each suboccipital muscle indicated the anatomical landmark, aiding subsequent steps of the procedure. However, variations of the course and depth of the V3 lead to a high risk of iatrogenic injury during V3 exposure, especially with the single layer (myocutaneous) flap.[2] A bipolar cutting technique was not only used for harvesting donor arteries, such as the superficial temporal and occipital arteries, but also used for exposing the V3 during the far lateral approach.[25]
In this study, we used the V3 exposure technique similar to the technique described by Balik and Takizawa and used the bipolar cutting method described by Tokugawa et al.[25] to skeletonize the V3. We also described the anatomical relationship of the V3, the SOT, and the surrounding muscles. Safe and effective dissection techniques are described using clinical cases. We believe that when the anatomy of the suboccipital muscles relative to the V3 and occipital artery is well-studied, each suboccipital muscle acts as a roadmap to these important structures and facilitates a safe and fast exposure of these structures.[2] The arcuate foramen, which is an aperture formed by the presence of a complete bony bridge on the posterior arch of the atlas, may complicate V3 exposure. Ahn et al. reported an 8–13% incidence of the arcuate foramen.[1] In our study, no arcuate foramen was detected intraoperatively.
To the best of our knowledge, all previous studies regarding the course of V3, SOT, and suboccipital muscles were performed in cadavers; we found no clinical studies.[4,5,10,11,16,17,26,27,30] We evaluated the anatomy of the V3 relative to the SOT, SO, IO, and RCPM in clinical cases while the far lateral approach was performed to treat VA and PICA aneurysms. Thus, our study is the first to regard the anatomy of the V3 and surrounding muscles in clinical cases with detailed descriptions of surgical techniques.
Limitations of study
There are several limitations to our study. First, this study was a retrospective and descriptive one. Therefore, no comparative group for an accurate evaluation of the benefits of the technique was available. Second, a relatively small number of patients were studied because the operative video during V3 exposure was only recorded in cases when OA-PICA was performed. Finally, the measurements were not intraoperatively performed at the time of surgery but were measured from the operative videos.
CONCLUSION
Most of the V3, including the V3 bulge, were located beneath the SO and the inferomedial part of V3 located within the SOT. Elevation of the SO is the most-likely step to injure the V3; this step should be performed carefully using the bipolar cutting technique. To the best of our knowledge, this is the first study to describe the identification of the V3 relative to the SOT in clinical cases.
Footnotes
How to cite this article: Sriamornrattanakul K, Akharathammachote N, Chonhenchob A, Mongkolratnan A, Niljianskul N, Phoominaonin IS, et al. Course of the V3 segment of the vertebral artery relative to the suboccipital triangle as an anatomical marker for a safe far lateral approach: A retrospective clinical study. Surg Neurol Int 2022;13:304.
Contributor Information
Kitiporn Sriamornrattanakul, Email: kitiporn6823@gmail.com.
Nasaeng Akharathammachote, Email: nasaeng@hotmail.com.
Areeporn Chonhenchob, Email: areepornc@gmail.com.
Atithep Mongkolratnan, Email: doc.thep@gmail.com.
Nattawut Niljianskul, Email: nattawut@nmu.ac.th.
I-Sorn Phoominaonin, Email: popo.japan@gmail.com.
Chanon Ariyaprakai, Email: bear.ariyaprakai@gmail.com.
Somkiat Wongsuriyanan, Email: wong_sk2007@yahoo.com.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
REFERENCES
- 1.Ahn J, Duran M, Syldort S, Rizvi A, D’Antoni AV, Johal J, et al. Arcuate foramen: Anatomy, embryology, nomenclature, pathology, and surgical considerations. World Neurosurg. 2018;118:197–202. doi: 10.1016/j.wneu.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Balik V, Takizawa K. Safe and bloodless exposure of the third segment of the vertebral artery: A step-by-step overview based on over 50 personal cases. Neurosurg Rev. 2019;42:991–7. doi: 10.1007/s10143-019-01158-5. [DOI] [PubMed] [Google Scholar]
- 3.Bertalanffy H, Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815–21. doi: 10.1097/00006123-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Castillo C, Viñas FC, Gutikhonda M, Diaz FG. Microsurgical anatomy of the suboccipital segment of the vertebral artery. Neurol Res. 1998;20:201–8. doi: 10.1080/01616412.1998.11740507. [DOI] [PubMed] [Google Scholar]
- 5.Cengiz SL, Cicekcibasi A, Kiresi D, Kocaogullar Y, Cicek O, Baysefer A, et al. Anatomic and radiologic analysis of the atlantal part of the vertebral artery. J Clin Neurosci. 2009;16:675–8. doi: 10.1016/j.jocn.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira E, Rhoton AL, Jr, Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24:293–352. doi: 10.1016/0090-3019(85)90042-4. [DOI] [PubMed] [Google Scholar]
- 7.George B, Laurian C. Surgical possibilities in the third portion of the vertebral artery (above C2), Anatomical study and report of a case of anastomosis between subclavian artery and vertebral artery at C1-C2 level. Acta Neurochir Suppl (Wien) 1979;28:263–9. [PubMed] [Google Scholar]
- 8.George B. Extracranial vertebral artery anatomy and surgery. Adv Tech Stand Neurosurg. 2002;27:179–216. doi: 10.1007/978-3-7091-6174-6_5. [DOI] [PubMed] [Google Scholar]
- 9.Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559–62. doi: 10.3171/jns.1986.64.4.0559. [DOI] [PubMed] [Google Scholar]
- 10.La Rocca G, Altieri R, Ricciardi L, Olivi A, Della Pepa GM. Anatomical study of occipital triangles: The ‘inferior’ suboccipital triangle, a useful vertebral artery landmark for safe postero-lateral skull base surgery. Acta Neurochir (Wien) 2017;159:1887–91. doi: 10.1007/s00701-017-3300-3. [DOI] [PubMed] [Google Scholar]
- 11.Lang J, Kessler B. About the suboccipital part of the vertebral artery and the neighboring bone-joint and nerve relationships. Skull Base Surg. 1991;1:64–72. doi: 10.1055/s-2008-1056982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loukas M, Tubbs RS. An accessory muscle within the suboccipital triangle. Clin Anat. 2007;20:962–3. doi: 10.1002/ca.20452. [DOI] [PubMed] [Google Scholar]
- 13.Matsushima T, Kawashima M, Masuoka J, Mineta T, Inoue T. Transcondylar fossa (supracondylar transjugular tubercle) approach: Anatomic basis for the approach, surgical procedures, and surgical experience. Skull Base. 2010;20:83–91. doi: 10.1055/s-0029-1242193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushima T, Matsukado K, Natori Y, Inamura T, Hitotsumatsu T, Fukui M. Surgery on a saccular vertebral artery-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: Surgical results and indications for using two different lateral skull base approaches. J Neurosurg. 2001;95:268–74. doi: 10.3171/jns.2001.95.2.0268. [DOI] [PubMed] [Google Scholar]
- 15.Matsushima T, Natori Y, Katsuta T, Ikezaki K, Fukui M, Rhoton AL. Microsurgical anatomy for lateral approaches to the foramen magnum with special reference to transcondylar fossa (supracondylar transjugular tubercle) approach. Skull Base Surg. 1998;8:119–25. doi: 10.1055/s-2008-1058570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meybodi AT, Lawton MT, Benet A. Sequential extradural release of the V3 vertebral artery to facilitate intradural V4 vertebral artery reanastomosis: Feasibility of a novel revascularization technique. Oper Neurosurg (Hagerstown) 2017;13:345–51. doi: 10.1093/ons/opw015. [DOI] [PubMed] [Google Scholar]
- 17.Meybodi AT, Rincon-Torroella J, El-Sayed IH, Lawton MT, Benet A. Early localization of the third segment of the vertebral artery: The atlanto-mastoid line. Oper Neurosurg (Hagerstown) 2016;12:350–9. doi: 10.1227/NEU.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 18.Muralimohan S, Pande A, Vasudevan MC, Ramamurthi R. Suboccipital segment of the vertebral artery: A cadaveric study. Neurol India. 2009;57:447–52. doi: 10.4103/0028-3886.55610. [DOI] [PubMed] [Google Scholar]
- 19.Nayak SR, Swamy R, Krishnamurthy A, Dasgupta H. Bilateral anomaly of rectus capitis posterior muscles in the suboccipital triangle and its clinical implication. Clin Ter. 2011;162:355–6. [PubMed] [Google Scholar]
- 20.Ota N, Tanikawa R, Miyama M, Miyazaki T, Kinoshita Y, Matsukawa H, et al. A Contralateral transcondylar fossa approach with bilateral V3 segment exposure for repairing complex vertebral artery aneurysms. World Neurosurg. 2017;99:340–7. doi: 10.1016/j.wneu.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Rhoton AL., Jr The far-lateral approach and its transcondylar, supracondylar, and paracondylar extensions. Neurosurgery. 2000;47:S195–209. doi: 10.1097/00006123-200009001-00020. [DOI] [PubMed] [Google Scholar]
- 22.Sen CN, Sekhar LN. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197–204. doi: 10.1097/00006123-199008000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Sriamornrattanakul K, Akharathammachote N. The intersection between the sternocleidomastoid and splenius capitis as the anatomical landmark to facilitate occipital artery harvest: A retrospective clinical study. World Neurosurg. 2022;157:e364–73. doi: 10.1016/j.wneu.2021.10.096. [DOI] [PubMed] [Google Scholar]
- 24.Tayebi Meybodi A, Zhao X, Borba Moreira L, Lawton MT, Lang MJ, Labib M, et al. The inferior nuchal line as a simple landmark for identifying the vertebral artery during the retrosigmoid approach. Oper Neurosurg (Hagerstown) 2020;18:302–8. doi: 10.1093/ons/opz152. [DOI] [PubMed] [Google Scholar]
- 25.Tokugawa J, Ogura K, Yatomi K, Kudo K, Hishii M, Tanikawa R, et al. Bipolar cutting method: Another technique for harvesting donor artery with histological investigation. Oper Neurosurg (Hagerstown) 2018;14:16–9. doi: 10.1093/ons/opx086. [DOI] [PubMed] [Google Scholar]
- 26.Ulm AJ, Quiroga M, Russo A, Russo VM, Graziano F, Velasquez A, et al. Normal anatomical variations of the V3 segment of the vertebral artery: Surgical implications. J Neurosurg Spine. 2010;13:451–60. doi: 10.3171/2010.4.SPINE09824. [DOI] [PubMed] [Google Scholar]
- 27.Wanibuchi M, Fukushima T, Zenga F, Friedman AH. Simple identification of the third segment of the extracranial vertebral artery by extreme lateral inferior transcondylar-transtubercular exposure (ELITE) Acta Neurochir (Wien) 2009;151:1499–503. doi: 10.1007/s00701-009-0360-z. [DOI] [PubMed] [Google Scholar]
- 28.Wen HT, Rhoton AL, Jr, Katsuta T, de Oliveira E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg. 1997;87:555–85. doi: 10.3171/jns.1997.87.4.0555. [DOI] [PubMed] [Google Scholar]
- 29.Wongsuriyanan S, Sriamornrattanakul K. Blind-alley formation and occipital artery-posterior inferior cerebellar artery bypass for the treatment of unclippable vertebral artery aneurysms with posterior inferior cerebellar artery involvement. World Neurosurg. 2020;138:e539–50. doi: 10.1016/j.wneu.2020.02.174. [DOI] [PubMed] [Google Scholar]
- 30.Youssef AS, Uribe JS, Ramos E, Janjua R, Thomas LB, van Loveren H. Interfascial technique for vertebral artery exposure in the suboccipital triangle: The road map. Neurosurgery. 2010;67(Suppl 2):355–61. doi: 10.1227/NEU.0b013e3181f741f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Further dissection of the suboccipital muscle to expose the V3 segment of the vertebral artery.


