Abstract
Background
Oral anti-cancer treatments such as adjuvant endocrine therapies (AET) for breast cancer survivors are commonly used but adherence is a challenge. Few low-touch, scalable interventions exist to increase ET adherence.
Purpose
To evaluate the acceptability, feasibility, and initial efficacy of a low-touch, remotely-delivered values plus AET education intervention (REACH) to promote AET adherence.
Methods
A mixed-methods trial randomized 88 breast cancer survivors 1:1 to REACH or Education alone. Wisepill real-time electronic adherence monitoring tracked monthly AET adherence during a 1-month baseline through 6-month follow-up (FU) (primary outcome). Patient-reported outcomes were evaluated through 3- and 6-month FU (secondary). Multiple indices of intervention feasibility and acceptability were evaluated. Qualitative exit interviews (n = 38) further assessed participants’ perceptions of feasibility/acceptability and recommendations for intervention adaptation.
Results
The trial showed strong feasibility and acceptability, with an eligible-to-enrolled rate of 85%, 100% completion of the main intervention sessions, and “good” intervention satisfaction ratings on average. For Wisepill-assessed AET adherence, REACH outperformed Education for Month 1 of FU (p = .027) and not thereafter. Participants in REACH maintained high adherence until Month 4 of FU, whereas in Education, adherence declined significantly in Month 1. Conditions did not differ in self-reported adherence, positive affective attitudes, future intentions, or necessity beliefs. REACH trended toward less negative AET attitudes than Education at 3-month FU (p = .057) reflecting improvement in REACH (p = .004) but not Education (p = .809). Exploratory moderator analyses showed that average to highly positive baseline AET affective attitudes and oncologist-patient communication each predicted higher adherence following REACH than Education; low levels did not. Participants identified recommendations to strengthen the interventions.
Conclusions
REACH, a low-touch values intervention, showed good feasibility and acceptability, and initial promise in improving objectively-assessed AET adherence among breast cancer survivors (relative to education alone). Future research should target improving REACH’s tailoring and endurance.
Keywords: Breast cancer, Endocrine therapy, Adherence, Acceptance and commitment therapy, Values, Real-time adherence monitoring
This study showed that having breast cancer survivors reflect on who and what they care about (their values), and design a personal values sticker for their anti-cancer medication pillbox, was feasible, and led them to more regularly take the medication than providing education about it.
Breast cancer is a leading cause of cancer death for women worldwide [1]; however, mortality has declined over time due to earlier detection and the widespread introduction of treatments such as adjuvant endocrine therapy (AET). AET is an adjuvant treatment for approximately 80% of breast cancer survivors with hormone receptor-positive breast cancers [2]; AET lowers the risk of breast cancer recurrence by approximately 40–50%, consequently reducing mortality [3, 4]. Current guidelines recommend taking one AET pill daily for 5 to 10 years [5]. Despite its beneficial effects, less than half of breast cancer survivors prescribed AET adhere to the medication in the dose, duration, and frequency prescribed [3, 6, 7]. Failure to adhere to AET for five years or suboptimal day-to-day adherence is associated with greater breast cancer recurrence and death [3, 4], highlighting the importance of adherence. The pronounced benefits of AET, coupled with high nonadherence, warrant the development of innovative and disseminable interventions to improve adherence.
Among breast cancer survivors, research suggests that being middle-aged (vs younger or older) and non-Latina white are associated with greater AET adherence [7, 8], whereas higher medication cost, comorbidity, and side effects are associated with lower adherence [9–12]. Psychosocial correlates of AET adherence are potentially malleable and have been shown to be important predictors of adherence to long-term medication regimens for other illnesses [13–15]. Two recent reviews revealed that more positive affective attitudes or emotions toward AET, greater self-efficacy, greater perceived need for the medication, and a more positive patient-healthcare provider relationship are each associated with greater AET adherence among breast cancer survivors [12, 16]. The present intervention thus targets an important, potentially modifiable predictor of AET adherence—affective attitudes or emotions toward AET.
The most recent Cochrane review of intervention studies to increase medication adherence revealed that only 1 of 182 studies focused on a sample with cancer [17], and a recent review of 49 studies on medication adherence in medically diverse populations did not include any in cancer [18]. Further, a recent meta-analysis and systematic review of the limited extant interventions to promote AET adherence among breast cancer survivors revealed overall null effects [19, 20]; however, interventions that were comprised of more than education (e.g., that incorporated motivational or bi-directional elements) demonstrated statistically significant, moderate effects, which is consistent with adherence intervention research in other chronic medical conditions, such as HIV [21]. The authors concluded that “participants may benefit from enhanced motivation [interventions]…presented in an individually tailored way” (p. 260; [19]). That stated, the few successful interventions were intensive, complex, and costly, rendering them poor candidates for dissemination. Among the more scalable, unidirectional interventions (which showed null effects), none were tested that included content beyond education or appointment reminders.
The current study addresses the critical need for interventions that can enhance AET adherence in individually tailored ways and provide more than education, yet also remain highly efficient and scalable. To address these gaps, we developed an innovative, low-touch (e.g., little to no human interaction), personalized values approach for promoting AET adherence among breast cancer survivors. In two meta-analyses across a variety of health behaviors, interventions that affirmed personal values were linked to a reliable, albeit small, aggregate effect on intentions to change behavior and on actual behavior change (e.g., diet, alcohol use) (e.g., [22]). Affirming values also facilitates greater openness to health-promotion information, reduces a sense of threat [23], and increases positive other-directed feelings [24], and thus might help cancer survivors to appreciate the importance of AET adherence.
Other values-based behavior change studies have drawn on Acceptance and Commitment Therapy (ACT; [25, 26]), a widely studied intervention that leverages personal values to motivate behavior change in personally meaningful directions. Within a cancer survivor population, an ACT telehealth intervention for colorectal cancer survivors led to greater physical activity, weight loss, and vegetable consumption over the following 6 to 12 months, compared to usual care [27]. Personal values in ACT represent autonomously chosen and enduring, as opposed to imposed and transitory, sources of positive reinforcement [25, 26] and thus offer the potential to support the sustained behavior change required to adhere to AET for the recommended period. REACH values content was thus informed by ACT as well as self-affirmation theory [23].
Although interventions that affirm or leverage personal values have been used to promote engagement in a range of health behaviors, to our knowledge, only one major trial has targeted medication adherence and none has targeted medication adherence among adults diagnosed with cancer. This one major randomized trial focused on improving medication adherence among African American adults with hypertension by affirming personal values and increasing positive affect [28]. This intervention significantly increased hypertension medication adherence (relative to education alone), mediated by increases in self-efficacy [29].
The Current Study
The current study seeks to address important gaps in the AET literature and build on the nascent evidence base on values-focused interventions for adherence behaviors by conducting a pilot randomized controlled trial of a novel, brief values intervention (REACH or Resources and Education for Adherence to Cancer Hormonal therapy) to support AET adherence. REACH aims to increase daily adherence to AET by increasing positive affective attitudes toward AET and reducing negative ones, through an intervention that connects patients to their personal sources of value/meaning for taking the medication and has them design a personal values-based ‘cue-to-action’ sticker for their AET pillbox. The current trial compares REACH, which includes the values intervention plus AET education, to an AET education-only control condition. REACH is delivered online and by post-mail, representing a remotely-delivered intervention. This study represents the first evaluation of the REACH intervention, potentially addressing a vital gap in low-cost, innovative strategies to increase AET adherence.
REACH extends research on interventions to promote AET adherence in four ways: first, by targeting breast cancer survivors who evidenced difficulties with AET adherence (as opposed to all breast cancer survivors taking AET); second, by using exclusively online and post-mail intervention delivery, which offers strong potential for scalability; third, by using multiple values-based strategies to promote AET adherence that are novel in content and scope (in that they extend beyond the education-focused unidirectional interventions tested previously); and fourth, by evaluating AET adherence through 6-month follow-up with Wisepill, a new-generation wireless medication adherence monitoring device. Wireless medication adherence monitoring devices to date have largely been used to monitor infectious disease treatment (i.e., HIV, tuberculosis), and thus this represents a novel application of this technology to cancer treatment. Use of electronic monitoring devices alone (e.g., without additional feedback) has been shown to not significantly affect medication adherence [30]. Thus, Wisepill could be used to track AET adherence without concern that it would significantly interfere with the REACH intervention of interest.
The current mixed-methods report evaluates the feasibility, acceptability, and preliminary efficacy of REACH relative to education alone. This report builds on earlier, pre-trial pilot and feasibility testing with both patients and providers (see Supplementary Appendix), reflecting the NIH ORBIT model Stages Ia-b for developing, refining, and evaluating behavioral interventions [31]. The current report focuses on ORBIT Phase IIb, characterized by pilot trials. In this randomized pilot trial, multiple indices of intervention feasibility appropriate to this phase were assessed, including aiming that at least 80% of study-eligible breast cancer survivors who contacted the study team enroll in the study, and among study enrollees, that at least 80% complete their assigned intervention. Two acceptability measures were assessed, with the goal of at least moderate scores on each, reflecting the low-touch nature of the interventions. Relative to the education-only control group, the REACH intervention was hypothesized to improve monthly AET adherence assessed by Wisepill through 6-month follow-up (primary outcome), as well as self-reported adherence, positive and negative affective attitudes about AET, intentions to adhere to AET in the future, and AET nonpersistence (secondary outcomes). Exploratory baseline moderators of the primary outcome, informed by known predictors of AET adherence [32], were examined, including positive and negative affective AET attitudes and oncologist-patient communication quality. Finally, we integrated qualitative findings from structured exit interviews conducted with a subset of participants, based on thematic coding of their perceptions of feasibility, acceptability, and suggestions for how to further refine the interventions.
Methods
Study Design
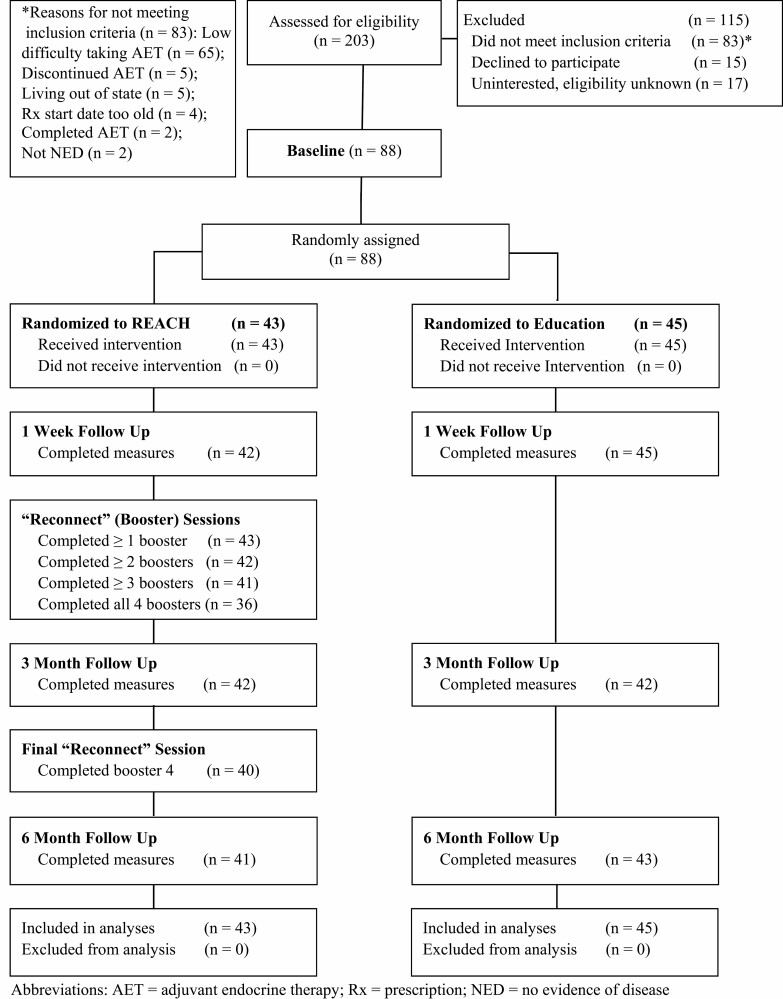
In this trial, 88 hormone receptor-positive breast cancer survivors with at least moderate difficulty adhering to AET (see Screener) were randomly assigned 1:1 to the REACH (Education+Values) intervention (n = 45) or an Education only control group (n = 43); see Fig. 1 CONSORT. Randomization was stratified by AET type (tamoxifen vs. aromatase inhibitors [AI]) using a computer-generated permuted block sequence that was uploaded into REDCap and not accessible to the study team prior to randomization. Thus, the team only accessed the randomization for the particular participant and not for future participants. The study was preregistered on Clinicaltrials.gov (NCT03980093). Wisepill-assessed adherence was monitored during a month-long baseline through 6-month follow-up. Patient-reported secondary outcomes were assessed at baseline (Pre [pre-intervention]), 1-week post-intervention (Post), 3 months after the intervention (3 m FU), and 6-month follow-up (6 m FU). Participants were compensated $20 for completing each outcome measures survey, plus a $5 bonus for completing it within 36 hours of receipt; they were not compensated for using Wisepill or for completing the intervention. The University of Colorado Boulder Institutional Review Board (IRB) approved the study (protocol #18-0234) and served as the IRB of record.
Fig. 1.
CONSORT diagram.
Participants
Study participants (Table 1) were recruited through Pueblo, Boulder, and Colorado Springs clinics of Rocky Mountain Cancer Centers (RMCC), the largest network of community oncology practices in Colorado, through targeted study mailings and flyers placed in clinics. Clinic providers/staff did not generally discuss the study with patients nor did they refer or recruit patients to the study. Recruitment occurred between February 2019 and January 2020. Study inclusion criteria were: (1) Women, age 21 or over, diagnosed with stages 0 to 3 hormone receptor-positive breast cancer, and finished with primary treatment; (2) Prescribed AET (including AI and tamoxifen) within the past 2.5 years, had already taken AET for at least 1 month, and had been prescribed to take it for at least 1 more year; (3) Reported difficulty taking their AET prescription (see Screener) or reported missing 2 or more pills in the past month; (4) Had internet access. Professional research coordinators at the Master’s-level or at the postbaccalaureate-level with 5 or more years of research experience screened and consented participants by phone. In both the screener and consent form, participants were informed that the study goal was to help support them in taking their AET medication and to help them maximize the benefits of the medication.
Table 1.
Sample baseline sociodemographic and medical characteristics (n = 88)
| Total(n = 88) | REACH (n = 43) | Education (n = 45) | Between Group | ||
|---|---|---|---|---|---|
| t or χ 2 | p value | ||||
| Sociodemographic characteristics | |||||
| Female | 100.00% (88/88) | 100.00% (43/43) | 100.00% (45/45) | ||
| Age (in years, Range: 31–81) | M = 58.34 (SD = 10.37) | M = 57.77 (SD = 11.22) | M = 58.89 (SD = 9.58) | .51 | .62 |
| Race/Ethnicity | .211 | .65 | |||
| White/Caucasian & Non-Latina | 92.05% (81/88) | 90.70% (39/43) | 93.33% (42/45) | ||
| Hispanic/Latina | 3.41% (3/88) | 4.65% (2/43) | 2.22% (1/45) | ||
| Biracial | 1.14% (1/88) | 2.33% (1/43) | 0.00% (0/45) | ||
| Native American/ Alaskan Native | 1.14% (1/88) | 2.33% (1/43) | 0.00% (0/45) | ||
| Black/African American | 0.00% (0/88) | 0.00% (0/43) | 0.00% (0/88) | ||
| Other | 2.27% (2/88) | 0.00% (0/43) | 4.44% (2/45) | ||
| Education (median) | Bachelor’s degree | Associate degree | Bachelor’s degree | 1.45 | .15 |
| Household income (median) | $71,000–$80,000 | $71,000–$80,000 | $61,000–$70,000 | –.13 | .90 |
| Married or partnered | 76.14% (67/88) | 79.07% (34/43) | 73.33% (33/45) | .40 | .53 |
| Children (1 or more) | 76.14% (67/88) | 81.40% (35/43) | 71.11% (32/45) | 1.28 | .26 |
| Cancer treatment history | |||||
| Months from end of treatment (Range 1–36) | M = 13.83 (SD = 8.54) | M = 14.42 (SD = 9.38) | M = 13.33 (SD = 7.64) | –.64 | .52 |
| % who received: | |||||
| 1)Surgery | 1) 100.00% (88/88) | 1) 100.00% (43/43) | 1) 100.00% (45/45) | ||
| 2)Chemotherapy | 2) 34.09% (30/88) | 2) 39.53% (17/43) | 2) 28.89% (13/45) | 2)1.11 | 2).29 |
| 3)Targeted Therapy | 3) 10.23% (9/88) | 3)6.98% (3/43) | 3) 13.33% (6/45) | 3).97 | 3).33 |
| 4)Radiation | 4) 68.18% (60/88) | 4) 69.77% (30/43) | 4) 66.67% (30/45) | 4).10 | 4).76 |
| 5)Ovarian Suppression | 5) 6.82% (6/88) | 5) 6.98% (3/43) | 5) 6.67% (3/45) | 5).00 | 5)1.00 |
| % BL AET Type: | |||||
| 1)Aromatase Inhibitors | 1) 62.50% (55/88) | 1) 65.12% (28/43) | 1) 60.00% (27/45) | .25 | .62 |
| 2)Tamoxifen | 2) 37.50% (33/88) | 2) 34.88% (15/43) | 2) 40.00% (18/45) | ||
| Months between initial AET Rx date & enrollment | M = 13.11 (SD = 8.29) | M = 13.74 (SD = 8.21) | M = 12.51 (SD = 8.41) | –.70 | .49 |
| Cancer stage | |||||
| 0 | 5.68% (5/88) | 6.98% (3/43) | 4.44% (2/45) | .392 | .53 |
| I | 71.59% (63/88) | 67.44% (29/43) | 75.56% (34/45) | ||
| II | 18.18% (16/88) | 25.58% (11/43) | 11.11% (5/45) | ||
| III | 4.55% (4/88) | 0.00% (0/43) | 8.88% (4/45) |
Note: BL = baseline; AET = adjuvant endocrine therapy, Rx = prescription
1The χ2 compared the portion of white, non-Latina participants to minority-identified participants
2The χ2 compared cancer stage categories of 0–I to II–III
Interventions
The two self-paced online interventions were designed and distributed using Qualtrics [33]. The programs used interactive features to create a personalized and engaging experience.
Education intervention
The online Education intervention (called My Medication Guide) consisted of a single ~15-minute session that provided information about AET and the importance of AET adherence, including highlighting its role in preventing breast cancer recurrence. Branching mechanisms allowed participants to select which side effects they were most concerned about (e.g., hot flashes, joint or muscle pain, vaginal dryness, etc.) and then learn tips for managing them, creating a personalized experience. The intervention also encouraged participants to discuss their AET concerns and side effects with their oncology care team. Quiz questions checked for understanding and promoted engagement. Educational content was adapted from existing publicly available AET education materials (e.g., National Cancer Institute, www.cancer.gov) in collaboration with RMCC oncologists and pharmacists.
REACH (education+values) intervention
REACH (called Take Today, Thrive Tomorrow) consisted of a single 30- to 40-minute main online session and four 5- to 10-minute online ‘booster’ sessions called Reconnect sessions, administered at 1.5, 6, 12, and 20 weeks following the main online session. The main online session included three sets of values exercises informed by self-affirmation theory and ACT (see Table 2): (1) health-focused brief values affirmation followed by AET education, per above; (2) values-based perspective-taking exercises on taking AET; and (3) the creation of the personalized values sticker for the Wisepill box. In creating the values sticker, each participant selected a background color and uploaded a photo (recommended) or phrase that represented their primary motivation for taking AET. An example of the sticker with the text “I take these for:” was projected at the top of the screen with the uploaded photo or phrase below (Supplementary Fig. 1). The study team printed the sticker, post mailed it to the participant, and asked her to affix it to her Wisepill box. To confirm, the team asked participants to email or text a photo of the box with attached sticker.
Table 2.
REACH (education+values) main online session content
| Content | Intended function | |
|---|---|---|
| Part 1. Clarify and affirm values in domain of health and self-care | Participants identify values “that specifically motivate you to take care of yourself” using interactive content, and write freely about how the value is important to them, including examples of how it influenced what they did. | Clarify and affirm values prior to AET education, per studies showing more openness to health information after values affirmation [59, 60], but with a focus on health domain-specific (not general) values |
| Part 2. AET education | Described in Education Intervention (see Methods). | Educate participants about AET’s purpose, the importance of adherence, and how to manage common side effects; designed as an active control |
| Part 3. Values and perspective-taking | Participants complete interactive, ACT-based exercises that draw on values and perspective-taking linked to motivation for AET adherence. | Link core personal values to the value of adhering to AET, thereby bolstering personal motivation for AET adherence |
| Part 4. Creating a visual reminder | Participants select the top value that motivates them to take AET, upload a photo or phrase that captures this value, and design a sticker that features it to place on their Wisepill box. | Visually reinforce the link between participants’ values and adhering to AET by creating a positive, affective “cue to action” in the form of a values sticker |
Participants were emailed links to the online booster Reconnect Sessions, which applied ACT strategies such as a values journaling exercise and the Choice Point [34] to reinforce AET adherence. Each participant’s individual responses to the main REACH session were incorporated into her booster, reminding her of her own values and motivations for taking AET. The session also included an option to see an online graph of her monthly Wisepill adherence data, a study innovation.
Measures
Apart from the Screener, which was administered by phone, all measures were administered in REDCap [35], a secure online platform that was used in a HIPAA-compliant manner. Assessments were automated in REDCap. The study team was not blind to condition as they needed to field support calls re: Wisepill, but they did not administer any assessments.
Screener
The Screener inquired whether potential participants had missed taking any AET pills in the past month, and whether any factors (from a list) made taking AET difficult. The list was based on common reasons for AET nonadherence from the literature (e.g., [36–38]) and ranged from “simply forgot” to “it was expensive” to “didn’t feel prepared by my doctor to take it”; participants could also provide their own reasons. To be eligible, women had to either report at least one factor that made taking AET at least moderately difficult for them or missing 2 or more pills in the past month.
Feasibility Indices
As noted, the study’s feasibility goal was to have 80% of study-eligible breast cancer survivors who contacted the study team enroll in the study, and 80% of enrollees complete their online intervention’s main session. In REACH, a further goal was to have 80% of enrollees complete all four Reconnect sessions.
Acceptability Measures
The Client Satisfaction Questionnaire, a widely used 9-item measure of intervention satisfaction and acceptability, was slightly adapted to reflect the current interventions (1 to 4 Likert scale, current α = .95). The Intervention Feedback Questionnaire, a piloted acceptability measure [39] designed to be adapted to a given intervention, consisted of 8 items for REACH (1 to 5 Likert scale, α = .91) and 2 items for Education (α = .84). Both were administered at Post. The goal was at least moderate satisfaction on these two measures, a goal that reflected the low-touch nature of the interventions.
Primary Outcome
Wisepill-monitored monthly adherence
The study’s primary outcome was Wisepill-assessed AET adherence, tabulated as the percent of days that Wisepill was opened on a 30-day basis from the 1-month baseline period through each month of 6-month FU. Wisepill is a real-time electronic adherence monitoring device (4.33” x 1.77” x.47”) that when opened sends real-time wireless data to a protected online server. As noted, this represents a novel application of Wisepill to cancer treatment. In HIV populations, Wisepill evidences high validity for examining adherence comparable to other gold standard adherence measures and virologic indicators of adherence [40]. Wisepill has reliability similar to that of other adherence tools (e.g., MEMS caps) with greater feasibility and acceptability compared to MEMS caps, and has also been found to be superior to unannounced pill count and patient self-report [41, 42]. Wisepill was used as an assessment strategy and although it has an electronic reminder capacity, it was not activated in order to minimize the potential intervention effects of Wisepill. Participants were instructed to store their AET medication in the device; only observations where the device battery was functional were included.
Secondary Outcome Measures
Affective attitudes about AET
Previously developed scales assessing positive and negative emotions about AET [43] measured five positive (happy, calm, enthusiastic, comforted, accepting; baseline α = .81) and five negative (sad, annoyed, tense, reluctant, angry; baseline α = .72) emotions and the extent to which they “describe your feelings toward anti-hormonal therapy”. Responses ranged from 0 = does not describe, 1 = slightly describes, 2 = definitely describes and were scored by averaging items on each of the positive and negative scales.
Self-reported AET adherence
We used a previously validated medication adherence item [44] that asked: “How many anti-hormonal therapy pills have you missed over the past 30 days?” The response choices listed every number individually from 0 to 30 (i.e., 0, 1, 2….30). Self-report measures of endocrine therapy are moderately positively correlated with objective adherence outcomes [45] and this single item adherence measure demonstrated convergent validity with the widely used self-report Morisky Medication Adherence scale [44].
AET future intentions
An item about AET adherence was adapted from previous studies (e.g., [46]) to assess, “How likely is it that you will be taking your anti-hormonal therapy several years from now (that is, for the whole period that it’s prescribed)?” Response anchors were 1 = not at all likely, 4 = neither likely nor unlikely, 7 = very likely.
Wisepill-assessed AET nonpersistence
Using Wisepill, we assessed AET nonpersistence, defined as stopping AET for a period that lasted through 6m FU and was confirmed in the medical record as permanently discontinued. Participants who did not open their Wisepill box for at least 1 week were contacted to confirm whether or not they had stopped taking their medication.
Exploratory Moderators of the Primary Outcome (Wisepill-assessed Monthly Adherence)
The 6-item validated subscale (“component 1”) from the Quality of Community Questionnaire [47] assessed the quality of oncologist-patient communication, and was evaluated as a moderator of the primary outcome. Affective attitudes toward AET at baseline, using the scales described above, were also tested as moderators of the primary outcome.
Side Effects
We administered the Patient-Report Outcome Version of the Common Terminology for Adverse Events (PRO-CTCAE; [48]) at baseline, tailored to assess AET-specific side effects [43]. The total number of side effects rated as mild or greater in presence or severity was covaried in the outcome analyses, though findings were nearly identical with vs. without it.
Sample Size and Analytic Approach for Outcomes
A priori power calculations were conducted using Optimal Design Software Version 3.01 [49]. A sample size of 32 per condition at 6m FU was estimated to provide 80% power to detect a mean difference in slopes of 2.5 percentage points per month, which represents a medium effect size. Assuming 80% retention at 6m FU, we aimed to recruit 80 participants. Recruitment exceeded this goal by 10%, resulting in a final sample of 88 participants.
Baseline participant characteristics in the two conditions were compared using independent t tests for continuous and χ2 tests for categorical variables. The original plan called for analyzing the Wisepill adherence data using a linear mixed model. However, the observed monthly adherence values were strongly negatively skewed, with most values at or near 100%, resulting in serious violation of the normality assumption. Therefore we used a fractional logit model [50], which is designed for modeling fractional dependent variables that can take values on the interval [0,1]. The model included month (as a discrete variable), condition, and their interaction. Cluster robust variance estimation was used to account for repeated measures within participant. Self-reported AET adherence was similarly negatively skewed and analyzed using the same approach. Secondary outcomes were analyzed using linear mixed models with fixed effects for timepoint (Pre, Post, 3m FU, 6m FU), condition, and their interaction, and a random intercept for participant. As planned a priori, type of AET (tamoxifen vs. AI) and the presence of mild or greater side effects were covaried in all analyses. For the exploratory moderator analyses, each moderator was tested separately by including a three-way interaction between the moderator, time (month), and condition. Analyses were performed in Stata/SE 15.1.
Qualitative Exit Interviews
After participants finished the study, we received feedback about the interventions that was largely positive but diverse in content. We thus decided to conduct exit interviews to gain a more in-depth view of participants’ experiences in the study. We conducted exit interviews with 38 participants (n = 21 REACH, n = 17 Control) after the 6m FU (mean interview length = 37 minutes). Based on their Client Satisfaction Questionnaire scores and written reflections on their intervention, all participants were stratified into (largely) negative, neutral, or positive feedback categories; 23 interview participants were randomly selected within strata. As most responses were neutral to positive, this approach oversampled those with negative feedback but ensured that the full range of experiences were represented. As we wished to increase the interviewee sample size but had already sampled most individuals with negative experiences, another 15 participants were selected at random using a random number generator.
Interviews were conducted by phone by four trained post-baccalaureate interviewers using a structured interview guide adapted from prior adherence research [51], then transcribed verbatim. Analysis occurred in an iterative team-based process involving three independent coders using established qualitative content methods and reflexive team analysis ([52, 53]; see Supplementary Appendix for details); disagreements were resolved by consensus. The software program ATLAS.ti v9.1.1 was used for data management. The analysis reported here focuses on themes related to feasibility, acceptability, and suggested intervention adaptations, particularly for REACH, regarding dose, helpfulness, amount and quality of information, and any additional support that might be needed.
Results
See Table 1 for participant characteristics, which did not differ by condition.
Feasibility: Enrollment and Intervention Completion
As shown in Fig. 1, of the 103 study-eligible individuals who responded to the study mailings, 85.44% (88/103) enrolled, exceeding the study’s 80% recruitment feasibility goal. Further, 100% (88/88) of participants across both conditions completed the main online intervention session and 95.45% (84/88) were retained through 6m FU. Median time to completion for the main online intervention session was 13.83 minutes (interquartile range = 10.09–26.58 minutes) for Education and 34.59 minutes (interquartile range = 22.68–64.88 minutes) for REACH. Supplementary Table 1 lists the AET side effects participants indicated concern about during the AET Education portion, which were similar across conditions.
All REACH participants (43/43) put their values sticker on their Wisepill box and completed at least one Reconnect session; 83.72% (36/43) completed all four Reconnect sessions once or more (sessions could be repeated if desired), exceeding the 80% feasibility goal. Most (93.02%) chose to view their Wisepill adherence graph at least once (see Supplementary Table 2).
Acceptability: Intervention Satisfaction Ratings
At Post, participants in both conditions rated the interventions similarly on the Client Satisfaction Questionnaire (Education M = 2.84, SD = .73; REACH M = 2.95, SD = .58, t[85] = .75, p = .46), with scores indicating “good” satisfaction, on average. Participants in both conditions also gave similar ratings on the Intervention Feedback Questionnaire (Education M = 3.14, SD = 1.08; REACH M = 3.43, SD = .98, t[85] = 1.31, p = .19), with scores indicating that, on average, the interventions helped between a “a moderate amount” and “quite a bit”.
Baseline Differences
The conditions did not differ at baseline on the outcome variables, ps > .14 (Supplementary Table 3).
Primary Outcome: Wisepill-assessed AET Adherence
As shown in Table 3, compared to baseline, REACH led to significantly greater Wisepill-assessed AET adherence than Education in the first month of FU (condition by time interaction p = .027). Condition differences did not reach significance for FU months 2 to 6 (ps > .130). However, in Education, relative to baseline, Wisepill-assessed adherence declined significantly from baseline every month of FU, from Month 1 to 6. By contrast, in REACH, adherence remained stably high during FU Months 1 to 3, and did not decline significantly from baseline until Month 4.
Table 3.
Primary outcome findings for Wisepill-assessed adherence (percent of days that Wisepill was opened on a 30-day monthly basis)
| Time | Education only | REACH intervention | Condition Differences | |||||
|---|---|---|---|---|---|---|---|---|
| Mean % Monthly Adherence (SE) (n = 45) | Change from baseline (SE) | Within Education change from baseline p value | Mean % MonthlyAdherence (SE) (n = 43) | Change from baseline (SE) | Within REACH change from baseline p value | Between-condition difference in change from baseline (SE) | Between-condition difference in change from baseline p value | |
| Baseline | 95.17 (3.38) | 95.31 (3.46) | ||||||
| Month 1 | 87.79 (3.39) | –7.11 (3.41) | .017 | 96.32 (3.46) | 1.01 (1.45) | .506 | 8.12 (3.71) | .027 |
| Month 2 | 86.67 (3.39) | –8.23 (3.26) | .004 | 94.12 (3.43) | –1.02 (2.16) | .641 | 7.21 (3.92) | .131 |
| Month 3 | 88.49 (3.39) | –6.41 (3.50) | .035 | 89.85 (3.43) | –5.29 (3.83) | .125 | 1.22 (5.24) | .889 |
| Month 4 | 87.18 (3.39) | –7.72 (3.59) | .013 | 82.02 (3.43) | –13.12 (4.19) | <.001 | –5.40 (5.61) | .423 |
| Month 5 | 84.46 (3.41) | –10.66 (4.17) | .003 | 81.31 (3.46) | –13.97 (4.97) | .001 | –3.30 (6.57) | .669 |
| Month 6 | 82.77 (3.41) | –12.24 (4.35) | .001 | 80.28 (3.49) | –14.44 (4.70) | <.001 | –2.20 (6.50) | .757 |
Note: Controlled for AET type and AET side effects (see Methods). However, neither was associated with adherence in these models; p-values >.50
Change from baseline and difference in change from baseline were obtained as average marginal effects, averaged over covariates.
Secondary Outcomes
Secondary outcome findings are reported in Supplementary Tables 4–6 and described briefly below.
Self-reported AET adherence
As shown in Supplementary Table 4, self-reported adherence did not differ between conditions at any point, ps > .63. Within condition, both REACH and Education showed significant declines in self-reported adherence by 6m FU. Compared to Wisepill adherence, both groups overestimated their adherence on average, but Education participants overestimated their adherence more (M = 7.43%, 95% CI 3.57 to 11.29%) than REACH participants (M = 2.70%, 95% CI 0.91–4.49%), a significant condition difference, p = .03, t(84) = 2.21.
Affective attitudes toward AET
As presented in Supplementary Table 5, change in negative affective attitudes toward AET did not significantly differ between conditions at Post or 6m FU relative to baseline. At 3m FU, condition differences from baseline approached a greater drop in negative affective attitudes in REACH compared to Education, p = .057, paralleled by within-condition findings of improved negative attitudes toward AET in REACH at both 3m FU (p = .004) and 6m FU (p = .029) and no change in negative attitudes in Education at any point (ps > .62).
Per Supplementary Table 5, both REACH and Education showed significant or trend increases in positive affective attitudes toward AET at 3 and 6m FU, with Education also showing significant increases at Post. The conditions did not significantly differ in positive affective attitudes toward AET at any point, ps > .13.
AET future intentions
Future AET intentions did not significantly differ by condition at any point. Within condition, REACH showed no change from baseline whereas Education showed trend-level increases from baseline to Post and 3m but not 6m FU (Supplementary Table 6).
Wisepill-assessed AET nonpersistence
Across the entire sample, 6 individuals did not persist in taking AET, including 4 in REACH and 2 in Education. Due to the small number of AET nonpersisters, this outcome was not analyzed.
Exploratory Moderator Findings
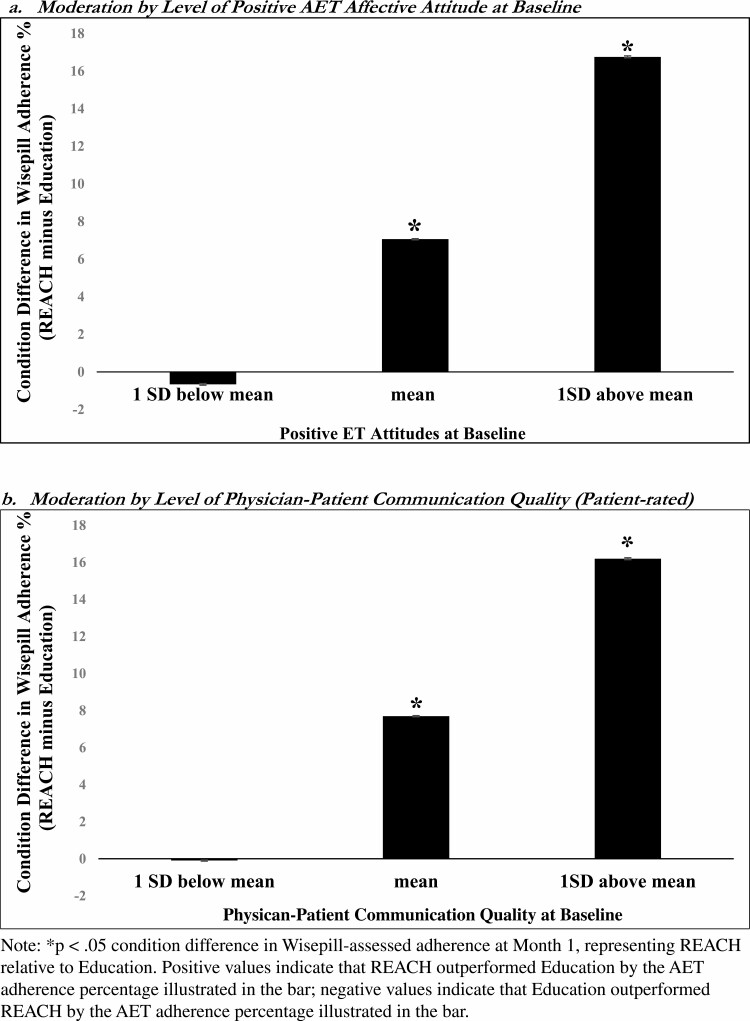
More positive affective attitudes toward AET (b = .44, SE = .18, CI95% = .09–.80, p = .01) and better oncologist-patient communication quality (b = .82, SE = .19, CI95% = .44–1.20, p < .001) at baseline moderated the condition effects on Wisepill-assessed adherence in Month 1 of FU. Specifically, compared to Education, REACH led to significantly higher AET adherence for those at average and higher (+1SD above mean) levels of these moderators (ps ≤.01) but not for those at lower levels (–1SD below, ps > .50), see Fig. 2. Negative affective attitudes toward AET at baseline did not moderate condition effects on adherence (b = –.10, SE = .20, CI95% = –.50–.30, p = .62).
Fig. 2.
Condition differences in Wisepill-assessed AET adherence at FU month 1 for different levels of the moderators (REACH minus education).
Qualitative Themes Identified in the Exit Interviews
Themes from the 38 exit interviews are highlighted first with regard to AET education, which was relevant to both conditions, followed by themes about the broader program, with a focus on REACH.
Suggested Adaptations to the Intervention’s Online Content or Delivery
The most common response to questions about suggested changes to the online program was that participants found the online interventions to be sufficient and wanted no changes (n = 10 total; n = 7 REACH): “I think it was quite user-friendly and easy to navigate and was written in language that is understood by the lay person…it was in depth enough.” (Education Participant 1007). However, some respondents recommended specific improvements to the online programs, particularly:
More information on how to manage side effects
Some participants (n = 8 total; n = 3 REACH) wished for more specific, concrete information about managing their AET side effects:
Maybe go deeper with the side effect as to what you can significantly do and try. Structure the materials that you provide in a way that would be more customized. (Education Participant 1060).
A smaller number (n = 3 total; n = 1 REACH) wanted the intervention to link to external resources on AET and breast cancer such as reputable websites or a list of useful books.
Suggested Changes to the Intervention in the Form of Provider Contact
To probe whether REACH or Education, neither of which included any provider contact, may have been more powerful or efficacious with such contact, participants in both conditions were asked whether they would have liked to review or discuss their responses to the online program with a supportive professional (and if so, to whom, how often, and about what).
No interest in speaking with a supportive professional
Many participants (n = 21 total; n = 13 REACH) indicated that they would not have wanted to speak with a supportive professional as they had sufficient resources already. However, some of them (n = 7 total; n = 4 REACH) noted that they thought speaking to a supportive professional would be helpful to other breast cancer survivors taking AET:
…I think for me personally no, but I can see how that could be a benefit for other people (REACH Participant 1008)
Interest in speaking with a supportive professional
A similar number of participants would have liked to discuss their responses with a supportive professional (n = 19 total; n = 9 REACH; two participants endorsed both an interest and a lack of interest in speaking with a supportive professional in reference to different dimensions of their experience; hence the totals for these two themes exceed n = 38):
I think sometimes it’s helpful when you talk to a person and they can validate what you’re experiencing. I know I love to talk to people more than everything being online...(REACH Participant 1065)
Yeah, I think that would have been helpful on the ones where I had more questions. Or, you know, had wanted to follow the research a little bit more. (REACH Participant 1054)
Among those interested in the program offering contact with a supportive professional, the majority (n = 14 total; n = 6 REACH) preferred phone calls as the preferred method of contact (compared to email or in-person). The most commonly reported ideal frequency for contact with a supportive professional (n = 7 total; n = 3 REACH) was once every 2 to 3 months. When probed with whom they would have liked to have spoken, some participants (n = 9 total; n = 4 REACH) wanted the program to include contact with a professional on the oncology care team, e.g., an oncologist or oncology nurse or nurse practitioner, while others (n = 6 total; n = 3 REACH) specified a desire for contact with a mental health professional.
Experience Reflecting on Values for Taking Endocrine Therapy (in REACH)
Personal values were explored in numerous ways in REACH; detailed responses are examined elsewhere [54]. Herein we summarize the overarching themes:
Linking AET to values was a positive experience
The majority of interviewed REACH participants reported that taking the time to think more deeply about their values/ reasons for taking AET was helpful, healthy, or positive (n = 12) and that seeing the values sticker on their AET pillbox was motivating, worthwhile, helpful, joyful, or cute (n = 18):
Oh I loved it. I looked forward to seeing it, looking at it every day…I mean you have a favorite picture of someone you love, it never gets old…it helped. You connect with why did I pick that sticker, what does it mean, it means my values, and it’s just that constant reinforcement. (1050)
It was good. Like I said I called it the guilt sticker. And it made me laugh, you know, but then seeing it every day really was a kick in the pants to do the right thing. (1054)
Linking AET to values was a negative or nonadditive experience
Less commonly, participants noted that they did not enjoy reflecting on their values in the main AET session (n = 2) because it made them feel guilty or worried, particularly if they also experienced challenging AET side effects. Some REACH participants (n = 5) did not enjoy seeing the values sticker for similar reasons or because the sticker was seen as redundant or unnecessary.
Well as the months progressed and I got worse and worse [AET side effects], it [the sticker] just kind of made me feel guilty, got me a little angry… I guess it was a guilt trip again, and especially because my initial motivator was my children, my husband, my grandkids, and then it was like I’m failing them. And then am I failing myself. (1049)
Just that I didn’t need to do this [sticker] and I had other things to do….Because the pillbox itself was reminder enough and I had in my mind the information [from the rest of the program]. (1032)
Discussion
This pilot randomized trial among breast cancer survivors took a mixed-methods approach to evaluating the feasibility, acceptability, and preliminary efficacy of REACH, a novel, low-touch, values intervention for promoting adherence to AET, related to an education-only control condition. To our knowledge, this represents the first evaluation of a values-based intervention to promote AET adherence among breast cancer survivors. Both interventions and the study assessments showed excellent feasibility and, given their low-touch nature, good acceptability. REACH led to significantly higher Wisepill-assessed AET adherence (the primary outcome) than did Education for the first month of the follow-up period. Moreover, REACH participants had no declines from baseline in Wisepill-assessed AET adherence for the first three months of the study, whereas Education participants showed immediate declines. These findings signify one of the first demonstrations that a low-touch psychological intervention can significantly improve objectively-assessed AET adherence among breast cancer survivors who on average were prescribed AET one year previously. However, REACH’s initial effect on AET adherence did not endure through the 6-month follow-up period, nor did it lead to superior outcomes on most secondary outcomes, suggesting that more support over time is needed.
Themes identified in the in-depth exit interviews conducted with 38 study participants pointed toward promising directions for providing that additional support, toward the goal of extending REACH’s benefits beyond three months. Most participants, particularly in REACH, found that the interventions were acceptable. However, when asked directly, a large portion expressed interest in discussing their responses to the online program with a supportive professional, most commonly desiring contact by phone once every 2–3 months with an oncology or mental health professional. Although a similar portion reported no interest in discussing their responses with a supportive professional, many among them spontaneously added that they thought this would be helpful for others. Also, especially in Education, some women requested more detailed information about managing side effects. Compared to the few known successful AET adherence interventions [19], which are high-touch and resource-intensive, adding infrequent phone calls from a supportive professional and providing more detailed online information and support for coping with side effects, particularly those that women were most concerned about (e.g., joint/muscle pain, hot flashes/night sweats in the current sample), could potentially improve the endurance of REACH’s effects while largely maintaining its low-touch nature. Thus, future studies should investigate whether adding these relatively low-touch and scalable strategies (e.g., infrequent phone check-ins and additional online information) provide sufficient support to extend the intervention effects.
In addition, the overwhelming majority of interviewed REACH participants reported that reflecting on their values for taking AET and using the values sticker were positive experiences, which converges with the quantitative acceptability ratings. However, a small minority expressed that reflecting on values or seeing their sticker made them feel guilty or worried, particularly if they also experienced bothersome AET side effects. In another paper, we will evaluate the specific values exercises in REACH that affected negative AET-associated emotions such as guilt. In future iterations of REACH, it may be important to address any guilt-producing values exercises by offering additional support to cope skillfully with the guilt or by removing them altogether given the low-touch nature of the intervention. Similarly, among women who experience chronic bothersome AET side effects, it may be important to include some provider contact to help address them.
REACH also led to somewhat greater reductions in negative affective attitudes toward AET than Education at 3-month follow-up, which reflected reductions in negative attitudes in REACH (at 3- and 6-month follow-up) but not in Education (at any point). This converges with the qualitative findings, in which many women interviewed in REACH expressed that pairing their medication pillbox with a values sticker created a more positive (or less negative) association with their medication. One possibility is that associative learning mechanisms undergirded these effects, such that the autonomously chosen positive/meaningful content of the values sticker became paired with, and thereby countered, the negative associations with the physician-mandated, side effect-associated AET medication. Negative affective attitudes toward AET, assessed in a similar manner as the attitude ratings in the current study, were associated with lower AET adherence in a large previous study [32], suggesting that this finding may signify an important impact of REACH. Finally, comparing Wisepill-assessed to self-reported adherence revealed that REACH led to less overreporting of AET adherence than Education, an unexpected finding. This finding suggests that REACH may have allowed participants to more honestly admit or perceive lapses in AET adherence. Overreporting/ overestimating medication adherence is clinically important as it leads to inaccuracies in patients’ and providers’ understanding of patients’ adherence. This potential benefit of REACH should be further investigated in future studies.
Behavioral interventions have been conceptualized as working in one of two ways: by capitalizing on participants’ relative strengths or by compensating for their relative weaknesses [55]. The exploratory moderator analyses were consistent with the notion that REACH, relative to Education control, worked through capitalization. Specifically, those who entered the study with average or higher (for this sample) perceived quality of communication with their oncologist or positive affective attitudes toward AET, showed significantly higher 1-month Wisepill-assessed AET adherence following REACH than Education, suggesting that REACH capitalized on their pre-existing strengths, whereas those who entered with low scores on these moderators did not benefit more from REACH than Education. This parallels the qualitative interview findings that a small minority of women in REACH did not enjoy the values sticker because they suffered from difficult AET side effects. Future iterations of REACH should address how to better support breast cancer survivors who report poorer oncologist communication, less positive associations with AET, or significant side effects, and to more enduringly capitalize on participants’ pre-existing strengths.
Study Limitations
This study had several limitations. First, although eligibility criteria required at least a modest degree of difficulty with AET adherence, most participants were still relatively highly AET adherent. High sample adherence reduced statistical power to detect condition differences; future studies should aim to recruit a less adherent sample. Participants also were informed that the study goal was the support them in taking and benefitting from their AET medication, which may have biased who consented to participate, or among study participants, may have somewhat boosted AET adherence behavior. Second, the conditions were matched for online delivery, no provider contact, and AET education content, but not for online ‘contact’ or booster sessions, a study limitation. That stated, both interventions were brief and remotely-delivered; thus, the impact of additional time online may not have been as impactful as additional time in a bi-directional intervention. Third, this study was designed as a pilot study and thus was not specifically powered to detect moderated effects; these findings should be considered exploratory. Fourth, electronic monitoring reflects a gold standard approach to assess medication adherence [56], yet such monitoring indicates only whether the pillbox was open and not whether the pill was taken, a limitation. Fifth, although the sample was diverse with respect to socioeconomic status and education, it was a largely white, non-Latina sample. It will be vital to extend future investigations to people from historically excluded groups. Finally, the study required internet access, which may have limited generalizability to lower-income, underserved individuals without access to the internet [57]. In future studies, it will be important to examine barriers to engaging in the intervention in these underserved communities and consider ways to reduce barriers for individuals who may not have internet access, for instance by providing internet-enabled loaner tablets and technical support in using them.
Conclusions
This mixed-methods, randomized pilot trial provides one of the first applications of a low-touch values intervention to promote AET adherence, and one of the first demonstrations that such an approach can improve objectively-assessed AET adherence. Findings suggested the feasibility and acceptability of the low-touch, low-intensity REACH intervention, with treatment effects on AET adherence sustaining through 3 months. Although the positive effects of the REACH intervention on AET adherence did not extend to the second half of the 6-month follow-up period or generalize to most secondary outcomes, the qualitative data provided important patient insights into what may be needed to intensify and extend its benefits while maintaining a relatively low-touch approach. As argued by adherence experts, “Simple adherence strategies that result in even small effect size at the individual level, when broadly implemented on a population level, may provide substantial cumulative public health benefit by significantly leveraging therapeutic efficacy” (p. 413; (58)). With a low-touch intervention, even modest improvements to AET adherence could translate to better clinical outcomes for breast cancer survivors at the population level. Thus, from a public health perspective, continuing to refine and evaluate low-touch, values-based approaches for promoting AET adherence such as REACH represents a worthwhile investment.
Supplementary Material
Acknowledgments
This study was funded by an National Institutes of Health/ National Cancer Institute grant R21CA218723 to the first author and by the National Institutes of Health/ National Cancer Institute for Advancing Translational Sciences Colorado CTSA grant UL1 TR002535 for the use of REDCap. At Rocky Mountain Cancer Centers, we thank Derek Burns, PharmD, and his former pharmacy team, as well as David Andorsky, M.D., Timothy Murphy, M.D., Glenn Balasky, and the social work team for their assistance and support of the study. At the University of Colorado Boulder, we thank Maya Rieselbach for her excellent qualitative coding and Maya, Anthony Pidanick and Nirali Murthy for their help in managing the study.
Contributor Information
Joanna J Arch, Department of Psychology and Neuroscience, University of Colorado Boulder, 345 UCB Muenzinger, Boulder, CO, 80309-0345, USA; Division of Cancer Prevention and Control, University of Colorado Cancer Center, Aurora, CO, USA.
Catherine M Crespi, Fielding School of Public Health, Department of Biostatistics, University of California Los Angeles, Los Angeles, CA, USA.
Michael E Levin, Department of Psychology, Utah State University, Logan, UT, USA.
Sarah R Genung, Department of Psychology and Neuroscience, University of Colorado Boulder, 345 UCB Muenzinger, Boulder, CO, 80309-0345, USA.
Madeline Nealis, Department of Psychology and Neuroscience, University of Colorado Boulder, 345 UCB Muenzinger, Boulder, CO, 80309-0345, USA.
Jill L Mitchell, Rocky Mountain Cancer Centers-Boulder, Boulder, CO, USA.
Emma E Bright, Department of Psychology and Neuroscience, University of Colorado Boulder, 345 UCB Muenzinger, Boulder, CO, 80309-0345, USA.
Karen Albright, Department of Medicine, Division of General Internal Medicine, University of Colorado School of Medicine, Aurora, CO, USA.
Jessica F Magidson, Department of Psychology, University of Maryland, College Park, MD, USA.
Annette L Stanton, Department of Psychology, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California Los Angeles, Los Angeles, CA, USA; Semel Institute for Neuroscience, Cousins Center for Psychoneuroimmunology, University of California Los Angeles, Los Angeles, CA, USA.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors (Joanna J. Arch, Catherine M. Crespi, Micheal E. Levin, Sarah R. Genung, Madeline Nealis, Jill L. Mitchell, Emma E. Bright, Karen Albright, Jessica F. Magidson, Annette L. Stanton) declare that they have no conflict of interest.
Ethical Approval: All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1. World Cancer Research Fund/American Institute for Cancer Research: Diet, Nutrition, Physical Activity and Breast Cancer. 2018, from dietandcancerreport.org [Google Scholar]
- 2. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: A prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hershman DL, Shao T, Kushi LH, et al. . Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists’ Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 5. Burstein HJ, Temin S, Anderson H, et al. . Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hershman DL, Kushi LH, Shao T, et al. . Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. [DOI] [PubMed] [Google Scholar]
- 9. Gadkari AS, McHorney CA. Medication nonfulfillment rates and reasons: Narrative systematic review. Curr Med Res Opin. 2010;26:683–705. [DOI] [PubMed] [Google Scholar]
- 10. Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. [DOI] [PubMed] [Google Scholar]
- 11. Toivonen K, Williamson T, Carlson L, Walker L, Campbell T. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: A systematic review. Cancers. 2021;13, 107:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambert LK, Balneaves LG, Howard AF, Gotay CC. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: An integrative review. Breast Cancer Res Treat. 2018;167:615–633. [DOI] [PubMed] [Google Scholar]
- 13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 14. DiMatteo MR. Social support and patient adherence to medical treatment: A meta-analysis. Health Psychol. 2004;23:207–218. [DOI] [PubMed] [Google Scholar]
- 15. DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42:200–209. [DOI] [PubMed] [Google Scholar]
- 16. Toivonen K, Williamson T, Carlson L, Walker L, Campbell T. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: A systematic review. Cancers 2021, 13, 107. Cancers. 2021;13:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieuwlaat R, Wilczynski N, Navarro T, et al. . Interventions for enhancing medication adherence. Cochrane Database of Syst Rev. 2014. Art. no.: CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kini V, Ho PM. Interventions to improve medication adherence: A review. Jama. 2018;320:2461–2473. [DOI] [PubMed] [Google Scholar]
- 19. Finitsis DJ, Vose BA, Mahalak JG, Salner AL. Interventions to promote adherence to endocrine therapy among breast cancer survivors: A meta-analysis. Psychooncology. 2019;28:255–263. [DOI] [PubMed] [Google Scholar]
- 20. Heiney SP, Parker PD, Felder TM, et al. . A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat. 2019;173:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5:193–203. [DOI] [PubMed] [Google Scholar]
- 22. Epton T, Harris PR, Kane R, van Koningsbruggen GM, Sheeran P. The impact of self-affirmation on health-behavior change: A meta-analysis. Health Psychol. 2015;34:187–196. [DOI] [PubMed] [Google Scholar]
- 23. Steele CM. The psychology of self-affirmation: Sustaining the integrity of the self. Adv Exp Soc Psychol. 1988; 21:261–302. [Google Scholar]
- 24. Crocker J, Niiya Y, Mischkowski D. Why does writing about important values reduce defensiveness? Self-affirmation and the role of positive other-directed feelings. Psychol Sci. 2008;19:740–747. [DOI] [PubMed] [Google Scholar]
- 25. Hayes SC, Strosahl KD, Wilson KG.. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York, NY: Guilford Press, 1999. [Google Scholar]
- 26. Hayes SC, Strosahl KD, Wilson KG.. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change, 2nd Ed. New York:NY Guilford Press, 2012. [Google Scholar]
- 27. Hawkes AL, Chambers SK, Pakenham KI, et al. . Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol. 2013;31:2313–2321. [DOI] [PubMed] [Google Scholar]
- 28. Ogedegbe GO, Boutin-Foster C, Wells MT, et al. . A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Arch Intern Med. 2012;172:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charlson ME, Wells MT, Peterson JC, et al. . Mediators and moderators of behavior change in patients with chronic cardiopulmonary disease: The impact of positive affect and self-affirmation. Transl Behav Med. 2014;4:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: A systematic review. Jama. 2014;312:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Czajkowski SM, Powell LH, Adler N, et al. . From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145:525–534. [DOI] [PubMed] [Google Scholar]
- 33. Qualtrics Software. Provo, Utah, USA, 2017, from https://www.qualtrics.com [Google Scholar]
- 34. Bailey A, Ciarrochi J, Harris R: The Weight Escape: How to Stop Dieting and Start Living. Boston, MA: Shambhala Publications, 2014. [Google Scholar]
- 35. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (Redcap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐ 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bright EE, Petrie KJ, Partridge AH, Stanton AL. Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res Treat. 2016;158:243–251. [DOI] [PubMed] [Google Scholar]
- 37. Doggrell SA. Adherence to oral endocrine treatments in women with breast cancer: Can it be improved? Breast Cancer Res Treat. 2011;129:299–308. [DOI] [PubMed] [Google Scholar]
- 38. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. [DOI] [PubMed] [Google Scholar]
- 39. Arch JJ, Mitchell JL. An acceptance and commitment therapy (ACT) group intervention for cancer survivors experiencing anxiety at re-entry. Psychooncology. 2016;25:610–615. [DOI] [PubMed] [Google Scholar]
- 40. Haberer JE, Kiwanuka J, Nansera D, et al. . Real-time adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS (London, England). 2013;27:2163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haberer JE, Kahane J, Kigozi I, et al. . Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis A, Sarsembayeva L, Gulyaev V, et al. . If you build it, will they use it? Preferences for antiretroviral therapy (ART) adherence monitoring among People Who Inject Drugs (PWID) in Kazakhstan. AIDS Behav. 2019;23:3294–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist. 2015;20:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walker HE, Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH. Perceptions, attributions, and emotions toward endocrine therapy in young women with breast cancer. J Adolesc Young Adult Oncol. 2016;5:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bright EE, Stanton AL. Correspondence between objective and self-reported endocrine therapy adherence among women with breast cancer. Ann Behav Med. 2019;53:849–857. [DOI] [PubMed] [Google Scholar]
- 46. Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: Attitudes, norms, self-efficacy, and temporal stability of intentions. Psychol Sport Exerc. 2010;11:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med. 2006;9:1086–1098. [DOI] [PubMed] [Google Scholar]
- 48. Basch E, Reeve BB, Mitchell SA, et al. . Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (Pro-Ctcae). JNCI. 2014;106:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spybrook J, Bloom H, Congdon R, et al. . Optimal Design for Longitudinal and Multilevel Research, Version 3.01 [Computer Software]. University of Michigan,2013, from http://hlmsoft.net/od/ [Google Scholar]
- 50. Papke LE, Wooldridge JM. Econometric methods for fractional response variables with an application to 401 (k) plan participation rates. J App Econ. 1996, 11:619–632. [Google Scholar]
- 51. Magidson JF, Joska JA, Belus JM, et al. . Project Khanya: results from a pilot randomized type 1 hybrid effectiveness-implementation trial of a peer-delivered behavioural intervention for ART adherence and substance use in HIV care in South Africa. J Int AIDS Soc. 2021;24 Suppl 2:e25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Graneheim UH, Lundman B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112. [DOI] [PubMed] [Google Scholar]
- 53. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. [DOI] [PubMed] [Google Scholar]
- 54. Slivjak ET, Arch JJ.. Evaluating the efficacy of common humanity enhanced exposure for individuals with social anxiety. Paper presented at the Association for Contextual Behavior Science World Conference 2020. (held virtually), 2020.
- 55. Cheavens JS, Strunk DR, Lazarus SA, Goldstein LA. The compensation and capitalization models: A test of two approaches to individualizing the treatment of depression. Behav Res Ther. 2012;50:699–706. [DOI] [PubMed] [Google Scholar]
- 56. Sutton S, Kinmonth AL, Hardeman W, et al. . Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med. 2014;48:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pew Research Center: Internet/ Broadband Fact Sheet. Retrieved October 8, 2021, from https://www.pewresearch.org/internet/fact-sheet/internet-broadband/. Accessed October 8, 2021.
- 58. Bosworth HB, Granger BB, Mendys P, et al. . Medication adherence: A call for action. Am Heart J. 2011;162:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Epton T, Harris PR. Self-affirmation promotes health behavior change. Health Psychol. 2008;27:746–752. [DOI] [PubMed] [Google Scholar]
- 60. Sherman DA, Nelson, LD., Steele CM. Do messages about health risks threaten the self? Increasing the acceptance of threatening health messages via Self-Affirmation. Pers Soc Psychol Bull. 2000;26:1046–1058. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.