ABSTRACT
Digital healthcare offers a huge range of possibilities and may improve the quality of patient care. The traditional paradigm of clinical history, examination, differential diagnosis and treatment may be improved by tools such as machine learning, mobile applications and sensors, wearables, and telehealth. The recent pandemic has accelerated the move towards this future, however, important concerns remain. These include ensuring that new technologies are assessed effectively and are introduced thoughtfully, data are unified into care records, and, ultimately, digital tools are underpinned with appropriate clinical support. Patients must benefit without worsening workload for the clinician. Old barriers may be overcome but new challenges will be faced, in particular, ensuring the promise of better care for those who lack access to smart phones and the internet will be crucial. Clinicians must continue to advocate for patients and their colleagues as we embrace the future of digital healthcare.
KEYWORDS: digital health, telehealth, smartphone, wearable, GIRFT
Introduction
The science fiction author William Gibson, who coined the term cyberspace, said, ‘The future is already here, it is just not very evenly distributed.’1 He meant that many of the tools that will transform our lives are already in place but not widely available or fully realised. In contrast to many, he wrote extensively of a dystopian future where technology doesn't necessarily lead to a better future, where many of the current inequalities are replicated or even exacerbated.
Healthcare is undergoing a digital transformation, accelerated by the COVID-19 pandemic, that will alter many of the fundamental building blocks of medical care. We know this road to progress may be bumpy, with many diversions. Clinicians need to be active participants in the journey, staying true to the core values that we have held dear for centuries to ensure we avoid some of the grimmer futures imagined by writers like William Gibson.
How will medicine change? What are the crucial drivers of those changes and how can we solve the problems with our current systems without making things worse? Critically, how can clinicians advocate for a better future for all our patients with healthcare enabled by technology rather than constrained by it.
How will medicine change?
The familiar process of history taking, examination, constructing a differential diagnosis, arranging investigations and planning treatment all based on theoretical knowledge, hard-earned through study and experience, has been the dominant paradigm for hundreds of years. Digital tools are changing this process in a myriad of ways that will fundamentally alter the clinician–patient relationship.
Clinicians are valued for their long training to acquire knowledge and its application to the patient. This is maintained by continuous professional development. The explosion in medical knowledge and increasing complexity of treatment makes it difficult for individuals to maintain knowledge in their own subspecialty let alone in the rest of medicine. A profound disconnect exists between this reality and the widely held perception of doctors as authoritative dispensers of knowledge and advice. A recent Royal College of Physicians (RCP) report calls for widespread genomics testing to tailor drug choice and dose depending on a patient's genetic makeup.2 The ability to individualise treatment with additional data sources (such as a wearable) will increase its complexity even further. How can a clinician keep all this information in their head? The answer is that they cannot and inevitably digital tools will be needed. This changes the clinician from being the authority to an interpreter of recommendations generated by algorithms that use the latest evidence integrated with genomics and other data. A fundamental shift in skills are needed, from knowledge acquisition to communication.
Black cab taxi drivers in London spend years memorising maps of and routes through the city's streets and traffic flow (the knowledge). Their livelihoods are threatened by, often part-time, casual drivers from ride-sharing apps who use mapping software and real-time traffic information to get to the destination quicker and cheaper. This is, of course, a waypoint until driverless cars replace them both. It's difficult not to draw analogies with this and the training required to be a clinician.
Taking a history is an art and felt to be the cornerstone of medicine. However, electronic questionnaires, with branching logic-changing questions dependent on the answers are already widely used. It is then a short leap to using these to generate differential diagnoses with further questions to refine the list before assigning probabilities to each diagnosis along with the most efficient investigation strategy. The use of chatbots may take this further, automating this process with the addition of voice pattern analysis to detect emotional undertones. Companies (such as Babylon) have invested significantly in the use of chatbots to diagnose patients in primary care with substantial financial investment raised to refine this technology. There will inevitably be weaknesses of these pathways but they are often different to those of traditional history taking, opening up the prospect of a synergy between the two methods. Importantly, these chatbots can be deployed any time the patients want rather than being constrained by clinician availability. They can help record symptoms over time, avoiding the recall bias of a one-off appointment with the clinician. There is a tendency to compare them with well-established methods that have evolved over decades and hold them to a higher standard, however, it is important to realise these new approaches are being rapidly iterated and improved. A comparison can be made with driverless cars that have travelled for millions of miles with a much lower accident rate when compared with human drivers who are responsible for tens of thousands of deaths. Despite this, resistance to their wider use remains because they are not perfect. As society becomes more comfortable with digital tools, the bar for entry is likely to be lowered as society appreciates the improvement seen with their enhanced capabilities.
Speech recognition has been present for decades but, with the improvement in computing power and machine learning, it can now be clinically useful during a consultation beyond simply dictating a letter. Imagine talking to a patient, the system transcribing as you go, then when you examine the patient's eardrums and say aloud, ‘Red, inflamed eardrum’, the system suggests otitis media as a diagnosis and then offers up a list of antibiotics from the formulary based on the patient's age and allergy status. This ambient speech recognition is generating significant excitement and has obvious applications in improving efficiency and clinical encounter documentation. One of the leaders in the field, Nuance, was recently bought by Microsoft for just under $20 billion.
The clinical examination is one of the defining characteristics of medicine, as shown by the ubiquity of the stethoscope as a mark of a doctor. Remote consultations have shown that many interactions can be done without any physical examination. However, digital tools exist to assist remote examination that range from software diagnosing that you may have COVID-19 by analysing the sound of your cough to your phone assessing gait stability. Dermatology is at the forefront of this trend with classification of skin lesions with image analysis to rapidly improve diagnostic accuracy. Google already has software that uses machine learning from its vast database of images to help check whether a mole may be cancerous.
Creating a differential diagnosis is currently based on knowledge, experience and a prior probability of an event. This may be prone to bias and defects in risk estimation. Software appropriately designed and implemented can overcome some of these barriers, and help suggest appropriate diagnoses and investigations. Decision-support software is becoming increasingly widespread and it is likely to increase further until it is superior to the individual clinician in many ways. However, it is currently difficult to imagine that it will replace that emotional connection vital for a therapeutic relationship.
Although clinicians are able to identify major influencers on prognosis, digital tools that integrate additional factors may increase prognostic accuracy. This could be important not only in predicting survival but also in other estimations, such as individual patient procedural success. One interesting study looked at the failure to attend a magnetic resonance imaging (MRI): over 60 factors potentially contributing to it were identified and then weighted to estimate the likelihood of not attending the scan.3 However, one must be careful of extrapolating from this kind of study; for example, in London, not owning a car may not be a strong predictor of non-attendance but, in a rural catchment area, it may be very important. Machine learning can identify patterns invisible to the human eye. There are already studies that can predict from a single 12-lead electrocardiography (ECG) the likelihood of detecting impaired left ventricular (LV) function on echocardiography or even the development of atrial fibrillation in the future.4,5
It is not just the mechanics of medicine that is changing, but also the model of medical care delivery. The success of companies like Babylon's ‘GP at hand’ shows that the demand is there for digital-first care and remote consultations with general practitioners (GPs), similar to the shift to virtual outpatients and virtual wards in secondary care. The rapid proliferation of these services suggests that there is a need for them; however, ultimately, only a small number of companies are likely to survive.
Mobile
Mobile phone ownership in western countries is ubiquitous, with close to over 90% of people <65 years old and up to 65% of people >65 years old owning a smartphone.6 These offer a tremendous opportunity for healthcare as a personal communication device that is carried constantly and can either actively or passively gather data related to a patient's health. Dedicated health applications have been developed that exploit sensors embedded into the phone. This has opened a huge opportunity to detect the development of disease earlier, track progress and may offer patient-centred intervention remotely at a low cost. The potential benefits of this are clear and there has been an explosion of applications (apps), with over 100,000 available on the Apple and Google app stores combined. A wide range of conditions have been addressed from the monitoring of Parkinson's disease with gait analysis and tap tests to cardiovascular care with heart rate, heart rhythm and blood pressure monitoring, and to apps that support mental health.
How patients and healthcare professionals have interacted with these apps has evolved organically over time. These tools may be hugely beneficial for the patient, however, this enthusiasm must be tempered with caution as to their validity and applicability. Assessment with well designed clinical trials remains a vital consideration. To support UK healthcare providers, NHS Digital has produced both guidelines and a formal process to assess and validate apps for use. The digital technology and assessment criteria (DTAC) set out national minimum standards criteria for clinical safety, data protection, technical security, interoperability, usability and accessibility.7
Only a handful of technologies are mature enough to have been through this process. One example is an app designed to use photoplethysmography with a smartphone camera to identify atrial fibrillation that has shown a good correlation with a single lead ECG.8 The contact tracing app was a novel concept that was rapidly prototyped within 3 months and deployed during the COVID-19 pandemic. This used the Bluetooth capability of a phone to assess people's proximity to each other and notify individuals anonymously if they had been in contact with someone infected with the virus. Only 28% of the population downloaded the app; despite this, around 600,000 infections were prevented between September 2020 and December 2020.9 Nevertheless, this interesting use of smartphone sensors integrated into an app has opened the door to delivering healthcare in a way that we would never have previously considered. The public, however, may need more convincing.
Wearables
Consumer wearable technology has been increasingly prevalent. These have typically been wristwatches, gloves, inner soles, headgear or rings.10 They gather physiological data (primarily passively) through embedded sensors. Data are then translated either on the device or when connected to a smartphone app. This has provided more data than ever for clinicians to interact with and digest, which may not be available on the standard electronic health record but fragmented over multiple apps.
Many of these devices were designed with goal-based metrics to maintain or improve fitness; however, increasingly, there is a move towards early disease detection, monitoring and intervention.10 Huge investment by large tech companies suggests recognition of an enormous potential revenue stream. The year-on-year growth for these devices has remained strong with 24% growth in the smartwatch market in 2021 with >40 million units delivered in the fourth quarter.11
Early clinical studies with wearables have highlighted the ability to generate massive datasets, however, disease detection has been low. The Apple Heart Study, with over 400,000 participants, identified 0.52% had an irregular heartbeat detected by their smartwatch and only 0.16% had confirmed atrial fibrillation.12 This is likely due to the included population being primarily affluent with a low baseline incidence of disease and limited risk factors. Despite this disappointing result, as these early adopters of wearables grow older, a greater burden of disease may be detected over time.
Who should guide the next steps based on these data? It could be the clinician, patient or an automated algorithm that suggests simple low-risk interventions to try before seeking medical advice. Guided-exercise programmes to support mental-health-related issues are an example of this and have shown early promise.13 These may filter out unnecessary doctors' visits but may also falsely reassure patients and, therefore, must be assessed cautiously.
Such huge volumes of accurate, passively collected, longitudinal physiological data may offer hugely valuable insights. As user interfaces improve, novel algorithms are developed and the relationship of these data to outcomes are better understood, the potential of wearables will continue to be unlocked. Who will be responsible for monitoring and explaining these data remains unresolved, do clinicians have the time to do so? Cost and practicality will also remain a factor for now, however, this should improve with time.
Telehealth
Telehealth is defined as the delivery and facilitation of health and health-related services (including medical care, provider and patient education, health information services, and self-care) via telecommunications and digital communication technologies. This concept is not new and has been used to support patient care since the 1950s.14 More recently, however, there has been a significant push in the UK to move to telehealth solutions, particularly in the wake of the COVID-19 pandemic. This has not only been the more traditional ‘telephone clinic’ but also the development of virtual wards.15,16
Prior to the pandemic, some services used telehealth routinely, such as remote monitoring for implantable cardiac devices. This was safe, identified clinical issues early and was well accepted by patients.17,18 More extensive use was being discussed to improve efficiency. The transition, however, was slow with a number of perceived and real barriers to implementation from patients, clinicians and organisations that needed to be addressed (Table 1).19
Table 1.
Barriers to telehealth implementation19
| Patient | Clinician | Organisation |
|---|---|---|
| Age | Technically challenged staff | Cost |
| Level of education | Resistance to change | Reimbursement |
| eHealth/computer literacy | Licensing | Legal liability |
| Internet bandwidth | Perception of impersonal care | Privacy/confidentiality |
| Unawareness | Information overload | Security of data |
| High expectations of users | Interoperability | Effectiveness |
| Apathy | Poor design | Age of existing equipment |
| No phone | Language barrier | Efficiency |
| Socioeconomic status | Profit status | |
| Gender | Organisation size | |
| Preference for personal care | Teaching status | |
| Implementation models |
The push for remote care during the pandemic overcame many of these issues, often with simple solutions. An example was virtual early discharge COVID-19 wards. These patients were either contacted on the phone or with video-calling software by a nurse or doctor. They were supplied with an oxygen saturation monitor and given details on who to call if they clinically deteriorated. This was found to be a safe, well-tolerated and cost-effective way of providing care to patients at home.20 Virtual clinics became universal to ensure ongoing care of patients under long-term follow-up and to ensure new referrals continued to be seen. Many of these then relied on novel remote testing tools to make a diagnosis. One remote arrhythmia clinic augmented their remote decision making by using a new Holter patch that was posted to the patient and then sent back to an external company who provided a report. Smartphone apps used photoplethysmography technology to assess heart rate and rhythm, with data sent for review via an online portal. This was also combined with remote patient-reported outcome measures gathered on web portals or smartphone apps, providing longitudinal symptom data, to measure the effect of interventions.15
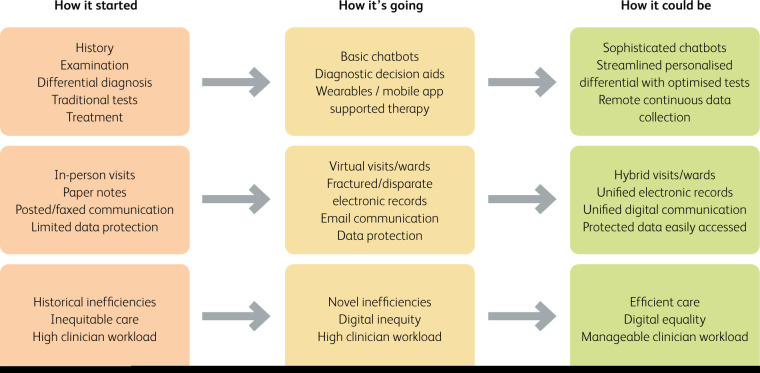
Fig 1.
Digital health: how it started, how it is going and how it could be. Three panels presenting traditional care (‘How it started’), current care (‘How it is going’) and a possible future care (‘How it could be’) paradigm.
This switch to largely remote clinics has started to drift back to a more conventional service, however, it is likely that a paradigm shift has been made.21 It is clear that virtual care is well tolerated by patients and, in some cases, preferred.22 Clinicians have found it effective and perhaps superior to a traditional care model.23 How this is reimbursed will impact the development of these services. An opportunity to integrate virtual care with more traditional pathways in a hybrid fashion is very appealing. Reducing the carbon footprint of healthcare while providing convenient, high-quality care to patients should be goal to which we aspire.
Current problems
There is a clear potential for digital solutions to make an enormous impact into long-standing issues within healthcare systems.
Costly and inefficient traditional models of care
Despite every effort, referral onto specialist care and communication back to the community remains both costly and inefficient. Simple interventions (such as digital advice and guidance, unified electronic care records, and careful selection of face-to-face patient meetings to reduce unnecessary and expensive transport costs) will have a huge impact.
Healthcare democracy
The opportunity to break down care barriers that exist due to socioeconomic status, disability and language is clear. Remote clinics, simple transfer of data, tests available for delivery or data acquired via smartphones/wearables at a low cost could dramatically improve access to care by centralised specialist units for remote underprivileged communities. Language barriers traditionally managed with telephone systems or booked interpreters may be dealt with by live auto-translation and clinic letters then sent out in the appropriate language. This may dramatically reduce the challenge of teasing out subtle clinical signs and increase confidence in making an appropriate clinical diagnosis and translating this to the patient with an appropriate management plan, reducing stress for all involved.
‘One strategy fits all‘ models of care
Personalised healthcare remains an important goal. As the volume and completeness of patient-level data improves, novel insights may be gained by marrying digital data with more complex clinical tests. Careful enquiry of these large datasets may pave the way to new therapies and risk stratification tools. Previous population observational studies, such as that from Framingham, remain the bedrock of many primary prevention interventions in modern medicine.24 The availability of larger datasets gathered at a fraction of the cost may allow us to take a similarly large step towards more personalised care.
Data acquisition, security, association and storage
Paper notes, plagued with security issues, illegible writing, missing data and challenges of data transfer are slowly being replaced by electronic systems, but these have a way to go. The lack of interconnectivity must be tackled to allow the creation of a unified patient dataset accessible by any patient or healthcare professional at any time. Appropriate data storage and security compliance with all relevant data protection remains of paramount importance but should not impact the ease of databut in time will be optimised for national healthcare systems. Tools, records may make things more challenging initially, but in time will be optimised for national healthcare systems. Tools, like the NHS app, represent an important first step in the right direction.
Digital inequality
The future of medicine will look very different to that of today but it is crucial that existing health inequalities are not maintained or even exacerbated. Having a digital-first strategy is pointless if many of our patients are denied access through lack of digital literacy, restricted access to devices and data, or from poverty. There are estimates that over 10 million people in the UK lack basic digital skills, these are often in groups with higher rates of illness and in need of greater support. The pandemic highlighted that, for many, the devices and internet connections needed to engage with the digital world are beyond their means; this digital poverty was recognised by many of the internet providers with programmes to help patients to access the internet. This need will continue but, currently, there is no comparable effort to address it. Many patients do not wish to or cannot access digital tools. This digital gap between those who can and cannot use digital platforms is, to some extent, inevitable and our systems need to be designed to minimise this impact and achieve a degree of equivalence.
Conclusion
Clinicians have a key role as patient advocates ensuring that our core ethical principles guide the development of new healthcare systems. Machine learning must not be allowed to exclude some groups in the population leading to bias and poor digital literacy shouldn't exclude patients from accessing high-quality healthcare. This should be familiar to all clinicians as we are the latest in a long line of clinicians advocating for our patients in a changing world. In the movie Terminator 2: Judgement Day, which paints a frightening dystopian future, the mantra is, ‘The future is not set. There is no fate but what we make for ourselves.’ Clinicians need to make sure that technology is our servant to deliver the best care for our patients and not our master separating us from them.
References
- 1.The Science in Science Fiction. NPR, 2018. www.npr.org/2018/10/22/1067220/the-science-in-science-fiction?t=1655297648031
- 2.Royal College of Physicians and British Pharmacological Society. Personalised prescribing: using pharmacogenomics to improve patient outcomes. RCP and BPS, 2022. [Google Scholar]
- 3.Nelson A, Herron D, Rees G, Nachev P. Predicting scheduled hospital attendance with artificial intelligence. NPJ Digit Med 2019;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–4. [DOI] [PubMed] [Google Scholar]
- 5.Attia ZI, Noseworthy PA, Lopez-Jimenez F, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 6.O'Dea S. UK: smartphone ownership by age from 2012–2021. Statista, 2021. www.statista.com/statistics/271851/smartphone-owners-in-the-united-kingdom-uk-by-age
- 7.NHS X. Digital Technology Assessment Criteria (DTAC). NHS. www.nhsx.nhs.uk/key-tools-and-info/digital-technology-assessment-criteria-dtac [Google Scholar]
- 8.Grunwez H, Evens S, Proesmans T, et al. Head-to-head comparision of proprietary PPG and single-lead ECG algorhythms to atrail fibrillation detection. Europace 2021;23(Suppl 3):euab116.524. [Google Scholar]
- 9.Department of Health and Social Care. NHS COVID-19 app alerts 1.7 million contacts to stop spread of COVID-19. DHSC, 2021. www.gov.uk/government/news/nhs-covid-19-app-alerts-17-million-contacts-to-stop-spread-of-covid-19 [Google Scholar]
- 10.Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: promises and barriers. PLoS Med 2016;13:e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim S. Smartwatch Market Grows 24% YoY in 2021, Records Highest Ever Quarterly Shipments in Q4. Counterpoint, 2022. www.counterpointresearch.com/global-smartwatch-market-2021
- 12.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeon G, Steel Z, Wells R, et al. A mental health-informed physical activity intervention for first responders and their partners delivered using Facebook: mixed methods pilot study. JMIR Form Res 2021;5:e23432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBain L, Morgan D. Telehealth, geography, and jurisdiction: Issues of health care delivery in Northern Saskatchewan. Canadian Woman Studies 2005;24:123–9. [Google Scholar]
- 15.Honarbakhsh S, Sporton S, Monkhouse C, et al. Remote clinics and investigations in arrhythmia services: what have we learnt during coronavirus disease 2019? Arrhythm Electrophysiol Rev 2021;10:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw A, Moodley M, McSporran K, et al. Safety and effectiveness of an integrated telehelth-led supported discahrges service for COVID-19. Thorax 2021;76:A1–205. [Google Scholar]
- 17.Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace 2008;10:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace 2010;12:674–9. [DOI] [PubMed] [Google Scholar]
- 19.Scott Kruse C, Karem P, Shifflett K, et al. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J Telemed Telecare 2018;24:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Malley E-J, Hansjee S, Abdel-Hadi B, Kendrick E, Lok S. A Covid-19 Virtual Ward Model: A Preliminary Retrospective Clinical Evaluation From a UK District General Hospital. Journal of Primary Care & Community Health 2022;13:21501319211066667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SY, Mehrotra A, Huskamp HA, et al. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern Med 2021;181:390–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad M, Chan S, Nguyen L, Srivastava S, Appireddy R. Patient perceptions of the benefits and barriers of virtual postnatal care: a qualitative study. BMC Pregnancy and Childbirth 2021;21:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akintomide E, Shah B, Sridharan S, et al. Clinical perception of effectiveness of virtual appointments and comparison with appointment outcomes at a specialist children's hospital. FHJ 2021;8:e660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS. 70-year legacy of the Framingham Heart Study. Nature Reviews Cardiology 2019;16:687–98. [DOI] [PubMed] [Google Scholar]
- 25.Cameron J. Terminator 2: Judgement Day [Feature film]. Tri-Star Pictures, 1991. [Google Scholar]