ABSTRACT
There is rapidly growing recognition of the important contribution of individually carried genetic factors to drug response variation (pharmacogenomics) for an increasingly wide range of drugs and of the resulting implications for healthcare across multiple specialisms. This concise overview of the March 2022 joint report of the Royal College of Physicians and the British Pharmacological Society on this topic outlines its coverage of aspects of scientific rationale (with examples), the so far largely unmet need for planned, systematic implementation and training within the UK NHS, and the key forward strategies required. They include a centrally funded, well defined developmental service design with implementation priorities, clinical decision support, clear clinical governance and ongoing research, public and patient engagement, and agreed, updated education and training packages.
KEYWORDS: drug response, pharmacogenomics, report overview, implementation, forward strategy
Introduction
Achieving optimal efficacy, safety and precision of prescribed medication depends on minimising differences in response between individuals. Alongside other sources of variation, the wide and important contribution to these differences of individual carriage of genetic variables, with the potential to adjust prescription accordingly for improved clinical outcomes and cost-effectiveness (pharmacogenomics), is now increasingly recognised in the UK and internationally. Translation and implementation into everyday practice has, so far, been slow and faces substantial challenges. This is despite the UK's leading track record and role in human genome characterisation and genomic medicine development.
The recently published joint Royal College of Physicians (RCP) and British Pharmacological Society (BPS) multidisciplinary, multi-specialty report addresses the issues involved, including scientific rationale, clinical practice and governance, service design, infrastructure and delivery, professional training, public awareness and ownership, and funding.1 Recommendations targeting much needed progress are presented. The report has attracted mainstream publicity.2
Scientific background, rationale and examples
The report focuses on the role and impact of inheritable nuclear germline and mitochondrial DNA variations. These have the potential to alter the handling (absorption, distribution, metabolism or excretion) of drugs (pharmacokinetics) and/or the scale/characteristics of the direct effect of drugs (pharmacodynamics) either on the target organ/system or on other tissues or immune mechanisms (Fig 1). These alterations can give rise to reduced or excessive effect of a drug on its intended target or accentuation of its anticipated side effects (type A adverse drug reactions (ADRs)); wider, serious, immune or non-immune side effects often independent of the response target (type B ADRs); or both type A and type B ADRs. The growth of evidence, importance and necessary application of pharmacogenomics within the day-to-day ambience of cross-cutting generalist/specialist practice is rapidly increasing.
Fig 1.
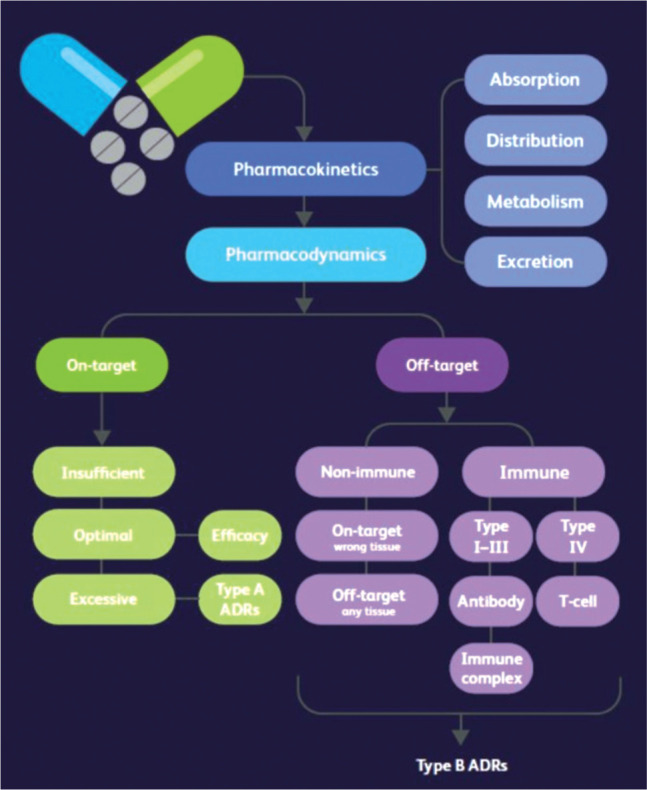
Pharmacokinetics and pharmacodynamics. Reproduced with permission from Royal College of Physicians and British Pharmacological Society. Personalised prescribing: Using pharmacogenomics to improve patient outcomes. RCP and BPS, 2022.
Some examples
Clinically actionable pharmacogenomic recommendations have now been developed by international guideline committees for over 90 prescribed drugs.3
The following examples show where such variations have potentially avoidable adverse impact (inefficacy or ADRs) with pharmacogenomic testing.
Codeine (pharmacokinetic basis)
The analgesic codeine is a prodrug metabolised to active morphine by the cytochrome P450 liver enzyme CYP2D6, the corresponding gene for which has many variants that subdivide recipients into poor (PM), intermediate (IM), extensive (EM) and ultra-rapid (UM) metabolisers.4 PM and IM exhibit reduced efficacy and can therefore benefit from CYP2D6 genotype-guided prescribing. Conversely, UM exhibit higher morphine exposure and corresponding risk, such that the summary of product characteristics now specifies codeine to be contraindicated both for paediatric patients undergoing (adeno)tonsillectomy for obstructive sleep apnoea and for patients of any age known to be CYP2D6 UM.5
Carbamazepine (pharmacodynamic basis)
The anticonvulsant carbamazepine (used for both epilepsy and neuropathic pain), although generally tolerated, can occasionally cause potentially serious type B ADRs, notably Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS-TEN). The population prevalence of the HLA allele HLA-B*15:02 in South-East Asia is strongly linked to SJS-TEN occurrence, as is HLA-A*31.01 in European and other populations.6 Prospective testing for the relevant allele can significantly reduce this.7
Abacavir
The occurrence of type B hypersensitivity (formerly 5%–7% of recipients) to this antiretroviral in HIV treatment closely mediated by the human leucocyte allele HLA-8*57:01 can now be largely eliminated by its avoidance in patients carrying this allele.8
Fluoropyrimidines (parenteral 5-fluorouracil and its oral (inactive) prodrugs capecitabine and tegafur)
Adverse reactions, such as myelosuppression and diarrhoea in recipients of this chemotherapy (gastrointestinal, breast, head and neck cancer) are linked in individuals to four identified variants of the DYPD gene encoding the enzyme dihydropyrimidine dehydrogenase which inactivates 5-fluorouracil.9 DYPD-genotype-guided prescribing can reduce serious ADR risk.10
Clopidogrel and aminoglycoside antibiotics
Similar genotype-guided prescribing is demonstrably relevant to mitigating thrombotic occurrence with the antiplatelet drug clopidogrel and sensorineural hearing loss with aminoglycoside antibiotics.11,12
Current progress in implementation
The range of specialties where the already mentioned and many other pharmacogenomic instances apply is clearly very wide. In addition, because individual patients carry their pharmacogenomic germline test status with them throughout their lifespan, continuity of individual healthcare in every integrated care setting requires effective, prompt multi-professional, cross-specialty, cross-agency access to this information and to clear implementation guidance (Fig 2).
Fig 2.
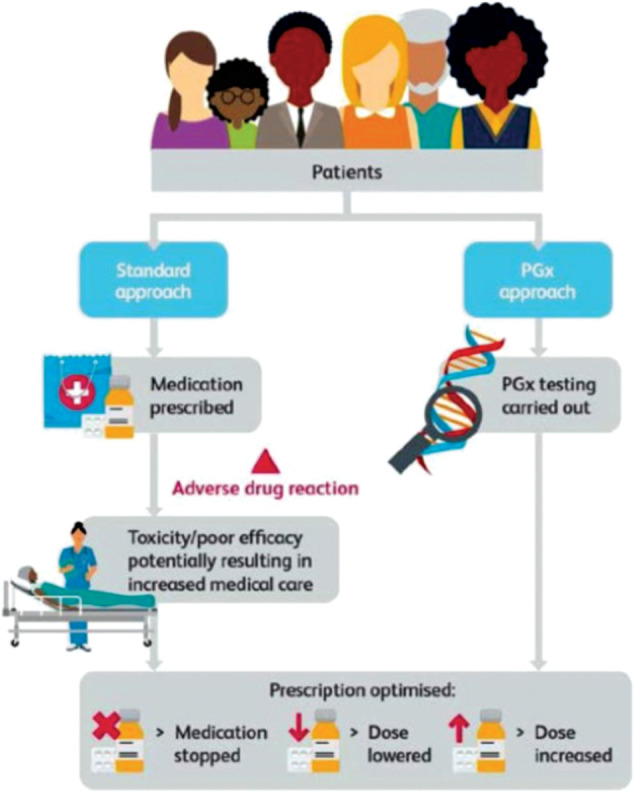
Pharmacogenomics compared with the standard approach to prescribing. PGx = pharmacogenomics. Reproduced with permission from Royal College of Physicians and British Pharmacological Society. Personalised prescribing: Using pharmacogenomics to improve patient outcomes. RCP and BPS, 2022.
Such guidance, in turn, requires consistent ongoing translational research evidence generation and updating. The evidence will include firm clarification (based on the pharmacogenomic issue concerned) of the optimal approach preferable in context: notably pre-emptive checking of a panel of pharmacogenomic tests; a focus solely on the variant of interest or on a whole exome or genome; or whether to test ahead of prescribing or following an adverse event / apparent inefficacy.
Both internationally and in the NHS, consistent strategic implementation is not yet in place (so, for example, while HLA testing is now mandatory in the NHS before initiating treatment with abacavir, routine screening for HLA-B*15:02 in population groups potentially susceptible to SJS-TEN with carbamazepine is currently not in place).13 General barriers to such implementation have included the need for more research, previous high testing costs, limitation of tests to specialist settings, unmet healthcare professional training needs and lack of routine inclusion of pharmacogenetic information in routine healthcare sources (such as electronic systems).
There are, however, substantial growing markers of progress. The UK has a leading research and development track record in genomics, including (for example) the development of sequencing technologies, population cohort studies (notably the UK Biobank project) and the 100,000 Genomes Project coordinated by Genomics England. Substantial findings have emerged to enable safer and more effective treatment in cancer cases (eg DPYD testing with fluoropyrimidines, now commissioned by NHS England) as well as in rare diseases.14 The NHS Genomic Medicine Service (GMS) has been launched (2018) in England (with similar facilities in the devolved nations) to deliver coordinated regional testing capability across the UK, followed by seven GMS alliances (2020) in England to drive structured embedding of genomics practice into mainstream clinical care.
Internationally, progress has also been made but, so far, more limited to highly specialist consortia promoting clinical research and implementation science.
Current education and training
In the light of ongoing development and progress, the need for growing awareness of pharmacogenomics with appropriately tailored education and training across all healthcare professionals in the NHS is strongly affirmed in the report (in tandem, not least, with a growing patient awareness and choice about individual ‘genetic code matching’ in medication selection and prescription). Training to appropriate levels will need to include understanding the core concepts and examples; their relevance in different specialties and settings; and clinical competence for those directly involved in clinical decision making and care delivery, including access to sources of information and available guidance (Fig 3).
Fig 3.
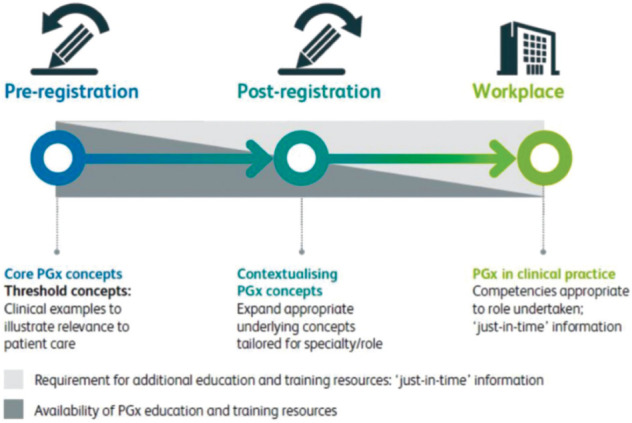
Pharmacogenomics education and training requirements. PGx = pharmacogenomics. Reproduced with permission from Royal College of Physicians and British Pharmacological Society. Personalised prescribing: Using pharmacogenomics to improve patient outcomes. RCP and BPS, 2022.
A review by Health Education England's Genomics Education Programme of current curricula highlights the widespread sparsity or absence of pharmacogenomics from pre-registration standards and post-registration curricula across the healthcare professional spectrum. Where available curricula do include pharmacogenomics (eg seven subspecialty curricula of the 35 explored in the RCP), the content focus has been towards introductory core concept coverage rather than applied patient-facing clinical practice.
Forward strategies
Under a series of key headings, the report outlines in depth the iterative developmental strategies now required and the challenges involved, concluding with clear recommendations for forward progress in the UK.
The following are five definitive requirements.
Service design, implementation and clinical decision support
-
Identification of planning priorities based, for example, on:
experience with existing services (eg fluoropyrimidines)
strength of research evidence
frequency, anticipated population incidence and severity of clinical outcomes
operational system feasibility and agility
required testing modalities
existing and future professional workforce requirements: numbers and role definitions.
Ongoing review of the optimal category of genetic testing approach for development: single gene testing, panel gene testing (which pre-empts future prescribing, so probably the optimal current clinical and cost strategy) or whole exome / whole gene sequencing.
Integrated primary–secondary care system provision of patient genomic data reporting (via electronic health records), accessible prescribing guidance information (perhaps, for example, including the British National Formulary) and readily available multidisciplinary specialist support (including clinical pharmacologists and pharmacists) remotely online and/or on site.
Service funding
A UK NHS-wide centrally funded, equitable service, enabling long-term patient follow-up, targeting the most clinically cost-effective gene–drug pairs, monitoring delivery standards and thereby delivering maximum long-term efficiency.
Clinical governance and research
A funded parallel ongoing strategic basic scientific and translational pharmacogenomic research programme to ensure continued knowledge growth around existing and new gene-drug pairs, public health benefit, and progress in service provision and uptake. This should involve collaboration across healthcare, academia (including all relevant disciplines (life and social sciences, humanities, and health economics)) and industry.
Systematic central and local clinical and service audit and quality improvement projects to sustain and raise practice standards across service providers.
Awareness, information gathering and case documentation of legal implications of evolving risk/benefit issues (eg unclarity or local variability in emerging standards; or debatable clinician decisions on whether and when to undertake genetic testing).
Recognition and pursuit of some known existing opportunities across a number of specialties (Table 1).1
Table 1.
Opportunities highlighted by the working party1
| Specialty | Area of further research/opportunities |
|---|---|
| Allergy | Immediate- and delayed-type hypersensitivity reactions |
| Anaesthesia | Exploration of any genetic component to ‘accidental awareness’ during anaesthesia |
| Cardiology | Wider subspecialty integration of pharmacogenomics |
| Diabetes and endocrinology | Monogenic diabetes therapy / variability in response to metformin / agranulocytosis in response to thyroid-supressing medications / adrenal suppression in response to corticosteroids |
| Gastroenterology and hepatology | Upper gastrointestinal ulceration / therapies for inflammatory bowel disease / drug-induced liver injury |
| Haematology | Phenotypic vs genotypic testing |
| Infectious diseases | Antibiotics and antivirals: dosing and adverse effects |
| Neurology | Personalised approach to anti-epileptic therapy / multiple sclerosis therapies |
| Obstetrics and gynaecology | Fertility treatments / teratogenicity |
| Oncology | Efficacy of dose-reduced therapeutics in response to pharmacogenomic testing and evidence to guide dose reduction and escalation |
| Ophthalmology | Steroid-induced glaucoma / treatments for macular degeneration |
| Paediatrics | International consortium to enable research in pharmacogenomics in children |
| Pharmacy | Role of pharmacists in all settings, and community pharmacies in implementation of pharmacogenomics |
| Primary care | Implementation of pharmacogenomics into primary care, decision support systems |
| Psychiatry | Optimising antidepressant therapy / identifying those at highest risk of adverse effects from antidepressant and antipsychotic therapies |
| Renal | Immunosuppressants in renal disease and renal transplantation / antihypertensives / antibiotics |
| Respiratory | Asthma treatment optimisation / targeted pulmonary fibrosis and cystic fibrosis therapeutics |
| Rheumatology | Osteonecrosis of the jaw in response to bisphosphonates / immunosuppressants and biologics |
Clear public and patient communication and engagement
Inclusion of patient representation throughout service development and design.
Public and patient confidence building through good service management, clear lines of public communication, and secure data storage and confidentiality.
Clarity (national standardised recommendations) and expertise in the handling of information and patient consent to pharmacogenetic testing (criteria essentially comparable to any other laboratory test-driven decision driving selection of best treatment (eg biochemical or haematological)), capacity, information and freedom from coercion.
Education and training packages
Comprehensive resolution of the needs and deficiencies outlined earlier by means of:
a contemporary audit of current curricula, and gap analysis of what is missing
embedding of pharmacogenomics into future training curricula using a layered approach to enable healthcare professionals to obtain ‘just in time’ information, short courses or formal qualifications, depending on their targeted professional remit.
Conclusion
The report's summary for patients and the public (equally pertinent to this journal's readership, including all healthcare professionals) states:
People are living longer today than ever before. But an ageing population means more and more of us are likely to live with long-term health conditions that require medication. This means the number of drugs we are taking is increasing...
Using pharmacogenomic testing more widely has the potential to keep people healthier for longer, improving their NHS care and outcomes. Unwanted side effects from prescription drugs cost the NHS £530 million annually in hospital admissions. Getting it right the first time could help save the NHS money and resources...
Together, the Royal College of Physicians and the British Pharmacological Society joint working party on pharmacogenomics have set out a plan to try to overcome these barriers...
The ultimate goal is to make pharmacogenomic prescribing a reality for everyone within the NHS. This will empower healthcare professionals to deliver better, more personalised, care.
Acknowledgements
The report acknowledges the dedication and work of all working party members and consultees, and is dedicated to the memory of Prof Donal O'Donoghue, who championed the creation of the working party and co-chaired it at its inception.
References
- 1.Royal College of Physicians and British Pharmacological Society . Personalised prescribing: Using pharmacogenomics to improve patient outcomes. RCP and BPS, 2022. www.rcp.ac.uk/projects/outputs/personalised-prescribing-using-pharmacogenomics-improve-patient-outcomes [Google Scholar]
- 2.Gallagher J. Matching drugs to DNA is ‘new era of medicine’. BBC, 2022. www.bbc.co.uk/news/health-60903839.amp [Accessed 18 April 2022]. [Google Scholar]
- 3.PharmGKB . Clinical guideline annotations. PharmGKB. www.pharmgkb.org/guidelineAnnotations [Accessed 18 April 2022]. [Google Scholar]
- 4.Thorn C, Klein T, Altman R. Codeine and morphine pathway. Pharmacogenetics and genomics 2009;19:556–8. [DOI] [PubMed] [Google Scholar]
- 5.Datapharm . Codeine phosphate 30mg tablets: Summary of product characteristics. Datapharm, 2020. www.medicines.org.uk/emc/product/2375/smpc#gref [Accessed 14 August 2021]. [Google Scholar]
- 6.Yip V, Marson A, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clin Pharmacol Ther 2012;92:757–65. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Lin J, Lu C, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011;364:1126–33. [DOI] [PubMed] [Google Scholar]
- 8.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;358:568–79. [DOI] [PubMed] [Google Scholar]
- 9.Meulendijks D, Henricks LM, Sonke GS, et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:1639–50. [DOI] [PubMed] [Google Scholar]
- 10.Henricks LM, Lunenburg CATC, de Man FM, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 2018;19:1459–67. [DOI] [PubMed] [Google Scholar]
- 11.CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010;304:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott JH, Wolf J, Hoshitsuki K, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for the use of aminoglycosides based on MT-RNR1 genotype. Clin Pharmacol Ther 2022;111:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The UK Collaborative HIV Cohort Study Steering Committee. HLA B*5701 status, disease progression, and response to antiretroviral therapy. AIDS (London, England) 2013;27:2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NHS England . Clinical commissioning urgent policy statement: Pharmacogenomic testing for DPYD polymorphisms with fluoropyrimidine therapies. NHS, 2020. www.england.nhs.uk/publication/clinical-commissioning-urgent-policy-statement-pharmacogenomic-testing-for-dpyd-polymorphisms-with-fluoropyrimidine-therapies [Accessed 10 September 2021]. [Google Scholar]


