ABSTRACT
Background:
Microfluidics (MF), an advanced sperm sorting technology results in the extraction of spermatozoa with higher DNA integrity and lower DNA damage compared to existing conventional sperm sorting methods.
Aims:
The aim of the present study is to assess the efficiency of MF and to isolate the best spermatozoa for intracytoplasmic sperm injection (ICSI) over the density gradient (DG) technique.
Study Setting and Design:
We recruited couples who choose the oocyte donation programme for this study to eliminate confounding factors associated with oocyte quality.
Materials and Methods:
Sperm was processed by MF (n = 180) and DG (n = 151). ICSI was performed and positive pregnancy, miscarriage and clinical pregnancy rates were compared.
Statistical Analysis:
All variables were analysed using Graph Pad Prism 5. The unpaired two-tailed t-test was used to assess the significance. A value of P < 0.05 was considered statistically significant.
Results:
There was no significant difference in pregnancy rates between the groups. However, a clear demarcation is seen in terms of clinical pregnancy rates, where the DG group achieved higher clinical pregnancies (91.7%) compared to the MF group (80.7%). Further, we compared miscarriage rates and biochemical pregnancies, and found a significantly higher miscarriage and biochemical pregnancy rate in the MF group (14.5% and 4%, respectively) compared to the DG group (6% and 1%, respectively).
Conclusions:
Based on the available literature, we anticipated a higher clinical pregnancy rate with MF compared with conventional processing. Our results show MF does not have any add-on positive effect on clinical pregnancy rate.
KEYWORDS: Density gradient, DNA integrity, intracytoplasmic sperm injection, miscarriage rate, sperm DNA damage
INTRODUCTION
Alterations in sperm parameters such as count, motility and morphology, leads to the inability to achieve a successful pregnancy in a normal fertile female which directs to the diagnosis of male factor infertility. In patients with significantly altered sperm parameters, intracytoplasmic sperm injection (ICSI) is the chosen method of treatment for conception. A significant step in assisted reproductive technology (ART) for male factor infertility is the selection of a sperm separation technique in an ICSI cycle. The pure population of functionally competent and motile spermatozoa can be obtained by various methods such as density gradient (DG) centrifugation and swim-up procedures. This selection method should consider determinants such as progressive motility, morphology, DNA integrity and maturity of spermatozoa for successful pregnancy outcomes.[1,2] It has been suggested that the sperm selection method which can mimic the natural selection process of spermatozoa in the female reproductive tract for fertilisation will result in a better population of spermatozoa for ART.
Conventional sperm preparation techniques such as swim-up and DG are popular and highly practiced. Although conventional methods are cost-effective and recover high motile spermatozoa, they also possess disadvantages of low yield, generation of reactive oxygen species (ROS) and causing significant damage to sperm DNA during the process.[2] In addition, these techniques perform better in expert hands and the possibility of errors in the procedure exists with the inexperienced.
The newer generation sperm selection methods which work based on microfluidics (MF) technology gained momentum as they extract spermatozoa with good motility and lower DNA fragmentation index (DFI).[3,4] MF devices mimic the natural physiological way of sperm selection replicating swimming through a variable micro-fluidic environment in the female reproductive system. MF operates on the principle of fluid dynamics in a space-constricted environment. In an MF device, motile sperm would be able to deviate from their initial stream-of-flow, cross the inter-streamline, and be isolated and enriched.[5] The complete liquid manipulation inside the micro channel system isolates motile and high-quality spermatozoa that are most likely to fertilise the eggs successfully. Sorting relies on the sperm's own ability to swim out of the fluid stream and move through the micro channels, thus reducing the risk of stress-induced damage. In natural conception, biologically, spermatozoa swim through a variable fluidic environment in the female reproductive system that may serve as a barrier to filter out the abnormal from the normal sperm. Although many of the characteristics of the oviductal environment are impossible to reproduce during sperm sorting, some features such as unidirectional laminar or gradient flow can be achieved in a MF environment. The idea is to mimic these features and minimise the risk of damage to the spermatozoa, that is, in a sense, a biomimetic sorting mechanism based on sperm motility.
Evidence-based studies show a significant variation between classical sperm processing techniques and MF in terms of sperm DNA integrity, the high number of euploid embryos and high positive pregnancy rates.[6,7] With this background, we wanted to assess the efficiency of MF sperm sorter over DG processing in donor oocyte-recipient cycles.
SUBJECTS AND METHODS
Study subjects
The study has been approved by Institutional Ethics Committee with the approval number 06/IIRC-MFH/2021 and conducted according to the principles outlined in the Declaration of Helsinki 2013. Written informed consent was obtained from the study participants. Couples opting for egg donation with poor ovarian reserve due to advanced age, with previous multiple (IVF) Invitro Fertilization failures or poor response in previous ovarian stimulation cycles were recruited into the study to eliminate the possibility of oocyte factors. If transvaginal ultrasound of women showed any evidence of submucous myomas, polyps and synechiae, they were excluded from the study. Endometrial biopsy was performed before starting the treatment cycle to eliminate the possibility of endometrial tuberculosis, chronic endometritis and the presence of natural killer cells which could negatively affect implantation. The minimum endometrial thickness during the embryo transfer (ET) was >7 mm with Doppler showing zone-3 flow.
The male partner's semen parameters were analysed in the laboratory following WHO 2010 guidelines. All men were asked to stop smoking and lifestyle changes were made pre-treatment. Only those men with a normal karyotype were selected for the study. Routine semen culture was performed to rule out infection before ICSI. All men were tested for sperm DFI by sperm chromatin structure assay (SCSA).[8] After approximately 2 months of oral antioxidants supplements before the treatment cycle. An abstinence period of 24 h was advised to all men before they produced the semen sample on the day of ICSI.[9,10] On the day of ICSI, two samples were collected 1–2 h apart and the second sample was analysed and used in both groups. Sperm samples taken up for processing and were randomly allocated into MF (n = 180) and DG group (n = 151). Needed sample size for the study was calculated at a confidence level of 95% and margin error 5%. As per the calculation, a minimum of 123 participants in MF and 109 participants in DG s group is required.
Sperm DNA fragmentation test by flow cytometry sperm chromatin structure assay
The Frozen semen samples were thawed and immediately transferred to Tris 10 mM, EDTA 1 mM, NaCl 100 mM buffer (TNE buffer). The samples were stained for flow cytometry assay as described previously.[8,9,10,11,12] The stained samples were assessed in the Fluorescence-Activated Single Cell Sorting (FACS CALIBURTM) flow cytometer (BD Biosciences, San Jose, CA, USA). Approximately 5000 sperm were analysed at an event rate of 100–250 events/s. Each sample was analysed in duplicate and these replicates of the data were utilised for the percentage of sperm with measurably increased red fluorescence (sperm with fragmented DNA) and those with high DNA stainability (HDS) using proprietary SCSA soft®. The results were generated as a clinical report expressed as (%DFI) and (%HDS).
Donor stimulation and oocyte retrieval
Oocyte donor stimulations were done by starting with programming using oral contraceptive pills or norethisterone. On day 2 of their current menstrual cycle depending on the individual case recombinant hormones follicle-stimulating hormone, luteinising hormone and human menopausal gonadotrophin were used for stimulation. The parameters considered for dose calculation were age, body mass index (BMI), right and left ovaries antral follicle count, volume and vascularity.
For all the donors’ fixed antagonist protocol was followed. From day 5 of the stimulation follicular growth was monitored under transvaginal ultrasonography and once the follicles were 17–18 mm, 0.3 mg decapeptyl was given as a trigger for final oocyte maturation. The oocyte retrieval was planned for 35 h after the trigger.
Oocytes were collected and cultured in single-step medium layered under mineral oil for a period of 2–3 h. Cumulus cells were stripped and oocytes were graded for maturity and each recipient received an average of 8 mature oocytes.
Sample preparation using discontinues density gradients
The two-step DG was prepared in a conical tube. First, 0.8 ml of the 80% (V/V) DG lower layer media was placed at the bottom of the tube. Then, the 40% (V/V) DG top layer media was gently layered over the 80% media following which the semen sample was layered on top of it. The tube was centrifuged for 10–12 min at 300 g. A soft pellet of motile spermatozoa formed at the bottom of the tube. The pellet was later washed with Origio sperm wash medium for 5 min. The sample was kept at 35°C and was used for ICSI within 2 h post-processing.
Sperm selection by microfluidics
The MF sperm sorter (Qualis) was fixed in a 60 mm culture dish (Falcon) and the streamlines in the MF channels were created by loading 100 μl of sperm washing medium into A, B, C and D chambers with a sterile micropipette. Immediately, the medium was removed from all four chambers with the micropipette. Chambers C and D were then loaded with 20 μl of sperm washing media and chambers B and A were loaded with 100 μl of sperm washing media and 65 μl of thoroughly mixed semen, respectively. The slide was then kept in the incubator at 37°C for 30 min. The contents in the A and B chambers flew parallel to each other and exit through their opposite wells, i.e., A/D, B/C. Spermatozoa sorted depending on their ability to swim across these two streams. Highly motile spermatozoa swam and deposited in outlet C, whereas immotile sperm kept flowing towards outlet D. After 30 min, spermatozoa (25–30 μl) from outlet C were collected with the sterile micropipette and loaded into the Polyvinylpyrrolidone (PVP) droplet of ICSI dish and used for injection.
Intracytoplasmic sperm injection and culture till day 5
During ICSI, from the processed sample, sperm was selected under high magnification based on its motility and morphology. Once injected, the oocytes were transferred to a culture dish with 30 μl drops of one-step media under oil. A maximum of four oocytes were cultured in each drop. The culture dish was placed in a conventional incubator and cultured at 37°C, 6.5% CO2 and 5.0% O2 for 5 days.
After a fertilisation check on Day 1, on Day 5 of culture, the embryos were screened and the blastocysts were graded as per Gardener and School Craft grading.[13] For all the study patients, two best quality fully expanded blastocysts with the minimum quality of 3BB were used for transfer. The remaining blastocysts were vitrified. The frozen ETs were not part of the present study.
Preparation of endometrium and embryo transfer
Programming of the cycle of the recipient of the donor oocyte was done by norethisterone given for a period of 7 days from the 23rd -day of the cycle. Ultrasound scan is done to rule out a cyst and decapeptyl is given for 10 days. Serum estradiol and progesterone levels are checked on day 2 and tablet estrodiol valerate 12 mg in divided doses is given for 12 days. If scan reveals an endometrium thickness is >7.0 mm with good endo texture injection progesterone (gestone 50 mg) is started once a day on 1st day and increased to 100 mg once a day for 4 days. One day before ET i.e., on day 5 of gestone, serum progesterone level was checked. Endometrial thickness was monitored for compaction. If progesterone levels were found to be <30 an additional gestone 50 mg was added and continued till the day of pregnancy.
ET was performed with labotech ET catheter 19 cm under ultrasound guidance and embryos were deposited at the position of maximum implantation potential. The pregnancy test was advised 14 days post-ET.
Post ET 8% crinone vaginal gel once at bedtime and tablet duphaston 10 mg twice a day was added and continued till the day of the pregnancy test.
Pregnancy assessment
Beta-human chorionic gonadotropin hormone (β-HCG) >200 units, 14 days after the ET in blood was considered positive pregnancy. Clinical pregnancy was confirmed on visualisation of an intrauterine gestational sac on ultrasound at 8 weeks. A miscarriage was documented when there was spontaneous expulsion of the products of conception from the uterus after the conformation of a gestational sac on ultrasound. A biochemical pregnancy was defined as bleeding and a fall in the β-HCG after a positive β-HCG but before confirmation of the pregnancy on ultrasound.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 version (GraphPad Software). Mean and standard deviations were calculated between defined groups. t-test was performed, followed by Mann–Whitney U and χ2 tests to assess the statistical variations between groups. Unpaired t-test was applied to obtain P values. A value of P < 0.05 was considered statistically significant.
RESULTS
Demographics
The mean age, BMI, history of smoking, semen parameters and sperm DFI (SCSA) with standard deviations of both groups are shown in Table 1. There is no noticeable variation in the basic characteristics between the groups except the average sperm motility. The average sperm motility in MF and DG is 17.66 ± 9.78 versus 13.54 ± 7.15 (P < 0.05), respectively.
Table 1.
Demographics of study popsation
| Sperm processing technique | Age (years) | BMI of female | Male use of tobacco (no/yes) | Fever in 3 months (male) | Sperm count (million) | DFI (%) | HDS (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Female | Male | Female | Male | Count | Motility | |||||
| MF (n=180) | 33.32±5.54 | 37.41±5.40 | 25.25±4.36 | 27.34±7.22 | 148/32 | Not significant | 54.26±19.03 | 17.66±9.78* | 18.24±10.45 | 11.32±5.73 |
| DG (n=151) | 31.14±3.70 | 36.14±4.21 | 25.68±4.61 | 27.59±7.45 | 128/23 | Not significant | 56.47±18.88 | 13.54±7.15* | 19.25±13.13 | 11.59±6.65 |
*P value between sperm motility in microfluidics and density gradient is significant<0.05. BMI=Body mass index, DFI=DNA fragmentation index, HDS=High DNA stainability, MF=Micro fluidics, DG=Density gradient
Clinical pregnancy rates
All the participants who opted oocyte donation cycle were part of the study to avoid oocyte-related confounding factors that may influence the study outcome. There was no significant difference in the percentage of positive pregnancies (MF 69% vs. DG 65%) and negative pregnancies (MF 31% vs. DG 35%) between both groups. However, a marked variation is observed in the clinical pregnancy rate, where is as the DG group achieved higher clinical pregnancies (92%) compared to MF group (81%) [Table 2].
Table 2.
Outcome of pregnancy in microfluidics and density gradient
| Sperm processing technique | Positive pregnancy, n (%) | Negative pregnancy, n (%) | Fate of positive pregnancy | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Clinical pregnancy, n (%) | Abortion, n (%) | Biochemical pregnancy, n (%) | Blighted ovum, n (%) | |||
| MF (n=180) | 124 (69) | 56 (31) | 100 (81) | 18 (14) | 5 (4) | 0 but 1 ectopic (1) |
| DG (n=151) | 97 (65) | 54 (35) | 89 (91.7) | 6 (6) | 1 (1) | 1 (1) |
MF=Micro fluidics, DG=Density gradient
The abortion rate and biochemical pregnancy rates were significantly higher in the MF group (14% and 4%, respectively) compared to DG group (6% and 1%, respectively) (P = 0.01) [Table 2].
Miscarriage rate
We tried to analyse the possible reasons for the higher abortion rate in the MF group. The possible role of women's age, infections and any other uterine factors which effects the implantation and contribute to the continuation of pregnancy were analysed in both groups. The mean age of women who had miscarriage and clinical pregnancy in the MF group is 32.82 ± 6.14 versus 32.79 ± 5.38, respectively [Figure 1] and the DG group is 37.00 ± 6.78 versus 30.76 ± 3.51. The variation in MF was not found to be significant (P = 0.7), but the mean age difference between abortion and clinical pregnancy group in DG cohort was found to be statistically significant (P = 0.007) where the mean age of the abortion group is higher than in clinical pregnancy group [Figure 2]. Two out of 18 women who suffered from miscarriage in the MF group had endometrial tuberculosis and both had undertaken anti-tuberculosis treatment before the ET. Uterine factors were considered. We analysed endometriosis, adenomyosis, fibroids, unicornuate and bicornuate uterus and did not find any significant role.
Figure 1.
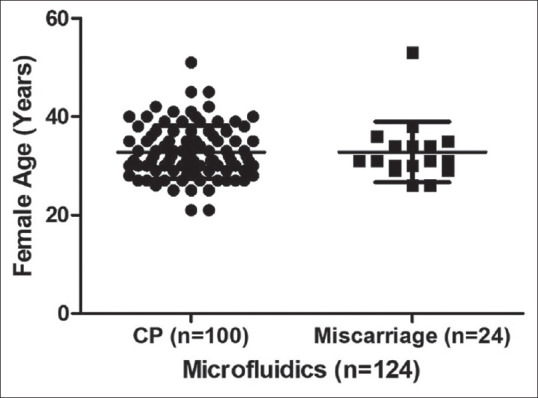
Comparison of female age between clinical pregnancy and miscarriage groups in microfluidics. No significant variation is observed. Miscarriage group included abortion, biochemical pregnancy, blighted ovum and ectopic pregnancy. CP = Clinical pregnancy
Figure 2.
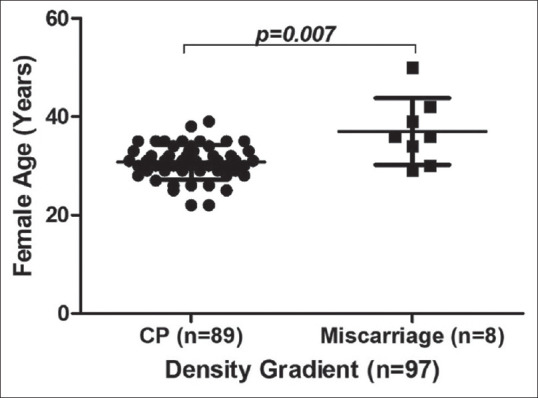
Comparison of female age between clinical pregnancy and miscarriage groups in density gradient. A significant variation is observed. Miscarriage group included abortion, biochemical pregnancy, blighted ovum and ectopic pregnancy. CP = Clinical pregnancy
We evaluated the DFI variation between the miscarriage group and clinical pregnancy group in both cohorts (Flow chart-1). There is no significant variation in both MF cohort (DFI % mean 17.87 ± 9.5 versus 17.34 ± 8.9 for miscarriage group and clinical pregnancy group, respectively) [Figure 3] and DG cohort (DFI% mean 18.50 ± 10.39 vs. 17.95 ± 12.20 for abortion group and clinical pregnancy group, respectively) [Figure 4].
Figure 3.
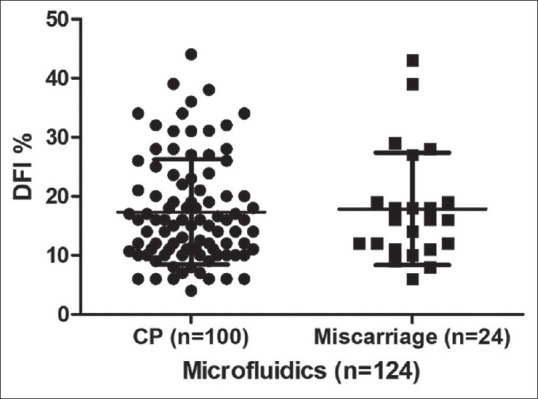
Comparison of sperm DFI percentage between CP group and miscarriage group in microfluidics. Miscarriage group included abortion, biochemical pregnancy, blighted ovum and ectopic pregnancy. DFI = DNA fragmentation index, CP = Clinical pregnancy
Figure 4.
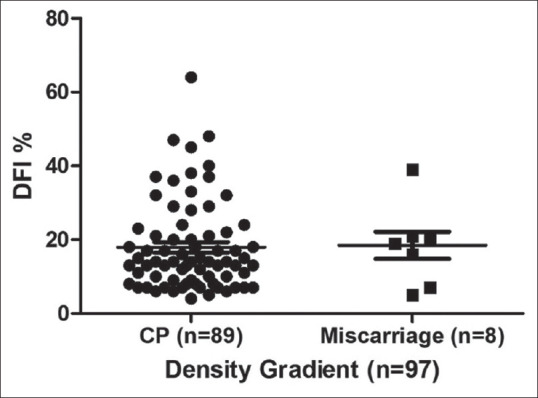
Comparison of sperm DFI percentage between CP group and miscarriage group in Density gradient. Miscarriage group included abortion, biochemical pregnancy, blighted ovum and ectopic pregnancy. DFI = DNA fragmentation index, CP = Clinical pregnancy
DISCUSSION
Sperm isolation techniques play an important role in Assisted Reproductive Technology (ART) to achieve higher fertilisation and good embryo development rates. The selection of the technique is usually dependent on sperm count and motility. Double DG is the most widely used procedure to reduce the generation of ROS and to improve the selection of sperm with lesser DNA damage for ART.
With advancements in reproductive technology and improved understanding of sperm physiology and the molecular reasons for ICSI failure such as sperm DNA fragmentation, evidence-based studies show high sperm DFI has a positive correlation with poor fertilisation and higher miscarriages rates post IVF and ICSI cycles. Hence, the focus shifted to sperm separation techniques to recover less damaged, physiologically potent and genetically competent spermatozoa. Reports have shown that iatrogenic factors like sperm centrifugation must be avoided to prevent sublethal damage to sperm and expose the DNA to the higher concentration of ROS[14] MF is a technology that manipulates small proportions of fluids in microchannels based on the fluid mechanics principal. This technology isolates morphologically healthy sperm from the raw ejaculate by laminar flow by creating gradients hypothesised to be similar to natural selection. It does not require any chemical or mechanical manipulation as in DG. The raw sample is simply introduced into the inflow from where the morphologically potent and motile spermatozoa swim through the channel and get collected into the outflow from where the final sample is isolated. Hence, selection of sperm using MF allows us to isolate sperm with high DNA integrity compared to other separation techniques.[15] The advantage of MF is that it is a single-step protocol and does not require centrifugation which hence reduces ROS generation and other adverse effects on sperm.[16] There is evidence in the literature stating that since MF yields genomically intact spermatozoa whole use in ART results in a higher number of euploid embryos.[6]
We recruited female partners who required oocyte donation for various fertility reasons with ICSI of husband's sperm to minimise the confounding factors related to oocyte defects. All the oocyte donors were young and belonged to evident fertile population. For better cycle performance and to make use of benefits of newer technology, based on the literature we decided using MF in our patients to reduce aneuploidy rate and increase chances of successful pregnancy further. We anticipated MF group with higher pregnancies and lower miscarriage rates. Although the positive pregnancy rates are higher in the MF group the miscarriage rates were also significantly higher compared to DG. The potential confounding variables for miscarriages were already taken care of while recruiting the subjects into the study. Sperm DFI of the MF group was comparable to DG group. In addition, the minor degree of defects in the spermatozoa DNA fragmentation is shown to be repaired through ooplasmic repair mechanism.[17] Therefore, we assume since our oocytes were obtained from young donors the ooplasmic mechanisms would have prevented the possible male genomic defects. The toxicity tests reports for each batch of MF chip did not reveal significant observations. Sperm membrane integrity is one of the most potential parameters that determines the pregnancy rate. Studies reveal that in sperm samples with disrupted membrane integrity measured using the HOS test, the use of MF did not result in any improvement of membrane integrity.[18] However, it needs validation in a larger cohort to conclude. In the present study, we anticipated high rate of successful term pregnancies over conventional methods; nevertheless, we observed a significant miscarriage rate in the group of patients under MF sperm sorting. There is lot of controversies and inconsistent evidence on the association between sperm DNA integrity and clinical pregnancy. Moreover, in ICSI, sperm DNA will have very little effect on fertilisation in the early stages, the prominent effect of damaged DNA will be exhibited only during blastulation and embryo implantation.[19,20,21] We minimised the confounding factors which possibly affect the miscarriage rate to understand the efficacy of MF sperm selection better. The selection of sperm based on motility and morphological characteristics for ICSI from a population of sperm with >50% DFI (SCSA) resulted in a similar pregnancy rate compared to sperm with <15% DFI (SCSA). However, there was an increased miscarriage rate in the >50% DFI group.[22,23] In our study, the percentage of DFI damage in both groups was nearly similar. Hence, we believe that MF may be useful only when DFI damage is high and in general, does not improve outcome.
CONCLUSIONS
In this study, we aimed to assess the efficiency of MF sperm sorter in improving the pregnancy rates over conventional sperm sorting methods for ICSI. We did not find any association of DFI or female age with miscarriage. We anticipate patients who suffered from miscarriages might have other factors in the sperm which have a greater association with pregnancy loss. Importantly, sperm proteomics in individual samples also interferes with pregnancy outcomes.[24] Simply using the principle of MF in selecting less DNA-damaged sperm is not enough to improve outcomes. MF sperm sorting is one of the newer technologies and results are still emerging. There is a necessity for further multicentred large cohort studies and validations on the clinical usage and safety of MF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability statement
The data used in the study is available with the corresponding author and the authors are willing to share the data upon reasonable request.
REFERENCES
- 1.Agarwal A, Selvam MKP. Advanced Sperm Processing/Selection Techniques. In: Zini A, Agarwal A, editors. A Clinician's Guide to Sperm DNA and Chromatin Damage. Springer, Cham; 2018. https://doi.org/10.1007/978-3-319-71815-6_28. [Google Scholar]
- 2.Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557–63. doi: 10.1007/s10815-012-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–93. doi: 10.1093/humrep/dey239. [DOI] [PubMed] [Google Scholar]
- 4.Samuel R, Feng H, Jafek A, Despain D, Jenkins T, Gale B. Microfluidic – Based sperm sorting & analysis for treatment of male infertility. Transl Androl Urol. 2018;7(Suppl 3):S336–47. doi: 10.21037/tau.2018.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosrati R, Graham PJ, Zhang B, Riordon J, Lagunov A, Hannam TG, et al. Microfluidics for sperm analysis and selection. Nat Rev Urol. 2017;14:707–30. doi: 10.1038/nrurol.2017.175. [DOI] [PubMed] [Google Scholar]
- 6.Parrella A, Choi D, Keating D, Rosenwaks Z, Palermo G. A microfluidic device for selecting the most progressively motile spermatozoa yields a higher rate of euploid embryos. Fertil Steril. 2018;110:e342. [Google Scholar]
- 7.Parrella A, Xie P, Keating D, Rosenwaks Z, Palermo G. Microfluidic selection of spermatozoa retains chromatin integrity and yields higher pregnancy rates. Fertil Steril. 2018;110:E343. [Google Scholar]
- 8.Mettler AD, Govindarajan M, Srinivas S, Mithraprabhu S, Evenson D, Mahendran T. Male age is associated with sperm DNA/chromatin integrity. Aging Male. 2019;23:822–9. doi: 10.1080/13685538.2019.1600496. [DOI] [PubMed] [Google Scholar]
- 9.Periyasamy AJ, Mahasampath G, Karthikeyan M, Mangalaraj AM, Kunjummen AT, Kamath MS. Does duration of abstinence affect the live-birth rate after assisted reproductive technology? A retrospective analysis of 1,030 cycles. Fertil Steril. 2017;108:988–92. doi: 10.1016/j.fertnstert.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–10. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Evenson DP. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56–75. doi: 10.1016/j.anireprosci.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Evenson DP. Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl Androl Urol. 2017;6(Suppl 4):S495–500. doi: 10.21037/tau.2017.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988;9:367–76. doi: 10.1002/j.1939-4640.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 15.Shirota K, Yotsumoto F, Itoh H, Obama H, Hidaka N, Nakajima K, et al. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil Steril. 2016;105:315–21.e1. doi: 10.1016/j.fertnstert.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Barroso G, Valdespin C, Vega E, Kershenovich R, Avila R, Avendaño C, Oehninger S. Developmental sperm contributions: Fertilization and beyond. Fertil Steril. 2009;92:835–48. doi: 10.1016/j.fertnstert.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Menezo Y. L’ovocyte réparateur de l’ADN du spermatozoïde Oocyte capacity to repair DNA damage induced in sperm. J Gynecol Obstet Biol Reprod (Paris) 2006;35:2S19–23. [PubMed] [Google Scholar]
- 18.Gonzalez-Castro RA, Carnevale EM. Use of microfluidics to sort stallion sperm for intracytoplasmic sperm injection. Anim Reprod Sci. 2019;202:1–9. doi: 10.1016/j.anireprosci.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Speyer BE, Pizzey AR, Ranieri M, Joshi R, Delhanty JD, Serhal P. Fall in implantation rates following ICSI with sperm with high DNA fragmentation. Hum Reprod. 2010;25:1609–18. doi: 10.1093/humrep/deq116. [DOI] [PubMed] [Google Scholar]
- 20.Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005;10:370–5. doi: 10.1016/s1472-6483(10)61798-1. [DOI] [PubMed] [Google Scholar]
- 21.Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod. 2001;16:918–24. doi: 10.1093/humrep/16.5.918. [DOI] [PubMed] [Google Scholar]
- 22.Dar S, Grover SA, Moskovtsev SI, Swanson S, Baratz A, Librach CL. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50%) Fertil Steril. 2013;100:75–80. doi: 10.1016/j.fertnstert.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56:1081–4. doi: 10.1016/s0090-4295(00)00770-6. [DOI] [PubMed] [Google Scholar]
- 24.Rahman MS, Lee J, Kwon W, Pang M. Sperm proteomics: Road to male fertility and contraception. Int J Endocrinol. 2013;2013:360986. doi: 10.1155/2013/360986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the study is available with the corresponding author and the authors are willing to share the data upon reasonable request.


