ABSTRACT
Background:
Polycystic ovarian syndrome (PCOS) is the predominant endocrine abnormality in premenopausal women characterised by hyperandrogenism, ovulatory dysfunction, metabolic disturbances such as, insulin resistance and obesity.
Aim:
The key objective of this study was to evaluate the efficacy of Aloe vera as a treatment drug to identify the factors among female Swiss albino mice (PCOS) models displaying ovarian and metabolic abnormalities and serum sex steroids that are associated with insulin sensitivity.
Study Setting and Design:
The laboratory breed 4 months adult virgin female Swiss albino mice showing regular estrous cycle and weighing about 20–30 g were employed in present study under CPCSEA guidelines with ethical clearance from the institutional ethical committee. These mice were categorized into two groups with 10 mice in each as control and test animals.
Materials and Methods:
The mice which were categorised into two groups with 10 mice in each. Carboxy methyl cellulose control and letrozole were induced for 21 days followed by 30 days treatment with distilled water for control, Diane as standard drug and Aloe vera gel formulation. Body weight, estrous cycle, biochemical estimations, hormone assay and histopathology were the parameters studied.
Statistical Analysis:
IBM SPSS version 20.0 was used for the mean and standard error calculations of biochemical and antioxidants estimation.
Results:
During the induction period, we observed a significant increase in the body weight, decrease in the number of estrous cycle, and the concomitant increase in diestrous. The biochemical estimations showed changes in the regular activity; hormonal imbalance was observed in hormone assay and cyst formation was also observed in the histopathological study. After the treatment of Aloe vera and Diane, all the parameters showed considerable good results.
Conclusion:
The findings of the current study provide a baseline data for designing further investigations on the therapeutic benefits of Aloe vera as an adjunct therapy in the management of PCOS.
KEYWORDS: Aloe vera, Endocrine Dysfunction, Hormone, Polycystic Ovarian Syndrome
INTRODUCTION
The polycystic ovarian syndrome (PCOS) is the most common endocrine abnormality in women with normal serum follicle-stimulating hormone (FSH) and estradiol levels with symptoms ranging from anovulation, obesity, clinical hyperandrogenism and insulin resistance, among 80% of the anovulatory patients. Although PCOS is a very common cause of anovulatory female infertility,[1,2] the mechanism of anovulation remains unknown. Anovulation in PCOS is characterised by apparent arrest of antral follicle development in the 5–10 mm stage and subsequently failure to enter the pre-ovulatory phase of the cycle,[3] even though endogenous serum levels of FSH are slightly lower than in the early follicular phase of the normal cycle.[4] It is likely that, this phenomenon occurs due to the lack of cyclicity rather than being a primary abnormality in PCOS. This is borne out by the observation that the rise of FSH which follows an induced ovulatory cycle is not usually sufficient to ensure spontaneous follicle maturation and hence, treatment must be given repeatedly until conception occurs. From time immemorial, several medicinal plants and their constituents have been used to prevent and treat various diseases. Medicinal plants such as Ashwagandha, Mimosa, Aloe vera etc., have been employed in the treatment of PCOS by some researchers earlier.[5]
The study aims to demonstrate the efficacy of the Aloe vera gel (AVG) formulation as a possible therapeutic agent in the prevention and management of PCOS. The study involves the exploration of laboratory breed female Swiss albino PCOS mice models possessing ovarian and/or metabolic abnormalities to evaluate the effect of Aloe vera as a treatment drug and also to identify the characteristics of serum sex steroids that are associated with insulin sensitivity. Hence, the female mice were continuously exposed to the aromatase inhibitor letrozole to induce a hyperandrogenic state.
MATERIALS AND METHODS
Aloe vera gel preparation
Fresh, mature (3–5-year-old) Aloe vera leaves [Figure 1] were taken and washed with water, incised with the sterilised knife and allowed to stand for 2 h to remove the aloin. Later, the gel was removed by separating the epidermis and sonicated to get a homogenous gel. Further, to the prepared gel, turmeric Curcuma longa L.(3–5 g/l) and lemon juice (Citrus limon 1%) were added as natural preservatives. This was further stored at 4°C. The dose of 0.1 ml/day was given as the treatment.
Figure 1.

Aloe Vera (BARBADENSIS MILL)
Chemicals
Carboxy methyl cellulose
Carboxy methyl cellulose (CMC) (1% aqueous solution) was dissolved in warm distilled water, used as vehicle for oral treatment (2 ml/kg body weight).
Letrozole
Letrozole is tablet that contains 2.5 mg of the active substance letrozole. It was procured from a pharmaceuticals shop, color yellow oxide of iron (0.5 mg/kg body weight) and (0.1 mg/kg body weight).
Diane-35
Each tablet contains the active substance ethinyl estradiol (0.035 mg) and cyproterone acetate (2 mg) and other ingredients such as lactose monohydrate, maize starch, povidone 25, magnesium sterate, sucrose, povidone 700,000, macrogole 6000 calcium carbonate precipitated, talc, glycerol 85%, titanium dioxide ferric oxide pigment yellow, ferric oxide pigment red and mountanglycole wax (0.2 mg/kg body weight).
Test animal
The present study was executed under CPCSEA guidelines with ethical clearance from the Institutional Ethical Committee, Department of Zoology, Karnatak University, Dharwad, (639/02/A/CPCSEA). Laboratory breed 4 months adult virgin female Swiss albino mice weighing about 20–30 g and showing regular estrous cycle of 4–5 days were used under standard animal housing condition (temperature controlled 22°C ± 3°C facility and maintained with 12 h light/dark cycle) with unlimited access to pellet diet and water ad libitum throughout the study. The mice were divided into two experimental groups: Control group of animals (n = 10) received orally 1% aqueous solution of CMC and another group of animals (n = 10) were treated orally with Letrozole (0.5 mg/kg body weight) daily for 21 days. Animals were housed in a separate cage containing sterile paddy husk as bedding material. Daily phases of estrous cycle and body weight were recorded throughout the experiment. Biochemical estimations were performed to the PCOS-induced mice to check the glucose sensitivity test, cholesterol estimation, antioxidant assay of superoxide dismutase (SOD) enzyme activity, hormone assay of luteinising hormone (LH), FSH and testosterone followed by histological studies were also carried to check the cyst formation in PCOS mice. Letrozole treated experimental mice, which demonstrated irregular estrous cycle, altered biochemical estimations, hormone assay, and cyst formation in histology results were considered as PCOS-positive animals and used for further study. These PCOS-positive animals were sub-divided into three groups; PCOS untreated (control) mice, AVG formulation treated mice, and standard drug (Diane) treated mice. The treatment of formulated AVG was given orally 1 ml/mice/day, daily for 30 days with Diane 0.2 mg/kg body weight/day as standard drug and during the treatment; regular estrous cycle along with body weight was recorded. At the end of the treatment, all mice were sacrificed for biochemical estimations, antioxidant assay, hormone assay and histological studies to observe the recovery after treatment.
Estrous cycle
The vaginal smear was examined as described earlier.[6] Few drops of 0.9% saline water were introduced carefully in the vagina by means of specially made pasture pipettes. The saline water was sucked quickly back into the pipette by releasing pressure of the rubber bulb of the pipette. The smear was carefully taken on a clean plain glass slide and observed under microscope for various stages of estrous cycle. Whenever necessary the smear was stained with haematoxyline and eosin to favour the clear identification of cells.
Biochemical estimations
Glucose sensitivity test
The total glucose content of serum was estimated by the method described previously,[7] wherein the serum was separated by centrifugation. The optical density was measured at 620 nm. Glucose concentration was expressed as mg/ml of serum.
Cholesterol estimation
Estimation of cholesterol content of tissue was estimated by the method described earlier.[8] Tissue of known weight was homogenized in 10 ml of 3:1 alcohol ether mixture and the homogenate were centrifuged for 10 min. The supernatant was collected in test tube and dried in a water bath. The dried residue was dissolved in 5 mL chloroform, and then 1 mL of acidic anhydride mixture (20 mL of acidic anhydride is mixed with 1 ml of concentrated sulfuric acid) was added and kept in the dark room for 15 min for colour development. Simultaneously blank and standards were run and the optical density was measured at 660 nm. Cholesterol concentration was expressed as microgram/mg tissue.
Antioxidant assay
Superoxide dismutase enzyme activity
SOD enzyme activity was assayed by a standard method described previously.[9] The assay is based on the principle that SOD inhibits the reduction of nitro blue tetrazolium with reduced nicotinamide adenine dinucleotide mediated by phenazine methosulphate under aerobic conditions. Blood plasma was separated by centrifugation and washed erythrocytes thrice with normal saline. Distilled water was added equal to the volume of plasma separated from erythrocyte pellet. Required reagents were added to a set of test tube, labelled as control and test. The increase in the absorbance at 560 nm for 2 min after every 30s was recorded.
Hormone assay
The hormone assay employed the enzyme-linked fluorescent assay principle with a final fluorescent detection.[10] The sample was taken and transferred into the well containing alkaline phosphatase labelled anti LH, FSH and testosterone antibodies (conjugate). The sample/conjugate mixture was cycled in and out of the solid phase receptacle (SPR) several times to increase the reaction speed. The antigen binds to the antibodies coated on the SPR and to the conjugate forming a 'sandwich.’ Unbound components were eliminated during the washing steps. During the final detection step, the substrate (4-methyl-umeliferyl phosphate) was cycled in and out of the SPR. The conjugate enzyme catalyses the hydrolysis of this substrate into a fluorescent product (4-methyl-umbelliferone); the fluorescent was measured at 450 nm. The intensity of the fluorescent is proportional to the concentration of the antigen present in the sample. At the end of the assay, results were automatically calculated by the instrument in relation to the calibration curve stored in memory and then printed out.
Histology
The ovaries were fixed in an aqueous Bouin's fluid for 24 h. and dehydrated by placing them in 30%, 50%, 70%, 90%, and 100% alcohol gradations, cleared in benzene, and embedded in paraffin wax. Sections of 5 μm thickness were obtained and stained with haematoxyline eosin as per standard.[11] Randomly chosen 10 good sections from each ovary in each group were observed under the microscope.
Statistical analysis
IBM SPSS version 20.0 was used for the mean and standard error calculations of hormone levels, glucose and cholesterol estimations along with superoxide dismutase estimation.
RESULTS
The phytocomponents present in the Aloe vera facilitated mice, treated with letrozole 0.5 mg/kg body weight gained significant body weight when compared with CMC control mice models. Further, there was also recovery in the body weight of mice on treatment with Aloe vera and Diane when compared with PCOS positive control [Table 1]. Correspondingly, decrease in the number of estrous cycles with concomitant increase in the duration of diestrus was observed among mice treated with letrozole when compared with the corresponding parameters of the control mice. However, the mice treated with Diane and Aloe vera showed a significant increase in the number of estrous cycles with concomitant decrease in the duration of diestrous when compared with the corresponding parameters of the PCOS positive control mice. PCOS positive mice exhibited the rise in the concentration of glucose and cholesterol, whereas the PCOS positive mice treated with Aloe vera and Diane showed a recovery in the glucose and cholesterol concentration [Table 2]. The SOD activity found considerably low in the serum of letrozole treated mice in relative to that of CMC control mice. Amongst all, the mice treated with Diane and Aloe vera had a recovery in the serum SOD activity when compared with that of PCOS positive control [Figure 2].
Table 1.
Effect of letrozole and Aloe vera treatment on the body weight of mice
| Groups | Treatment | Body weight (g) | Change in body weight (g) | |
|---|---|---|---|---|
|
| ||||
| Initial | Final | |||
| I | CMC control | 28.78 | 30.82 | 2.0±0.40 |
| II | CMC +0.5 let | 28.58 | 31.26 | 2.6±0.53 |
| III | PCOS control | 28.44 | 30.66 | 2.22±0.44 |
| IV | Diane | 29.34 | 31.20 | 1.86±0.37 |
| V | Aloe vera | 28.34 | 30.30 | 1.96±0.39 |
PCOS: Polycystic ovarian syndrome, CMC: Carboxy methyl cellulose
Table 2.
Effect of letrozole and Aloe vera treatment on biochemical contents in mice
| Groups | Treatment | Biochemical contents | ||
|---|---|---|---|---|
|
| ||||
| Cholesterol (µg/g) | Glucose (µg/ml) | |||
| I | CMC control | 104.8±0.32 | 43.2±0.32 | 87.1±0.35 |
| II | CMC +0.5 Let | 269.8±0.42 | 70.8±0.23 | 124.3±0.25 |
| III | PCOS control | 245.2±0.39 | 152.3±0.45 | 203.5±0.45 |
| IV | Diane | 158.4±0.25 | 125.4±0.31 | 84.2±0.38 |
| V | Aloe vera | 175.4±0.28 | 134.1±0.52 | 101.4±0.21 |
PCOS: Polycystic ovarian syndrome, CMC: Carboxy methyl cellulose
Figure 2.
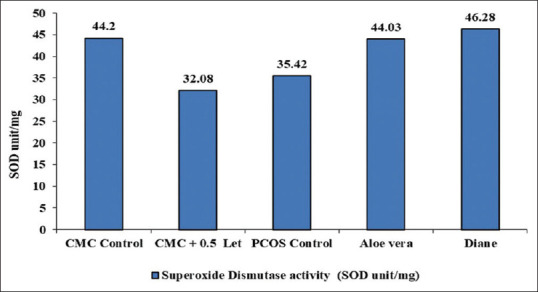
Effect of letrozole and Aloe vera treatment on superoxide dismutase enzyme activity in mice (SOD unit/mg). SOD: Superoxide dismutase, PCOS: Polycystic ovarian syndrome, CMC: Carboxy methyl cellulose
The effect of Letrozole on the reproductive hormones showed an increase in the levels of serum LH and testosterone but decrease in serum FSH. Hence, the PCOS positive mice treated with Diane and Aloe vera presented a significant decrease in serum LH and Testosterone level with the recovery in the serum FSH level [Figure 3]. The T. S of ovary of the CMC control mice showed the components with delineated structures. Cortex presented follicles with different stages of maturation. There were primordial, primary follicle, secondary follicle and Graafian follicles unruptured. Stroma is made up of round to spindle theca cells, blood vessels were normal, the surface epithelium was lined by low cuboidal to flattened cells. The T. S of ovary from 0.5 mg/kg body weight letrozole treated mice had an occasional Graafian follicle and numerous follicular cysts associated with hyperplasia of theca cells (Hyperthecosis) along with stromal nodules. The vagaries exhibited by ovaries of letrozole treated mice could be due to pathologic effect of letrozole [Figure 4]. The T. S of an ovary of PCOS-positive control mice showed follicular cyst (fluid filled sacs), whereas the T. S of an ovary from Aloe vera treated mice displayed follicular cyst, medulla, and Graafian follicle. Finally, the T. S of an ovary from Diane treated mice had medulla, cortex and follicular cyst. Therefore, the results propose that there was considerable recovery of histopathological symptoms in mice treated with Aloe vera 0.1 mL/day/mice gel and Diane as standard drug when compared with PCOS positive control [Figure 5].
Figure 3.
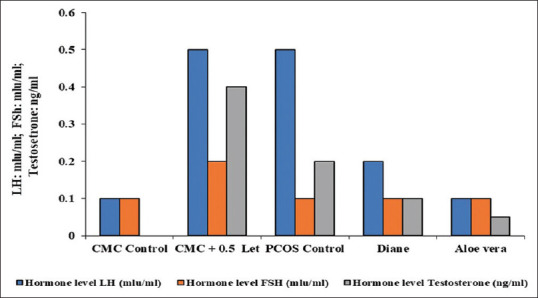
Effect of letrozole and Aloe vera treatment on reproductive hormones in mice. PCOS: Polycystic ovarian syndrome, CMC: Carboxy methyl cellulose, LH: Luteinizing hormone
Figure 4.
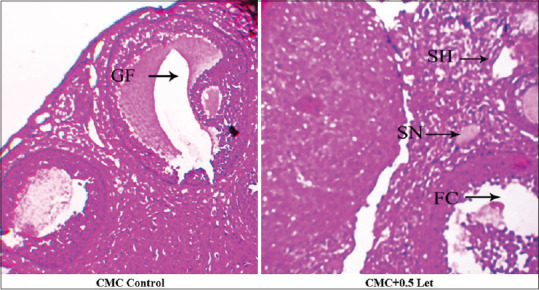
Ovarian Histopathology of Letrozole induced PCOS in Mice. CMC Control: T.S of ovary section showing graafian follicles in CMC control mice; CMC+0.5 letrozole: T.S of ovary section showing numerous follicular cysts and stromal nodule, stromal hyperplasia (Follicular hyperthecosis stromal nodule)
Figure 5.
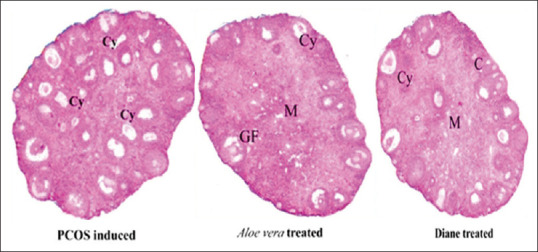
Ovarian Histopathology of after Aloe vera Treatment in PCOS induced Mice. PCOS induced: T.S of Ovary section showing Follicular cysts; Aloe vera treated: T.S of ovary section showing Medulla, Graafian follicle and Follicular cyst; Diane treated: T.S of ovary section showing Medulla, Cortex and Follicular cyst
DISCUSSION
PCOS has a numerous clinical manifestation, including oligomenorrhea and hyper-and rogenism, leading to metabolic disfunction.[12] In the present study, we investigated biochemical and clinical characters of PCOS in mouse models. We found a significant weight gain in Letrozole-induced PCOS mice as compared to control mice which was attributable to deposition of abdominal fat. A remarkable increase in the body weight was noticed which was significantly higher than the control mice after 21 days of treatment with Letrozole. This increase was possibly due to that treatment which affected the metabolic pathway toward hypoglycemic condition resulted in the stimulation of growth hormone secretion from anterior pituitary gland that encourages the secretion of insulin-like growth factors ‘(IGF-I) and II.’[13] This hormone is necessary to stimulate skeletal muscle growth, regulate lipolysis and promote cellular uptake of amino acids.[14] This result is in agreement with other researchers who reported a significant increase in body weight of female mice[15] and male mice[16] treated with TT extract. IGF-I and II play important role in increasing protein synthesis and decreasing protein catabolism.[17] When the PCOS induced mice were treated with AVG, there was no further increase in the body weight this is in accordance with earlier observation.[18,19]
As estrogen synthesis is inhibited by the use of inhibitor in our model, the androgen production will be higher than estrogen production which will affect LH: FSH hormonal balance. This causes irregular estrous cyclicity (causing amenorrhea) show increased diestrous index. This observation coincides with earlier experiment.[20] AVG treated PCOS mice also showed similar kinds of rhythm in estrous cyclicity as found in normal control mice and the Diane group suggesting reversion toward normal physiology.[19] Reversion of estrous cyclicity to normal following AVG treatment could be attributed to phytochemical components present in the gel that maintains steroid status, enabling fertility status to be regained.
PCOS is positively correlated with insulin resistance. Our PCOS mice model was examined for glucose sensitivity test finding that these mice exhibited hyperglycemic tendencies leading to full hyperglycemia and metabolic syndrome (shows insulin resistance), the similar results observed in earlier work.[21] Thus, insulin resistance may be a consequence of increased truncal fat and high levels of free fatty acids. Similarly, the present study found that AVG treated PCOS mice to have returned to normal glycemic condition from their hyperglycemic condition as per the observation. This may be attributable to nutritionally rich phytosterols and phytophenols present in the plant.[22,23]
Women with PCOS are hyper-androgenemic which is associated with alterations in circulating lipids and lipoprotein levels resulting in dyslipidemia. Characteristically, PCOS patients have elevated cholesterol levels.[24,25] The patients with PCOS tend to be obese, probably due to high lipid and cholesterol content. Similar effects were seen after the induction of PCOS in mice from the current study. It was observed that the PCOS-induced mice showed elevated cholesterol levels which decreased significantly when the PCOS induced mice were treated with AVG.[19] The working of our PCOS model was confirmed by assaying serum testosterone and LH levels, which were found to be increased significantly when compared with normal mice indicating hyperandrogenism. This result concurs with the earlier experiments.[26] Earlier findings have reported that being a non-steroidal aromatase inhibitor Letrozole blocks the conversion of testosterone to oestradiol. This leads to the reduction in oestrogen production.[27,28] Similar results were also seen in the present study. The PCOS-induced mice showed decreased levels of oestrogen. Repetitive administration of AVG led to a significant increase in oestrogen levels, thereby significant reduction in serum testosterone and LH levels were observed. This is in accordance with the earlier observations.[29]
The biochemical results were also supported by histopathological observations of light microscopy. It is reported that the histopathological study of PCOS induced mice show the formation of cysts in the ovary.[30] The ovarian cortex shows the presence of atretic follicles and the formation of more than two cysts in the ovary. The cysts show the attenuated layer of granulosa cells and hyperplasia of thecal layer.[31,32] Atretic follicles exhibit massive degeneration and sloughing–off, of the central granulosa layer into the antrum thus the follicles become atretic with the presence of dying cells and debris in the antrum. In PCOS condition, the corpora lutea does not form or the number of corpora lutea are diminished indicating anovulation and the frequency of estrous cycle is almost nil in PCOS mice.[33] In PCOS high level, androgen concentrations are responsible for anovulation by direct effect on the ovary. Androgen-induced follicular atresia is thought to occur by entry of androgens into the granulosa layer of pre-antral follicles, where they bind to the cell receptors and cause the cell death. Androgens cause deterioration of follicles by increasing the number of pycnotic granulosa cells and degenerating oocytes.[33]
The observations seen in the current research work when the mice were induced with PCOS by Letrozole also corroborate earlier findings. The histopathological observations of the AVG treated group showed marked recovery of the ovarian tissue with the presence of normalized structure of antral follicle. The light microscopic observations also revealed the presence of many well-defined antral follicles in the process of normalizing. The follicles showed normal granulosa layer with defined thecal layers. The follicles also showed the presence of a clear antrum free of any cell debris. The presence of corpora lutea was also seen after plant treatment suggesting that treatment AVG restores normal estrous cyclicity. The ovarian cortex showed the presence of many follicles in the various stages of development indicating normal oogenesis. The present protocol was adjusted to produce PCOS-like mice models which may not be suitable to generate similar results in different mice models or rodent models. The present study also borrowed single strain mice with limited number, which could be a limitation.
CONCLUSION
Preliminary phytochemical data found that Aloe vera gel is rich in phytophenols and phytosterols that could be active components in controlling hyperglycemic conditions and modulating steroidogenesis. However, no reports of phytosterols and phytophenols affecting steroidogenic enzymes have been published. We hypothesize that these phytocomponents act at various stages of the steriodogenic enzyme cascade and modulate the activity back to normal. Data from our “in vivo” study indicate that Aloe vera gel acts directly on key enzymes like 13βHSD decreasing enzyme activity and modulating flux toward estradiol formation. However, the specific phytocomponent acting on the enzyme system needs to be identified.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293:355–9. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hull MG. Epidemiology of infertility and polycystic ovarian disease: Endocrinological and demographic studies. Gynecol Endocrinol. 1987;1:235–45. doi: 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- 3.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–8. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 4.Thanagari BS, Fefar DT, Prajapati KS, Jivani BM, Thakor KB, Patel JH, et al. Haemato-biochemical alterations induced by diclofenac sodium toxicity in Swiss albino mice. Vet World. 2012;5:417–9. [Google Scholar]
- 5.Kobayashi T, Kobayashi T, Kato J, Minaguchi H. Fluctuations in monoamine oxidase activity in the hypothalamus of rat during the estrous cycle and after castration. Endocrinol Jpn. 1964;11:283–90. doi: 10.1507/endocrj1954.11.283. [DOI] [PubMed] [Google Scholar]
- 6.Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956;220:583–93. [PubMed] [Google Scholar]
- 7.Abel LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–66. [PubMed] [Google Scholar]
- 8.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 9.Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, et al. Intermittent calcitriol therapy in secondary hyperparathyroidism: A comparison between oral and intraperitoneal administration. Kidney Int. 1998;54:907–14. doi: 10.1046/j.1523-1755.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Dickerson EH, Cho LW, Maguiness SD, Killick SL, Robinson J, Atkin SL. Insulin resistance and free androgen index correlate with the outcome of controlled ovarian hyperstimulation in non-PCOS women undergoing IVF. Hum Reprod. 2010;25:504–9. doi: 10.1093/humrep/dep393. [DOI] [PubMed] [Google Scholar]
- 11.Tapanadechopone P, Hassell JR, Rigatti B, Couchman JR. Localization of glycosaminoglycan substitution sites on domain V of mouse perlecan. Biochem Biophys Res Commun. 1999;265:680–90. doi: 10.1006/bbrc.1999.1714. [DOI] [PubMed] [Google Scholar]
- 12.Gazzah AC, El Golli Bennour E, Bouaziz C, Abid S, Ladjimi M, Bacha H. Sequential events of apoptosis induced by zearalenone in cultured hepatocarcinoma cells. Mycotoxin Res. 2010;26:187–97. doi: 10.1007/s12550-010-0053-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang MX, Yin Q, Xu X. A rat model of polycystic ovary syndrome with insulin resistance induced by letrozole combined with high fat diet. Med Sci Monit. 2020;26:e922136. doi: 10.12659/MSM.922136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YJ, Tsushima T, Onoda N, Minei S, Sanaka M, Nagashima T, et al. Expression of messenger RNA of insulin-like growth factors (IGFs) and IGF binding proteins (IGFBP1-6) in placenta of normal and diabetic pregnancy. Endocr J. 1996;43 Suppl:S89–91. doi: 10.1507/endocrj.43.suppl_s89. [DOI] [PubMed] [Google Scholar]
- 15.Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:999–1004. doi: 10.1210/jc.2007-2117. [DOI] [PubMed] [Google Scholar]
- 16.Maharjan R, Nagar PS, Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2010;1:273–9. doi: 10.4103/0975-9476.74090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: From pathophysiology to treatment. Reprod Biomed Online. 2009;19:552–63. doi: 10.1016/j.rbmo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Kurabayashi T, Yahata T, Quan J, Tanaka K. Association of polymorphisms in the beta2 and beta3 adrenoceptor gene with polycystic ovary syndrome. J Reprod Med. 2006;51:389–93. [PubMed] [Google Scholar]
- 19.Desai BN, Maharjan RH, Nampoothiri LP. Aloe barbadensis Mill.formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacognosy Res. 2012;4:109–15. doi: 10.4103/0974-8490.94736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manneras L, Lystig T, Holmang A, Ottosson-Lonn M, Stener-Victorin E. Continuous Administration of Dihydrotestosterone or Letrozole to Immature Female Rats Results in Polycystic Ovary Syndrome Characteristics at Adult Age. Proceedings 9th European Congress of Endocrinology. 2007;14 [Google Scholar]
- 21.Baravalle C, Salvetti NR, Mira GA, Pezzone N, Ortega HH. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch Med Res. 2006;37:830–9. doi: 10.1016/j.arcmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch Med Res. 2004;35:103–8. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Brawer JR, Munoz M, Farookhi R. Development of the polycystic ovarian condition (PCO) in the estradiol valerate-treated rat. Biol Reprod. 1986;35:647–55. doi: 10.1095/biolreprod35.3.647. [DOI] [PubMed] [Google Scholar]
- 24.Rezvanfar MA, Rezvanfar MA, Ahmadi A, Saadi HA, Baeeri M, Abdollahi M. Mechanistic links between oxidative/nitrosative stress and tumor necrosis factor alpha in letrozole-induced murine polycystic ovary: Biochemical and pathological evidences for beneficial effect of pioglitazone. Hum Exp Toxicol. 2012;31:887–97. doi: 10.1177/0960327111426589. [DOI] [PubMed] [Google Scholar]
- 25.Sasikala SL, Shamila S. Unique rat model exhibiting biochemical fluctuations of letrozole induced polycystic ovary syndrome and subsequent treatment with allopathic and ayurvedic medicines. J Cell Tissue Res. 2009;9:2013. [Google Scholar]
- 26.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 27.Yang AM, Cui N, Sun YF, Hao GM. Letrozole for female infertility. Front Endocrinol (Lausanne) 2021;12:676133. doi: 10.3389/fendo.2021.676133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007;105(Suppl 1):7–17. doi: 10.1007/s10549-007-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–22. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 30.Annie L, Gurusubramanian G, Roy VK. Inhibition of visfatin by FK866 mitigates pathogenesis of cystic ovary in letrozole-induced hyperandrogenised mice. Life Sci. 2021;276:119409. doi: 10.1016/j.lfs.2021.119409. [DOI] [PubMed] [Google Scholar]
- 31.Noorafshan A, Ahmadi M, Mesbah SF, Karbalay-Doust S. Stereological study of the effects of letrozole and estradiol valerate treatment on the ovary of rats. Clin Exp Reprod Med. 2013;40:115–21. doi: 10.5653/cerm.2013.40.3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi D, Vine DF. Animal models of polycystic ovary syndrome: A focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012;98:185–93. doi: 10.1016/j.fertnstert.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93:69. doi: 10.1095/biolreprod.115.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


