Abstract
Introduction
SuperAgers are individuals over age 80 with superior episodic memory, at a level consistent with individuals 20 to 30 years their junior and who seem to show resistance to age‐related neurofibrillary degeneration. Here we examine whether low genetic risk for Alzheimer's disease (AD) contributes to SuperAgers’ unusually high episodic memory performance in advanced age.
Methods
The AD polygenic hazard score (PHS) was calculated for each SuperAger and cognitively normal participant and compared between groups.
Results
A total of 37 SuperAgers (73% female, mean [standard deviation] 82.7 [2.8] years old) and 35 controls (54% female, 83.7 [4.3] years old) were included. There was no significant difference in the AD PHS between SuperAgers and cognitively normal controls.
Discussion
Unusually successful cognitive aging cannot be simply explained by low polygenic risk for AD as assessed by common genetic variants. However, rare variants and common protective genetic factors may contribute to resistance or resilience.
Highlights
SuperAging cannot be simply explained by low polygenic risk for Alzheimer's disease.
Rare variants and common protective genetic factors may contribute to SuperAging.
A protective factors polygenic score may uncover mechanisms for SuperAging.
Keywords: aging, Alzheimer's disease, dementia, episodic memory, polygenic risk, resilience, resistance, successful aging
1. INTRODUCTION
Unusually successful cognitive aging (SuperAging) may reflect underlying resistance (i.e., avoidance of expected negative factor) to age‐associated cognitive decline and the neuropathologic markers of Alzheimer's disease (AD). Indeed, SuperAgers have a slower rate of atrophy 1 and less AD pathology 2 than their cognitively normal peers. However, it is unknown whether SuperAgers vary in their genetic protection from AD relative to their cognitively normal elderly peers.
The apolipoprotein E (APOE) ε4 allele is known to modulate AD risk and may be underrepresented in SuperAgers. 2 A recently developed polygenic hazard score (PHS) captures an individual's risk for AD as an aggregate of their risk across 31 common variants and APOE and better predicts the age of AD onset than does APOE alone. 3 The PHS is associated with the hallmark AD neuropathologic changes, neuritic plaques and neurofibrillary tangles, and both clinical progression and cognitive decline in clinically normal individuals. 4 , 5 We examined whether polygenic risk for AD was significantly lower in SuperAgers compared to similarly aged cognitively normal peers (controls). If SuperAgers show exceptionally low PHS relative to controls it would suggest this may be a mechanism by which they resist age‐related decline in memory, while high PHS scores would support that there may be an undiscovered mechanism by which SuperAgers are resilient (i.e., ability to overcome effects of a negative factor) to the previously established AD polygenic risk metric.
2. METHODS
2.1. Participants
SuperAgers (n = 37), defined by age and stringent neuropsychological performance criteria, were drawn from the Northwestern SuperAging Program. 2 , 6 Briefly, SuperAgers were at least 80 years old and performed at or above normative values for average 50‐ to 65‐year‐olds on delayed recall of the Rey Auditory Verbal Learning Test (RAVLT) and within one standard deviation of the average normative range for their age, or better, on other cognitive tests including the 30‐item Boston Naming Test, Trail Making Test Part B and Category Fluency Test (Animals).
The control group (n = 35) was drawn from the University of California, San Diego Shiley‐Marcos Alzheimer's Disease Research Center. Inclusion was limited to individuals with available genetic data who were at least 80 years old with average performance for their age on cognitive measures. The California Verbal Learning Test (CVLT), edition I or II, was used in place of the RAVLT in this group.
All participants gave written and informed consent for their participation in their respective research projects and for the sharing of data.
1. RESEARCH IN CONTEXT
Systematic Review: The existing literature was reviewed (e.g., PubMed) and cited. Previous studies have examined the utility of a polygenic hazard score (PHS) for Alzheimer's disease (AD) risk. Here we assess whether genetic protection from AD explains the superior episodic memory performance of SuperAgers relative to their cognitively normal elderly peers.
Interpretation: In this cross‐sectional study, there was no significant difference in polygenic risk for AD between SuperAgers in advanced age and their similarly aged cognitively normal peers. Thus, unusually successful cognitive aging beyond the eighth decade cannot be simply explained by low polygenic risk for AD as assessed by common genetic variants.
Future Directions: Protective genetic factors are emerging that highlight the potential utility for the development of a protective polygenic score.
2.2. Genetic data
Genetic data for all participants were accessed through the National Alzheimer's Coordinating Center (NACC). Genetic data were preprocessed with PLINK to exclude samples with a missingness rate greater than 10% and to perform strand flips as necessary. Pre‐imputation quality controls removed duplicate sites, non–single nucleotide polymorphism (SNP) sites, monomorphic sites, and SNPs with a call rate <90%. The imputation was performed using the Michigan Imputation Server 7 with the Haplotype Reference Consortium reference panel 8 (hg19). Post‐imputation the data were filtered to exclude genotype calls with an estimated posterior genotype probability <.9.
2.3. PHS calculation
The PHS was calculated as described for all participants. 3 Briefly, potentially AD‐associated SNPs were selected in the International Genomics of Alzheimer's Project (IGAP) cohort at P < 10−5. These SNPs were then integrated into a stepwise Cox proportional hazards model using a subset of the Alzheimer's Disease Genetics Consortium (ADGC) phase 1 genetic data, excluding individuals from the NACC. This stepwise procedure identified 31 SNPs, which are listed in the original report, 3 that most improved the model prediction. The PHS used in the current study was calculated for each participant as the vector product of that individual's genotype for the 31 SNPs and the corresponding parameter estimates from the ADGC phase 1 Cox proportional hazard model, in addition to the APOE effects.
2.4. Statistical analysis
Differences in demographic characteristics and raw neuropsychological test scores between the SuperAger and control groups were examined using Pearson's chi‐squared tests or Mann–Whitney U‐tests as appropriate. The difference in PHS between groups was examined with the Mann–Whitney U‐test. Given the current sample size and previously published PHS effect size, there was sufficient power to detect a difference in PHS between groups.
3. RESULTS
The SuperAger and control groups were similar in age, years of education, sex, and APOE ε4 allele carrier status (Table 1). Compared to controls, the SuperAgers performed better on the 30‐item Boston Naming Test, Category Fluency Test, and Trail Making Test Part B (P < .01; Table 1). Episodic memory performance followed inclusion criteria (above), and thus, was not compared between groups.
TABLE 1.
Demographic characteristics and raw neuropsychological test scores displayed by cohort
| SuperAgers (N = 37) | Controls (N = 35) | P‐value | |
|---|---|---|---|
| Age, years | 82.7 (2.8) | 83.7 (4.3) | .46 |
| Women, N (%) | 27 (73) | 19 (54) | .16 |
| White, N (%) | 37 (100) | 35 (100) | |
| Education, years | 16.1 (2.5) | 15.9 (2.9) | .88 |
| APOE genotype frequency, N (%) | .86* | ||
| 2/3 | 5 (14) | 5 (14) | |
| 3/3 | 24 (65) | 24 (69) | |
| 2/4 | 0 (0) | 2 (6) | |
| 3/4 | 8 (22) | 4 (11) | |
| Neuropsychological measures | |||
| RAVLT, delayed recall | 11.65 (1.7) | – | |
| CVLT‐I, delayed recall | – | 8.45 (3.1) | |
| CVLT‐II, delayed recall | – | 8.00 (2.5) | |
| 30‐item Boston Naming Test | 28.32 (2.7) | 26.67 (2.7) | <.001 |
| Category fluency (animals) | 22.51 (4.7) | 18.37 (5.8) | <.001 |
| Trail Making Test (Part B) | 86.70 (38.8) | 113.97 (54.2) | <.01 |
Note: Reported as mean (standard deviation) unless otherwise noted. P‐values based on Pearson's chi‐squared tests or Mann–Whitney U tests as appropriate. RAVLT delayed recall normative range for 50‐ to 60‐year‐olds is 9 to 15. CVLT‐I delayed recall normative range (standard score –1 to 1) for 75‐ to 80‐year‐olds is 9 to 10 (6–13) for women and 7 to 9 (4–13) for men. CVLT‐II delayed recall normative range (standard score –1 to 1) for 80‐ to 89‐year‐olds is 9 (6–12) for women and 6 to 7 (3–9) for men.
*P‐value reflects APOE ε4 allele carrier status.
Abbreviations: APOE, apolipoprotein E; CVLT, California Verbal Learning Test; RAVLT, Rey Auditory Verbal Learning Test.
Average PHS (centered) was less than zero for both the SuperAger and control groups, indicating lower than average population AD risk. However, there was no significant difference in PHS between groups (Figure 1, P = .34). In addition, analysis by sex revealed no significant differences in PHS between groups in 46 women (27 SuperAgers –0.17 [0.73]; 19 controls –0.24 [0.93]; P = .84) or in 26 men (10 SuperAgers –0.06 [0.75]; 16 controls –0.41 [0.88]; P = .22).
FIGURE 1.
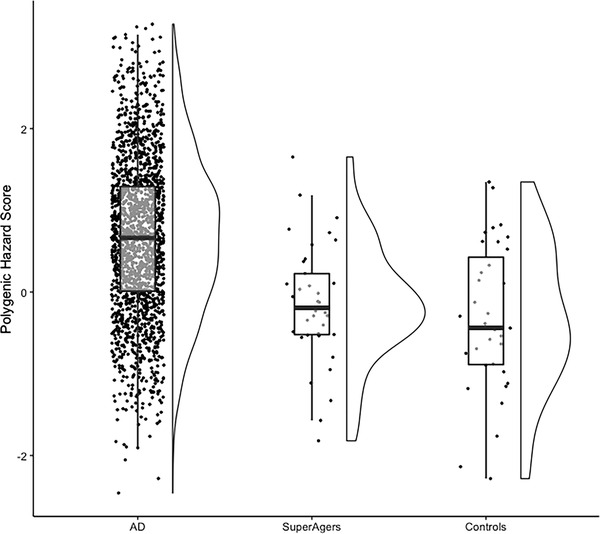
SuperAgers do not show a significantly lower Alzheimer's disease (AD) polygenic hazard score (PHS) than controls. There was no significant difference in PHS between the SuperAger (mean [standard deviation] –0.14 [0.73]) and control (–0.32 [0.90]) groups (P = .34). To contextualize these results, we have included a pathologically defined AD comparison group (N = 1405) from a study that examined the relationship between the PHS and pathological diagnostic categories. AD cases included here are a conservative subset of those that met at least intermediate or high AD neuropathologic change and at least Braak stage V 13
4. DISCUSSION
Despite superior episodic memory performance, SuperAgers did not have significantly reduced polygenic risk for AD compared to their cognitively normal elderly peers. This suggests determinants of superior memory performance in older age cannot be solely explained by having unusually low risk for AD as assessed by common genetic variants.
There was a wide range in PHS within each group, consistent with the notion that low polygenic risk for AD may be a critical factor for some SuperAgers, but is insufficient to fully explain their youthful memory phenotype. Due to observed differences in AD genetic risk across racial and ethnic groups, these findings are largely limited to White, non‐Hispanic individuals. Given the known heterogeneity in the genetic architecture of AD across age, future work may wish to evaluate the polygenic risk profile of older (≥80 years) age of onset AD cases compared to controls and SuperAgers. Additionally, repeating the PHS development using a larger, more recent genome‐wide association study of AD may increase statistical power for detecting differences between groups. Rare genetic mutations that would not be captured by the current approach have been identified that confer protection against AD. 9 Other variants may also be important for promoting healthy brain aging and superior cognitive performance. Our previous exome‐wide analysis suggests inheritance of polymorphisms of the MAP2K3 gene is different between SuperAgers and controls. 10 Likewise, a variant of the KLOTHO gene that promotes longevity has been associated with enhanced cognition 11 and, even in those who carry an APOE ε4 allele, a reduced risk for AD. 12 Taken together, youthful performance beyond the eighth decade cannot be simply explained by low polygenic risk for AD as assessed by common genetic variants. Protective genetic factors are emerging that highlight the potential utility for the development of a polygenic score focused on protective factors.
CONFLICTS OF INTEREST
Authors Emily Rogalski, Sarah J. Banks, Sandra Weintraub, James B. Brewer, M.‐Marsel Mesulam, Barbara E. Spencer, Anders M. Dale, and Changiz Geula receive grant support from NIH. Anders M. Dale is a founder of and holds equity interest in CorTechs Labs, La Jolla, California, and serves on its scientific advisory board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. Dr. James B. Brewer has served on advisory boards for Elan, Bristol‐Myers Squibb, Avanir, Novartis, Genentech, and Eli Lilly and holds stock options in CorTechs Labs, Inc. and Human Longevity, Inc. Ms. Beth Makowski‐Woidan reports no conflicts. Author disclosures are available in the supporting information.
AUTHOR CONTRIBUTIONS
Dr. Emily Rogalski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Barbara E. Spencer, Sarah J. Banks, and Emily Rogalski. Analysis and interpretation of data: Barbara E. Spencer, Sarah J. Banks, and Emily Rogalski. Drafting of the manuscript: Barbara E. Spencer, Sarah J. Banks, and Emily Rogalski. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Barbara E. Spencer, Sarah J. Banks, and Emily Rogalski.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
Research reported in this publication was supported, in part, by the National Institutes of Health's National Institute on Aging, by R01AG045571, R56AG04557, R01AG067781, P30 AG13854 (Northwestern's Alzheimer's Disease Research Center), and P30‐AG062429 (University of California, San Diego Alzheimer's Disease Research Center). The NACC database is funded by NIA/NIH grant U01 AG016976. NACC data are contributed by the NIA‐funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428‐01 (PI James Leverenz, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421‐01 (PI Bradley Hyman, MD, PhD), P30 AG062422‐01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429‐01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715‐01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Spencer BE, Banks SJ, Dale AM, et al. Alzheimer's polygenic hazard score in SuperAgers: SuperGenes or SuperResilience? Alzheimer's Dement. 2022;8:e12321. 10.1002/trc2.12321
REFERENCES
- 1. Cook AH, Sridhar J, Ohm D, et al. Rates of cortical atrophy in adults 80 years and older with superior vs average episodic memory. JAMA. 2017;317(13):1373‐1375. 10.1001/jama.2017.0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogalski EJ, Gefen T, Shi J, et al. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci. 2013;25(1):29‐36. 10.1162/jocn_a_00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age‐associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14(3):e1002258. 10.1371/journal.pmed.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan CH, Hyman BT, Tan JJX, et al. Polygenic hazard scores in preclinical Alzheimer disease. Ann Neurol. 2017;82(3):484‐488. 10.1002/ana.25029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan CH, Fan CC, Mormino EC, et al. Polygenic hazard score: an enrichment marker for Alzheimer's associated amyloid and tau deposition. Acta Neuropathol. 2018;135(1):85‐93. 10.1007/s00401-017-1789-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogalski E, Gefen T, Mao Q, et al. Cognitive trajectories and spectrum of neuropathology in SuperAgers: the first ten cases. Hippocampus. 2019;29(5):458‐467. 10.1002/hipo.22828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das S, Forer L, Schönherr S, et al. Next‐generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284‐1287. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarthy S, Das S, Kretzschmar W, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nature Genetics. 2016;48(10):1279‐1283. 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arboleda‐Velasquez JF, Lopera F, O'Hare M, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med. 2019;25(11):1680‐1683. 10.1038/s41591-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huentelman MJ, Piras IS, Siniard AL, et al. Associations of MAP2K3 gene variants with superior memory in SuperAgers. Front Aging Neurosci. 2018;10:155. 10.3389/fnagi.2018.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014;7(4):1065‐1076. 10.1016/j.celrep.2014.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD for the Alzheimer's Disease Neuroimaging Initiative . Association of Klotho ‐VS Heterozygosity with risk of Alzheimer disease in individuals who carry APOE4 . JAMA Neurol. 2020;77(7):849. 10.1001/jamaneurol.2020.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spencer BE, Jennings RG, Fan CC, Brewer JB. Assessment of genetic risk for improved clinical‐neuropathological correlations. Acta Neuropathol Commun. 2020;8:160. 10.1186/s40478-020-01033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


