Abstract
The hydroalcoholic extracts of the Turkish traditional coffee samples from 18 commercial brands were tested for their neurobiological effects through enzyme inhibition based on enzyme-linked immunosorbance microtiter assays against acetylcholinesterase, butyrylcholinesterase, and tyrosinase, linked to Alzheimer’s and Parkinson’s diseases. The extracts were also subjected to several antioxidant test systems to define their antiradical, metal-chelation capacity, and reducing power. Total phenol and flavonoid contents in the extracts were delineated by spectrophotometric methods, while chlorogenic acid in the coffee samples was quantified by high-pressure liquid chromatography. The extracts displayed low to moderate inhibition (from 2.13 ± 0.01% to 36.12 ± 1.07% at 200 μg/mL) against the tested enzymes, whereas they had notable 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity up to 56.15 ± 2.03% at 200 μg/mL. The extracts exerted a remarkable ferric-reducing antioxidant power values, while chlorogenic acid was found to range between 0.288 ± 0.005% and 2.335 ± 0.010%.
Keywords: antioxidant activity, cholinesterase inhibition, chlorogenic acid, high performance liquid chromatography, Turkish coffee
1. Introduction
Drinking Turkish coffee is an important part of the culture and indoor/outdoor social activities in Turkey. Coffee was introduced to Turks approximately five centuries ago. During the Ottoman period, an Ottoman governor to Yemen who was brought back to Istanbul introduced the coffee beans to the Ottoman capital in 1543 (http://www.turkish-coffee.org/turkish_coffee.htm). Turkish coffee is also famous and consumed widely in the same style in the Balkan and Middle East countries. It is traditionally prepared from the seeds of Coffea arabica L. (Rubiaceae) using a special type of narrow-topped small boiling pot called a cezve.
Neurodegenerative diseases are among the deadly disorders affecting elderly population. In particular, Alzheimer’s (AD) and Parkinson’s disease (PD) have been found to have a multifactorial and progressive nature and no complete cure is yet available for either disease. Therefore, intensive research is being conducted on finding new drug candidates of natural or synthetic origins for the treatment of AD and PD, whose pathogeneses are still mostly unclear. The cholinergic hypothesis has been the most accepted theory for AD, whereby a marked deficit in level of acetylcholine (ACh) has been shown in the AD patients. Thus, inhibition of the enzyme acetylcholinesterase (AChE), which can break down ACh in the brain, has been a widely used treatment strategy against AD [1]. Relevantly, butyrylcholinesterase (BChE; also known as pseudocholinesterase or plasma cholinesterase) is a nonspecific cholinesterase that has ability to hydrolyze many different choline esters including ACh and, therefore, inhibition of BChE is also important for AD treatment [2]. Since dementia is also associated with PD, cholinesterase inhibitors are also of interest for PD treatment [3]. Moreover, neuromelanoma associated with enzymes such as tyrosinase (TYR) and tyrosine hydroxylase has been suggested to play a role in increased susceptibility to both PD and melanoma [4]. Hence, inhibition of TYR has emerged as a possible new strategy towards PD.
In our enduring research on finding new cholinesterase and TYR inhibitors of natural origin, we have recently investigated neuroprotective properties of a number of traditional herbal coffees consumed in Turkey such as terebinth coffee as well as some other herbal coffees prepared from the seeds of carob, black cumin, date, and tumble thistle [5,6]. In the current study, our target was to evaluate neurobiological effects of the hydroalcoholic extracts (ethanol, 80%) prepared from 18 commercial brands of Turkish coffee sold in Turkey and northern Cyprus. For this purpose, cholinesterase and TYR inhibitory activity of the extracts was tested using enzyme-linked immunosorbance microtiter assays. As oxidative stress has been stated to contribute to neurodegeneration [7], antioxidant activity of the coffee extracts was tested using 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and N,N-dimethyl-p-phenylendiamine (DMPD) radical scavenging activity, metal-chelation capacity, ferric-reducing antioxidant power (FRAP), and phosphomolibdenum-reducing antioxidant power (PRAP) assays. Total phenol and flavonoid contents in the extracts were calculated spectrophotometrically. In addition, the extracts were subjected to high-pressure liquid chromatography (HPLC) analysis for quantification of chlorogenic acid, a phenolic acid widely found in coffee beans.
2. Methods
2.1. Coffee samples
The medium-roasted type of Turkish coffee samples belonging to 18 commercial brands (Kaffka, Shazili, Alkan, Hiscafe, Madenci, Asli, Arif, Con, Hisar, Mehmet Efendi, Oza, Oza light, Dibek, Action, Nescafe Falci, Ozerlat, Cezbeli, and Ulker) were purchased from supermarkets in Turkey or northern Cyprus during 2011.
2.2. Extraction procedure
Each coffee sample was weighed precisely in a digital balance (Shimadzu, Kyoto, Japan) and, then, extracted with ethanol (80%) at room temperature over 3 days by shaking occasionally. The hydroalcoholic phases were filtered through a regular filter paper and evaporated in vacuo until dryness to give the crude coffee ethanol extracts.
2.3. AChE and BChE inhibitory activity assays
AChE and BChE inhibitory activity of the extracts was measured by slightly modified spectrophotometric method of Ellman et al [8]. Electric eel AChE (Type-VI-S, EC 3.1.1.7; Sigma, St Louis, MO, USA) and horse serum BChE (EC 3.1.1.8; Sigma, St. Louis, MO, USA) were used, while acetylthiocholine iodide and butyrylthiocholine chloride (Sigma) were employed as substrates of the reaction, respectively. 5,5′-Dithio-bis(2-nitrobenzoic) acid (Sigma) was used for the measurement of the anticholinesterase activity. All reagents and conditions were as described in our previous publication [5]. Hydrolysis of acetylthiocholine iodide/butyrylthiocholine chloride was monitored by the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of 5,5′-dithio-bis(2-nitrobenzoic) acid with thiocholines, catalyzed by enzymes at 412 nm utilizing a 96-well microplate reader (VersaMax; Molecular Devices, Sunnyvale, CA, USA). The measurements and calculations were evaluated by using Softmax PRO 4.3.2.LS software (Softmax Moleculer Devices, Downingtown, USA). Percentage of inhibition of AChE/BChE was determined by comparison of rates of reaction of samples relative to blank (ethanol in phosphate buffer, pH = 8) using the formula:
| (1) |
where E is the activity of enzyme without test sample and S is the activity of enzyme with test sample. The experiments were done in triplicate. Galanthamine (Sigma) was used as the reference.
2.4. TYR inhibitory activity assay
Inhibition of TYR (EC 1.14.1.8.1, 30 U, mushroom tyrosinase; Sigma) was determined using the modified dopachrome method with L-DOPA as substrate [9]. The assays were conducted in a 96-well microplate using an enzyme-linked immunosorbance assay microplate reader (VersaMax) to measure absorbance at 475 nm. An aliquot of the extracts dissolved in dimethyl sulfoxide with 80 μL of phosphate buffer (pH 6.8), 40 μL of tyrosinase, and 40 μL of L-DOPA were put in each well. Results were compared with control (dimethyl sulfoxide). Baicalein (Sigma) was used as the reference. The percentage tyrosinase inhibition (I%) was calculated as follows:
| (2) |
2.5. Antioxidant activity assays
2.5.1. DPPH radical scavenging activity
The stable DPPH radical scavenging activity was determined by the method of Blois [10]. The samples dissolved in methanol (75%) were mixed with DPPH solution (1.5 × 10−4M). The amount of DPPH remaining was measured at 520 nm using a Unico 4802 UV–visible double beam spectrophotometer (Unico, Dayton, NJ, USA). Gallic acid was employed as the reference. Inhibition of DPPH in percent (I%) was calculated as:
| (3) |
where Ablank is the absorbance of the control reaction (containing all reagents except the test sample), and Asample is the absorbance of the extracts/reference. Analyses were run in triplicate and the results were expressed as average values with standard error of the mean (SEM).
2.5.2. DMPD radical scavenging activity
The principal of the assay is based on reduction of the purple-colored radical DMPD+ (N,N-dimethyl-p-phenylendiamine). According to the method [11], a reagent comprising of 100mM DMPD, 0.1M acetate buffer (pH = 5.25), and 0.05M ferric chloride solution, which lead to formation of DMPD radical, was freshly prepared and the reagent was equilibrated to an absorbance of 0.900 ± 0.100 at 505 nm. Then, the reagent was mixed with 50 μL of the extract dilutions and absorbance was taken at 505 nm using a Unico 4802 UV-visible double beam spectrophotometer. Quercetin was employed as the reference and the experiments were done in triplicate. The results were calculated according to the same formula given for DPPH radical scavenging test and expressed as average values with SEM.
2.6. Metal-chelation effect
The metal-chelating effect of the samples via ferrous ion was estimated in accordance with Chua et al’s method [12]. Briefly, various dilutions of the extracts were incubated with 2mM FeCl2 solution. The reaction was initiated by the addition of 5mM ferrozine into the mixture and left standing at ambient temperature for 10 minutes. The absorbance of the reaction mixture was measured at 562 nm using a Unico 4802 UV–visible double beam spectrophotometer. The ratio of inhibition of ferrozine–Fe2+ complex formation was calculated as:
| (4) |
where Ablank is the absorbance of the control reaction (containing only FeCl2 and ferrozine), and Asample is the absorbance of the extracts/reference. Analyses were run in triplicate and the results were expressed as average values with SEM. The reference was EDTA in this assay.
2.6.1. FRAP assay
The ferric-reducing power of the extracts was tested using the assay of Oyaizu [13]. Different concentrations of the extracts were mixed with 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of potassium ferricyanide. Later, the mixture was incubated at 50°C for 20 minutes and, then, trichloroacetic acid (10%) was added. After the mixture was shaken vigorously, this solution was mixed with distilled water and ferric chloride (0.1%). After 30 minutes of incubation, absorbance was read at 700 nm using a Unico 4802 UV–visible double beam spectrophotometer. Analyses were made in triplicate. Increased absorbance of the reaction meant increased reducing power and compared to that of chlorogenic acid as the reference.
2.6.2. PRAP assay
In order to perform PRAP assays on the samples, each dilution was mixed with 0% phosphomolybdic acid solution in ethanol (w/v) [14]. The solution was subsequently subjected to incubation at 80°C for 30 minutes and the absorbance read at 600 nm using a Unico 4802 UV–visible double beam spectrophotometer. Analyses were run in triplicate. Increased absorbance of the reaction meant increased reducing power and compared to that of quercetin as the reference.
2.7. Statistical analysis
Data obtained from in vitro enzyme inhibition and antioxidant experiments were expressed as the mean ± SEM. Statistical differences between the reference and the sample groups were evaluated by one way analysis of variance. Dunnett’s multiple comparison tests were used as post hoc tests. A p value < 0.05 was considered to be significant (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
2.8. Determination of total phenol and flavonoid contents in the extracts
Phenolic content of the extracts was determined in accordance with the Folin–Ciocalteau method [15]. In brief, a number of dilutions of gallic acid were obtained to prepare a calibration curve. The extracts and gallic acid dilutions were mixed with 750 μL of Folin–Ciocalteau reagent and 600 μL of sodium carbonate in test tubes. The tubes were then vortexed and incubated at 40°C for 30 minutes. Absorption was measured at 760 nm in a Unico 4802 UV–visible double beam spectrophotometer.
Total flavonoid content of the extracts was calculated by aluminum chloride colorimetric method [16]. Briefly, a number of quercetin dilutions was obtained to prepare a calibration curve. Then, the extracts were mixed with ethanol (95%), aluminum chloride reagent, 0.1 mL of sodium acetate as well as distilled water. Following incubation for 30 minutes at room temperature, absorbance of the reaction mixtures was measured at wavelength of 415 nm with a Unico 4802 UV–visible double beam spectrophotometer. The total phenol and flavonoid contents of the extracts were expressed as gallic acid and quercetin equivalents (mg/g extract), respectively.
2.9. Quantification of chlorogenic acid in the coffee extracts by HPLC
Analyses were performed using an Agilent Technologies 1200 series HPLC (Agilent, Santa Clara, CA, USA), including a binary pump, vacuum degasser, autosampler, and diode array detector. The analysis method of Somporn et al [17] was applied herein with some modifications. Chromatographic separations were performed on ACE-5 C18 column (5 μm, 250 mm; 4.6 mm). A mobile phase consisting of 0.4% aqueous solution of acetic acid (A) and acetonitrile (B) was used for separation with a gradient elution at a flow rate of 0.5 mL/ min. Gradient elution was performed as follows: from 0 minutes to 30 minutes, linear gradient from 5% to 12% solvent B; from 30 minutes to 50 minutes, from 12% to 20% solvent B; from 50 minutes to 60 minutes, from 20% to 35% solvent B; from 60 minutes to 70 minutes, from 35% to 70% solvent B; from 70 minutes to 75 minutes, linear gradient from 70% to 5% solvent B, and a re-equilibration period of 2.5 min with solvent B used between individual runs. Detection was at 350 nm. The injection volume was 20 μL. Column temperature was set to 40°C. Identification of the chlorogenic acid was made by comparing the retention times and UV spectra of the peaks in the coffee samples and the standard. Standard solution was then added to the sample; increase in the intensity of the peaks verified the identification. All of the calculations concerning the quantitative analysis were performed with external standardization by measurement of peak areas. Standard solutions of chlorogenic acid (0.3 mg/ mL) were prepared in methanol. Each injection was achieved in triplicate to establish reproducibility of detector response at each concentration level. Five concentrations of chlorogenic acid (0.3–0.003 mg/mL) were subjected to regression analysis in order to calculate calibration equation and correlation (Table 1). Limits of detection and limits of quantitation were established at a signal to noise ratio of 3 and 9, respectively.
Table 1.
Calibration equation, limit of detection (LOD) and limit of quantification (LOQ) values.
| Standard curve | r2 | LOD (μg/mL) | LOQ (μg/mL) | |
|---|---|---|---|---|
| Chlorogenic acid | y = 8260.7x – 16.93 | 0.9991 | 0.1209 | 0.4030 |
3. Results
3.1. Enzyme inhibitory activities of the extracts
AChE, BChE, and TYRO inhibitory activity of the hydroalcoholic extracts obtained from the 18 Turkish coffee samples (Kaffka, Shazili, Alkan, Hiscafe, Madenci, Asli, Arif, Con, Hisar, Mehmet Efendi, Oza, Oza light, Dibek, Action, Nescafe Falci, Ozerlat, Cezbeli, and Ulker) is tabulated in Table 2. Among the extracts tested at 200 μg/mL, only seven showed inhibition against AChE (Madenci, Con, Mehmet Efendi, Oza, Oza light, Dibek, and Action) at low level (from 7.93 ± 0.59% to 23.42 ± 1.39%), while 14 extracts had BChE inhibition to some extent (from 6.78 ± 0.68% to 36.12 ± 1.07%). They also exerted low inhibition rates for TYR (from 3.95 ± 1.69% to 15.72 ± 0.30%).
Table 2.
Inhibitory effects [% inhibition ± standard error of the mean (SEM; n = 3)] of the coffee extracts against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and tyrosinase (TYR).
| Extracts | % Inhibition ± SEM at 200 μg/mL | ||
|---|---|---|---|
|
| |||
| AChE | BChE | TYR | |
| Kaffka | —b | 6.95 ± 2.14**** | 15.51 ± 2.25**** |
| Shazili | — | 12.46 ± 1.69**** | — |
| Alkan | — | — | 3.95 ± 1.69**** |
| Hiscafe | — | 6.89 ± 1.34**** | 2.13 ± 0.01**** |
| Madenci | — | 3.99 ± 0.98**** | 15.72 ± 0.30**** |
| Asli | 20.90 ± 1.48**** | 14.34 ± 0.88**** | — |
| Arif | — | 36.12 ± 1.07**** | 7.11 ± 1.01**** |
| Con | 9.94 ± 3.15**** | 6.87 ± 0.18**** | 3.37 ± 1.56**** |
| Hisar | — | — | — |
| Mehmet Efendi | 7.46 ± 1.32**** | 13.39 ± 0.87**** | — |
| Oza | 23.42 ± 1.39**** | 21.68 ± 1.24**** | 7.68 ± 0.80**** |
| Oza light | 21.05 ± 2.30**** | 16.05 ± 3.11**** | 11.38 ± 0.01**** |
| Dibek | 7.93 ± 0.59**** | 18.85 ± 1.67**** | 12.95 ± 0.20**** |
| Action | 15.90 ± 0.61**** | 13.32 ± 1.71**** | 8.11 ± 1.60**** |
| Nescafe Falci | — | 6.78 ± 0.68**** | — |
| Ozerlat | — | — | — |
| Cezbeli | — | — | — |
| Ülker | — | 11.51 ± 0.37**** | — |
| Galanthaminea | 92.87 ± 1.01 | 78.89 ± 0.09 | |
| α-Kojic acidb | 78.89 ± 0.09 | ||
— = no inhibition.
p < 0.05;
p < 0.01;
p < 0.001,
p < 0.0001.
Reference for AChE and BChE inhibition.
Reference for TYR inhibition.
3.2. Antioxidant activity of the extracts
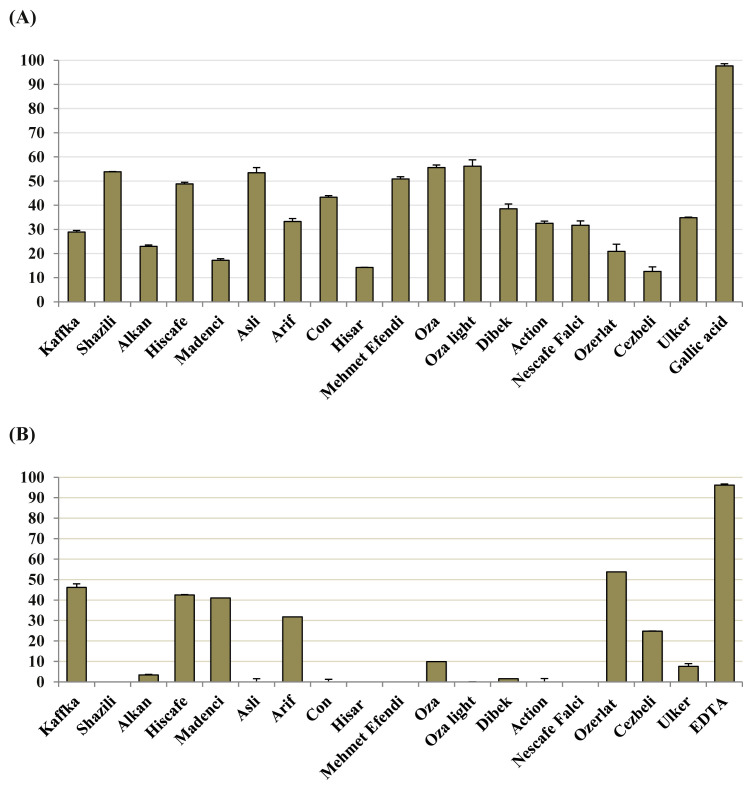
In association with their neuroprotective effect, antioxidant activity of the coffee extracts was firstly tested by DPPH and DMPD radical scavenging tests at 2000 μg/mL. None of the extracts had ability to scavenge DPMD, whereas low to moderate scavenging effect against DPPH (from 12.55 ± 0.19% to 56.15 ± 2.01%) was caused by the extracts (Fig. 1). Among the coffee extracts, the Ozerlat sample possessed the best metal-chelation capacity (53.79 ± 0.05%; Fig. 1). By contrast, the extract of Oza light displayed the highest FRAP value, followed by Asli and Mehmet Efendi brands (Table 3).
Fig. 1.
(A) 2,2’-diphenyl-1-picrylhydrazyl radical scavenging activity (%scavenging ± standard error of the mean) and reference (gallic acid). (B) Metal-chelation capacity (%chelation ± standard error of the mean) and reference (EDTA) of the coffee ethanol extracts at 2000 μg/mL.
Table 3.
Ferric- (FRAP) and phosphomolybdenum-reducing antioxidant power (PRAP) of the coffee extracts.
| Extracts | FRAP at 2000 μg/mL (absorbance at 700 nm ± SEM)a | PRAP at 2000 μg/mL (absorbance at 600 nm ± SEM) |
|---|---|---|
| Kaffka | 0.602 ± 0.002**** | 0.227 ± 0.008**** |
| Shazili | 0.994 ± 0.007*** | 0.202 ± 0.001**** |
| Alkan | 0.573 ± 0.011**** | 0.199 ± 0.001**** |
| Hiscafe | 0.958 ± 0.001*** | 0.213 ± 0.006**** |
| Madenci | 0.605 ± 0.013**** | 0.189 ± 0.001**** |
| Asli | 1.027 ± 0.004*** | 0.224 ± 0.004**** |
| Arif | 0.801 ± 0.009**** | 0.230 ± 0.001**** |
| Con | 0.889 ± 0.005**** | 0.219 ± 0.001**** |
| Hisar | 0.505 ± 0.008**** | 0.149 ± 0.006**** |
| Mehmet Efendi | 0.996 ± 0.004*** | 0.225 ± 0.004**** |
| Oza | 0.948 ± 0.011*** | 0.267 ± 0.004**** |
| Oza light | 1.073 ± 0.009*** | 0.242 ± 0.004**** |
| Dibek | 0.892 ± 0.015**** | 0.194 ± 0.001**** |
| Action | 0.633 ± 0.005**** | 0.181 ± 0.001**** |
| Nescafe falci | 0.712 ± 0.012**** | 0.180 ± 0.004**** |
| Ozerlat | 0.672 ± 0.005**** | 0.180 ± 0.001**** |
| Cezbeli | 0.558 ± 0.011**** | 0.163 ± 0.030**** |
| Ülker | 0.832 ± 0.013**** | 0.180 ± 0.001**** |
| Quercetinb | 1.534 ± 0.04 | 0.989 ± 0.003 |
p < 0.05;
p < 0.01;
p < 0.001,
p < 0.0001.
SEM = standard error of the mean (n = 3).
Higher absorbance indicates higher antioxidant power.
Reference for FRAP and PRAP assays.
3.3. Total phenol and flavonoid contents and chlorogenic acid percentages in the extracts
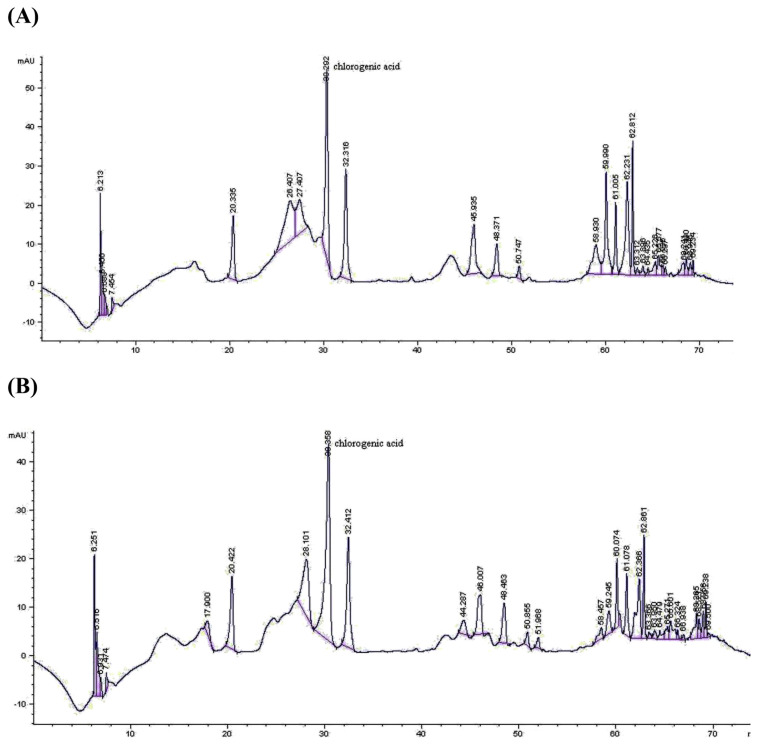
According to our data obtained for total phenol and flavonoid contents in the extracts as shown in Table 4, Action brand was found to be richest in total phenol amount as gallic acid equivalent (238.92 ± 0.76 mg/g extract), while the highest total flavonoid content (161.43 ± 2.03 mg/g extract) was in the Mehmet Efendi brand extract. Chlorogenic acid amounts analyzed by HPLC showed a great variation amongst the coffee extracts from 0.288 ± 0.005% (Ozerlat brand) up to 2.271 ± 0.004% (Madenci brand; Table 4, Fig. 2).
Table 4.
Total phenol and flavonoid, and chlorogenic acid contents in the coffee extracts.
| Extracts | Total phenol contenta ± SEMb | Total flavonoid contentc ± SEM | Chlorogenic acid contents (%) ± SEM by HPLCd |
|---|---|---|---|
| Kaffka | 170.90 ± 1.78 | 49.08 ± 1.04 | 1.915 ± 0.005 |
| Shazili | 131.47 ± 1.53 | 137.05 ± 3.55 | 0.829 ± 0.032 |
| Alkan | 203.11 ± 2.54 | 58.34 ± 1.77 | 0.785 ± 0.017 |
| Hiscafe | 132.58 ± 2.01 | 116.25 ± 2.54 | 1.904 ± 0.009 |
| Madenci | 210.85 ± 1.80 | 72.33 ± 3.30 | 2.271 ± 0.004 |
| Asli | 120.34 ± 2.54 | 153.54 ± 1.01 | 0.468 ± 0.002 |
| Arif | 141.03 ± 0.54 | 108.90 ± 1.27 | 0.883 ± 0.006 |
| Con | 160.11 ± 1.27 | 119.12 ± 2.54 | 2.335 ± 0.010 |
| Hisar | 178.09 ± 0.76 | 57.44 ± 2.51 | 0.127 ± 0.001 |
| Mehmet Efendi | 160.11 ± 1.27 | 161.43 ± 2.03 | 1.067 ± 0.007 |
| Oza | 126.09 ± 0.98 | 154.79 ± 2.28 | 0.169 ± 0.001 |
| Oza light | 123.04 ± 1.27 | 151.03 ± 1.52 | 1.222 ± 0.010 |
| Dibek | 130.78 ± 2.56 | 116.78 ± 0.76 | 1.527 ± 0.002 |
| Action | 238.92 ± 0.76 | 113.74 ± 0.51 | 0.538 ± 0.018 |
| Nescafe falci | 178.28 ± 2.54 | 113.92 ± 1.77 | 0.437 ± 0.012 |
| Ozerlat | 136.17 ± 1.53 | 83.08 ± 0.76 | 0.288 ± 0.005 |
| Cezbeli | 217.68 ± 0.76 | 73.76 ± 0.75 | 1.682 ± 0.004 |
| Ulker | 144.81 ± 2.54 | 117.69 ± 2.54 | 1.081 ± 0.018 |
HPLC = high-pressure liquid chromatography; SEM = standard error of the mean (n = 3).
Data expressed in mg equivalent of gallic acid to 1 g of extract.
Standard error mean (n = 3).
Data expressed in mg equivalent of quercetin to 1 g of extract.
High-pressure liquid chromatography.
Fig. 2.
Representative high-pressure liquid chromatograms of the coffee ethanol extracts from (A) Con and (B) Madenci brands.
4. Discussion
Coffee is a common beverage in the world and Turkish coffee made with the seeds of C. arabica is consumed a great deal in Anatolia and neighboring countries. Only a few studies have reported on neuroprotective effects of the coffee extracts via different mechanisms [18,19]. However, according to our literature survey, cholinesterase or TYR inhibitory effect of coffee samples has not been demonstrated up to date. By contrast, many researchers published about antioxidant activity of the coffee extracts. Among them, Karakaya et al [20] revealed moderate radical scavenging effect of the Turkish coffee against 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), which is consistent with our present data. Another study performed on Turkish coffee sample consumed in Egypt [21] showed 33.2 ± 0.02% of DPPH radical scavenging effect, which is similar to our result in the equivalent experiment. Interestingly, in the same study [21], radical scavenging effect of the Turkish coffee decreased as incubation duration increased.
Chlorogenic acid (the ester of caffeic acid and quinic acid) has been considered to be a kind of marker or characteristic compound to coffee as the most abundant phenolic acid found in coffee, which has been confirmed by the vast number of publications on chlorogenic acid content in coffee samples [22]. It was also shown to have neuroprotective effect through different mechanisms, e.g., antiamnesic activity in mice, anti-AChE activity in vitro and ex vivo [23]. Therefore, we particularly determined amount of chlorogenic acid in our coffee brands considering that it might be somewhat important in relation to neuroprotection. Nevertheless, we previously found chlorogenic acid to be ineffective against AChE up to 100 μg/mL [24]. Thus, it is someway controversial to conclude that chlorogenic acid may contribute to AChE inhibitory effect of the coffee extracts screened herein. By contrast, we also revealed chlorogenic acid with 30.8 ± 0.81% of BChE inhibition at the same concentration [25], which may lead to the assumption that this phenolic acid might be donating to BChE inhibitory action of the coffee extract to some extent. Hence, it can be speculated that chlorogenic acid may contribute to neuroprotective effect through its strong antioxidant potential, rather than its low cholinesterase-inhibitory action.
By contrast, chlorogenic acid seems to be more likely to be responsible for antioxidant activity of the extracts since it was shown to have strong antioxidant properties by several researchers [23,26,27], which is consistent with our previous data on chlorogenic acid [23]. Although chlorogenic acid was earlier stated to be a metal chelator [28], it did not exert metal-chelation capacity in our former study [24]. Thus, we can speculate that chlorogenic acid as well as other polyphenols such as caffeic acid commonly found in coffee beans could be the major contributors to the antioxidant activity of the coffee extracts whose contribution is changeable according to method applied.
Doubtlessly, roasting, a vital stage in coffee production for getting aroma, flavor, and color of the coffee, as well as storage duration are very imperative factors for phytochemistry and antioxidant activity of coffee. During the roasting procedure, phenolic compounds are known to degrade and/or to bind to polymer structures [29]. Besides, chlorogenic acid amount was reported to be strongly linked to antioxidant activity of the coffee after roasting [30]. Cammerer and Kroh [29] also stated that chlorogenic acid content increases as temperature rises. Gomez-Ruiz et al [31] demonstrated that increasing roasting degrees applied to coffee beans caused a reduction in radical scavenging activity. This finding was also supported by the results of Del Castillo et al [32]. Nevertheless, another report made a conclusion that dark coffees exerted better peroxyl radical scavenging activity than the green coffee beans [33]. Roasting caused the degradation of chlorogenic acid and formation of melanoidins, while antioxidant activity remained mostly the same after roasting [34], but the larger caffeine content in Robusta coffee led to the occurrence of higher antioxidant activity.
In addition to chlorogenic acid and other polyphenols, coffee beans contain other components that exhibit antioxidant capacity such as caffeine. Caffeine (1,3,7-trimethylxanthine) was revealed to possess high antioxidant activity [35,36] as well as AChE-inhibiting action [37], whereas we earlier reported caffeine with no inhibitory action cholinesterases [25]. Relevantly, Yilmaz et al [37] stated that no relation existed between caffeine content and AChE and BChE inhibitory effect of the coffee samples.
It should be noted that the nutritional quality of coffee is highly related to its phytochemical content, which also affects pharmacological efficacy of the plant.
Our findings obtained in this study reveal that Turkish coffee extracts at medium-roasted degree prepared from 18 commercial brands had no, low, or modest level of AChE, BChE, and TYR inhibition, while they exerted notable DPPH radical scavenging activity and FRAP values. The percentage of chlorogenic acid showed a great variation from brand to brand on which multifactors such as botanical origin, roasting duration, storage conditions, and temperature could play a role. To the best of our knowledge, we herein disclose the first report on neurobiological activity of Turkish coffee extracts via AChE, BChE, and TYRO inhibition and antioxidant methods including DMPD radical scavenging, metal-chelation capacity, and PRAP. This is also the first study investigating chlorogenic acid quantities of these Turkish coffee brands.
Acknowledgments
F.S. Senol delivers her sincere appreciation to the Scientific and Technological Research Council of Turkey (TUBITAK) for the scholarship provided for her Ph.D. program.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1. Orhan G, Orhan I, Subutay-Oztekin N, Ak F, Sener B. Contemporary anticholinesterase pharmaceuticals of natural origin and their synthetic analogues for the treatment of Alzheimer’s disease. Rec Patents CNS Drug Disc. 2009;4:43–51. doi: 10.2174/157488909787002582. [DOI] [PubMed] [Google Scholar]
- 2. Cokugras AN. Butyrylcholinesterase: structure and physiological importance. Turk J Biochem. 2003;28:54–61. [Google Scholar]
- 3. Fernandez HH. Updates in the medical management of Parkinson disease. Cleveland Clin J Med. 2012;79:28–35. doi: 10.3949/ccjm.78gr.11005. [DOI] [PubMed] [Google Scholar]
- 4. Pan T, Li X, Jankovic J. The association between Parkinson’s disease and melanoma. Int J Cancer. 2011;128:2251–60. doi: 10.1002/ijc.25912. [DOI] [PubMed] [Google Scholar]
- 5. Orhan IE, Senol FS, Gulpinar AR, Sekeroglu N, Kartal M, Sener B. Neuroprotective potential of some terebinth coffee brands and the unprocessed fruits of Pistacia terebinthus L. and their fatty and essential oil analyses. Food Chem. 2012;130:882–8. [Google Scholar]
- 6. Sekeroglu N, Senol FS, Orhan IE, Gulpinar AR, Kartal M, Sener B. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Food Res Int. 2012;45:197–203. [Google Scholar]
- 7. Di Carlo M, Giacomazza D, Picone P, Nuzzo D, San Biagio PL. Are oxidative stress and mitochondrial dysfunction the key players in the neurodegenerative diseases? Free Rad Res. 2012;46:1327–38. doi: 10.3109/10715762.2012.714466. [DOI] [PubMed] [Google Scholar]
- 8. Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 9. Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 10. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 11. Schlesier K, Harvat M, Bohm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Rad Res. 2002;36:177–87. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- 12. Chua MT, Tung YT, Chang ST. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophleum. Biores Technol. 2008;99:1918–25. doi: 10.1016/j.biortech.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 13. Oyaizu M. Studies on products of Browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jap J Nutr. 1986;44:307–15. [Google Scholar]
- 14. Falcioni G, Fedeli D, Tiano L, Calzuola I, Mancinelli L, Marsili V, Gianfranceschi GL. Antioxidant activity of wheat sprouts extract in vitro: inhibition of DNA oxidative damage. J Food Sci. 2002;67:2918–22. [Google Scholar]
- 15. Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–58. [Google Scholar]
- 16. Woisky R, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apicol Res. 1998;37:99–105. [Google Scholar]
- 17. Somporn C, Kamtuo A, Theerakulpisut P, Siriamornpun S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor) Int J Food Sci Technol. 2011;46:2287–96. [Google Scholar]
- 18. Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008;582:2655–62. doi: 10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 19. Trinh K, Andrews L, Krause J, Hanak T, Lee D, Gelb M, Pallanck L. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J Neurosci. 2010;30:5525–32. doi: 10.1523/JNEUROSCI.4777-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karakaya S, El SN, Tas AA. Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr. 2001;52:501–8. [PubMed] [Google Scholar]
- 21. Ramadan-Hassanien MF. Total antioxidant potential of juices, beverages and hot drinks consumed in Egypt screened by DPPH in vitro assay. Grasas Aceites. 2008;59:254–9. [Google Scholar]
- 22. Oliveira-Neto JR, Rezende SG, De Fátima Reis C, Benjamin SR, Rocha ML, De Souza Gil E. Electrochemical behavior and determination of major phenolic antioxidants in selected coffee samples. Food Chem. 2016;190:506–12. doi: 10.1016/j.foodchem.2015.05.104. [DOI] [PubMed] [Google Scholar]
- 23. Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010;649:210–7. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 24. Orhan IE, Altun ML, Sever-Yilmaz B, Saltan G. Anti-acetylcholinesterase and antioxidant assets of the major components (salicin, amentoflavone, and chlorogenic acid) and the extracts of Viburnum opulus and Viburnum lantana and their total phenol and flavonoid contents. J Med Food. 2011;14:434–40. doi: 10.1089/jmf.2010.0053. [DOI] [PubMed] [Google Scholar]
- 25. Orhan I, Kartal M, Tosun F, Sener B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z Naturforsch. 2007;62:829–32. doi: 10.1515/znc-2007-11-1210. [DOI] [PubMed] [Google Scholar]
- 26. Jung HA, Park JC, Chung HY, Kim J, Choi JS. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch Pharm Res. 1999;22:213–8. doi: 10.1007/BF02976549. [DOI] [PubMed] [Google Scholar]
- 27. Wu L. Effect of chlorogenic acid on antioxidant activity of Flos lonicerae extracts. J Zhejiang Univ. 2007;8:673–9. doi: 10.1631/jzus.2007.B0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kono Y, Kashine S, Yoneyama T, Sakamoto Y, Matsui Y, Shibata H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci Biotechnol Biochem. 1998;62:22–7. doi: 10.1271/bbb.62.22. [DOI] [PubMed] [Google Scholar]
- 29. Cammerer B, Kroh LW. Antioxidant activity of coffee brews. Eur Food Res Technol. 2006;223:469–74. [Google Scholar]
- 30. Anese M, Nicoli MC. Antioxidant properties of ready-to-drink coffee brews. J Agric Food Chem. 2003;51:942–6. doi: 10.1021/jf025859+. [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Ruiz JA, Ames JM, Leake DS. Antioxidant activity and protective effects of green and dark coffee components against human low density lipoprotein oxidation. Eur Food Res Technol. 2008;227:1017–24. [Google Scholar]
- 32. Del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem. 2002;50:3698–703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- 33. Vignoli JA, Bassoli DG, Benassi MT. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–8. [Google Scholar]
- 34. Chu YF, Chen Y, Brown PH, Lyle BJ, Black RM, Cheng IH, Ou B, Prior RL. Bioactivities of crude caffeine: antioxidant activity, cyclooxygenase-2 inhibition, and enhanced glucose uptake. Food Chem. 2012;131:564–8. [Google Scholar]
- 35. Demirtas C, Ofluoglu E, Hussein A, Pasaoglu H. Effects of caffeine on oxidant-antioxidant mechanisms in the rat liver. Gazi Med J. 2012;23:13–8. [Google Scholar]
- 36. Karadsheh N, Kussie P, Linthicum DS. Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives. Toxicol Lett. 1991;55:335–42. doi: 10.1016/0378-4274(91)90015-x. [DOI] [PubMed] [Google Scholar]
- 37. Yilmaz PK, Hacibekiroglu I, Kolak U. Effect of roasting on antioxidant and anticholinesterase capacities of coffee. J Food Nutr Res. 2014;53:232–9. [Google Scholar]