Abstract
Oyster-derived polysaccharides (OPS) have been shown to modulate the T helper (Th)1/Th2 immunobalance toward the Th1-dominant direction in antigen-primed splenocytes. In the present study, we hypothesized that OPS might attenuate intestinal inflammation associated with food allergy, a Th2-dominant immune disorder. BALB/c mice were sensitized twice with ovalbumin (OVA) absorbed to alum and then repeatedly challenged with intragastric OVA to induce intestinal allergic responses. The mice were administered by gavage with OPS and/or vehicle (distilled water) once/d during the two sensitization phases, and once every other day during the challenge phase. Administration with OPS attenuated OVA challenge-elicited diarrhea, and the infiltration of mast cells in the intestine. OPS demonstrated a protective effect on the reduced ratio of villus length over crypt depth of the intestine in allergic mice. Furthermore, OPS administration markedly attenuated the intestinal expression of the Th2 signature cytokine interleukin-4 (IL-4). Collectively, these results demonstrated the in vivo antiallergic activity of OPS, which is associated with the suppression of allergen-induced intestinal Th2 responses and mast cell activation.
Keywords: Crassostrea gigas, food allergy, oyster, polysaccharides, T helper
1. Introduction
The Pacific oyster Crassostrea gigas (Thunberg) is a common seafood widely cultured in the Penghu islands and Taiwan’s coastal areas. Previous reports suggest that the Pacific oyster is of nutritional value, as it contains amino acids, carbohydrates, lipids, minerals, polyunsaturated fatty acids, and proteins [1,2]. In addition, functional studies demonstrated that constituents of the Pacific oyster exhibited a number of potential health-promoting properties, including anti-hyperlipidemia, antioxidation, and immunomodulation [3–6]. Of relevance to the present study, we previously investigated the immunomodulatory effect of the oyster-derived poly-saccharides (OPS) on antigen-specific immune responses. Direct exposure of ovalbumin (OVA)-primed murine splenocytes to OPS enhanced the cell metabolic activity and proliferation, and the expression of T helper (Th)1 signature cytokine and transcription factor, namely interferon (IFN)-γ and T-bet, respectively. By contrast, OPS attenuated the expression of the Th2 cell-related cytokine interleukin (IL)-4 [7]. OPS-mediated Th1-enhancing effects were further confirmed in vivo, as evidenced by the upregulation of IFN-γ production and T-bet mRNA expression in splenocytes isolated from mice sensitized with OVA and administered with OPS. Together these results demonstrate the immunomodulatory effects of OPS on T cell responses, in which the T cell immunobalance was skewed to the Th1-dominant direction.
Food allergy is an immunological disorder to various dietary proteins. Common food allergens include eggs, milk, peanuts, shellfishes, and tree nuts [8,9]. Hypersensitivity reactions associated with food allergy may range from moderate gastrointestinal disturbance to life-threatening anaphylaxis [10,11]. Current management of food allergy primarily relies on allergen avoidance. Effectiveness of symptomatic pharmacotherapy is limited to mainly control inflammatory responses associated with allergic attacks [12]. The immunopathology of food allergy is characterized by an aberrant T cell immunobalance to dietary allergens, in which the differentiation of antigen-specific Th cells is skewed to the Th2 phenotype [13]. IL-4, the signature cytokine expressed by Th2 cells, is a key mediator promoting the maturation of mast cells that is responsible for triggering hypersensitivity reactions [14].
On the basis of our previous results showing that OPS modulated the T cell immunobalance toward the Th1-dominant direction in OVA-primed splenocytes, and promoted Th1-type immunity in OVA-sensitized mice [7], we hypothesized that OPS might be effective in modulating food allergy, a predominantly Th2-type immune disorder. We report here that oral administration with OPS attenuates intestinal allergic inflammation associated with food allergy.
2. Methods
2.1. Reagents
All reagents and chemicals were purchased from Sigma Chemicals (St. Louis, MO, USA) unless otherwise described. Anti-mouse IL-4 rat immunoglobulin G1 (IgG1) was purchased from BioLegend (San Diego, CA, USA). Reagents used for immunohistochemical (IHC) staining were purchased from AbCam Inc. (Cambridge, MA, USA) and BioGenex Laboratories (San Ramon, CA, USA).
2.2. Preparation of OPS
The OPS used in the present study was extracted from fresh oysters (C. gigas). After drying and weighing, the oysters were incubated with hot water at 80°C for 4 hours, cooled down to room temperature (RT), and then centrifuged. The supernatant was mixed with an equal volume of 95% ethanol to precipitate polysaccharides. After centrifuging at 12,000 g for 30 minutes, the pellet was collected and freeze-dried to obtain OPS. The OPS contained 238.2 g of β-glucans/kg, as determined by measuring its nondigestible portion to α-glucosidase. The molecular weight of OPS has been determined to be approximately 435 kDa. The amount of glucose and protein in OPS was also determined to be 979.8 g/kg and <20.0 g/kg, respectively, confirming the purity of OPS.
2.3. Animals
Male BALB/c mice, 4–6 weeks of age, were obtained from BioLasco (Ilan, Taiwan). On arrival, mice were randomized, transferred to plastic cages with a sawdust bedding (5–6 mice/ cage) and quarantined at least for 1 week before experimentation. The mice were given standard laboratory food and water ad libitum, except for the days receiving OVA challenge (described below). The animal room was maintained at the temperature of 25 ± 2°C and a relative humidity of 50 ± 20%, with a 12 hour light/dark cycle.
2.4. Protocol of animal experiments
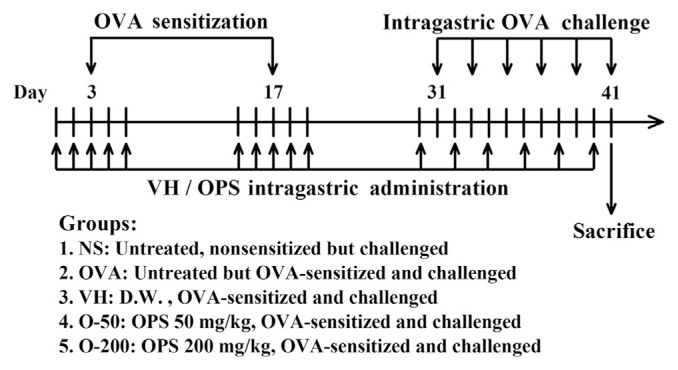
A previously described murine model of food allergy induced by OVA was employed [15,16]. BALB/c mice were randomly divided into the following five groups (5–6 mice/group; Fig. 1): (1) NS group: untreated, nonsensitized but OVA-challenged; (2) OVA group: untreated but OVA-sensitized and challenged; (3) VH group: vehicle-treated (distilled water; 0.1 mL/ mouse) and OVA-sensitized and challenged; (4) O-50 group: OPS-treated (50 mg/kg; 0.1 mL/mouse) and OVA-sensitized and challenged; and (5) O-200 group: (200 mg/kg; 0.1 mL/ mouse) and OVA-sensitized and challenged. Except for the NS group, the mice were sensitized by an intraperitoneal injection of 50 μg OVA absorbed to 1 mg alum on Day 3, and then boosted with a double dose on Day 17. To induce allergic responses, the mice were repeatedly challenged with OVA (50 mg/0.3 mL in saline/mouse) by gavage every other day from Day 31 to Day 41. Before each intragastric OVA challenge, mice were deprived of food for 3 hours. Allergic diarrhea was visually monitored for 3 hours after challenge. Mice demonstrating profuse liquid stools were recorded as diarrhea-positive animals. The diarrheal occurrence (%) was calculated according to the following formula:
Fig. 1.
Protocols of oyster-derived polysaccharides (OPS) administration and ovalbumin (OVA) sensitization and challenge. BALB/c mice were randomly divided into five groups (5–6 mice/group) as described in the figure. The mice in the VH, O-50, and O-200 groups were administered daily OPS (50 mg/kg or 200 mg/kg) and/or VH (distilled water; D.W.) for five doses during the sensitized phase, and once every other day for six doses during the challenge phase. Except for the NS group (untreated, nonsensitized but OVA-challenged), the mice were sensitized by an intraperitoneal injection of OVA and alum on Day 3 and boosted on Day 17. To induce allergic diarrhea, the mice were repeatedly challenged with OVA by gavage every other day from Day 31 to Day 41. For histological examination, all mice were sacrificed 3 hours after the last OVA challenge on Day 41.
OPS and/or VH (distilled water) were administered by gavage once/d during the two sensitization phases from Day 1 to Day 5 and from Day 15 to Day 19, and once every other day during the challenge phase from Day 30 to Day 40. The dosing of OPS on alternate days during the challenge phase avoided delivering OPS and OVA on the same day. The dose of OPS was chosen according to our previous results showing the immunomodulatory activity of OPS in OVA-sensitized mice [7]. For histological examination, all mice were sacrificed 3 hours after the last OVA challenge on Day 41. The animal experiments were approved by the Institutional Animal Care and Use Committee of the National Taiwan University, Taipei, Taiwan.
2.5. Necropsy and intestinal tissue preparation
The duodenum was excised and fixed in 10% neutral buffered formalin for 24 hours. The tissues were embedded in paraffin and cut into 4–5 μm sections that were stained with hematoxylineosin (H&E) and toluidine blue for routine histopathology and identification of mast cells, respectively. Tissue sections were also subjected to IHC staining for the detection of IL-4.
2.6. IHC staining
The tissue sections were first deparaffinized by immersing the slides in xylene for 5 minutes three times at RT. The slides were rehydrated by immersing sequentially in 100%, 95%, 90%, 80%, and 60% ethanol for 5 minutes each, and then washed three times with 1× Tris-based solution (TBS) for 5 minutes at RT. The rehydrated slides were immersed in Trilogy at 121°C for 15 minutes for antigen retrieval. The slides were then immersed in preheated Trilogy at 80°C for 10 minutes and cooled down immediately in 1× TBS for 10 minutes. The endogenous peroxidase activity was quenched with 3% H2O2 in methanol for 10 minutes at RT, then washed with 1× TBS and blocked with normal goat serum for 1 hour at RT in the dark. All of the following operations were placed in the dark. The anti-mouse IL-4 monoclonal antibody was applied on to each section overnight at 4°C. After washing, slides were treated with super enhancer for 1 hour at RT, and then incubated with poly-horseradish peroxidase (HRP) reagent for 1 hour at RT. For visualization, slides were treated with the HRP substrate 3-amino-9-ethylcarbazole followed by hematoxylin counter staining for an appropriate time at RT. Slides were visualized under a light microscope.
2.7. Morphometric analysis and IHC staining quantification
The villus length and crypt depth on H&E stained slides were measured using the Image-Pro Plus version 5.1 morphometric analysis software (Media Cybernetics, Inc., Silver Spring, MD, USA). The number of mast cells was counted manually after staining with toluidine blue. The number of IL-4+ cells (the red color) was identified in contrast to the background counter stain (the blue color) using Image-Pro Plus version 5.1 morphometric analysis software (Media Cybernetics, Inc.). Three measurements/tissue section and 4–6 sections/group were analyzed at 200-fold magnification.
2.8. Statistical analysis
Data of diarrhea occurrence were expressed as a percentage and analyzed using the Chi-square test to compare the difference between the OPS-treated group and the VH control. All other quantitative data are expressed as the mean ± standard error for each experimental group. The student t test was used to analyze the statistical difference between the treatment groups (OPS) and control group (VH). A p value < 0.05 was defined as statistical significance.
3. Results
3.1. OPS attenuated the occurrence of allergic diarrhea
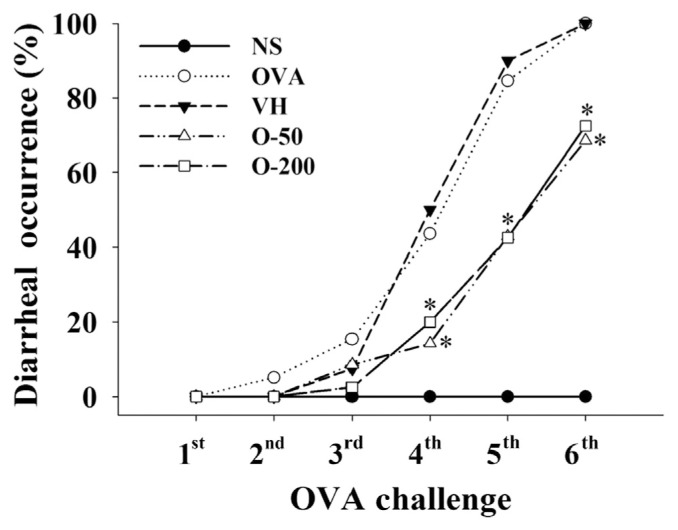
We first examined the effect of OPS on the occurrence of allergic diarrhea in mice with a food allergy. As expected, allergic diarrhea was successfully induced in all of the OVA-sensitized mice, but not in the NS group. The rate of diarrhea reached 100% following the sixth OVA challenge in the OVA and VH groups (Fig. 2). Administration with OPS (50 mg/ kg and 200 mg/kg) partially, but significantly attenuated the occurrence of diarrhea from the fourth to the sixth OVA challenge. Both doses of OPS showed a comparable effect, in which the diarrhea rate in OPS-treated groups was approximately 30% less than that in the VH group (Fig. 2). In addition, OPS administration neither altered the body weight of mice nor induced obvious gross lesions at necropsy (data not shown), suggesting that the employed dosing regimens were safe to the animals.
Fig. 2.
Oyster-derived polysaccharides (OPS) attenuated the occurrence of allergic diarrhea. Mice were treated as described in Fig. 1. Allergic diarrhea was visually monitored for 3 hours after every challenge. Mice demonstrating profuse liquid stools were recorded as diarrhea positive animals. Results are pooled samples from eight independent experiments (n = 35–40). *p < 0.05 compared to the VH group [vehicle-treated (distilled water; 0.1 mL/mouse)]. NS = nonsensitized; OVA = ovalbumin; VH = vehicle.
3.2. Effect of OPS on morphologic alterations of the duodenum of allergic mice
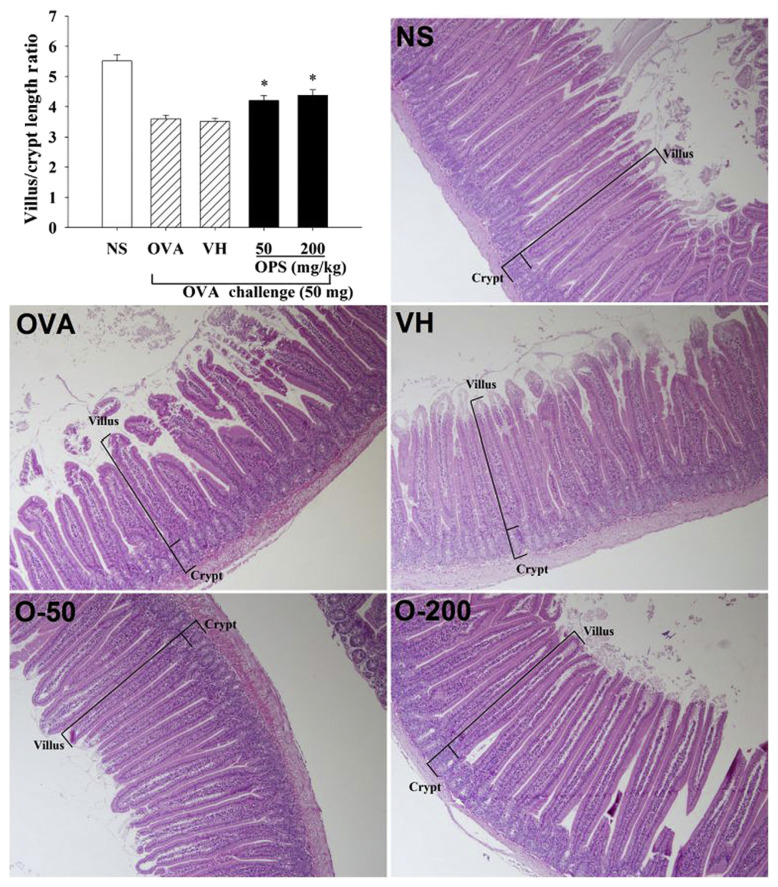
The above results showed that OPS attenuated the occurrence of allergic diarrhea. We next examined the influence of OPS on the duodenal morphology using histological examination. Results from H&E staining showed irregular shapes of the villi and hyperplasia in the crypts in both OVA and VH groups (Fig. 3), demonstrating the alterations of normal duodenal morphology associated with food allergy. To quantify the alterations, the villus length and crypt depth were measured and the ratio of villus/crypt was calculated. Compared to the NS group, the ratio was significantly reduced in allergic mice (Fig. 3; OVA vs. NS). Administration with OPS (50 mg/kg and 200 mg/kg) prevented the morphologic changes, and restored the reduced villus/crypt ratio (Fig. 3; OPS vs. VH).
Fig. 3.
Oyster-derived polysaccharides (OPS) restored the diminished ratio of villus length over crypt depth in the duodenum. Mice were treated as described in Fig. 1. Tissue sections of duodenum were prepared for standard hematoxylineosin (H&E) staining. The villus length and crypt depth were measured using the Image-Pro Plus version 5.1 morphometric analysis software (100× field; Media Cybernetics, Inc., Silver Spring, MD, USA). Data are expressed as the mean ± standard error of 27-1 samples per group pooled from six independent experiments. *p < 0.05 compared to the VH group [vehicle-treated (distilled water; 0.1 mL/mouse)]. NS = nonsensitized; OVA = ovalbumin.
3.3. Effect of OPS on the infiltration and degranulation of mast cells in the duodenum
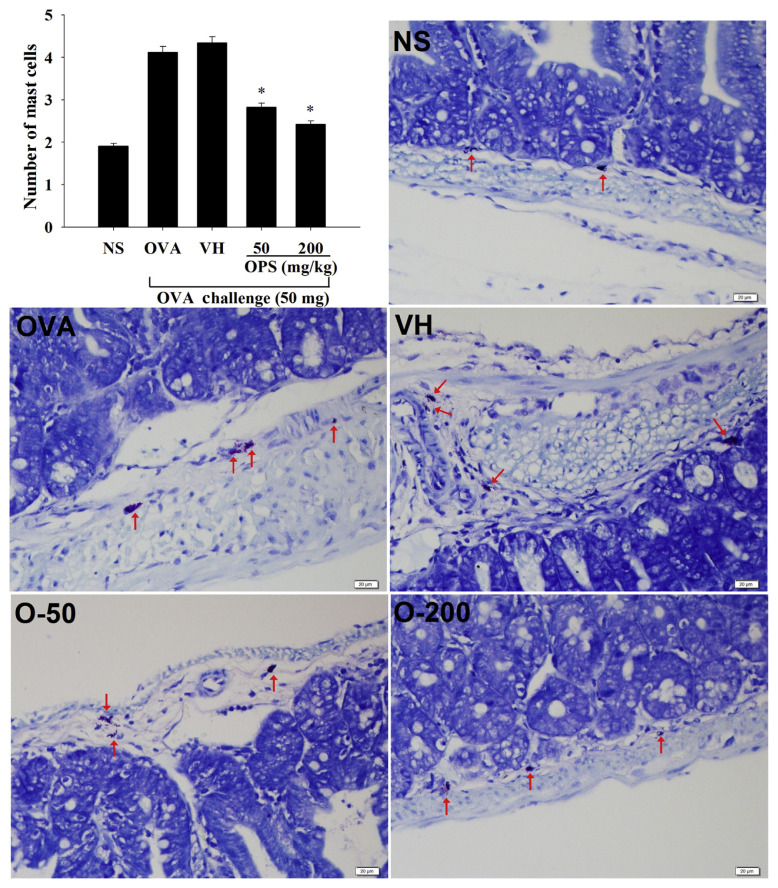
Inflammatory mediators released by mast cells are responsible for triggering inflammatory symptoms in type I hypersensitivity. The infiltration of mast cells in the duodenum was examined using toluidine blue staining. Both doses of OPS (50 mg/kg and 200 mg/kg) significantly attenuated the number of infiltrated mast cells (Fig. 4).
Fig. 4.
Effect of oyster-derived polysaccharides (OPS) on the infiltration of mast cells. Mice were treated as described in Fig. 1. Tissue sections of duodenum were stained with toluidine blue for identification of mast cells. The number of mast cells (red arrows) was counted under 200× field. Data are expressed as the mean ± standard error of 32 samples per group pooled from six independent experiments. *p < 0.05 compared to the VH group [vehicle-treated (distilled water; 0.1 mL/ mouse)]. NS = nonsensitized; OVA = ovalbumin; VH = vehicle.
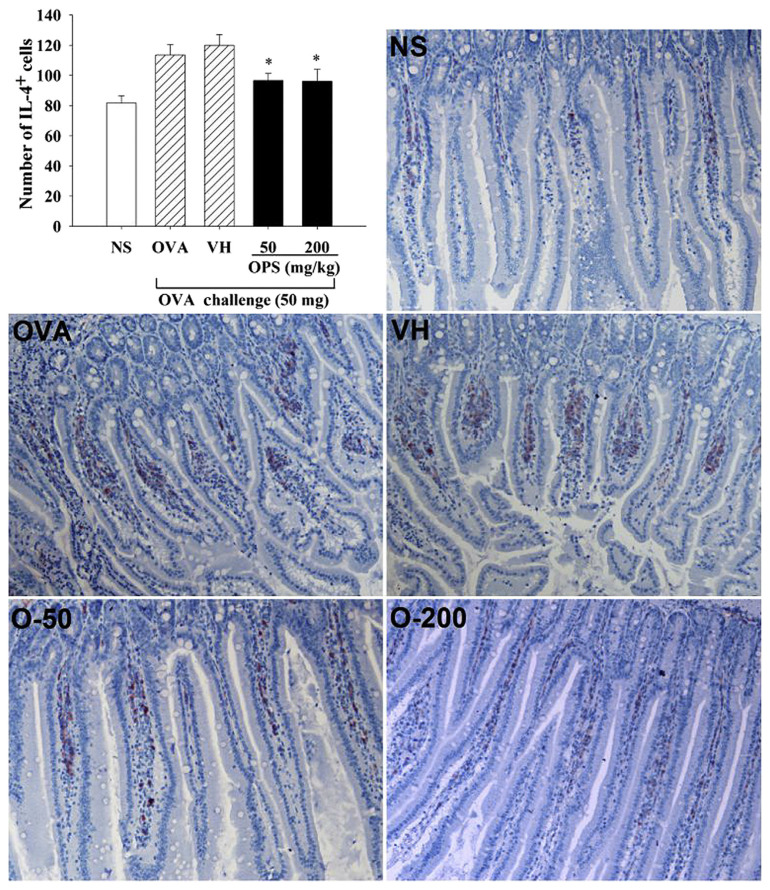
3.4. Effect of OPS on the infiltration of IL-4+ cells in duodenum
Th cells play a pivotal role in the immunopathology of type I hypersensitivity such as food allergy. To scrutinize the effect of OPS on the functionality of Th cells in the inflammatory site, we examined the duodenal expression of IL-4, a Th2 signature cytokine. The number of IL-4+ cells in the duodenum was markedly elevated in the OVA group, compared to the NS group, confirming the Th2 profile of food allergy. The elevated number of IL-4+ cells was significantly attenuated in allergic mice administered with OPS (Fig. 5; OPS vs. VH).
Fig. 5.
Effect of oyster-derived polysaccharides (OPS) on the infiltration of interleukin-4 (IL-4)+ cells in duodenum. Mice were treated as described in Fig. 1. Immunohistochemical (IHC) staining was performed to detect IL-4+ cells in the duodenum. The image was analyzed and the positive signal (the red color) was quantified using the Image-Pro Plus version 5.1 morphometric analysis software (200× field; Media Cybernetics, Inc., Silver Spring, MD, USA). Data are expressed as the mean ± standard error of 20–22 samples per group pooled from four independent experiments. *p < 0.05 compared to the VH group [vehicle-treated (distilled water; 0.1 mL/mouse)]. NS = nonsensitized; OVA = ovalbumin; VH = vehicle.
4. Discussion
Polysaccharides, in particular β-glucans, exhibit immunomodulatory activities that may be beneficial for the management of allergic disorders. For example, intake of β-glucans decreased the level of IL-4 and IL-5 and the eosinophil count in the nasal lavage fluid of patients with allergic rhinitis [17]. β-Glucans purified from Aureobasidium pullulans attenuated food allergic reactions in a murine model of food allergy [18]. Based on our previous results showing that OPS modulated the Th1/Th2 immunobalance toward the Th1-dominant direction [7], the present study investigated the potential antiallergic effect of OPS in a murine model of food allergy. In this model, OVA-sensitized mice were repeatedly challenged with OVA by gavage to induce allergic diarrhea and intestinal inflammation. Our results showed that OPS administration partially, but significantly attenuated the occurrence of allergen-induced diarrhea. To the best of our knowledge, the present study provides the first evidence to show that polysaccharides extracted from the common seafood oyster are immunoactive to attenuate food allergic responses.
Histological examination was employed to investigate the antiallergic effect of OPS against intestinal inflammation. The shape of villi became irregular, and the crypts showed hyperplasia in allergic mice, resulting in a decreased ratio of the villus length over the crypt depth. In allergic mice administered with OPS, the degree of hyperplasia of crypts was reduced, and the ratio of villus/crypt was restored. These results clearly demonstrated that in line with the attenuation of allergic diarrhea, administration with OPS exhibited a protective effect on the morphologic alterations associated with allergic inflammation in the intestine.
To further investigate the effect of OPS on allergic inflammation, the duodenal sections were stained with toluidine blue for detection of mast cells. The number of mast cells was markedly increased in the duodenum of allergic mice, confirming the infiltration of mast cells in the inflamed site of allergy. Notably, the infiltration of mast cells was attenuated by the treatment with OPS. More importantly, both doses of OPS significantly reduced the number of infiltrated mast cells. It is well known that inflammatory mediators released by mast cells trigger allergic reactions. Hence, our results suggest that OPS may be potentially used to alleviate mast cell-mediated inflammatory disorders such as food allergy. Further studies using other appropriate models of inflammation are warranted to address this notion.
It is well established that allergen-primed Th2 cells are critical for the development of type I hypersensitivity [19]. We therefore examined in the duodenum the expression of IL-4, a signature Th2 cytokine. Consistent with the Th2 nature of the food allergy model [20], the number of IL-4+ cells was markedly increased in allergic mice. Administration with OPS significantly decreased the number of IL-4+ cells, suggesting that OPS-mediated attenuation of allergic inflammation might be mediated by a reduced activation of Th2 cells in the inflammatory site. Furthermore, these results confirm our hypothesis that OPS possesses immunomodulatory activities against Th2 cell-mediated allergic reactions. Notably, the antiallergic effects between the two doses of OPS were comparable, implying that OPS at a dose greater than 50 mg/kg might reach a plateau on the allergen-induced intestinal inflammation. It is possible that dosages lower than 50 mg/kg may be effective against food allergy, which warrants further investigation.
Collectively, the present study demonstrated the in vivo antiinflammatory activities of OPS against food allergy, including the attenuation of allergic diarrhea, the protection of the duodenal morphology, and the suppression of mast cell and Th2 cell functionality. Results from the present study suggest a potential for OPS to be exploited as a functional natural product for the management of Th2-dominant disorders, such as food allergy.
Acknowledgments
This work was supported by the grant 103-2321-B-002-008 from the Ministry of Science and Technology, Executive Yuan, Taiwan.
Funding Statement
This work was supported by the grant 103-2321-B-002-008 from the Ministry of Science and Technology, Executive Yuan, Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Bac P, Pages N, Dhalluin S, Tapiero H. Protective effect of Crassostrea gigas extract on audiogenic seizures in magnesium deficient mice. Biomed Pharmacother. 1998;52:162–5. doi: 10.1016/s0753-3322(98)80205-9. [DOI] [PubMed] [Google Scholar]
- 2. Pennarun AL, Prost C, Haure J, Demaimay M. Comparison of two microalgal diets. 1. Influence on the biochemical and fatty acid compositions of raw oysters (Crassostrea gigas) J Agric Food Chem. 2003;51:200–10. doi: 10.1021/jf020548k. [DOI] [PubMed] [Google Scholar]
- 3. Achour A, Lachgar A, Astgen A, Chams V, Bizzini B, Tapiero H, Zagury D. Potentialization of IL-2 effects on immune cells by oyster extract (JCOE) in normal and HIV-infected individuals. Biomed Pharmacother. 1997;51:427–9. doi: 10.1016/s0753-3322(97)82320-7. [DOI] [PubMed] [Google Scholar]
- 4. Yoshikawa T, Naito Y, Masui K, Fujii T, Boku Y, Nakagawa S, Yoshida N, Kondo M. Free radical-scavenging activity of Crassostera gigas extract (JCOE) Biomed Pharmacother. 1997;51:328–32. doi: 10.1016/S0753-3322(97)88050-X. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka K, Ikeda I, Kase A, Koba K, Nishizono S, Aoyama T, Imaizumi K. Effects of feeding oyster, Crassostrea gigas, on serum and liver lipid levels in rats. J Nutr Sci Vitaminol (Tokyo) 2003;49:100–6. doi: 10.3177/jnsv.49.100. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe M, Fuda H, Jin S, Sakurai T, Ohkawa F, Hui SP, Takeda S, Watanabe T, Koike T, Chiba H. Isolation and characterization of a phenolic antioxidant from the Pacific oyster (Crassostrea gigas) J Agric Food Chem. 2012;60:830–5. doi: 10.1021/jf2038532. [DOI] [PubMed] [Google Scholar]
- 7. Cheng JY, Ng LT, Lin CL, Jan TR. Pacific oyster-derived polysaccharides enhance antigen-specific T helper (Th)1 immunity in vitro and in vivo. Immunopharmacol Immunotoxicol. 2013;35:235–40. doi: 10.3109/08923973.2012.751398. [DOI] [PubMed] [Google Scholar]
- 8. Burks W, Helm R, Stanley S, Bannon GA. Food allergens. Curr Opin Allergy Clin Immunol. 2001;1:243–8. doi: 10.1097/01.all.0000011021.73682.01. [DOI] [PubMed] [Google Scholar]
- 9. Fogg MI, Spergel JM. Management of food allergies. Expert Opin Pharmacother. 2003;4:1025–37. doi: 10.1517/14656566.4.7.1025. [DOI] [PubMed] [Google Scholar]
- 10. Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2003;111:S540–7. doi: 10.1067/mai.2003.134. [DOI] [PubMed] [Google Scholar]
- 11. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. quiz 20. [DOI] [PubMed] [Google Scholar]
- 12. Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, Garssen J, Knol EF, Van Hoffen E. Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: key role for IL-10. J Allergy Clin Immunol. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13. Helm RM, Burks AW. Mechanisms of food allergy. Curr Opin Immunol. 2000;12:647–53. doi: 10.1016/s0952-7915(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 14. Cardoso CR, Provinciatto PR, Godoi DF, Ferreira BR, Teixeira G, Rossi MA, Cunha FQ, Silva JS. IL-4 regulates susceptibility to intestinal inflammation in murine food allergy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G593–600. doi: 10.1152/ajpgi.90431.2008. [DOI] [PubMed] [Google Scholar]
- 15. van Halteren AG, van der Cammen MJ, Biewenga J, Savelkoul HF, Kraal G. IgE and mast cell response on intestinal allergen exposure: a murine model to study the onset of food allergy. J Allergy Clin Immunol. 1997;99:94–9. doi: 10.1016/s0091-6749(97)70305-1. [DOI] [PubMed] [Google Scholar]
- 16. Huang CH, Ku CY, Jan TR. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med. 2009;75:1300–5. doi: 10.1055/s-0029-1185578. [DOI] [PubMed] [Google Scholar]
- 17. Kirmaz C, Bayrak P, Yilmaz O, Yuksel H. Effects of glucan treatment on the Th1/Th2 balance in patients with allergic rhinitis: a double-blind placebo-controlled study. Eur Cytokine Netw. 2005;16:128–34. [PubMed] [Google Scholar]
- 18. Kimura Y, Sumiyoshi M, Suzuki T, Sakanaka M. Inhibitory effects of water-soluble low-molecular-weight beta-(1,3-1,6) d-glucan purified from Aureobasidium pullulans GM-NH-1A1 strain on food allergic reactions in mice. Int Immunopharmacol. 2007;7:963–72. doi: 10.1016/j.intimp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19. Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]