Abstract
Most studies of tea trees have focused on their ornamental properties, there are fewer published studies on their medical values. The purpose of this study was to compare the chemical constituents and the biological potential of the water extract of leaves in eight species of Camellia including Camellia sinensis. Among eight Camellia species, Camellia sasanqua showed potent anticancer activities in prostate cancer PC3 cells. In addition to catechins, the major component, eugenyl β-primeveroside was detected in C. sasanqua. Eugenyl β-primeveroside blocked the progression of cell cycle at G1 phase by inducing p53 expression and further upregulating p21 expression. Moreover, eugenyl β-primeveroside induced apoptosis in PC3 prostate cancer cells. Our results suggest that C. sasanqua may have anticancer potential.
Keywords: apoptosis, Camellia sasanqua, eugenyl β-primeveroside, G1 arrest, prostate cancer
1. Introduction
Prostate cancer is the most commonly diagnosed disease and the second leading cause of cancer-related deaths among men in the USA [1]. Despite recent advances in surgery, radiation therapy, hormonal therapy, and medical management, clinical management of advanced prostate cancer is still challenging. Therefore, chemoprevention involving naturally occurring compounds should be devoted for developing preventive strategies to reduce incidence and morbidity of prostate cancer [2].
The genus Camellia (Theaceae) attracts considerable attention due to its great economic value, broad geographic distribution, and remarkable species diversity. The main economic value of Camellia is the production of tea from the young leaves of Camellia sinensis. Tea is one of the most widely consumed nonalcoholic beverages in the world and shows marked benefits to human health, such as providing antioxidant activity and reducing the risk of cancer [3]. Moreover, the extract from Camellia oleifera seed has been primarily used as cooking oil [4]. In addition, other species such as Camellia sasanqua, Camellia reticulata, and Camellia japonica are cultivated in temperate regions worldwide as ornamentals.
The quality of the processed tea product depends on the chemical composition of the tea. These chemical compositions affecting taste and mouth feel of tea are also speculated to provide potential health benefits [5]. The compounds determining taste and astringency of tea include catechins, theaflavins, flavonoids, and thearubigins. Some major catechins present in tea are: (−)-epigallocatechin gallate (EGCG); (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), (−)-epicatechin (EC), (+)-catechin (C), (−)-gallocatechin (GC), (−)-gallocatechin gallate (GCG), and catechin gallate (CG) [6]. Catechins constitute about 25% dry weight of fresh tea leaf; total catechin content varies widely depending on species, light variation, altitude of growing, location, clonal variation, and season [7–10].
Numerous studies have reported the biological activities of C. sinensis [11], but few studies have discussed the biological potential of the other species of Camellia. In the present paper, we compared eight species of Camellia with C. sinensis. To investigate the health benefits of eight species of Camellia, the leaves were directly extracted with hot water. To assess the effect of anticancer activities, the experiments were performed using PC3 human prostate cancer cells. The objectives of this paper are to: (1) analyze the content of caffeine and catechins of various species of Camellia; and (2) evaluate the bioactivities of various species of Camellia in PC3 prostate cancer cells.
2. Materials and methods
2.1. Chemicals and reagents
EGCG, EGC, ECG, EC, C, GC, GCG, CG, 3-(4,5-dimethylthiaxol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), propidium iodide (PI), and antibodies for β-actin were purchased from Sigma Chemical Co (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) and sodium dodecyl sulfate (SDS) were purchased from Merck Co (Darmstadt, Germany). Antibodies for caspase-3, caspase-9, poly(ADP-ribose) polymerase (PARP), p21, and p53 were purchased from Cell Signaling Technology (Beverly, MA, USA).
2.2. Preparation of samples
Fresh leaves were obtained from the Tea Flower Manor (Ping Xi, Taipei, Taiwan). There are eight species of Camellia in this study including C. oleifera Abel., C. sasanqua Thunb., C. tenuifolia (Hay) Cohen-Stuart, C. japonica cv. “Carp’s Eye”, C. japonica cv. “Cantonese Pink”, C. japonica cv. “Early Spring”, C. japonica cv. “Kramer’s Supreme”, and C. japonica cv. “Nine Bends”. Samples of fresh leaves (1 g) were placed in boiling distilled water (100 mL) for 30 minutes and then the infusion was filtered through a 0.45 μm polyvinylidene difluoride (PVDF) filter (Millipore, Bedford, MA, USA). The filtrate was analyzed with high-performance liquid chromatography (HPLC) as described below. The filtrate was dried under reduced pressure using a rotavapor to afford powdered crude extract and kept in a refrigerator at −20°C until use. In the following experiments, the extraction was dissolved in DMSO (100 mg/mL) and diluted to the desired concentrations.
2.3. Reverse-phase HPLC analysis of catechins and caffeine
The composition of catechins and caffeine in different species of Camellia leaves were analyzed by reverse-phase HPLC using a Waters 600E system controller [10,12]. The Waters 484 tunable absorbance detector was used to detect the constituents at 280 nm, and all peaks were plotted and integrated by a Waters 745 data module. The HPLC set consisted of a 250 mm × 4.6 mm i.d., 5 μm Cosmosil 5 C18-MS packed column (Nacalai Tesque, Kyoto, Japan). The extracts of different species of Camellia leaves were filtered through a 0.45 μm filter disk and then injected into the column. The concentrations of catechins and caffeine working solutions were 100 μg/mL. Each authentic standard compound (500 ng, catechins and caffeine) was injected. Briefly, the mobile phase set consists of a mixture of methanol–water–formic acid (19.5:82.5:0.3) at the flow rate of 1 mL/min. Identification of caffeine or individual catechins was based on the comparison of the retention times of unknown peaks to those of reference authentic standards. The amount of each constituent in different species of Camellia leaves extract was determined by the integrated data provided by the Waters data module.
2.4. Cell culture
All cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The human prostate cancer cell line used in this study was PC3. We also used the PZ-HPV-7 cell line, which was derived from epithelial cells cultured from the normal peripheral zone of the prostate and were transformed by HPV18 E6 transforming region oncoprotein, expressed negative for prostate specific antigen. Human prostate PC3 cancer cells were grown in RPMI-1640 media (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin-streptomycin (Invitrogen). PZ-HPV-7 was cultured in Keratinocyte Serum Free Medium (Invitrogen), supplied with bovine pituitary extract and epidermal growth factor. These cells were grown at 37°C in a humidified atmosphere of 5% CO2.
2.5. Cell viability
The cell viability was measured by MTT assay [13]. PC3 cells were dispensed in 24-well plates at a density of 2 × 104 cells per well. After overnight incubation, PC3 cells were treated with various concentrations of samples at 37°C for 48 hours. At the end of experiments, 40 μL MTT solution (2 mg/mL) was added to each well for 2 hours. The formazan crystal formed was dissolved in DMSO. Results were measured using a microplate reader at 590 nm. Experiments were carried out in triplicate.
2.6. Western blot analysis
PC3 cells treated with various crude extracts were lysed at the time indicated. Equal amount of cell lysates were electrophoresed on SDS-polyacrylamide gels. The proteins were transferred to the PVDF membrane and incubated with primary antibodies recognizing caspase-3, caspase-9, PARP, p21, p53, and β-actin. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for 60 minutes and developed using enhanced chemiluminescence (ECL; Millipore, Watford, Herts, UK) [14].
2.7. Statistics
All values are expressed as mean ± standard deviation. Each value is the mean of at least three separate experiments in each group. All data were analyzed with a paired t test. Asterisks indicate that the value is significantly different from that of the control (* p < 0.05; ** p < 0.01; *** p < 0.001).
3. Results
3.1. Inhibition of PC3 cell proliferation by different species of Camellia
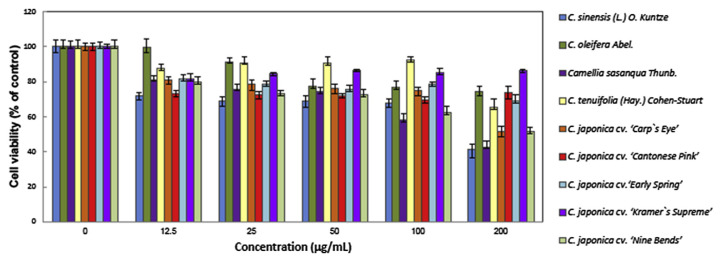
The cytotoxic effect of eight species of Camellia and C. sinensis was first evaluated in human prostate cancer PC3 cells. As shown in Fig. 1, treatment with 12.5–200 μg/mL different species of Camellia extracts caused a dose-dependent decrease of cell viability. Our result showed that in addition to C. sinensis, C. sasanqua has good antiproliferative activity in PC3 cells.
Fig. 1.
Effect of different species of Camellia (C.) extracts on the proliferation of human prostate cancer PC3 cells. After incubation with different concentrations of Camellia extracts at 37°C for 48 hours, the effect on cell growth was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The percentage of viable cells was compared with that of the vehicle-only control. This experiment was repeated three times. Bars represent the standard deviation.
3.2. Induction of apoptosis by different species of Camellia
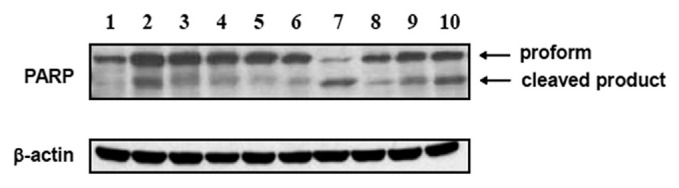
The different species of Camellia possess potential cytotoxic effect, so we next examined whether they could induce apoptosis in PC3 cells. The caspase-3 substrate PARP will be cleaved when cells undergo apoptosis. Therefore, we examined the presence of these cleavage products by western blotting. Our result showed that different species of Camellia extracts induced apoptosis in PC3 cells. Moreover, it appeared that the potency of C. sasanqua is higher than that of C. sinensis in PARP cleavage (Fig. 2).
Fig. 2.
Effect of different species of Camellia extracts on the cleavage of poly(ADP-ribose) polymerase (PARP) in PC3 cells. PC3 cells were treated with 200 μg/mL of Camellia water extracts from: (1) dimethyl sulfoxide only as control; (2) C. sinensis; (3) C. oleifera Abel.; (4) C. tenuifolia (Hay.) Cohen-Stuart; (5) C. japonica cv. “Carp’s Eye”; (6) C. japonica cv. “Cantonese Pink”; (7) C. sasanqua Thunb.; (8) C. japonica cv. “Early Spring”; (9) C. japonica cv. “Kramer’s Supreme”; (10) C. japonica cv. “Nine Bends” at 37°C for 48 hours. Immunoblotting was used to measure the preformed and cleavage products of PARP.
3.3. HPLC analysis of catechins and caffeine in different species of Camellia
We compared the compositions of catechins and caffeine in eight different species of Camellia with C. sinensis by HPLC. As shown in Table 1, the composition of catechins and caffeine in variety species of Camellia are different from that of C. sinensis both qualitatively and quantitatively. Most species of Camellia contain caffeine and catechins, although caffeine is undetectable in C. japonica cv. “Cantonese Pink”. It is worthy to note that our previous studies and others have demonstrated that EGCG was the most abundant catechin and performed the major bioactivity for anticancer effect in C. sinensis [15,16]. However, the most active catechin, EGCG was lower in eight species of Camellia than C. sinensis. Moreover, C. oleifera Abel. possessed the greatest amount of CG, and C. tenuifolia (Hay.) Cohen-Stuart possessed the greatest amount of C and EC among these different species of Camellia. Interestingly, although the catechins were lower, some species such as C. sasanqua still had potent bioactivity.
Table 1.
Levels of caffeine and catechins in various fresh Camellia leaves.
| Gallic | GC | EGC | Caffeine | C | EC | EGCG | GCG | ECG | CG | |
|---|---|---|---|---|---|---|---|---|---|---|
| C. sinensis (L.) O. Kuntze | 1.4623 ± 0.07 | 0.051 ± 0.002 | 0.3475 ± 0.009 | 4.4967 ± 0.112 | 0.018 ± 0.002 | 0.3329 ± 0.018 | 2.9362 ± 0.092 | 0.1922 ± 0.011 | 0.7902 ± 0.026 | 0.0098 ± 0.002 |
| C. oleifera Abel. | 0.4649 ± 0.01 | 0.031 ± 0.001 | 0.0367 ± 0.003 | 0.1524 ± 0.001 | 0.1487 ± 0.005 | 0.1082 ± 0.002 | 0.0495 ± 0.001 | 0.1107 ± 0.026 | 0.1985 ± 0.007 | 0.2599 ± 0.000 |
| C. sasanqua Thunb. | 0.3193 ± 0.01 | 0.0095 ± 0.001 | 0.0627 ± 0.002 | 0.1260 ± 0.001 | 0.0722 ± 0.001 | 0.0315 ± 0.001 | 0.0132 ± 0.001 | 0.0112 ± 0.002 | 0.1052 ± 0.004 | 0.1904 ± 0.008 |
| C. tenuifolia (Hay.) Cohen-Stuart | 0.5945 ± 0.005 | 0.0462 ± 0.001 | 0.0082 ± 0.001 | 0.5136 ± 0.003 | 2.0405 ± 0.026 | 0.7278 ± 0.012 | 0.0262 ± 0.004 | 0.0551 ± 0.008 | 0.0384 ± 0.001 | 0.0691 ± 0.001 |
| C. japonica cv. “Carp’s Eye” | 0.1259 ± 0.009 | ND | 0.0176 ± 0.005 | 0.0253 ± 0.004 | ND | 0.0957 ± 0.013 | 0.0174 ± 0.004 | 0.0393 ± 0.002 | 0.0565 ± 0.003 | 0.0603 ± 0.006 |
| C. japonica cv. “Cantonese Pink” | 0.7698 ± 0.008 | ND | 0.0589 ± 0.001 | ND | ND | 0.0616 ± 0.001 | 0.0011 ± 0.001 | 0.0177 ± 0.001 | 0.0648 ± 0.002 | 0.0145 ± 0.001 |
| C. japonica cv. “Early Spring” | 0.1197 ± 0.003 | ND | 0.0528 ± 0.002 | 0.0424 ± 0.003 | 0.0274 ± 0.001 | 0.4375 ± 0.013 | 0.0700 ± 0.008 | 0.0841 ± 0.001 | 0.0674 ± 0.003 | 0.0951 ± 0.002 |
| C. japonica cv. “Kramer’s Supreme” | 0.0609 ± 0.001 | ND | 0.0365 ± 0.001 | 0.0237 ± 0.001 | 0.0174 ± 0.001 | 0.0287 ± 0.002 | 0.0126 ± 0.001 | 0.0324 ± 0.003 | 0.0566 ± 0.000 | 0.0669 ± 0.003 |
| C. japonica cv. “Nine Bends” | 0.1207 ± 0.002 | ND | 0.0324 ± 0.003 | 0.0191 ± 0.001 | ND | 0.0232 ± 0.008 | 0.0347 ± 0.001 | 0.0164 ± 0.001 | 0.0348 ± 0.009 | 0.0553 ± 0.002 |
Values (mg/g) = mean ± standard deviation (n = 3).
C = (+)-catechin; CG = catechin gallate; EC = (−)-epicatechin; ECG, (−)-epicatechin gallate; EGC = (−)-epigallocatechin; EGCG = (−)-epigallocatechin gallate; GC = (−)-gallocatechin; GCG = (−)-gallocatechin gallate; ND = not detectable.
3.4. Profile of constituents in C. sasanqua
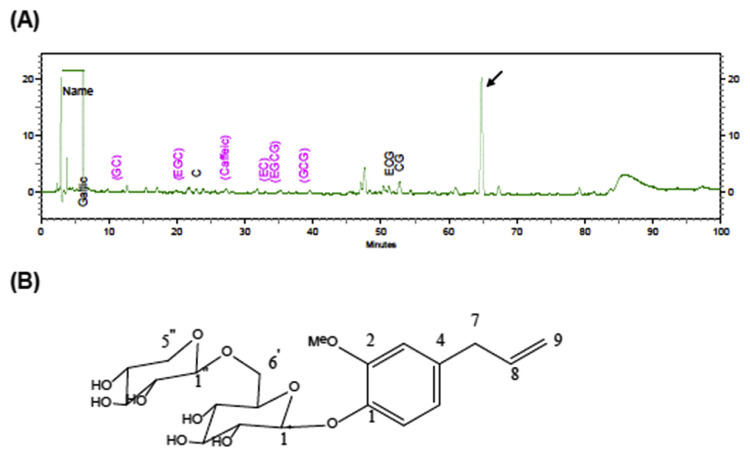
It is likely that bioactive molecules other than catechins exist in C. sasanqua, so we next identified the chemical constituents of C. sasanqua by liquid chromatography/tandem mass spectrometry analyses. As shown in Fig. 3A, several constituents in addition to catechins have been identified in C. sasanqua. The major peak was determined as eugenyl β-primeveroside (Fig. 3B). Eugenyl β-primeveroside was found to have a molecular formula of C21H29O11 based on an electrospray ionization mass spectrometry (ESI-MS) ion at m/z 457 [M + H]+. Analysis of the heteronuclear multiple-bond correlations (HMBCs) were used to connect the correlation spectroscopy (COSY) fragments and assign the quaternary carbons (supplementary Fig. 1). Specifically, HMBCs from the anomeric hydrogens resonating at δH 4.83 (H-1′) and 4.29 (H-1″) to δC 146.2 (C-1) and 69.66 (C-6′) completed the sugar portion of the glycoside moiety. The remainder of the fragments were assigned on the basis of HMBCs from the methylene hydrogens at C-6′ (δH4.07 and 3.75) to δC 77.4 (C-5′), 71.36 (C-4′), and 105.29 (C-1″). Further HMBC correlations from the C-5″ methylene hydrogens to the C-1″ (δC105.29), C-3″ (δC 77.59), C-4″ (δC 71.18; supplementary Fig. 1). The molecular formula and 1H and 13C nuclear magnetic resonance spectroscopic data of the isolated compound was identical to eugenyl β-primeveroside as described previously [17].
Fig. 3.
(A) Isocratic high-performance liquid chromatography separation of C. sasanqua; (B) structures of eugenyl β-primeveroside.
3.5. Eugenyl β-primeveroside induces PC3 cells G1 arrest and apoptosis
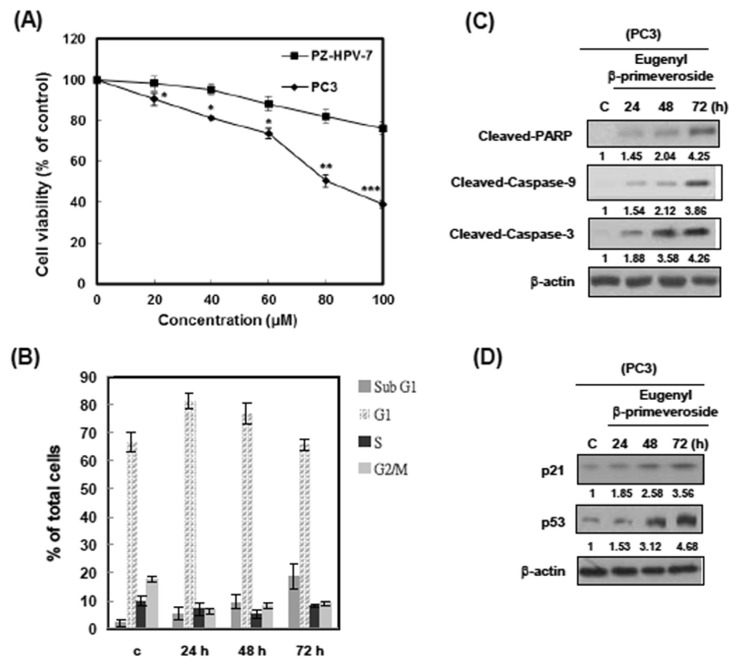
To investigate the bioactivity of eugenyl β-primeveroside in prostate cells, we treated PC3 cells with different concentrations of eugenyl β-primeveroside for 48 hours, and assessed cell proliferation by MTT assay. As shown in Fig. 4A, the growth of PC3 cells was inhibited by eugenyl β-primeveroside in a dose-dependent manner. Importantly, eugenyl β-prime-veroside was less toxic to normal cell type PZ-HPV-7 (Fig. 4A). These data indicate that eugenyl β-primeveroside induces selective cytotoxicity in human prostate cancer cells but less in normal cells. To examine whether eugenyl β-primeveroside produced cytotoxic effects, we used a PI stain to measure flow cytometrically. After treatment with 80μM eugenyl β-prime-veroside, the cell cycle of PC3 arrested in the G1 phase and the cells underwent apoptosis (Fig. 4B). Moreover, we examined the expression of G1 related cell cycle control proteins and apoptosis related proteins on western blot analysis. PC3 cells were treated with 80μM eugenyl β-primeveroside with indicated durations. Western blot analysis indicated that treatment of PC3 cells with 80μM eugenyl β-primeveroside resulted in a clear apoptosis within 72 hours, showing cleavages for PARP, upregulation of caspase-9 and caspase-3 in western blot analyses (Fig. 4C). After 24 hours of eugenyl β-primeveroside treatment, we could observe increased levels of p21 and p53 in PC3 cells (Fig. 4D). These results demonstrate that eugenyl β-primeveroside induced G1 arrest and apoptosis in PC3 cells.
Fig. 4.
Eugenyl β-primeveroside induced cell cycle arrest and apoptosis in PC3 cells. (A) PC3 and PZ-HPV-7 cells were treated with various concentrations of eugenyl β-primeveroside at 37°C for 48 hours. The effect on cell growth was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, and the percentage of cell proliferation was calculated by defining the absorption of cells without eugenyl β-primeveroside as 100%. This experiment was repeated three times. Bars represent the standard deviation. Asterisks indicate that the value is significantly different from that of the control (*p < 0.05; **p < 0.01; ***p < 0.001). (B) PC3 cells were treated with 80μM of eugenyl ββ-primeveroside for the indicated duration and analyzed for PI-stained DNA content by flow cytometry. The indicated percentages are the mean of three independent experiments, each in duplicate. Bars represent the standard deviation. (C) PC3 cells were treated with vehicle (dimethyl sulfoxide), eugenyl β-primeveroside (80μM) for the indicated time. Cells were then harvested and lysed for the detection of cleaved PARP, Caspase-9, Caspase-3 and β-actin protein expression. (D) PC3 cells were treated with vehicle (dimethyl sulfoxide), eugenyl β-primeveroside (80μM) for the indicated time. Cells were then harvested and lysed for the detection of p21, p53 and β-actin protein expression. Western blot data presented are representative of those obtained in at least three separate experiments. Immunoblots were quantified, and relative expression to control is indicated.
4. Discussion
Cancer preventive activity of tea and tea constituents has been studied in many different models of carcinogenesis [18]. In this study, we used the MTT assay and PARP cleavage to compare the capability of eight species of Camellia and C. sinensis to inhibit proliferation and induce apoptosis in human prostate cancer PC3 cells. Interestingly, our results demonstrated that some Camellia species other than C. sinensis showed a significant effect on anti-proliferation and apoptosis induction. C. sasanqua showed a particularly good anti-proliferative effect similar to that in C. sinensis. The induction of apoptosis by C. sasanqua was even more powerful than C. sinensis (Fig. 2).
According to previous reports, the major components in tea leaves are caffeine and catechins. Catechins have been extensively studied for their cancer preventive effects [19]. Accumulating evidence has shown that green tea catechins, like EGCG, have strong antioxidant activity and affect several signal transduction pathways relevant to cancer development [11]. In this report, the occurrence of catechins (namely gallic acid, GC, EGC, C, EC, EGCG, GCG, ECG, and CG) and caffeine in eight species of Camellia has been demonstrated by HPLC analysis (Table 1). The levels of catechins and caffeine in most species of Camellia are lower than that of C. sinensis. The amount of caffeine is lower in eight species of Camellia than C. sinensis as indicated in Table 1.
C. sasanqua is a species of Camellia native to China and Japan, and usually grows at an altitude of 900 m. The leaves of C. sasanqua are used to make tea while the seeds or nuts are used to make tea seed oil, which is used for lighting, lubrication, cooking, and cosmetic purposes [20–22]. A previous study [23] reported that a novel 3,4-seco-triterpene alcohol, named sasanquol, was isolated from the nonsaponifiable lipid fraction of sasanqua seed oil. Sasanquol has been found to inhibit TPA-induced ear inflammation in mice [23].
The levels of catechins and caffeine in most species of Camellia are lower compared to that of C. sinensis, however, C. sasanqua showed the best apoptotic inducing activity toward PC3 cells among different species of Camellia (Fig. 2). It is suggested that some other molecules besides catechins in C. sasanqua are responsible for its cytotoxic effect. We have therefore isolated and identified eugenyl β-primeveroside as a major component of C. sasanqua. Eugenol, a volatile phenylpropene that is widely distributed across the plant kingdom, exhibits various physiological activities such as antimicrobial [24] and acaricidal [25] activities. In the crop tomato, eugenol is often stored as glycosides, representing an aroma precursor, and is an important factor determining the quality of the tomato fruits [26]. Eugenyl β-primeveroside has been isolated from a methanolic extract of rose flowers using multilayer coil countercurrent chromatography [17]. Moreover, eugenyl β-primeveroside has also been isolated from the fresh leaves of C. sasanqua [27]. However, there is no report on the medical function of eugenyl β-primeveroside.
Our studies demonstrated that eugenyl β-primeveroside strongly inhibited PC3 cell proliferation and blocked cell cycle progression at the G1 phase transition. To better understand the molecular mechanisms underlying eugenyl β-primeveroside-induced cell growth inhibition and cell cycle arrest, we determined the effect of eugenyl β-primeveroside on the expression of key regulatory proteins. It has been well established that p21 and p53 play important roles in the regulation of cell cycle progression [28]. Our results demonstrated that a significant upregulation in p21 and p53 occurred during the G1-phase arrest in PC3 cells treated with eugenyl β-primeveroside. To the best of our knowledge, this is the first report that eugenyl β-primeveroside exhibits antiproliferative activity against cancer cells.
In conclusion, the use of naturally occurring compounds in the development of antitumor agents has become a critical topic in the scientific and industrial communities. Many studies have reported the biological activities of C. sinensis, but few have discussed the biological potential of other Camellia species. In this study, C. sasanqua was discovered to exhibit antiproliferative activity against human prostate cancer cells and its major component eugenyl β-primeveroside induced G1 arrest and apoptosis in PC3 cells. Taken together, it is suggested that C. sasanqua could be developed as an agent for the management of human prostate cancers.
Acknowledgments
This study was supported by the National Science Council NSC 95-2320-B-039-009, and 95-2320-B-039-044-MY2.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2015.06.005.
Funding Statement
This study was supported by the National Science Council NSC 95-2320-B-039-009, and 95-2320-B-039-044-MY2.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2. Bommareddy A, Eggleston W, Prelewicz S, Antal A, Witczak Z, McCune DF, et al. Chemoprevention of prostate cancer by major dietary phytochemicals. Anticancer Res. 2013;33:4163–74. [PubMed] [Google Scholar]
- 3. Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3:592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- 4. Lee CP, Shih PH, Hsu CL, Yen GC. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2007;45:888–95. doi: 10.1016/j.fct.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5. Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–7. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- 6. Pan MH, Chiou YS, Wang YJ, Ho CT, Lin JK. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011;2:101–10. doi: 10.1039/c0fo00174k. [DOI] [PubMed] [Google Scholar]
- 7. Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8. Lin X, Chen Z, Zhang Y, Gao X, Luo W, Li B. Interactions among chemical components of Cocoa tea (Camellia ptilophylla Chang), a naturally low caffeine-containing tea species. Food Funct. 2014;5:1175–85. doi: 10.1039/c3fo60720h. [DOI] [PubMed] [Google Scholar]
- 9. Cimpoiu C, Cristea VM, Hosu A, Sandru M, Seserman L. Antioxidant activity prediction and classification of some teas using artificial neural networks. Food Chem. 2011;127:1323–8. doi: 10.1016/j.foodchem.2011.01.091. [DOI] [PubMed] [Google Scholar]
- 10. Lin JN, Lin HY, Yang NS, Li YH, Lee MR, Chuang CH, et al. Chemical constituents and anticancer activity of yellow camellias against MDA-MB-231 human breast cancer cells. J Agric Food Chem. 2013;61:9638–44. doi: 10.1021/jf4029877. [DOI] [PubMed] [Google Scholar]
- 11. Yu Y, Deng Y, Lu BM, Liu YX, Li J, Bao JK. Green tea catechins: a fresh flavor to anticancer therapy. Apoptosis. 2014;19:1–18. doi: 10.1007/s10495-013-0908-5. [DOI] [PubMed] [Google Scholar]
- 12. Syu KY, Lin CL, Huang HC, Lin JK. Determination of theanine, GABA, and other amino acids in green, oolong, black, and Puerh teas with dabsylation and high-performance liquid chromatography. J Agric Food Chem. 2008;56:7637–43. doi: 10.1021/jf801795m. [DOI] [PubMed] [Google Scholar]
- 13. Chen WC, Hsu KY, Hung CM, Lin YC, Yang NS, Ho CT, et al. The anti-tumor efficiency of pterostilbene is promoted with a combined treatment of Fas signaling or autophagy inhibitors in triple negative breast cancer cells. Food Funct. 2014;5:1856–65. doi: 10.1039/c4fo00145a. [DOI] [PubMed] [Google Scholar]
- 14. Liu YJ, Lin YC, Lee JC, Kuo SC, Ho CT, Huang LJ, et al. CCT327 enhances TRAIL-induced apoptosis through the induction of death receptors and downregulation of cell survival proteins in TRAIL-resistant human leukemia cells. Oncol Rep. 2014;32:1257–64. doi: 10.3892/or.2014.3317. [DOI] [PubMed] [Google Scholar]
- 15. Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–65. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 16. Pae M, Wu D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: mechanisms and applications. Food Funct. 2013;4:1287–303. doi: 10.1039/c3fo60076a. [DOI] [PubMed] [Google Scholar]
- 17. Straubinger M, Knapp H, Watanabe N, Oka N, Washio H, Winterhalter P. Three novel eugenol glycosides from rose flowers, Rosa damascena Mill. Nat Prod Lett. 1999;13:5–10. [Google Scholar]
- 18. Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharm Des. 2013;19:6141–7. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–31. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 20. Feás X, Estevinho LM, Salinero C, Vela P, Sainz MJ, Vázquez-Tato MP, et al. Triacylglyceride, antioxidant and antimicrobial features of virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules. 2013;18:4573–87. doi: 10.3390/molecules18044573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akihisa T, Arai K, Kimura Y, Koike K, Kokke WCMC, Shibata T, et al. Camelliols A-C, three novel incompletely cyclized triterpene alcohols from sasanqua oil (Camellia sasanqua) J Nat Prod. 1999;62:265–8. doi: 10.1021/np980336a. [DOI] [PubMed] [Google Scholar]
- 22. Akihisa T, Koike K, Kimura Y, Sashida N, Matsumoto T, Ukiya M, et al. Acyclic and incompletely cyclized triterpene alcohols in the seed oils of theaceae and gramineae. Lipids. 1999;34:1151–7. doi: 10.1007/s11745-999-0466-5. [DOI] [PubMed] [Google Scholar]
- 23. Akihisa T, Yasukawa K, Kimura Y, Yamanouchi S, Tamura T. Sasanquol, a 3,4-seco-triterpene alcohol from sasanqua oil, and its anti-inflammatory effect. Phytochemistry. 1998;48:301–5. doi: 10.1016/s0031-9422(97)01107-2. [DOI] [PubMed] [Google Scholar]
- 24. Zhang P, Zhang E, Xiao M, Chen C, Xu W. Enhanced chemical and biological activities of a newly biosynthesized eugenol glycoconjugate, eugenol α-D-glucopyranoside. Appl Microbiol Biotechnol. 2013;97:1043–50. doi: 10.1007/s00253-012-4351-2. [DOI] [PubMed] [Google Scholar]
- 25. Pasay C, Mounsey K, Stevenson G, Davis R, Arlian L, Morgan M, et al. Acaricidal activity of eugenol based compounds against scabies mites. PLoS One. 2010;5:e12079. doi: 10.1371/journal.pone.0012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tikunov YM, de Vos RC, González Paramás AM, Hall RD, Bovy AG. A role for differential glycoconjugation in the emission of phenylpropanoid volatiles from tomato fruit discovered using a metabolic data fusion approach. Plant Physiol. 2010;152:55–70. doi: 10.1104/pp.109.146670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koeduka T, Suzuki S, Iijima Y, Ohnishi T, Suzuki H, Watanabe B, et al. Enhancement of production of eugenol and its glycosides in transgenic aspen plants via genetic engineering. Biochem Biophys Res Commun. 2013;436:73–8. doi: 10.1016/j.bbrc.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 28. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]