Abstract
In the present study, 2,2-diphenyl-1-picrylhydrazyl radical scavenging, α-amylase and α-glucosidase inhibition activities, and total phenolic contents of n-hexane, ethyl acetate, and methanol extracts of various parts of Allium paradoxum, Buxus hyrcana, Convolvulus persicus, Eryngium caucasicum, Heracleum persicum, Pimpinella affinis, Parrotia persica, Primula heterochroma, Pyrus boissieriana, Ruscus hyrcanus, and Smilax excelsa were investigated. These plants, which mostly serve as food flavoring, were collected from Hyrcania region, Sari, Iran. Some extracts of H. persicum, S. excels, P. boissieriana, P. persica, and P. heterochroma exhibited significant antidiabetic activities in α-amylase and α-glucosidase assays, more effective than acarbose (concentrations that cause 50% inhibition = 75.7 μg/mL and 6.1 μg/mL against α-amylase and α-glucosidase, respectively). Also, C. persicus, P. boissieriana, and P. heterochroma showed strong antioxidant activities, compared with butylated hydroxytoluene (concentration that causes 50% inhibition = 16.7 μg/mL). In conclusion, this study can recommend these plants as good candidates for further investigations to find potent antidiabetic natural products or probable lead compounds. Statistical analysis showed significant correlation between the 2,2-diphenyl-1-picrylhydrazyl scavenging activity and total phenolic contents (r = 0.711, p < 0.001).
Keywords: 2, 2-diphenyl-1-picrylhydrazyl, α-amylase, α-glucosidase, antidiabetics, phenolic content
1. Introduction
Plants have developed an array of defense strategies (antioxidant systems) to manage oxidative stress. In these systems, there is a wide variety of antioxidants [e.g., ascorbic acid, gluthione, uric acid, tocopherol, carotenoids, and (poly)phenols], which are different in their composition, mechanism, and site of action [1]. Antioxidants have significant inhibition roles, not only on undesirable changes in the flavor and nutritional quality of food, but also on tissue damage in various human diseases such as inflammation, cancer, and atherosclerosis [2]. Moreover, having antioxidant activity in addition to pharmaceutical properties, such as antidiabetic, anticarcinogenic, and antialzheimeric activities, can be a special function to obtain multifunctional drugs. Recently, there has been an increased interest globally to discover natural antioxidants with low or no side effects for use in preventive medicine and the food industry [3].
Noninsulin-dependent diabetes mellitus or type-II diabetes mellitus is one of the most common and serious metabolic disorders with abnormally high blood glucose levels (hyper-glycaemia) due to defects in insulin secretion, or action, or both [4]. Hydrolysis of dietary carbohydrates such as starch is the major source of glucose in the blood. Because α-glucosidase and pancreatic α-amylase play a critical role in carbohydrate digestion and glycoprotein processing, inhibitors of these enzymes might be used to treat diabetes, human immunodeficiency virus, Gaucher’s disease, cancers, and Alzheimer’s disease [5–7]. Some inhibitors, such as acarbose, miglitol (a deoxynojirimycin derivative), and voglibose, are widely used clinically in combination with diet to control blood glucose levels of patients [8,9]. To prevent or decline the side effects of these drugs and also to provide more candidates of drug choices, it is still essential to seek new α-glucosidase inhibitors for further drug development. In recent years, many efforts have been made to approach glucosidase inhibitors from natural sources for antidiabetes treatment [10,11].
One of the major hypotheses proposed to explain the hyperglycaemia-induced onset of diabetic complications is that it is a result of the impairment in the equilibrium between reactive oxygen species capacity and antioxidant defence capacity [12–14]. Accordingly, using antioxidant agents can be helpful for scavenging various reactive oxygen species and prevention of diabetes mellitus.
In this work, antioxidant and antidiabetic activities of different extracts of Allium paradoxum (M.B.) G.Don (Liliaceae), Buxus hyrcana Pojark. (Buxaceae), Convolvulus persicus L. (Convolvulaceae), Eryngium caucasicum Trautv. (Apiaceae), Heracleum persicum Desf. ex Fischer (Apiaceae), Pimpinella affinis Ledeb. (Apiaceae), Parrotia persica C.A. Mey (Hamamelidaceae), Primula heterochroma Stapf (Primulaceae), Pyrus boissieriana Buhse (Rosaceae), Ruscus hyrcanus Woron. (Asparagaceae), and Smilax excelsa L. (Smilacaceae) were investigated. These plants were collected in Sari, Hyrcania region, Iran. The Hyrcania (Caspian) region, that covers an area of 1,925,125 ha, extends throughout the south coast of the Caspian Sea in the northern part of Iran [15]. In this region, people use these plants as food flavoring, and antiflatulence, antimicrobial, antifever, and antidiabetic natural sources (Table 1).
Table 1.
Plant species, their traditional uses, and biological properties.
| Plant species | Medicinal properties | Traditional uses | Voucher No.a | Family |
|---|---|---|---|---|
| Allium paradoxum | Antihemolytic [17], antioxidant [18], protective against gentamicin-induced nephrotoxicity [19] | Food flavoring, antiacne, antidigestive disorders [16] | 2961 | Liliaceae |
| Buxus hyrcana | Anti-plasmodial activity [20], acetylcholinesterase-inhibitor [21,22], immunosuppressive [23], antioxidant, anti-HIV [24], antifungal [25] | Antimalaria, antipneumonia, antihair loss, antirheumatism, laxative, febrifuge, anti-infection, analgesic, antiheadache, antiepileptic, aperient [16,20] | 1529 | Buxaceae |
| Convolvulus persicus | — | — | 6005 | Convolvulaceae |
| Eryngium caucasicum | Antioxidant [28] | Food flavoring [16,26,27] | 6159 | Apiaceae |
| Heracleum persicum | Anticonvulsant [32], antitumor, antibacterial [31], antifungal [34], anti-inflammatory, analgesic [35], antioxidant [36,37], cytotoxic [38] | Food flavoring [16,29], analgesic [30], antiseptic [31], antiepilepsy [32], antimicrobial [33], antiflatulence, antidyspepsia [16] | 9869 | Apiaceae |
| Pimpinella affinis | — | Food flavoring, antispasmodic, narcotic, expectorant, diuretic, antimigraine, antimicrobial, antiasthma, carminative, anti-cholera [16] | 3148 | Apiaceae |
| Parrotia persica | Antioxidant [39], antibacterial [40] | Food coloring and food flavoring, antifever [39,40] | 8370 | Hamamelidaceae |
| Primula heterochroma | Antihemolytic [41] | Food flavoring [16] | 3136 | Primulaceae |
| Pyrus boissieriana | Antioxidant [24] | Food flavoring, anti-infection, narcotic, anticramp, antihypertensive [16] | 4607 | Rosaceae |
| Ruscus hyrcanus | — | Diuretic, appetizer, antilaxative, vasoconstrictor, antibleeding, antinephritis, anti-infection, aperient, antivaricose, laxative [16] | 9407 | Asparagaceae |
| Smilax excelsa | Antioxidant [42], cytotoxic, antimicrobial [43] | Food flavoring, diuretic, sudatory, antieczema [16,42] | 2973 | Smilacaceae |
Voucher specimens were deposited in the herbarium of Nowshahr botanical garden, Nowshahr, Iran.
Antioxidant activities of n-hexane, ethyl acetate, and methanol extracts of various parts of these plants were examined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. Also, the total phenolic content of these herbal plants was determined. In addition, inhibition activities of the extracts against pancreatic α-amylase and α-glucosidase were investigated.
2. Methods
2.1. Reagents
All the chemicals were purchased from Sigma–Aldrich Chemie Gmbh (Munich, Germany) and Merck (Darmstadt, Germany) companies. The chemicals were of analytical grades.
2.2. Plant materials
Different parts of A. paradoxum, B. hyrcana, C. persicus, E. caucasicum, H. persicum, P. affinis, P. persica, P. heterochroma, P. boissieriana, R. hyrcanus, and S. excelsa were collected from lowland to submountain forest areas of Sari, Mazandaran province, Iran in April and May 2011. Voucher specimens were deposited in the herbarium of Nowshahr botanical garden, Nowshahr, Iran (Table 1). The plant materials were dried at room temperature and ground to a powder in a blender.
2.3. Solvent extraction of the plants
The protocol for extraction of the plants was sequential extraction using three different solvents with different polarities, starting with the most nonpolar. The dried and fine plant parts (100 g) were extracted with 400 mL n-hexane by maceration (48 hours × 2). By addition of 400 mL ethyl acetate to the dried plant’s surplus, ethyl acetate extract was obtained (48 hours × 2). The methanol extract was obtained by the same method (48 hours × 1). Each extract was then concentrated under reduced pressure at approximately 40°C to obtain n-hexane, ethyl acetate, and methanol fractions. The percentage of extract yield was calculated as (dry extract weight/dry starting material weight) × 100 (Table 2).
Table 2.
Results of extraction yields, DPPH radical scavenging activity, total phenolic contents, and α-amylase and α-glucosidase inhibition activity of the medicinal plants.
| Plant species | Plant part used | Extract yield (%W/W) | DPPH scavenging activity (%)a | Total phenolic content (mg GAE/g DW) | α-Amylase inhibition (%)b | α-Glucosidase inhibition (%)c | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Allium paradoxum | Aerial parts | Hex. | 1.2 | 27.6 ± 0.7d | 23.0 ± 1.4 | 45.9 ± 5.5d | 15.3 ± 1.3d |
| EA. | 1.9 | 22.3 ± 0.3d | 53.0 ± 3.1 | 17.8 ± 2.0f | NI | |||
| Met. | 4.0 | 47.1 ± 0.3d | 85.6 ± 2.9 | 33.5 ± 5.0d | 27.8 ± .06d | |||
| Bulb | Hex. | 0.3 | 43.7 ± 0.7d | 39.6 ± 4.5 | 36.8 ± 1.6f | 37.7 ± 1.3e | ||
| EA. | 0.3 | 68.3 ± 0.1d,* | 118.7 ± 6.3 | 12.9 ± 3.4d | 9.2 ± 1.5d | |||
| Met. | 1.8 | 41.6 ± 0.4d | 110.1 ± 4.1 | NI | NI | |||
| 2 | Buxus hyrcana | Leaf | Hex. | 1.9 | 20.8 ± 0.6d | 15.3 ± 3.3 | 15.2 ± 2. 1e | 42.9 ± 2.3e |
| EA. | 1.6 | 33.8 ± 0.1d | 101.6 ± 5.1 | NI | NI | |||
| Met. | 5.4 | 47.8 ± 0.8d | 148.2 ± 4.1 | 33.3 ± 2.0e | 12.3 ± 1.1e | |||
| 3 | Convolvulus persicus | Aerial parts | Hex. | 1.8 | 19.4 ± 1.1d | 39.0 ± 4.7 | 4.7 ± 2.2f | NI |
| EA. | 2.2 | 37.9 ± 0.8e | 55.2 ± 2.8 | 8.7 ± 1.0e | NI | |||
| Met. | 5.1 | 88.3 ± 0.6d,* | 134.7 ± 6.2 | NI | NI | |||
| Root | Hex. | 0.7 | 48.8 ± 0.8d | 65.4 ± 3.2 | 17.7 ± 3.7d | 2.9 ± 1.0d | ||
| EA. | 1.6 | 56.6 ± 1.4d,* | 77.0 ± 2.2 | 42.9 ± 0.5d | 13.0 ± 1.5d | |||
| Met. | 7.1 | 55.3 ± 0.9d,* | 81.9 ± 4.2 | 20.6 ± 3.1e | 6.7 ± 2.0e | |||
| 4 | Eryngium caucasicum | Aerial parts | Hex. | 1.0 | 29.7 ± 0.7d | 7.1 ± 0.6 | 9.6 ± 2.7f | NI |
| EA. | 1.8 | 24.4 ± 1.7d | 14.4 ± 3.3 | 11.9 ± 1.3e | NI | |||
| Met. | 5.0 | 31.2 ± 2.1d | 86.2 ± 5.0 | 15.9 ± 4.8f | NI | |||
| 5 | Heracleum persicum | Aerial parts | Hex. | 1.4 | 26.2 ± 0.4d | 42.0 ± 1.7 | 78.5 ± 3.9d,* | 66.4 ± 1.6d,* |
| EA. | 1.5 | 76.6 ± 0.7d,* | 167.2 ± 2.3* | 38.9 ± 1.0e | 3.8 ± 1.3f | |||
| Met. | 3.4 | 41.5 ± 0.3d | 113.2 ± 5.8 | 30.8 ± 5.0f | NI | |||
| Root | Hex. | 2.1 | 5.2 ± 0.4d | 58.9 ± 1.7 | 41.9 ± 2.7e | 84.5 ± 1.2e,* | ||
| EA. | 1.9 | 28.7 ± 0.6e | 90.8 ± 4.4 | 10.5 ± 3.3e | NI | |||
| Met. | 4.2 | 19.1 ± 0.1d | 78.2 ± 5.1 | NI | NI | |||
| 6 | Pimpinella affinis | Leaf | Hex. | 1.2 | 29.0 ± 0.6d | 31.0 ± 3.6 | 81.3 ± 3.7d,* | 26.4 ± 1.3e |
| EA. | 1.0 | 31.6 ± 0.9d | 54.3 ± 2.1 | 26.2 ± 2.4f | NI | |||
| Met. | 2.6 | 91.5 ± 1.2d,* | 155.5 ± 5.5* | 24.5 ± 3.0e | 0.3 ± 2.1f | |||
| Root | Hex. | 1.3 | 11.5 ± 0.6d | 20.6 ± 4.4 | 5.5 ± 0.6f | 33.6 ± 3.3e | ||
| EA. | 2.2 | 36.2 ± 0.6d | 37.1 ± 3.3 | 76.4 ± 1.0e,* | 3.5 ± 1.0a | |||
| Met. | 3.8 | 32.3 ± 0.9e | 90.5 ± 3.5 | 27.6 ± 5.1f | NI | |||
| 7 | Parrotia. persica | Leaf | Hex. | 0.4 | 27.5 ± 1.3d | 37.7 ± 2.5 | 25.6 ± 2.3e | 35.7 ± 0.4e |
| EA. | 0.6 | 96.0 ± 1.0d,* | 145.7 ± 8.3 | 45.6 ± 4.7e | 99.3 ± 2.0d,* | |||
| Met. | 5.1 | 95.9 ± 0.7d,* | 506.5 ± 11.3* | 38.3 ± 1.4e | 99.6 ± 2.8d,* | |||
| 8 | Primula. heterochroma | Leaf | Hex. | 0.5 | 15.4 ± 0.3d | 24.9 ± 5.8 | 14.7 ± 1.3e | NI |
| EA. | 0.8 | 39.9 ± 0.8d | 82.5 ± 1.3 | 37.6 ± 4.3e | NI | |||
| Met. | 4.3 | 95.5 ± 0.7d,* | 223.7 ± 5.0* | 31.0 ± 3.9a | 97.8 ± 1.9d,* | |||
| Root | Hex. | 0.4 | 13.7 ± 1.4d | 32.8 ± 2.8 | NI | NI | ||
| EA. | 0.8 | 56.5 ± 0.5d,* | 153.4 ± 4.5* | 4.5 ± 2.9f | 61.8 ± 1.2d,* | |||
| Met. | 5.7 | 95.1 ± 0.8d,* | 165.7 ± 6.3* | 12.7 ± 2.1e | 98.7 ± 3.3d,* | |||
| 9 | Pyrus boissieriana | Leaf | Hex. | 0.9 | 27.8 ± 0.7d | 21.8 ± 5.1 | 1.6 ± 3.1e | 20.4 ± 1.0e |
| EA. | 1.6 | 84.5 ± 0.5d,* | 174.6 ± 3.8* | 2.0 ± 1.1d | NI | |||
| Met. | 3.5 | 94.3 ± 0.4d,* | 414.5 ± 9.5* | 30.2 ± 4.0d | 51.6 ± 1.3e,* | |||
| Stem | Hex. | 0.3 | 25.1 ± 1.1e | 15.7 ± 4.0 | 44.1 ± 2.5f | 99.2 ± 4.1d,* | ||
| EA. | 0.6 | 94.0 ± 1.7d,* | 312.6 ± 6.4* | 25.2 ± 1.0d | 93.4 ± 1.6d,* | |||
| Met. | 3.8 | 94.7 ± 0.4d,* | 549.5 ± 8.3* | 56.5 ± 4.4e,* | 99.1 ± 2.6d,* | |||
| 10 | Ruscus hyrcanus | Aerial parts | Hex. | 0.7 | 19.2 ± 0.9d | 12.6 ± 2.3 | 13.9 ± 3.2e | 19.1 ± 1.3e |
| EA. | 0.8 | 32.4 ± 0.7d | 94.2 ± 4.2 | 20.4 ± 1.8e | NI | |||
| Met. | 3.5 | 25.0 ± 2.4d | 83.1 ± 2.7 | 8.8 ± 1.3d | 7.3 ± 0.6e | |||
| Root | Hex. | 0.2 | 4.8 ± 0.5d | 57.1 ± 3.3 | 15.3 ± 1.0f | 30.1 ± 1.3d | ||
| EA. | 0.5 | 35.3 ± 0.8d | 170.9 ± 5.2* | 1.6 ± 2.1f | NI | |||
| Met. | 6.8 | 10.5 ± 0.5e | 42.6 ± 3.4 | 10.8 ± 4.2f | NI | |||
| 11 | Smilax excelsa | Leaf | Hex. | 1.2 | 22.1 ± 0.3d | 19.3 ± 2.7 | 78.2 ± 3.0d,* | 15.1 ± 1.0e |
| EA. | 1.3 | 22.4 ± 1.4d | 97.9 ± 3.5 | 76.1 ± 4.6d,* | 2.7 ± 1.1d | |||
| Met. | 3.9 | 47.1 ± 0.3d | 239.0 ± 5.4* | 98.5 ± 3.3e,* | 1.5 ± 1.4d | |||
| Stem | Hex. | 0.7 | 43.7 ± 0.7e | 7.1 ± 2.1 | 10.8 ± 2.9f | 29.6 ± 2.2d | ||
| EA. | 1.0 | 68.3 ± 0.8d,* | 134.1 ± 6.5 | 98.5 ± 3.1e,* | 58.9 ± 0.4e,* | |||
| Met. | 2.9 | 41.7 ± 0.7d | 226.7 ± 4.3* | 38.5 ± 1.7d | 32.1 ± 2.4f | |||
| BHT | — | — | — | 99.6 ± 1.1g | — | — | — | |
| Acarbose | — | — | — | — | — | 74.9 ± 1.3h | 41.7 ± 0.7i | |
The extracts were tested at concentrations of a200 μg/mL for DPPH scavenging, b238.1 μg/mL for α-amylase inhibitory, and c3.3 μg/mL for α-glucosidase inhibitory assays.
Values are presented as the mean ± standard deviation of three independent experiments.
p < 0.001,
p < 0.01,
p < 0.05 as compared with control.
The best results.
BHT = butylated hydroxytoluene; DPPH = 2,2-diphenyl-1-picrylhydrazyl; DW = dry weight; EA = ethyl acetate; GAE = gallic acid equivalent; Hex. = n-hexane; Met. = methanol; NI = not identified.
2.4. 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
The ability of plant extracts to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals was determined according to the method described by Chiu et al [44]. A 50-μL aliquot of 500 μg/mL of test sample in dimethyl sulfoxide (DMSO) was added to 200 μL of 100μM DPPH solution in methanol. After an incubation period of 30 minutes at room temperature in the darkness, the decrease in the absorbance (Abs) was measured at 517 nm. Butylated hydroxytoluene (BHT) was used as a positive control for this assay. Experiments were carried out three times. The percentage inhibition was calculated using the following equation:
DPPH scavenging activities of various concentrations of the most effective extracts were assessed to determine concentration that causes 50% inhibition (IC50).
2.5. Determination of total phenolic contents
Total phenolic contents of the extracts were determined using the Folin–Ciocalteu method [45]. A 2.5-μL sample of each extract (1000 μg/mL in DMSO) was added to 12.5 μL of Folin–Ciocalteu reagent and 195 μL of phosphate buffer (75mM, pH 7.0). After 3 minutes, 50 μL of Na2CO3 (7.0%) was added and incubated at room temperature for 2 hours. After 2 hours the absorbance was measured at 765 nm. Standard curve was prepared by using different concentrations of gallic acid. Total phenolic content was expressed as mg gallic acid equivalent (GAE)/g dry weight. Analyses were done in triplicate.
2.6. Pancreatic α-amylase inhibition assay
The pancreatic α-amylase inhibition assay was performed according to the literature procedure [46]. Briefly, 50 μL of samples (1000 μg/mL in DMSO) were added to 150 μL starch solution (containing 1% starch and 17mM NaCl). The reaction was initiated by adding 10 μL α-amylase (26 U/mL) to the reaction mixture. After 30 minutes, the reaction was stopped by adding 20 μL of NaOH solution (2N). Subsequently, 20 μL of dinitrosalicylic acid reagent (44mM 3,5-dinitrosalicylic acid, 106mM potassium sodium tartarate, 40mM NaOH) was added to the reaction mixture. The mixtures were heated at 100°C for 20 minutes. After cooling to room temperature, Abs was recorded at 540 nm using a spectrophotometer. Standard curve was prepared by using different concentrations of maltose after addition of dinitrosalicylic acid reagent to determine equal absorption of the produced maltose. Acarbose was used as a positive control for this assay. All samples were analyzed in triplicate. The percentage inhibition was calculated using follow the equation.
In this equation, Abs540 (sample) is the absorption of maltose produced from starch by the enzyme at 540 nm in the presence of the extract, and Abs540 (control) is the equal absorption of the produced maltose by the enzyme at 540 nm in the absence of the extract.
α-Amylase inhibitory activities of various concentrations of the most effective extracts were assessed to determine IC50.
2.7. α-Glucosidase inhibition assay
The α-glucosidase inhibition assay was performed according to the literature procedure [47]. A 40-μL aliquot of α-glucosidase solution (1 U/mL) was added to 1 μL of sample solution (500 μg/mL in DMSO) and 69 μL of 0.1M sodium phosphate buffer (pH = 7.0). After 15 minutes’ incubation at 37°C, 40 μL of substrate solution (5mM p-nitrophenyl α-D-glucopyranoside) was added to the reaction mixture and incubated at 37°C for 30 minutes. Then, the reaction terminated by adding 150 μL of 0.1M Na2CO3. The Abs was determined at 405 nm using a spectrophotometer and the percentage inhibition was calculated using the following equation. Acarbose was used as a positive control for this assay. All samples were analyzed in triplicate.
In this equation, Abs405 (sample) is the absorption of the produced p-nitrophenol from p-nitrophenyl α-D-glucopyranoside by the enzyme in 405 nm in the presence of the extract, and Abs405(control) is the absorption of produced p-nitrophenol by the enzyme in 405 nm in the absence of the extract.
α-Glucosidase inhibitory activities of various concentrations of the most effective extracts were assessed to determine IC50.
2.8. Statistical analysis
All assays were performed at least in triplicate and the data were expressed as mean ± standard deviation. Statistical analyses were carried out using SPSS version 19.0 (SPSS Inc., Armonk, NY, USA). One-way analysis of variance followed by Tukey’s multicomparison test was used for comparing the results among treatments. Differences were considered significant at p < 0.01. IC50 values were determined by plotting a percent of inhibition versus concentration curve for positive controls in α-amylase, α-glucosidase, and DPPH radical scavenging assays.
3. Results
The extraction yield from 11 aromatic plants by three solvents (57 extracts of n-hexane, ethyl acetate, and methanol) is represented in Table 2.
The present study was designed to investigate the bioactive properties of the aforementioned plants. These properties included DPPH radical scavenging activity, total phenolic contents, and also α-amylase and α-glucosidase inhibition activity. The results of these assays are shown in Table 2, in which the best are marked with asterisks (*).
The DPPH radical scavenging assay measures the reduction of DPPH radical by hydrogen-donating or electron-transferring antioxidants due to the formation of the non-radical form, DPPH-H. The extracts were tested at a concentration of 200 μg/mL. The results showed that ethyl acetate and methanol extracts of leaves of P. persica (96.0% and 95.9%, respectively), methanol extract of leaves of P. affinis (91.5%), methanol extracts of leaves and roots of P. heterochroma (95.5% and 95.1%, respectively) and ethyl acetate and methanol extracts of leaves and stems of P. boissieriana (84.5%, 94.3%, 94.0%, and 94.7%, respectively) exhibited strong antioxidant activities. Generally, ethyl acetate and methanol extracts of P. boissieriana and P. heterochroma had the best antioxidant activities (Table 2). BHT was used as standard antioxidant (99.6%).
According to the total phenolic assay, methanol extracts of P. persica, P. heterochroma, P. boissieriana, and S. excelsa were rich in phenolic compounds. It can be concluded that highly polar solvents are more effective in extracting phenolic compounds from plant materials than the less polar solvents, as has already been reported [48,49]. The best results are marked with asterisks (*) in Table 2.
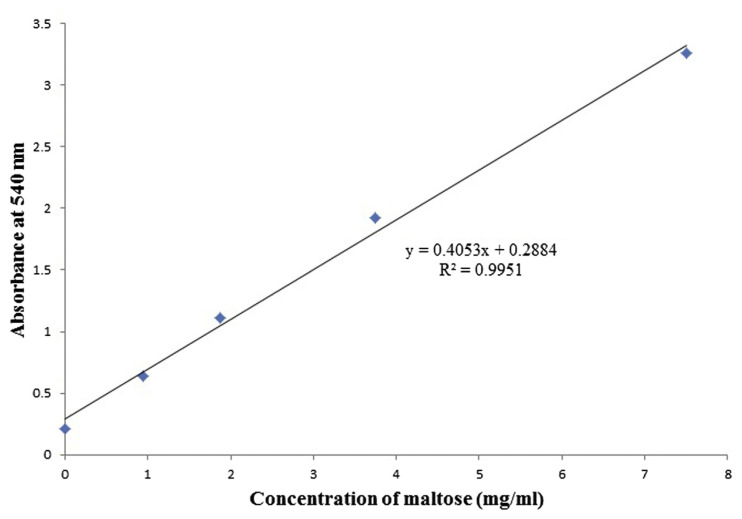
The maltose standard curve for α-amylase inhibitory assay was plotted using various concentrations of maltose (Fig. 1). The extracts were tested at a concentration of 238.1 μg/mL for inhibition of α-amylase. A significant inhibition was observed with n-hexane, ethyl acetate, and methanol extracts of leaves of S. excelsa (78.2%, 76.1%, and 98.5%, respectively). Also, ethyl acetate extract of stems of S. excelsa was found to have high inhibition activity (98.5%). n-Hexane extracts of P. affinis (leaves) and H. persicum (aerial parts) showed enzyme inhibition of 81.3% and 78.5%, respectively. Some of the plant extracts including n-hexane extract of P. heterochroma, ethyl acetate extract of B. hyrcana, and methanol extracts of A. paradoxum, C. persicus, and H. persicum showed negative values, which indicates that no inhibition occurred at 238.1 μg/mL. In this assay, the positive control, acarbose, showed a 74.9% inhibitory effect (Table 2).
Fig. 1.
Maltose standard curve for α-amylase inhibitory assay.
α-Glucosidase inhibitory activities of the plants were assessed using 3.3 μg/mL of the different extracts. High levels of α-glucosidase inhibition were observed in n-hexane, ethyl acetate, and methanol extracts of stems of P. boissieriana (99.2%, 93.4% and 99.1%, respectively). Also, ethyl acetate and methanol extracts of P. persica and P. heterochroma was found to have high inhibitory activity (99.3%, 99.6%, 61.8%, and 98.7%, respectively). The results were compared with those of acarbose (41.7%; Table 2).
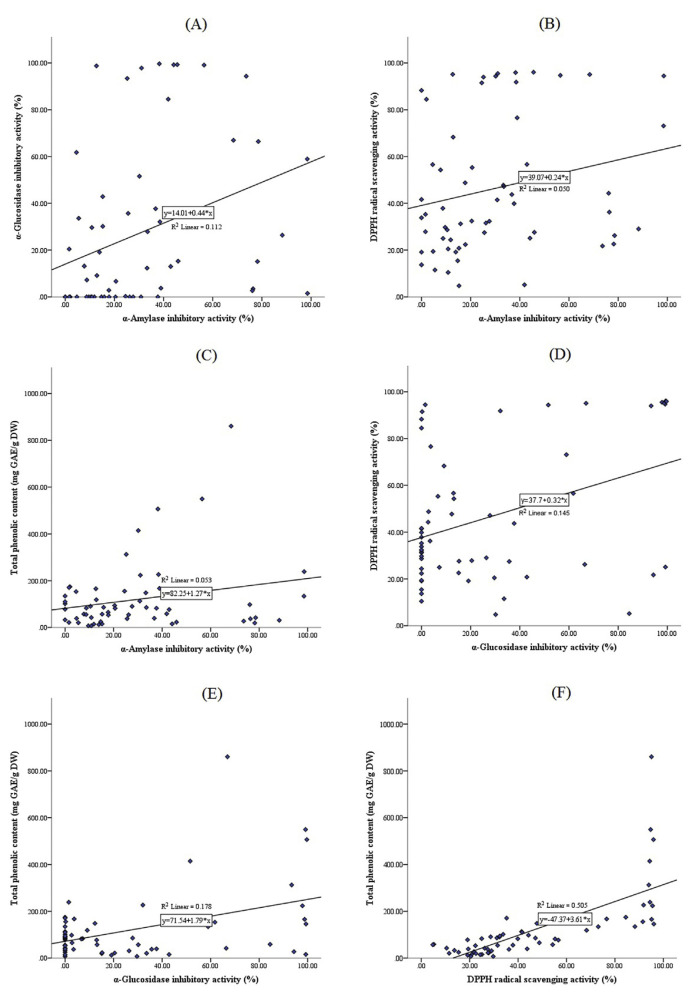
The statistical analysis showed significant correlation between α-amylase and α-glucosidase inhibitory activities (r = 0.335, p < 0.01) and also between DPPH scavenging activity and total phenolic contents (r = 0.711, p < 0.001) for all of the extracts. There is a good correlation between α-glucosidase inhibitory activity and DPPH scavenging activity (r = 0.381, p < 0.01), and also between α-glucosidase inhibitory activity and total phenolic contents (r = 0.422, p < 0.001). However, there were no significant correlations between α-amylase inhibitory activity with DPPH radical scavenging (r = 0.223, p < 0.1) and total phenolic contents (r = 0.229, p < 0.1) [50,51]. All the correlation curves are shown in Fig. 2.
Fig. 2.
Correlations between: (A) α-amylase and α-glucosidase inhibitory activities; (B) α-amylase inhibitory activity and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity; (C) α-amylase inhibitory activity and total phenolic contents; (D) α-glucosidase inhibitory activity and DPPH radical scavenging activity; (E) α-glucosidase inhibitory activity and total phenolic contents; and (F) DPPH radical scavenging activity and total phenolic contents.
The IC50 values of the most effective extracts in DPPH scavenging, α-amylase and α-glucosidase assays, are shown in Table 3. As indicated, all the plant extracts exhibited lower DPPH scavenging activity than BHT. Among them, methanol extract of C. persicus roots (IC50 = 38.9 μg/mL), methanol extract of P. heterochroma leaves, and ethyl acetate and methanol extracts of its roots (IC50 = 41.7 μg/mL, 37.9 μg/mL, and 30.1 μg/mL, respectively), and methanol extract of P. boissieriana stems (IC50 = 39.3 μg/mL) showed better antioxidant activities. All these extracts exhibited high concentration-dependent activity in the DPPH scavenging assay.
Table 3.
Concentrations that cause 50% inhibition (IC50) values of high-effective extracts in DPPH radical scavenging, and α-amylase and α-glucosidase inhibitory assays.
| Plant species | Plant part used | Extract | DPPH scavenging activity (IC50 μg/mL) | α-Amylase inhibition (IC50 μg/mL) | α-Glucosidase inhibition (IC50 μg/mL) |
|---|---|---|---|---|---|
| Alium paradoxum | Bulb | EA. | 61.3 ± 0.9 | > 238.1 | > 20.0 |
| Convolvulus persicus | Aerial parts | Met. | 94.9 ± 1.4 | > 238.1 | > 20.0 |
| Root | EA. | 52.7 ± 0.7 | > 238.1 | > 20.0 | |
| Met. | 38.9 ± 1.6 | > 238.1 | > 20.0 | ||
| Heracleum persicum | Aerial parts | Hex. | > 200.0 | 41.7 ± 3.4* | 5.2 ± 0.5* |
| EA. | 119.4 ± 6.2 | > 238.1 | > 20.0 | ||
| Root | Hex. | > 200.0 | 59.3 ± 2.9* | 2.9 ± 0.1* | |
| Pimpinella affinis | Leaf | Hex. | > 200.0 | 114.7 ± 8.9 | 12.9 ± 0.2 |
| Met. | 74.9 ± 1.9 | > 238.1 | > 20.0 | ||
| Root | EA. | > 200.0 | 104.5 ± 6.7 | > 20.0 | |
| Parrotia persica | Leaf | EA. | 66.0 ± 2.6 | > 238.1 | 8.4 ± 0.8 |
| Met. | 57.1 ± 3.0 | > 238.1 | 6.9 ± 0.5 | ||
| Primula heterochroma | Leaf | Met. | 41.7 ± 1.4 | > 238.1 | 8.1 ± 0.4 |
| Root | EA. | 37.9 ± 1.3 | > 238.1 | 5.9 ± 0.7 | |
| Met. | 30.1 ± 2.8 | > 238.1 | 6.7 ± 0.1 | ||
| Pyrus boissieriana | Leaf | EA. | 92.1 ± 3.1 | > 238.1 | > 20.0 |
| Met. | 47.2 ± 4.0 | > 238.1 | 4.7 ± 0.8* | ||
| Stem | Hex. | > 200.0 | > 238.1 | 3.2 ± 0.9* | |
| EA. | 47.8 ± 3.5 | > 238.1 | 2.3 ± 0.3* | ||
| Met. | 39.3 ± 1.3 | 186.9 ± 4.5 | 2.5 ± 0.6* | ||
| Smilax excelsa | Leaf | Hex. | > 200.0 | 99.3 ± 3.3 | 18.6 ± 1.9 |
| EA. | > 200.0 | 143.7 ± 5.8 | > 20.0 | ||
| Met. | > 200.0 | 89.4 ± 3.9 | > 20.0 | ||
| Stem | EA. | 119.6 ± 0.1 | 73.9 ± 3.4* | 3.9 ± 0.4* | |
| BHT | — | — | 16.7 ± 0.2 | — | — |
| Acarbose | — | — | — | 75.7 ± 2.4 | 6.1 ± 0.3 |
IC50 values lower than the standard positive control.
BHT = butylated hydroxytoluene; DPPH = 2,2-diphenyl-1-picrylhydrazyl; EA = ethyl acetate; Hex. = n-hexane; Met. = methanol.
As shown in Table 3, the n-hexane extracts of H. persicum aerial parts and roots and the ethyl acetate extract of S. excelsa stems exhibited significant inhibitory activities against α-amylase and α-glucosidase, even more effective than acarbose. Also, the methanol extract of P. boissieriana leaves and all the extracts of its stems inhibited α-glucosidase better than acarbose. The methanol extract of P. persica leaves and the ethyl acetate and methanol extract of P. heterochroma roots possess similar inhibitory activity against α-glucosidase to acarbose.
Generally, all these extracts exhibited moderate to high concentration-dependent response in α-amylase and α-glucosidase inhibitory assays.
4. Discussion
This study indicates that among the 11 plants studied, the extracts of H. persicum, S. excelsa, P. boissieriana, P. persica and P. heterochroma showed comparable activities against α-amylase and α-glucosidase to acarbose. In addition, C. persicus, P. boissieriana, and P. heterochroma showed significant antioxidant activities. Therefore, these four plants can be recommended as good natural sources for further investigations of antidiabetic drugs and antioxidants.
P. boissieriana and P. persica are wild trees in Hyrcania region. Native people do not use P. boissieriana as a medicinal plant, however, its antioxidant activity has been reported [24]. P. persica has been used as food coloring, food flavoring, and antifever [39,40]. Also, its antibacterial and antioxidant activities were measured. Easy access to these two plants marks them as good sources of antidiabetic natural products. P. heterochroma is a decorating plant in the northern part of Iran. Local people use leaves of S. excelsa as a food flavoring and some studies report its antioxidant and antimicrobial activities [42,43]. Now it can be regarded as a candidate for the control of diabetes mellitus. In the northern part of Iran, native people widely use leaves of P. affinis as a flavoring agent. Also, H. persicum is one of the most important plants in Iranian traditional medicine that has been used as an antiepilepic, carminative, antimicrobial, and pain killer plant [30–35]. A few studies have reported biological activities of H. persicum, such as antifungal, antimicrobial, and antioxidant [31,34,36,37]. Therefore, this paper can be a guideline for researchers in the field of pharmacology to make more investigations about these plants from other points of view. Also, the results can be useful for nutrition scientists.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;8:217–33. [Google Scholar]
- 2. Cupta VK, Sharma SK. Plants as natural antioxidants. Nat Prod Rad. 2006;5:326–34. [Google Scholar]
- 3. Shahidi F. Nutraceuticals, functional foods and dietary supplements in health and disease. J Food Drug Anal. 2012;20:226–30. [Google Scholar]
- 4. El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev. 2011;7:392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 5. Li G, He J, Zhang A, Wan Y, Wang B, Chen W. Toward potent α-glucosidase inhibitors based on xanthones: a closer look into the structure–activity correlations. Eur J Med Chem. 2011;46:4050–5. doi: 10.1016/j.ejmech.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6. Xie W, Tanabe G, Akaki J, Morikawa T, Ninomiya K, Yoshikawa M, Wu X, Muraoka O, Minematsu T. Isolation, structure identification and SAR studies on thiosugar sulfonium salts, neosalaprinol and neoponkoranol, as potent α-glucosidase inhibitors. Bioorg Med Chem. 2011;19:2015–22. doi: 10.1016/j.bmc.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 7. Hasbal G, Yilmaz-Ozden T, Can A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. J Food Drug Anal. 2014;23:57–62. doi: 10.1016/j.jfda.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asano N. Sugar-mimicking glycosidase inhibitors: bioactivity and application. Cell Mol Life Sci. 2009;66:1479–92. doi: 10.1007/s00018-008-8522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Standl E, Schnell O. Alpha-glucosidase inhibitors 2012—cardiovascular considerations and trial evaluation. Diab Vasc Dis Res. 2012;9:163–9. doi: 10.1177/1479164112441524. [DOI] [PubMed] [Google Scholar]
- 10. Jung M, Park M, Lee HC, Kang Y, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13:1203–18. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 11. Jin H, Zhang YJ, Jiang JX, Zhu LY, Chen P, Li J, Yao HY. Studies on the extraction of pumpkin components and their biological effects on blood glucose of diabetic mice. J Food Drug Anal. 2013;21:184–9. [Google Scholar]
- 12. Misbah H, Abdul Aziz A, Aminudin N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complem Altern Med. 2013;13:118. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 14. Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal. 2014;22:505–11. doi: 10.1016/j.jfda.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanbari F, Shataee S.ACRS, PS 2,38. Capability investigation of ASTER imagery for mixed Hardwood Forest types classification. Asian Conference on Remote Sensing; Colombo: Sri Lanka. November 2008; [Google Scholar]
- 16.Mozaffarian V. Identification of medicinal and aromatic plants of Iran. Iran: Farhang Moaser; 2012. [Google Scholar]
- 17. Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B. Antihemolytic and antioxidant activities of Allium paradoxum. Cent Eur J Bio. 2010;5:338–45. [PubMed] [Google Scholar]
- 18. Mohammadi-Motamed S, Naghibi F. Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem. 2010;119:1637–42. [Google Scholar]
- 19. Nabavi SF, Nabavi SM, Hajizadeh Moghaddam A, Naqinezhad A, Bigdellou R, Mohammadzadeh S. Protective effects of Allium paradoxum against gentamicin-induced nephrotoxicity in mice. Food Funct. 2012;3:28–9. doi: 10.1039/c1fo10173k. [DOI] [PubMed] [Google Scholar]
- 20. Esmaeili S, Naghibi F, Mosaddegh M, Sahranavard S, Ghafari S, Abdullah NR. Screening of antiplasmodial properties among some traditionally used Iranian plants. J Ethnopharmacol. 2009;121:400–4. doi: 10.1016/j.jep.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 21. Ata A, Van Den Bosch SA, Harwanik DJ, Pidwinski GE. Glutathione S-transferase- and acetylcholinesterase-inhibiting natural products from medicinally important plants. Pure Appl Chem. 2007;79:2269–76. [Google Scholar]
- 22. Babar ZU, Ata A, Meshkatalsadat MH. New bioactive steroidal alkaloids from Buxus hyrcana. Steroids. 2006;71:1045–51. doi: 10.1016/j.steroids.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed Mesaik M, Halim SA, Ul-Haq Z, Iqbal Choudhary M, Shahnaz S, Ayatollahi SAM, Murad S, Ahmad A. Immunosuppressive activity of buxidin and E-buxenone from Buxus hyrcana. Chem Biol Drug Des. 2010;75:310–7. doi: 10.1111/j.1747-0285.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 24. Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Pourmorad F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. Afr J Biotechnol. 2010;9:5212–7. [Google Scholar]
- 25. Ata A, Iverson CD, Kalhari KS, Akhter S, Betteridge J, Meshkatalsadat MH, Orhan I, Sener B. Triterpenoidal alkaloids from Buxus hyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry. 2010;71:1780–6. doi: 10.1016/j.phytochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 26. Khoshbakht K, Hammer K, Pistrick K. Eryngium caucasicum Trautv. cultivated as a vegetable in the Elburz Mountains (Northern Iran) Genet Resour Crop Ev. 2007;54:445–8. [Google Scholar]
- 27. Hashemabadi D, Kaviani B. Seasonal and geographical variations in the essential oils of Eryngium caucasicum Trautv. growing in Iran. Am Eurasian J Agric Environ Sci. 2012;8:212–5. [Google Scholar]
- 28. Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Jafari M. Free radical scavenging activity and antioxidant capacity of Eryngium caucasicum Trautv and Froripia subpinnata. Pharmacologyonline. 2008;3:19–25. [Google Scholar]
- 29. Sefidkon F, Dabiri M, Mohammad N. Analysis of the oil of Heracleum persicum L. (seeds and stems) J Essent Oil Res. 2004;16:296–8. [Google Scholar]
- 30. Firuzi O, Asadollahi M, Gholami M, Javidnia K. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 2010;122:117–22. [Google Scholar]
- 31. Naeini A, Khosravi AR, Chitsaz M, Shokri H, Kamlnejad M. Anti-Candida albicans activity of some Iranian plants used in traditional medicine. J Mycol Med. 2009;19:168–72. [Google Scholar]
- 32. Sayyah M, Moaied S, Kamalinejad M. Anticonvulsant activity of Heracleum persicum seed. J Ethnopharmacol. 2005;98:209–11. doi: 10.1016/j.jep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 33. Dehghan-Noudeh G, Sharififar F, Dehghan-Noodeh A, Moshafi MH, Ahmadi-Afzadi M, Behravan E, Aref M, Sakhtianchi R. Antitumor and antibacterial activity of four fractions from Heracleum persicum Desf. and Cinnamomum zeylanicum Blume. J Med Plants Res. 2010;4:2176–80. [Google Scholar]
- 34. Shokri H, Sharifzadeh A, Ashrafi-Tamai I. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J Mycol Med. 2012;22:211–6. doi: 10.1016/j.mycmed.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 35. Hajhashemi V, Sajjadi SE, Heshmati M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J Ethnopharmacol. 2009;124:475–80. doi: 10.1016/j.jep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 36. Souri E, Farsam H, Sarkheil P, Ebadi F. Antioxidant activity of some furanocoumarins isolated from Heracleum persicum. Pharm Biol. 2004;42:396–9. [Google Scholar]
- 37. Çoruh N, Celep AGS, Özgökçe F. Antioxidant properties of Prangos ferulacea (L.) Lindl, Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007;100:1237–42. [Google Scholar]
- 38. Moshafi MH, Sharififar F, Dehghan G, Ameri A. Bioassay screening of the essential oil and various extracts of fruits of Heracleum persicum Desf. and rhizomes of Zingiber officinale Rosc. using brine shrimp cytotoxicity assay. Iran J Pharm Res. 2009;8:59–63. [Google Scholar]
- 39. Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR. Determination of antioxidant activity, phenol and flavonoid content of Parrotia persica Mey. Pharmacologyonline. 2008;2:560–7. [Google Scholar]
- 40. Ahanjan M, Mohana DC, Raveesha KA, Azadbakht M. Antibacterial potential of extracts of leaves of Parrotia persica. Afr J Biotechnol. 2007;22:2526–8. [Google Scholar]
- 41. Alinezhad H, Zare M, Nabavi SF, Naqinezhad A, Nabavi SM. Assessing the protective effect of Primula heterochroma Stapf. extracts against sodium fluoride-induced hemolysis in rat erythrocytes. Fluoride. 2011;44:238–42. [Google Scholar]
- 42. Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008;110:571–83. [Google Scholar]
- 43. Ivanova A, Mikhova B, Kostova I, Evstatieva L. Bioactive chemical constituents from Smilax excelsa. Chem Nat Compd. 2010;46:295–7. [Google Scholar]
- 44. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 45. Nithiyanantham S, Varadharajan S, Siddhuraju P. Differential effects of processing methods on total phenolic content, antioxidant and antimicrobial activities of three species of Solanum. J Food Drug Anal. 2012;20:844–54. [Google Scholar]
- 46. Akkarachiyasit S, Yibchok-Anun S, Wacharasindhu S, Adisakwattana S. In vitro inhibitory effects of cyandin-3-rutinoside on pancreatic α-Amylase and its combined effect with acarbose. Molecules. 2011;16:2075–83. doi: 10.3390/molecules16032075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hwang IG, Kim HY, Woo KS, Hong JT, Hwang BY, Lee J, Jeong HS, Jung JK. Isolation and characterisation of an α-glucosidase inhibitory substance from fructose–tyrosine Maillard reaction products. Food Chem. 2011;127:122–6. [Google Scholar]
- 48. Fatin RJ, Wahab R, Daud JM, Sudin M, Rasat MS, Sulaiman O. Study on methanol extracts of Nauclea subdita (Korth) Steud. heartwood parts for the total phenolic contents and free radical scavenging activities. Curr Res J Biol Sci. 2012;4:600–7. [Google Scholar]
- 49. Hatipoĝlu G, Sökmen M, Bektaş E, Daferera D, Sökmen A, Demir E, Şahin H. Automated and standard extraction of antioxidant phenolic compounds of Hyssopus officinalis L. ssp. Angustifolius. Ind Crop Prod. 2013;43:427–33. [Google Scholar]
- 50. Firuzi O, Miri R, Asadollahi M, Eslami S, Jassbi AR. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran J Pharm Res. 2013;12:801–10. [PMC free article] [PubMed] [Google Scholar]
- 51. Andrade-Cetto A, Becerra-Jimenez J, Cardenas-Vazquez R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32. doi: 10.1016/j.jep.2007.10.031. [DOI] [PubMed] [Google Scholar]