Abstract
The contents of lead, cadmium, chromium, copper, and nickel were determined in 25 tea samples from China, including green, yellow, white, oolong, black, Pu’er, and jasmine tea products, using high-resolution continuum source graphite furnace atomic absorption spectrometry. The methods used for sample preparation, digestion, and quantificational analysis were established, generating satisfactory analytical precisions (represented by relative standard deviations ranging from 0.6% to 2.5%) and recoveries (98.91–101.32%). The lead contents in tea leaves were 0.48–10.57 mg/kg, and 80% of these values were below the maximum values stated by the guidelines in China. The contents of cadmium and chromium ranged from 0.01 mg/kg to 0.39 mg/kg and from 0.27 mg/kg to 2.45 mg/kg, respectively, remaining in compliance with the limits stipulated by China’s Ministry of Agriculture. The copper contents were 7.73–63.71 mg/kg; only 64% of these values complied with the standards stipulated by the Ministry of Agriculture. The nickel contents ranged from 2.70 mg/kg to 13.41 mg/kg. Consequently, more attention must be paid to the risks of heavy metal contamination in tea. The quantitative method established in this work lays a foundation for preventing heavy metal toxicity in human from drinking tea and will help establish regulations to control the contents of heavy metals in tea.
Keywords: Chinese tea, continuum source graphite furnace atomic absorption spectrometry, heavy metals, quantitative analysis
1. Introduction
Tea is one of the most widely consumed beverages in the world and is produced from the dried leaves of Camellia sinensis. Drinking tea might help reduce serum cholesterol, provide antiaging activities, and decrease the risks of both cardiovascular disease and cancer [1]. However, heavy metal contaminants might accumulate during tea growth, transportation, packaging, and processing. Heavy metals are harmful to human health. Lead (Pb), for instance, has carcinogenic properties, and it impairs both the respiratory and digestive systems and suppresses the immune system; this metal is particularly harmful in children, damaging their intelligence and nervous systems [2–5]. Cadmium (Cd) accumulates easily in the circulatory system, kidney (especially the renal cortex), lung, and heart, and is toxic to bones and gonads. These risks are recognized by the International Agency for Research on Cancer and the National Toxicology Program, and Cd has been classified as a Group 1 carcinogen [5–12]. Chromium (Cr) can exist in several oxidation states. Hexavalent chromium (VI) is highly soluble and mobile and is harmful to the skin, liver, kidney, and respiratory organs [13], causing various diseases, such as dermatitis, renal tubular necrosis, perforation of the nasal septum, and lung cancer [14]. Cr is also a Category 1 carcinogen according to the International Agency for Research on Cancer and the National Toxicology Program [11,12]. Excessive copper (Cu) intake can cause nausea, vomiting, kidney failure, blood cell damage, and central nervous system inhibition [15]. Nickel (Ni) is primarily accumulated in the spinal cord, brain, and organs due to its mutability and carcinogenicity [16]. Ni is also a Category 1 carcinogen [11,12].
Numerous techniques have been employed to analyze the heavy metal contents in tea, including flame atomic absorption spectrometry (FAAS) [17,18], graphite furnace atomic absorption spectrometry (GFAAS) [19,20], inductively coupled plasma atomic emission spectroscopy (ICP-AES) [21], and inductively coupled plasma mass spectrometry (ICP-MS) [22]. GFAAS is a useful tool for studying trace metals because it is highly accurate, sensitive, and selective. Ning et al [20] investigated the Pb, Cd, and Cu contents in 30 brands of Chinese Pu’er tea using GFAAS and observed that the Cu content (12.22–22.22 mg/kg) was the highest. Khakhathi et al [23] quantitatively determined the content of Cr(VI) in tea leaves and infusions of black, green, and herbal teas using GFAAS, and found that black tea contained more total Cr content than green and herbal teas.
High-resolution continuum source atomic absorption spectrometry (HRCS-AAS) achieves its high resolution using a double monochromator with a prism for predispersion and an echelle grating for highest resolution [24]. HRCS-AAS analysis employs only one light source (a xenon lamp) for all elements and all available wavelengths instead of several dozen of hollow cathode lamps used for conventional line source AAS. It has been used extensively to analyze trace heavy metals in foods, beverages, and other samples owing to its high sensitivity, good accuracy, and wide applicability. For example, Boschetti et al [25] quantified 10 elements in Brazilian red wines using HRCS-FAAS; the limit of detection (LOD) ranged from 0.005 mg/L to 4.4 mg/L. Previously, we determined the Pb content in human hair using the direct solid sampling HRCS-GFAAS [26] and found that the Pb levels increased from the root to the tip of the hair strands. The LOD and recovery of this method were 0.82 ng/g and 98.7–103.1%, respectively. Sun et al [27] determined the Pb, Cd, Cu, and Ni contents of the Tonghui River in Beijing, China, using cloud point extraction combined with HRCS-AAS and suggested that the concentration of heavy metals in the water might originate from the industrial factories near the river. In this study, the contents of Pb, Cd, Cr, Cu, and Ni in green, white, yellow, black, oolong, Pu’er, and jasmine tea were determined using HRCS-AAS.
2. Materials and methods
2.1. Instruments and reagents
A ContrAA 700 (Analytik Jena AG, Jena, Germany) high-resolution continuum source atomic absorption spectrometer equipped with a short-arc xenon lamp, a high-resolution double echelle grating monochromator, and a charge-coupled device array detector was used. An MPE-60 autosampler for liquid samples was installed on the GFAAS. High purity argon (99.999%) was supplied as the protective and purge gas. The method was optimized based on the proper atomic lines for Pb (283.306 nm), Cd (228.802 nm), Cr (357.869 nm), Cu (324.754 nm), and Ni (232.003 nm). A BT-214D electronic balance (Sartorius, Göttingen, Germany) was used to weigh the samples. An ETHOS A Microwave Digestion System (Milestone, Milano, Italy) was used for the samples digestion.
The certified reference material for Green Tea (GBW 10052) and the standard solutions (100 mg/L Pb, 1000 mg/L Cd, 1000 mg/L Cr, 1000 mg/L Cu, and 1000 mg/L Ni) were purchased from the National Research Center for Certified Reference Materials of China (Beijing, China). The working standard solutions were prepared daily through a stepwise dilution of the standard stock solutions using 0.5% (v/v) nitric acid (HNO3), ammonium phosphate monobasic (NH4H2PO4), and magnesium nitrate [Mg(NO3)2] (Sigma-Aldrich, St. Louis, MO, USA) were used as chemical modifiers for the determination of the Pb, Cd, Cr, Cu, and Ni levels. The reagents were of analytical grade, and all solutions were prepared using deionized water (18.2 MΩ/cm) produced using a PureLab Prime system (PALL, Washington, NY, USA). All containers and glassware were cleaned by soaking in the 5 mol/L HNO3 for at least 24 hours and rinsed three times with deionized water prior to use.
2.2. Sample preparation
After drying to a constant weight, the tea samples were ground into powder. Approximately 0.25 g of dried tea powder was weighed and added into the polytetrafluoroethylene digestion vessel with 7 mL of concentrated HNO3 and 1 mL of hydrogen peroxide (H2O2). Subsequently, the samples weredigested using a two-step temperature program. During the first step, the temperature was linearly increased to 190°C over 10 minutes; the maximum power of the rotating magnetron was 1000 W. During the second step, the temperature was maintained at 190°C for 30 minutes. After digestion and cooling, each solution was evaporated to ~2 mL and diluted with deionized water in a 50 mL volumetric flask for the GFAAS analysis. The results were reported as the average of three repeated measurements, and all digestions were conducted in triplicate.
2.3. Analysis conditions
The analytical working solutions included the following: 0 μg/L, 10.0 μg/L, 20.0 μg/L, 40.0 μg/L, 60.0 μg/L, and 80.0 μg/L for Pb and Ni; 0 μg/L, 0.2 μg/L, 0.4 μg/L, 0.6 μg/L, 0.8 μg/L, and 1.0 μg/L for Cd; 0 μg/L, 4.0 μg/L, 8.0 μg/L, 12.0 μg/L, 16.0 μg/L, and 20.0 μg/L for Cr; and 0 μg/L, 40.0 μg/L, 80.0 μg/L, 120.0 μg/L, 160.0 μg/L, and 200.0 μg/L for Cu. These solutions were prepared daily using appropriately diluted dilutions of the stock standard solutions. Next, 20 μL of the sample solution or the standard solution was transferred to GFAAS together with the modifiers. The conditions and temperature programs for determining the Pb, Cd, Cr, Cu, and Ni levels are shown in Table 1.
Table 1.
Heating program for the graphite furnace atomic absorption spectrometry analysis of lead, cadmium, chromium, copper, and nickel.
| Element | Chemical modifier | Drying (°C) | Pyrolysis (°C) | Atomization (°C) | Clean (°C) |
|---|---|---|---|---|---|
| Pb | 10 g/L NH4H2PO4 | 110 | 900 | 1500 | 2500 |
| Cd | 10 g/L NH4H2PO4 | 110 | 650 | 1400 | 2500 |
| Cr | 1 g/L Mg(NO3)2 | 110 | 1400 | 2300 | 2500 |
| Cu | 0.5 g/L Mg(NO3)2 | 110 | 1100 | 2000 | 2500 |
| Ni | 0.5 g/L Mg(NO3)2 | 110 | 1200 | 2300 | 2500 |
Cd = cadmium; Cr = chromium; Cu = copper; NH4H2PO4 = ammonium phosphate monobasic; Mg(NO3)2 = magnesium nitrate; Ni = nickel; Pb = lead.
3. Results and discussion
3.1. Conditions of microwave digestion
Wet digestion and microwave digestion are commonly used for sample digestion when analyzing trace metals with AAS. The latter was chosen for this study because of its advantages including less sample pollution, limited analyte evaporation, lower acid consumption, shorter digestion time, and significant blank value reduction [28]. The tea samples were mixed with the digestion agents (HNO3 and H2O2) and sealed under high pressure. In the acidic digestion mixture, H2O2 decomposed to form high-energy reactive oxygen, and the HNO3 degraded to form catalytically active NO2. Reactive oxygen and NO2 accelerate the oxidation process and improve the sample digestion. In this work, a mixture of HNO3–H2O2 (volume ratio 7:1) was used to digest the powdered tea samples to colorless and transparent liquid.
3.2. Optimization of temperature program
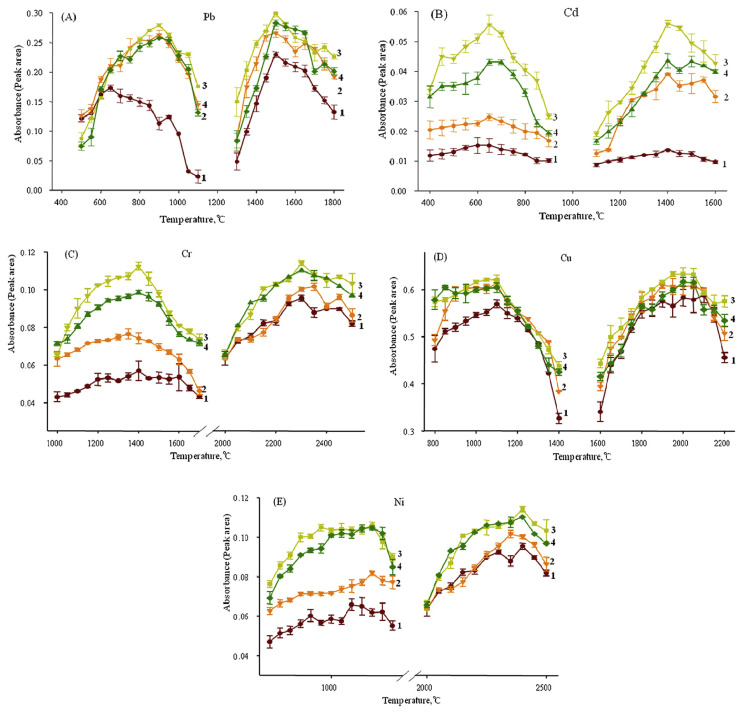
The temperature program of the GFAAS analysis included four steps, including drying, pyrolysis, atomizing, and clean. Using a ramped temperature program including slow heating for drying might prevent the loss of analytes. Pyrolysis removed all or most of the coexisting elements while minimizing analyte loss. Excessively high pyrolysis temperature may cause analyte loss, sensitivity reduction, and poor repeatability, while low pyrolysis temperature will generate high background absorption values [29]. During atomization, the target elements were turned into atomic vapor at the ground state. Appropriate atomization temperatures completely evaporate the analytes without leaving residue, and prolong the life of the graphite tubes. In this work, the pyrolysis and atomization temperatures were optimized for the five heavy metals. The pyrolysis and atomization temperature curves are shown in Fig. 1. For Pb, Cr, Cd, Cu, and Ni, the optimal pyrolysis temperatures were 900°C, 650°C, 1400°C, 1100°C, and 1200°C, respectively; and the optimal atomization temperatures were 1500°C, 1400°C, 2300°C, 2000°C, and 2300°C, respectively.
Fig. 1.
Pyrolysis and atomization temperature curves for lead, cadmium, chromium, copper, and nickel. 1, no matrix modifier; 2, 3, and 4: with 2 μL, 5 μL, and 10 μL of modifier, respectively. (10 g/L NH4H2PO4 solution for Pb and Cd; 1 g/L Mg(NO3)2 solution for Cr; 0.5 g/L Mg(NO3)2 solution for Cu and Ni). Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
Matrix modifiers are widely employed in heavy metal analysis with GFAAS because they can improve the thermal stability of the analytes and reduce the matrix interference by facilitating the volatilization of the sample matrix in the graphite tube [30]. A suitable matrix modifier is necessary for applying high pyrolysis temperatures to eliminate the matrix effects before atomization, as well as reduce interference and background signal [30]. NH4H2PO4 and Mg(NO3)2 are frequently used as chemical modifiers for determining heavy metals in food, and biological and environmental samples using GFAAS [31–35]. A method for measuring Cr(VI) and Cr(III) contents in water samples was developed using electrothermal atomic absorption spectroscopy (ETAAS) with Mg(NO3)2 as a modifier by de Blas et al [36]. Kim [33] determined Cd and Pb in wines using GFAAS with NH4H2PO4 and Mg(NO3)2 as the matrix modifiers. Vinas et al [32] used NH4H2PO4 as a matrix modifier when determining the Cd and Pb contents in food colorants. Zacharia et al [37] reported the determination of Pb in wine by ETAAS using palladium (II) nitrate and Mg(NO3)2 as the matrix modifiers. Acar [38] measured the level of Cd, Pb, Cu, iron, and zinc in Turkish dietary vegetable oils and olives using ETAAS and FAAS with a Sc+Ir+NH4H2PO4 mixture as the modifier. de Jesus et al [39] determined the Pb contents in vegetable-based foods by ETAAS using 5 μg of NH4H2PO4 as a chemical modifier. A report also showed that 5 μg of Pd (applied as nitrate) with 3 μg Mg(NO3)2 was the most efficient modifier while determining the Cd, Cr, Cu, and Pb contents in honey using GFAAS [40]. In our previous study [26], NH4H2PO4 was used as a modifier for determining Pb levels in human hair. In this work, a 10 g/L NH4H2PO4 solution was used as a modifier for the analysis of Pb and Cd; a 1 g/L Mg(NO3)2 solution was used for Cr, and a 0.5 g/L Mg(NO3)2 solution was used for Cu and Ni. After adding the modifiers, the pyrolysis temperatures were 900°C, 650°C, 1400°C, 1100°C, and 1200°C for Pb, Cd, Cr, Cu, and Ni; the atomization temperatures were 1500°C, 1400°C, 2300°C, 2000°C, and 2300°C, respectively (see Table 1). Fig. 1 shows the pyrolysis and atomization temperature curves of the five heavy metals after adding varying amounts of the appropriate modifier. The absorbance values observed after adding the modifiers were much higher compared with those samples without modifiers. The results indicated that the optimal amount of modifier was 5 μL for the five heavy metals.
3.3. Method validation
The freshly prepared working solutions (80 μg/L Pb, 1.0 μg/L Cd, 20 μg/L Cr 200 μg/L Cu, and 80 μg/L Ni) were diluted with 0.5% (v/v) HNO3 solution to obtain a series of working solutions for plotting the calibration curves: 10 μg/L, 20 μg/L, 40 μg/L, 60 μg/L, and 80 μg/L for Pb and Ni, 0.2 μg/L, 0.4 μg/L, 0.6 μg/L, 0.8 μg/L, and 1.0 μg/L for Cd, 4 μg/L, 8 μg/L, 12 μg/L, 16 μg/L, and 20 μg/L for Cr, and 40 μg/L, 80 μg/L, 120 μg/L, 160 μg/L, and 200 μg/L for Cu. The linearity of the calibration curves was evaluated based on the correlation coefficient (R2). Table 2 lists the linear and nonlinear calibration curves that were automatically generated using the Aspect CS software (Aspect CS1.5.6, Analytic Jena AG, Jena, Germany). Obviously, the R2 values of the nonlinear curves are higher than those of the linear curves. Therefore, nonlinear calibration curves (see Fig. 2) with R2 > 0.998 were selected for quantifying the Pb, Cd, Cr, Cu, and Ni in tea.
Table 2.
Equations and correlation coefficients for the standard curves of lead, cadmium, chromium, copper, and nickel.
| Element | Equation | Fitting method | Correlation coefficient |
|---|---|---|---|
| Pb | Y = (0.0173107 + 0.0062618x)/(1 + 0.0049140x) | Nonlinear | 0.9992 |
| Y = 0.0315368 + 0.0044330x | Linear | 0.9861 | |
| Cd | Y = (0.0016850 + 0.0309467x)/(1 + 0.0282792x) | Nonlinear | 0.9981 |
| Y = 0.0100180 + 0.0240163x | Linear | 0.9911 | |
| Cr | Y = (0.0072104 + 0.0072150x)/(1 – 0.0028717x) | Nonlinear | 0.9994 |
| Y = 0.0063864 + 0.0077033x | Linear | 0.9987 | |
| Cu | Y = (0.0069972 + 0.0020622x)/(1 – 0.0006243x) | Nonlinear | 0.9997 |
| Y = 0.0037828 + 0.0023314x | Linear | 0.9964 | |
| Ni | Y = (0.0017610 + 0.0034749x)/(1 + 0.0024445x) | Nonlinear | 0.9999 |
| Y = 0.0069248 + 0.0029018x | Linear | 0.9960 |
Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
Fig. 2.
The nonlinear standard curves for lead, cadmium, chromium, copper, and nickel. Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
To evaluate the influence of the entire procedure on the measured results, the recoveries of the five heavy metals were studied. Four quality control samples were prepared by adding various amounts (0 μL, 10 μL, 20 μL, and 30 μL) of the standard solution mixtures (containing 100 mg/L Pb, 1 mg/L Cd, 10 mg/L Cr, 100 mg/L Cu, and 50 mg/L Ni) to quadruplication of tea sample 12 (0.25 g for each), respectively. The concentrations of the five heavy metals in QC samples were analyzed six times to determine the relative standard deviation and the recovery of the method. The recovery was calculated as shown in formula (1). As listed in Table 3, the recoveries ranged from 97.2% to 101.1% for Pb, from 97.7% to 99.0% for Cd, from 98.4% to 102.3% for Cr, from 98.7% to 104.1% for Cu, and from 96.7% to 98.2% for Ni. The precision represented by relative standard deviation ranged from 1.7% to 6.5%.
Table 3.
Repeatability of the lead, cadmium, chromium, copper, and nickel determinations.
| Element | Addeda (μg/L) | Determined (μg/L) | Recovery (%) | RSDb (%) |
|---|---|---|---|---|
| Pb | 0 | 8.34 | — | 4.3 |
| 20 | 28.14 | 99.3 | 3.2 | |
| 40 | 46.99 | 97.2 | 1.7 | |
| 60 | 69.09 | 101.1 | 2.9 | |
| Cd | 0 | 0.16 | — | 6.5 |
| 0.2 | 0.35 | 98.4 | 5.7 | |
| 0.4 | 0.55 | 99.0 | 1.8 | |
| 0.6 | 0.74 | 97.7 | 4.4 | |
| Cr | 0 | 6.57 | — | 4.8 |
| 2 | 8.77 | 102.3 | 3.7 | |
| 4 | 10.40 | 98.4 | 2.6 | |
| 6 | 12.46 | 99.1 | 3.4 | |
| Cu | 0 | 77.93 | — | 3.6 |
| 20 | 101.95 | 104.1 | 6.2 | |
| 40 | 119.70 | 101.5 | 4.4 | |
| 60 | 136.14 | 98.7 | 2.4 | |
| Ni | 0 | 23.95 | — | 5.2 |
| 10 | 32.83 | 96.7 | 4.2 | |
| 20 | 42.85 | 97.5 | 3.7 | |
| 30 | 52.98 | 98.2 | 2.6 |
Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
Four quality control samples were prepared by adding various amounts (0 μL, 10 μL, 20 μL, and 30 μL) of the standard solution mixtures (containing 100 mg/L Pb, 1 mg/L Cd, 10 mg/L Cr, 100 mg/L Cu and 50 mg/L Ni) to quadruplication of tea sample 12 (0.25 g for each), respectively.
RSD: relative standard deviation.
| (1) |
To validate the accuracy of this method, the contents of the five heavy metals in a certified reference material of a green tea sample were determined using the same method as the tea samples. As shown in Table 4, the determined concentrations were in good agreement with the certified values. The recovery, precision, and accuracy indicate that the quantitative methods employed in this work are appropriate for measuring the trace amounts of heavy metal in tea.
Table 4.
Recovery and RSD for the determinations of lead, cadmium, chromium, copper, and nickel (n = 4).
| Reference material | Elements | Certified value (mg/kg) | Determined (mg/kg) |
|---|---|---|---|
| GBW 10052 green tea | Pb | 1.6 ± 0.2 | 1.61 ± 0.13 |
| Cd | 0.076 ± 0.004 | 0.077 ± 0.004 | |
| Cr | 0.92 ± 0.20 | 0.91 ± 0.01 | |
| Cu | 24 ± 1 | 23.83 ± 0.56 | |
| Ni | 5.4 ± 0.4 | 5.36 ± 0.10 |
Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead; RSD = relative standard deviation.
The LODs were calculated as three times the standard deviation of the reagent blank readings. The standard deviation was obtained from 20 analyses of the blank, which only contained 0.5% (v/v) HNO3 and modifiers, using the same temperature program as that used for the tea samples. The LODs were 1.04 μg/L for Pb, 0.098 μg/L for Cd, 1.24 μg/L for Cr, 3.44 μg/L for Cu, and 1.76 μg/L for Ni. The sensitivity of the method utilized in this work was higher than those achieved by FAAS or ICP-AES in previous studies. Salahinejad and Aflaki [41] determined the contents of some toxic and essential mineral elements in black tea leaves and infusions obtained from markets in Iran using ICP-AES, and the LOD ranged from 2 μg/L to 50 μg/L. Narin et al [42] applied FAAS to determine the Pb, Cd, Cr, Cu, and Ni contents in black tea produced in Turkey, and the detection limits were 100 μg/L, 50 μg/L, 110 μg/L, 60 μg/L, and 110 μg/L, respectively. The LOD of the method obtained in this work was comparable to those achieved in previous study using ICP-MS. Ahmed et al [43] determined the level of Pb in green tea using ICP-MS, and the LOD was 0.3 μg/L.
3.4. Application of the proposed method
Tea samples were analyzed using the proposed HRCS-GFAAS method. The contents of the five heavy metals in the tea samples are listed in Table S1 and graphically summarized in Fig. 3. Within the overall distribution of the five heavy metals in the 25 samples, Cu had the highest content, while Cd had the lowest. The average levels of Cu, Ni, Pb, Cr, and Cd were 27.53 mg/kg, 7.55 mg/kg, 3.04 mg/kg, 0.98 mg/kg, and 0.08 mg/kg, respectively. The highest total contents of the five heavy metals were 76.47 mg/kg found in green tea, 25.98 mg/kg in oolong tea, 44.98 mg/kg in black tea, 43.35 mg/kg in Pu’er tea, and 40.82 mg/kg in jasmine tea. The total contents of heavy metals in the green tea samples were higher than those in the other types of tea samples on average.
Fig. 3.
Lead, cadmium, chromium, copper, and nickel contents in tea samples determined with high-resolution continuum source-graphite furnace atomic absorption spectrometry. Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
The contents of Cu were 17.01–63.07 mg/kg in green tea, 7.73–20.49 mg/kg in oolong tea, 22.61–33.45 mg/kg in black tea, 21.06–31.35 mg/kg in Pu’er tea, and 19.21–30.35 mg/kg in jasmine tea. The lowest Cu content was found in oolong tea (sample Tieguanyin A), while the highest content was found in green tea (sample Maojian B). As shown in Fig. 4, green tea had a higher Cu level than the other types of tea, and oolong tea had the lowest Cu level on average. Table S2 compares the heavy metal contents in tea from different parts of the world. The Cu levels in the green tea from India and Japan ranged from 23.1 mg/kg to 36.5 mg/kg [44] and from Turkey Cu levels were reported to range from 6.39 mg/kg to 9.84 mg/kg [45] (see Table S2). These were much lower than the levels detected in this study. Han et al [46] reported that the levels of Cu in green, oolong, black, and other teas from China ranged from 2.06 mg/kg to 239.02 mg/kg, from 3.44 mg/kg to 29.82 mg/kg, from 2.04 mg/kg to 447.50 mg/kg, and from 4.49 mg/kg to 38.07 mg/kg, respectively. In this work, the level of Cu in white tea is 19.59 mg/kg, which is consistent with the literature values reported for 28 white tea samples from China and Japan (10–26 mg/kg) [47]. Although Cu is an essential element for human health, excessive intake can impair organs and systems in the human body, possibly causing serious symptoms, including nausea, vomiting, hemolytic jaundice, kidney failure, and central nervous system depression [15]. Therefore, the maximal content of Cu in tea is limited in some regulations. According to the regulations imposed upon tea in Japan (100 mg/kg) and the United States (150 mg/kg) [20], the determined levels of Cu in the tea samples were all below the limits. However, according to the Industrial Standard (NY/T 288-2012) of China’s Ministry of Agriculture (MOA) [48], the Cu content is limited to 30 mg/kg. The Cu contents in nine samples exceeded the statutory limit, including five green tea samples, one yellow tea sample, one black tea sample, one Pu’er tea sample, and one jasmine tea sample. The Cu contamination in these tea samples may be attributed to the excessive application of the Cu-bearing Bordeaux mixture to prevent plant diseases and the copper tools used during the manufacturing process [49].
Fig. 4.
Distribution of the lead, cadmium, chromium, copper, and nickel contents in the seven types of tea. Cd = cadmium; Cr = chromium; Cu = copper; Ni = nickel; Pb = lead.
Ni is nutritionally essential as a trace element for several animal species, microorganisms, and plants [50]. However, excessive Ni intake by consuming tea with Ni contents above a certain threshold is harmful to humans [51–53]. An important adverse health effect of Ni is a skin disorder known as “nickel-eczema” [54]. As shown in Fig. 4, white tea contained the highest level of Ni (13.41 mg/kg on average), followed by yellow tea (12.59 mg/kg on average). Sample 13 (Jinjunmei) in black tea had the lowest Ni level (2.70 mg/kg), and all oolong tea samples also had relatively low Ni levels (3.87 mg/kg on average). Nookabkaew et al [22] reported that the Ni content in black tea from Thailand ranged from 2.28 mg/kg to 9.19 mg/kg. Seenivasan et al [55] measured the Ni content in black tea from India (0.4–9.2 mg/kg), finding values similar to those (2.70–9.31 mg/kg) in the black tea in this work, while still higher than the black tea samples from Turkey (3.52–5.70 mg/kg) determined by Soylak et al [45]. The Ni levels in the oolong tea from Indonesia and China were 1.88–3.33 mg/kg [44], which were much lower than those determined in this work. The Ni in tea plants mainly originates from foliar and soil applications of low quality fertilizers and micro nutrients [56]. However, there are not enough regulations for the Ni levels in tea.
The Pb levels ranged from 0.48 mg/kg to 10.57 mg/kg and averaged 3.04 mg/kg. The lowest Pb content was found in jasmine tea (0.48 mg/kg), while the highest was found in green tea (10.57 mg/kg). As shown in Fig. 4, the average Pb content in the green tea (4.75 mg/kg) was approximately nine times that of the white tea (0.51 mg/kg on average) and twice that of the oolong tea (2.45 mg/kg on average). The Pb contents obtained in this study were significantly higher than those determined by Al-Othman et al [18], which ranged from 3.9 mg/kg to 8.7 mg/kg in the tea samples from Jazan and Jeddah, Saudi Arabia. Moreover, as listed in Tables S1 and S2, the Pb contents in green tea from Japan and Serbia were reported as 0.11–1.93 mg/kg and 0.350–3.53 mg/kg, respectively, which were also lower than those determined in this study [57–59]. The Pb contents in the black teas ranged from 1.88 mg/kg to 5.63 mg/kg, similar to those in black teas (1.46–5.64 mg/kg) from Turkey [60], lower than those from India and Iran [61–63], and higher than those from Bangladesh, Burundi, Japan, Ceylon, Russian, Serbia, and Saudi Arabia [58,59,64] (Table S2). Tea plants are possible to be contaminated by absorbing Pb from the soil, water, and atmosphere, accumulating high Pb levels in the tea leaves [64]. The Pb content in commercial tea leaves raises concerns for both consumers and producers. According to the limit for the Pb content in tea leaves stipulated by the Chinese National Standards (GB 2762-2012) [65], the Pb contents in five samples exceeded the maximum residue limits (5 mg/kg), including three green, one black, and one Pu’er tea sample. The high Pb contents in those five tea samples may be attributed to the use of older tea leaves [61] and contamination from dust particles during processing [56].
The mean Cr content was 0.98 mg/kg; the lowest content was found in oolong tea (0.27 mg/kg), and the highest was found in black tea (2.45 mg/kg). The determined Cr contents in oolong tea were similar to those obtained from South Africa (0.46–1.60 mg/kg) [23]. The Cr contents in black tea ranged from 0.90 mg/kg to 2.45 mg/kg, similar to those found in black tea from Turkey (1.16–2.87 mg/kg) [60]. On average, the Cr contents determined in this work were lower than those from South Africa, South India, and Bangladesh [23,55,58], but much higher than those from Iran [50] (see Table S2). According to the standard (NY 659-2003) enacted by the MOA of China [66], the maximum residue limit for Cr in tea is 5 mg/kg. The Cr contents in the 25 tea samples all satisfied this criterion.
The average Cd content was 0.08 mg/kg (range, 0.01–0.39 mg/kg). The lowest Cd level was found in black tea, while the highest was found in Pu’er tea. The Cd contents of six green tea samples, ranging from 0.04 mg/kg to 0.11 mg/kg, were similar to those in green tea (0.051–0.114 mg/kg) from China and Japan [58]. The Cd levels in oolong tea ranged from 0.04 mg/kg to 0.10 mg/kg, and were lower than those in the oolong tea samples (ND-0.23 mg/kg) reported by Han et al [46]. Phosphate fertilizer applied to soil is a significant contributor of Cd contamination [67]. According to the MOA standard (NY 659-2003) [66], the Cd limit in tea is 1 mg/kg. The determined contents of Cd in the 25 tea samples all satisfied this criterion.
4. Conclusion
In this study, the contents of Pb, Cd, Cr, Cu, and Ni in tea produced and marketed in China were analyzed using HRCS-GFAAS. The Pb, Cu, and Ni levels in green tea were much higher than those in the other types of tea; the highest total content (76.47 mg/kg) was found in the Maojian tea (sample 4). The Cd, Cr, Cu, and Ni levels and the total contents in oolong tea, particularly the Tieguanyin sample (sample 9), were much lower than those of the other types of tea. The Pb, Cd, Cr, Cu, and Ni contents in oolong tea fell below the limit stipulated in the related standards (GB 2762-2012, NY 659-2003, and NY/T 288-2012), while the Pb and Cu contents in the green tea samples, as well as a few black, Pu’er, and jasmine tea samples, exceeded the limit. Consequently, more attention must be paid to the health risks of heavy metal contamination in tea. The quantitation method established in this work will assist in establishing regulations to control the heavy metal contents in tea. Meanwhile, the results lay a foundation for preventing heavy metal toxicity in humans from drinking tea.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 21277001), the Beijing Municipal Education Commission Science and Technology Project (KZ201110005003), and the Beijing Nova Program (No. 2009B08).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2015.04.010
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 21277001), the Beijing Municipal Education Commission Science and Technology Project (KZ201110005003), and the Beijing Nova Program (No. 2009B08).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Chung FL, Schwartz J, Herzog CR, Yang YM. Tea and cancer prevention: Studies in animals and humans. J Nutr. 2003;133:S3268S–74. doi: 10.1093/jn/133.10.3268S. [DOI] [PubMed] [Google Scholar]
- 2. Preuss HG. A review of persistent, low-grade lead challenge: neurological and cardiovascular consequences. J Am Coll Nutr. 1993;12:246–54. doi: 10.1080/07315724.1993.10718306. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for lead. Atlanta: US Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- 4.Eisler R. Handbook of chemical risk assessment: health hazards to humans, plants and animals. New York: Lewis Publishers; 2000. [Google Scholar]
- 5. Borges DLG, Veiga MAMS, Frescura VLA, Welz B, Curtis AJ. Cloud-point extraction for the determination of Cd, Pb and Pd in blood by electrothermal atomic absorption spectrometry, using Ir or Ru as permanent modifiers. J Anal Atom Spectrom. 2003;18:501–7. [Google Scholar]
- 6. Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 7. Mortada WI, Sobh MA, El-Defrawy MM. The exposure to cadmium, lead and mercury from smoking and its impact on renal integrity. Med Sci Monit. 2004;10:CR112–6. [PubMed] [Google Scholar]
- 8. Satarug S, Ujjin P, Vanavanitkun Y, Nishijo M, Baker JR, Moore MR. Effects of cigarette smoking and exposure to cadmium and lead on phenotypic variability of hepatic CYP2A6 and renal function biomarkers in men. Toxicology. 2004;204:161–73. doi: 10.1016/j.tox.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 9. Davis AC, Wu P, Zhang XF, Hou XD, Jones BT. Determination of cadmium in biological samples. Appl Spectrosc Rev. 2006;41:35–75. [Google Scholar]
- 10. Rezende HC, Nascentes CC, Coelho NMM. Cloud point extraction for determination of cadmium in soft drinks by thermospray flame furnace atomic absorption spectrometry. Microchem J. 2011;97:118–21. [Google Scholar]
- 11.World Health Organization. International Agency for Research on Cancer. Monographs volumes 1–60, 1972–1994 and Supplement 7, 1987. Geneva: World Health Organization; 1994. [Google Scholar]
- 12.US Department of Health and Human Services. National Toxicology Program, Sixth Annual Report on Carcinogens. PA, USA: Diane Publishing Co; 1991. [Google Scholar]
- 13. Slavica R, Svetlana D. Determination of chromium in Mentha piperita L. and soil by graphite furnace atomic absorption spectrometry after sequential extraction and microwave-assisted acid digestion to assess potential bioavailability. Chemosphere. 2010;78:451–6. doi: 10.1016/j.chemosphere.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 14. Gad SC. Acute and chronic systemic chromium toxicity. Sci Total Environ. 1989;86:149–57. doi: 10.1016/0048-9697(89)90201-5. [DOI] [PubMed] [Google Scholar]
- 15. Hashem EY, Seleim MM, El-Zohry AM. Environmental method for spectrophotometric determination of copper(II) Green Chem Lett Rev. 2011;4:241–8. [Google Scholar]
- 16. Werfel U, Langen V, Eickhoff I, Schoonbrood J, Vahrenholz C, Brauksiepe A, Popp W, Norpoth K. Elevated DNA single-strand breakage frequencies in lymphocytes of welders exposed to chromium and nickel. Carcinogenesis. 1998;19:413–8. doi: 10.1093/carcin/19.3.413. [DOI] [PubMed] [Google Scholar]
- 17. Turker AR, Yalcinkaya O, Tunceli A. Application of column solid phase extraction of chromium for indirect determination of ascorbic acid by flame atomic absorption spectrometry. J Food Drug Anal. 2008;16:83–8. [Google Scholar]
- 18. Al-Othman ZA, Yilmaz E, Sumayli HMT, Soylak M. Evaluation of trace metals in tea samples from Jeddah and Jazan, Saudi Arabia by atomic absorption spectrometry. B Environ Contam Tox. 2012;89:1216–9. doi: 10.1007/s00128-012-0842-1. [DOI] [PubMed] [Google Scholar]
- 19. Lozak A, Soltyk K, Ostapczuk P, Fijalek Z. Determination of selected trace elements in herbs and their infusions. Sci Total Environ. 2002;289:33–40. doi: 10.1016/s0048-9697(01)01015-4. [DOI] [PubMed] [Google Scholar]
- 20. Ning PB, Gong CM, Zhang YM, Guo KK, Bai J. Lead, cadmium, arsenic, mercury and copper levels in Chinese Yunnan Pu’er tea. Food Addit Contam B. 2011;4:28–33. doi: 10.1080/19393210.2011.551945. [DOI] [PubMed] [Google Scholar]
- 21. Yang JI, Yeh DB, Kuo JM, Pan BS, Lee GC, Liu YH, Lai YJ. Detection of copper Ions in liquid foods and beverages based on an enzymatic method. J Food Drug Anal. 2012;20:83–7. [Google Scholar]
- 22. Nookabkaew S, Rangkadilok N, Satayavivad J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J Agr Food Chem. 2006;54:6939–44. doi: 10.1021/jf060571w. [DOI] [PubMed] [Google Scholar]
- 23. Khakhathi LM, Nikolay P, Svetlana P. Determination of chromium(VI) in black, green and herbal teas. Food Chem. 2011;129:1839–43. [Google Scholar]
- 24. Welz B, Lepri FG, Araujo RGO, Ferreira SLC, Huang MD, Okruss M, Becker-Ross H. Determination of phosphourus, sulfur and the halogens using high-temperature molecular absorption spectrometry in flames and furnaces – a review. Anal Chim Acta. 2009;647:137–48. doi: 10.1016/j.aca.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 25. Boschetti W, Rampazzo RT, Dessuy MB, Vale MGR, Rios AD, Hertz P, Manfroi V, Celso PG, Ferrao MF. Detection of the origin of Brazilian wines based on the determination of only four elements using high-resolution continuum source flame AAS. Talanta. 2013;111:147–55. doi: 10.1016/j.talanta.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 26. Zhao LJ, Ren T, Zhong RG. Determination of lead in human hair by high resolution continuum source graphite furnace atomic absorption spectrometry with microwave digestion and solid sampling. Anal Lett. 2012;45:2467–81. [Google Scholar]
- 27. Sun BS, Ren T, Zhao LJ, Zhong RG. Determination of trace lead in environmental water by cloud point extraction-high resolution continuum source graphite furnace atomic absorption spectrometry. Spectrosc Spect Anal. 2012;32:2847–52. [PubMed] [Google Scholar]
- 28. Fernandez-Martinez R, Rucandio I, Gomez-Pinilla I, Borlaf F, Garcia F, Larrea MT. Evaluation of different digestion systems for determination of trace mercury in seaweeds by cold vapour atomic fluorescence spectrometry. J Food Compos Anal. 2015;38:7–12. [Google Scholar]
- 29. Ren T, Zhao LJ, Zhong RG. Determination of aluminum by microwave digestion-high resolution continuum source graphite furnace atomic absorption spectrometry in wheat flour food. Spectrosc Spect Anal. 2011;31:3388–91. [PubMed] [Google Scholar]
- 30. Dobrowolski R, Adamczyk A, Otto M. Comparison of action of mixed permanent chemical modifiers for cadmium and lead determination in sediments and soils by slurry sampling graphite furnace atomic absorption spectrometry. Talanta. 2010;82:1325–31. doi: 10.1016/j.talanta.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 31. Lopez-Garcia I, Vinas P, Arroyo-Cortez J, Hernandez-Cordoba M. Rapid determination of lead and cadmium in sewage sludge samples using electrothermal atomic absorption spectrometry with slurry sample introduction. Fresenius J Anal Chem. 2000;367:727–32. doi: 10.1007/s002160000464. [DOI] [PubMed] [Google Scholar]
- 32. Vinas P, Pardo-Martinez M, Lopez-Garcia I, Hernandez-Cordoba M. Slurry atomization for the determination of arsenic, cadmium and lead in food colourants using electrothermal atomic absorption spectrometry. J Anal Atom Spectrom. 2001;16:1202–5. [Google Scholar]
- 33. Kim M. Determination of lead and cadmium in wines by graphite furnace atomic absorption spectrometry. Food Addit Contam. 2004;21:154–7. doi: 10.1080/02652030310001642762. [DOI] [PubMed] [Google Scholar]
- 34. Biasino J, Dominguez JR, Alvarado J. Hydrogen peroxide in basic media for whole blood sample dissolution for determination of its lead content by electrothermal atomization atomic absorption spectrometry. Talanta. 2007;73:962–4. doi: 10.1016/j.talanta.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 35. Kummrow F, Silva FF, Kuno R, Souza AL, Oliveira PV. Biomonitoring method for the simultaneous determination of cadmium and lead in whole blood by electrothermal atomic absorption spectrometry for assessment of environmental exposure. Talanta. 2008;75:246–52. doi: 10.1016/j.talanta.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 36. de Blas AJ, Alonso MC, Garcia-Sanchez A, Alvarez-Ayuso E. Chromium speciation in water by sorption on calcite and determination by electrothermal atomic absorption spectrometry. Commun Soil Sci Plant Anal. 2007;38:2091–101. [Google Scholar]
- 37. Zacharia AN, Zhuravlev AS, Chebotarev AN, Arabadgi MV. Direct determination of lead in wine materials by atomic absorption spectrometry using an electrothermal atomizer with a graphite filter-insert. J Appl Spectrosc. 2013;79:949–54. [Google Scholar]
- 38. Acar O. Evaluation of cadmium, lead, copper, iron and zinc in Turkish dietary vegetable oils and olives using electrothermal and flame atomic absorption spectrometry. Grasas y Aceites. 2012;63:383–93. [Google Scholar]
- 39. de Jesus RM, Junior MMS, Matos GD, Santos ADMP, Ferreira SLC. Validation of a digestion system using a digester block/cold finger system for the determination of lead in vegetable foods by electrothermal atomic absorption spectrometry. J AOAC Int. 2011;94:942–57. [PubMed] [Google Scholar]
- 40. Ajtony Z, Bencs L, Haraszi R, Szigeti J, Szoboszlai N. Study on the simultaneous determination of some essential and toxic trace elements in honey by multi-element graphite furnace atomic absorption spectrometry. Talanta. 2007;71:683–90. doi: 10.1016/j.talanta.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 41. Salahinejad M, Aflaki F. Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol Trace Elem Res. 2010;134:109–17. doi: 10.1007/s12011-009-8449-z. [DOI] [PubMed] [Google Scholar]
- 42. Narin I, Colak H, Turkoglu O, Soylak M, Dogan M. Heavy metals in black tea samples produced in Turkey. B Environ Contam Tox. 2004;72:844–9. doi: 10.1007/s00128-004-0321-4. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed O, Al-ansi S, Al-tufail M. Determination of lead in Saudi Arabian imported green tea by ICP-MS. E-J Chem. 2012;9:79–82. [Google Scholar]
- 44. Malik J, Szakova J, Drabek O, Balik J, Kokoska L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008;111:520–5. doi: 10.1016/j.foodchem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 45. Soylak M, Tuzen M, Souza AS, Korn MdGA, Ferreira SLC. Optimization of microwave assisted digestion procedure for the determination of zinc, copper and nickel in tea samples employing flame atomic absorption spectrometry. J Hazard Mater. 2007;149:264–8. doi: 10.1016/j.jhazmat.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 46. Han WY, Shi YZ, Ma LF, Ruan JY. Arsenic, cadmium, chromium, cobalt and copper in different types of Chinese tea. Bull Environ Contam Toxicol. 2005;75:272–7. doi: 10.1007/s00128-005-0748-2. [DOI] [PubMed] [Google Scholar]
- 47. McKenzie JS, Jurado JM, de Pablos F. Characterisation of tea leaves according to their total mineral content by means of probabilistic neural networks. Food Chem. 2010;123:859–64. [Google Scholar]
- 48.Industrial Standards of the Ministry of Agriculture of the People’s Republic of China. Green food-Tea. NY/T 288, 2012. 2012. [Google Scholar]
- 49. Jin CW, Du ST, Zhang K, Lin XY. Factors determining copper concentration in tea leaves produced at Yuyao County, China. Food Chem Toxicol. 2008;46:2054–61. doi: 10.1016/j.fct.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 50. Kasrai M, Shoushtarian MJ, Bozorgzadeh MH. Determination of trace elements in tea leaves by neutron activation analysis. J Radioanal Nucl Ch. 1977;41:73–9. [Google Scholar]
- 51.Reeves R, Baker AJM. Metal-accumulating plants. In: Raskin I, Ensley BD, editors. Phytoremediation of toxic metals: using plants to clean up the environment. USA: John Wiley; 2000. [Google Scholar]
- 52.Kabata-Pendias A, Pendias H. Trace elements in soils and plants. Boca Raton: CRC Press; 2001. [Google Scholar]
- 53. Peralta-Videa JR, Gardea-Torresdey JL, Gomez E, Tiemann KJ, Parsons JG, Carrillo G. Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environ Pollut. 2002;119:291–301. doi: 10.1016/s0269-7491(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 54. Christensen JM, Kristiansen J, Nielsen NH, Menne T, Byrialsen K. Nickel concentrations in serum and urine of patients with nickel eczema. Toxicol Lett. 1999;108:185–9. doi: 10.1016/s0378-4274(99)00088-0. [DOI] [PubMed] [Google Scholar]
- 55. Seenivasan S, Manikandan N, Muraleedharan NN. Chromium contamination in black tea and its transfer into tea brew. Food Chem. 2008;106:1066–9. [Google Scholar]
- 56. Karak T, Bhagat RM. Trace elements in tea leaves, made tea and tea infusion: a review. Food Res Int. 2010;43:2234–52. [Google Scholar]
- 57. Tsushida T, Takeo T. Zinc, copper, lead and cadmium contents in green tea. J Sci Food Agr. 1977;28:255–8. doi: 10.1002/jsfa.2740280306. [DOI] [PubMed] [Google Scholar]
- 58. Marcos A, Fischer A, Rea G, Hill SJ. Preliminary study using trace element concentrations and a chemometrics approach to determine geographical origin of tea. J Anal Atom Spectrom. 1998;13:521–5. [Google Scholar]
- 59. Slavica R, Vesna K. Diverse elements in herbal tea products consumed in Serbia using inductively coupled plasma mass spectrometry. Int J Food Prop. 2013;16:1–8. [Google Scholar]
- 60. Tokalioglu S, Kartal S. Bioavailability of soil-extractable metals to tea plant by BCR sequential extraction procedure. Instrum Sci Technol. 2004;32:387–400. [Google Scholar]
- 61. Natesan S, Ranganathan V. Content of various elements in different parts of the tea plant and in infusions of black tea from southern India. J Sci Food Agr. 1990;51:125–39. [Google Scholar]
- 62. Shokrzadeh M, Saberyan M, Saravi SSS. Assessment of lead (Pb) and cadmium (Cd) in 10 samples of Iranian and foreign consumed tea leaves and dissolved beverages. Toxicol Environ Chem. 2008;90:879–83. [Google Scholar]
- 63. Zazouli MA, Mohseni A, Maleki A, Saberian M, Izanloo H. Determination of cadmium and lead contents in black tea and tea liquor from Iran. Asian J Chem. 2010;22:1387–93. [Google Scholar]
- 64. Ashraf W, Mian AA. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. B Environ Contam Tox. 2008;81:101–4. doi: 10.1007/s00128-008-9402-0. [DOI] [PubMed] [Google Scholar]
- 65. National Standards of the People’s Republic of China. Maximum levels of contaminants in foods. GB 2762-2012. 2012 [Google Scholar]
- 66. Industrial Standards of the Ministry of Agriculture of the People’s Republic of China. Residue limits for chromium, cadmium, mercury, arsenic and fluoride in tea. NY 659-2003. 2003 [Google Scholar]
- 67. Cupit M, Larsson O, de Meeus C, Eduljee GH, Hutton M. Assessment and management of risks arising from exposure to cadmium in fertilisers-II. Sci Total Environ. 2002;291:189–206. doi: 10.1016/s0048-9697(01)01099-3. [DOI] [PubMed] [Google Scholar]