Abstract
Corn peptides (CPs) are reported to have many biological functions, such as facilitating alcohol metabolism, antioxidation, antitumor, antihypertension, and hepatoprotection. To develop a method for quality control, the high-performance liquid chromatography (HPLC) system was applied. Twenty-eight common peaks were found in all the CPs of corn samples from Enshi, China, based on which, a fingerprinting chromatogram was established for use in quality control in future research. Subsequently, the major chemical constituents of these common peaks were identified respectively using the HPLC-diode-array detection electrospray ionization tandem mass spectrometry (DAD-ESI-MS/MS) system, and 48 peptide fractions were determined ultimately. This was the first time for the majority of these peptides to be reported, and many of them contained amino acids of glutamine (Q), L and A, which might play an important role in the exhibition of the bioactivities of CPs. Many peptides had a similar primary structure to the peptides which had been proven to be bioactive such as facilitating alcohol metabolism, scavenging free radicals, and inhibiting lipid peroxidation. This systematical analysis of the primary structure of CPs facilitated subsequent studies on the relationship between the structures and functions, and could accelerate holistic research on CPs.
Keywords: corn peptides, electrospray ionization mass spectrometry (ESI-MS), high-performance liquid chromatography (HPLC), HPLC fingerprints, primary structure
1. Introduction
Bioactive peptides are specific protein fragments which play a significant role in the human body. Since the physiological importance of the casein-derived phosphorylated peptides was firstly reported by Mellander [1], thousands of bioactive peptides have been identified and described. These peptides may have specific or multiple biofunctions, such as the properties of anticancer, mineral binding, and antihypertension [2]. Peptides have prominent advantages in digestion and utilization, compared with protein. In accordance with the research of modern nutriology, the majority of proteins ingested by humans are decomposed into small peptides, composed of two to seven amino acids in the digestive tract, then the peptides are absorbed directly. Only a very small proportion of proteins are assimilated in the form of amino acids [3]. Other studies have indicated that the absorptive rate of intact peptides from protein hydrolysates may even be faster than free amino acids [4] and the energy consumption of assimilating the peptide is less than that of assimilating equivalent amino acids [5]. This particular superiority described above contributes to the high value of bioactive peptides in nutrition. Therefore, it will be of great significance in exploiting bioactive peptides with particular biological functions.
Corn peptides (CPs) are usually prepared through the hydrolysate of corn protein, which is treated as a low nutritional product [6]. CPs are verified to exhibit many biofunctions, such as facilitating alcohol metabolism [7,8], antioxidation [9], antitumor [10], antihypertension [11], and hepatoprotection [12–14] in many of our previous studies, and our laboratory has done extensive research on the characteristics of CPs prepared from the corns cultivated in Enshi, China. However, unlike pure chemical compounds, CPs are a complex mixture containing hundreds of fragments of peptides, and the quality of these corns in previous studies might be affected by different producing areas, varieties, and harvest time. These affects eventually led to the variation of CP components. Furthermore, the vagueness of effectively active ingredients severely limits the studies and application of CPs in the future. In order to carry on more overall and deeper research and facilitate the pace of stepping towards the commercial markets, the quality control of CPs is needed. It is impossible to utilize the traditional quality control method, which mainly comprises direct qualification and quantification of the natural components, because the reference compounds in the CPs are hard to obtain [15]. Besides, some of the biological activities of CPs are not expressed by a single compound, and the constituents in CPs may promote each other and jointly play a role in this system. Therefore, a comprehensive and integral system of quality assessment should be established. In recent studies, fingerprinting analysis has become one of the most efficient methods to identify the characteristic profiles of foods with complex elements and has been recommended by the World Health Organization and the State Food and Drug Administration (SFDA) of China for the characterization of Traditional Chinese Medicine [16]. Among different analytical methods, the technology of high performance liquid chromatography (HPLC) fingerprinting has been widely used in many studies of natural products, such as oolong tea [17] and Gynostemma pentaphyllum [18], and has shown good value in practical applications.
However, there are few reports about the systematic study of the primary structures of CPs, and the amount of constituents in CPs has not been identified to date. Thus, an urgent need to comprehensively analyze the structures of CPs still exists. High performance liquid chromatography–electrospray ionization mass spectrometry (HPLC–ESI-MS/MS) has been chosen as an efficient analytical tool, which was applied in peptide sequence identification [19].
This study aims to establish a chromatographic fingerprint method for the quality authentication of CPs from Enshi, China. The major component of CPs is to be identified so as to reveal the relationship between the structural characteristics and the physiological function in CPs.
2. Materials and methods
2.1. Chemicals, reagents, and materials
Sixteen batches of corn samples (Cultivar Xiangyu No.10) from Enshi Tujia Antonymous Region, China were obtained and summarized in Table 1. All the samples were cultivated in May 2013 and collected in the autumn of 2013. The raw materials were dried under the sun and kept in a refrigerator at 4°C. The voucher specimen (No. 131013) has been deposited in the College of Food Science and Technology, Huazhong Agricultural University, Wuhan, China. HPLC-grade acetonitrile was purchased from Fisher Chemical (Fair Lawn, NJ, USA), and HPLC-grade water was prepared using a Milli-Q system (Millipore Ibérica, Madrid, Spain). Trifluoroacetic acid (TFA) of HPLC grade was purchased from Sigma Chemical Co. (Saint Louis, MO, USA). All other chemicals and reagents were of analytical purity.
Table 1.
Habitats and batch numbers of the corn samples (cultivar Xiangyu No.10) from Tujia Antonymous Region, Enshi, China.
| Batch no. | Habitat | Cultivator | Batch no. | Habitat | Cultivator |
|---|---|---|---|---|---|
| S1 | Zhoujiatai, Enshi | Jilin Yi | S9 | Majiaao, Enshi | Qunhai Wang |
| S2 | Zhoujiatai, Enshi | Yinju Sun | S10 | Cheying village, Enshi | Yunkui Zhang |
| S3 | Huaguomu, Enshi | Yunxing, Zhang | S11 | Majiaao, Enshi | Qunsong Wang |
| S4 | Beijie, Enshi | Jilin Yi | S12 | Qiaoping, Enshi | Bin Zhang |
| S5 | Zhoujiatai, Enshi | Sanzhou Lu | S13 | Cheying village, Enshi | Yunkui Zhang |
| S6 | Guolitang village, Enshi | Yanxiang Yu | S14 | Qiaoping, Enshi | Changji Zhang |
| S7 | Beijie, Enshi | Jiping YI | S15 | Qiaotou, Enshi | Changji Zhang |
| S8 | Qiaoping, Enshi | Changfu Zhang | S16 | Yutangba village, Enshi | Wenfan, He |
2.2. Protein extraction
Raw materials were dried and ground into powder. The powder (100 g) was heated in a water bath twice with 650 mL of NaOH (0.1 mol/L) and 650 mL of 90% ethanol at 45°C, and the heating time was 2 hours each time. Then the mixture was centrifuged at 4000 rpm for 10 minutes. The supernatant containing the crude protein was combined and doubled using distilled water. The pH was adjusted to 6.3 to make the protein precipitate. The precipitate of protein was lyophilized.
2.3. Preparation of peptide
The powder of protein with distilled water (1:35, w/v) was heated on the boiling water bath for 30 minutes, and then went through the process of hydrolysis of Alcalase (19.2 U/g, Novozymes Co., Copenhagen, Denmark) (0.6%, w/w) at pH 8.0, 55°C for 4 hours. The hydrolysate of corn protein was transferred to an ultrafiltration system (Millipore Co., Bedford, MA, USA) and then the fraction (Mw < 5 kDa) of the CPs was concentrated by rotary evaporation. The concentrate was lyophilized into peptide powder, and was stored at −20°C in a refrigerator. Then the peptide powder was redissolved into a solution (10 mg/mL) using HPLC-grade water.
2.4. HPLC–diode array detection analysis instrumentation
The HPLC fingerprinting analysis was performed on a Waters e2695 HPLC–diode array detection (DAD) system (Waters, Milford, MA, USA), equipped with a diode array detector, a vacuum degasser, an autosampler, a binary pump, and a column compartment. The separations of the peptide solution were carried out on an Agilent C18 column (250 mm × 4.6 mm, 5 μm) at a flow rate of 1 mL/min. The temperature of the column was maintained at 30°C. A binary gradient elution system, comprising water (with 0.1% TFA, A) and acetonitrile (with 0.1% TFA, B), was applied as follows: 0.0–60.0 minutes, 10–60% B. After each run, an equilibration time of 20 minutes was set. The volume of injection was 10 μL and the DAD detector was set at 230 nm for analysis.
2.5. Similarity analysis
On the basis of the HPLC–DAD data, the similarity of these corn peptide samples were calculated using the software named Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine, Version 2004A, which was recommended by the SFDA of China. Simultaneously, a reference chromatogram, containing all the common peaks in the chromatograms of the 16 samples, was calculated using the median method, and then the similarities between the reference chromatogram and the 16 samples were calculated. In previous research, the similarities obtained in this way were regarded as significant parameters to distinguish the origins and varieties of natural products. Higher similarities usually implied closer relationships in producing area, variety, and chemical constitution [16,17].
2.6. HPLC–ESI-MS/MS analysis instrumentation
The HPLC system above was replaced by an Agilent1100 series HPLC–ESI-MS system (Agilent Technologies, Santa Clara, CA, USA) and the column and gradient elution system was in accord with those used in HPLC-DAD analysis. Mass spectrometry conditions were as follows: ESI+ ion source; drying gas temperature, 325°C; nebulizer, 40.00 psi; dry gas flow rate, 10.0 L/min; capillary voltage, 3500 V; and scan spectra from m/z 50 to 1100.
2.7. Identification of the primary structure of peptide
The MS/MS spectra were analyzed using the software LCMSD-Trap DataAnalysis to obtain the primary structure of peptide preliminary, then the MS database of SwissProt. 2013.06.27 provided by University of California, San Francisco (UCSF) Mass Spectrometry Facility was used to identify the accurate structure of peptides. The searching taxonomy was Zea mays (ZEA), digest parameter was set to no enzyme.
The peptide residues with some similar molecular weight were retrieved in the SwissProt.2013.06.27 database, respectively, because the structure of the peptide to be determined ultimately depended on whether the residue existed in the taxonomy ZEA or not. In general, no more than one peptide residue was eligible.
3. Results and discussion
3.1. Method validation of HPLC fingerprints
In order to evaluate the precision, Sample 12 was analyzed six times, successively. The stability was examined by measuring Sample 12 at different time points (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, and 12 hours) respectively. Five solutions made by the same sample (S12) were measured to check the repeatability. The similarities of all the tests were calculated using the software named Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine, Version 2004A and the results were all above 0.98, which indicated that the analysis method of HPLC fingerprint was valid and stable.
3.2. Similarity analysis of HPLC fingerprints
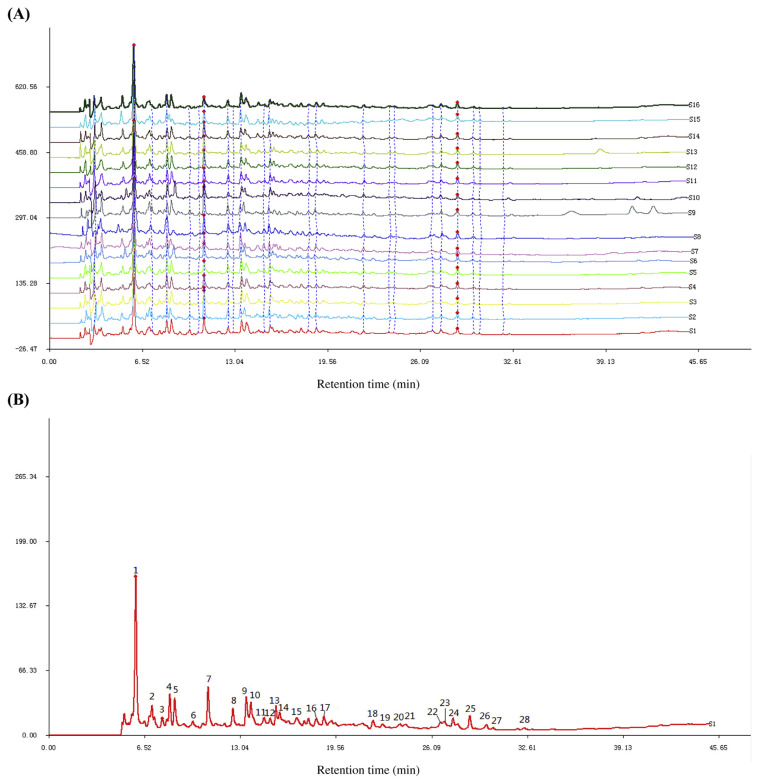
The HPLC fingerprints of CPs were obtained and are shown in Fig. 1A. It was found that these batches of samples had generally similar spectra which indicated the similar chemical compositions of these samples. Meanwhile, the reference chromatogram generated by the software is shown in Fig. 1B. There were 28 common peaks (existing in all chromatograms of the 16 batches of samples) which were marked (1–28) in the reference chromatogram. Peak 1 (tR = 5.942) was selected as the reference peak because it was the maximum peak in this chromatogram. The relative retention times (tR) and relative peak areas of these 28 common peaks are listed in Table 2. The similarities of the CPs were calculated. As shown in Table 3, compared with the reference chromatogram, the least similarity values of these samples were 0.837, and majority of the values were > 0.9, which indicated that the corn samples collected from a variety of areas had similar chemical compositions and this reference chromatogram could be applied as a standard HPLC fingerprint.
Fig. 1.
(A) HPLC fingerprint of 16 batches of corn peptides; (B) reference chromatogram of 16 batches of corn peptides. HPLC = high-performance liquid chromatography.
Table 2.
Relative peak areas and relative retention times of the common peaks of corn peptides.
| Peak no. | tR (min) | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | Average | RSD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak 1 | 5.924 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.000 |
| Peak 2 | 7.032 | 0.510 | 0.270 | 0.451 | 0.466 | 0.464 | 0.238 | 0.267 | 0.340 | 0.463 | 0.197 | 0.216 | 0.181 | 0.206 | 0.499 | 0.143 | 0.377 | 0.325 | 0.359 |
| Peak 3 | 7.729 | 0.204 | 0.189 | 0.192 | 0.186 | 0.205 | 0.341 | 0.223 | 0.122 | 0.168 | 0.200 | 0.241 | 0.205 | 0.264 | 0.359 | 0.161 | 0.189 | 0.215 | 0.290 |
| Peak 4 | 8.246 | 0.314 | 0.287 | 0.295 | 0.306 | 0.301 | 0.334 | 0.253 | 0.382 | 0.285 | 0.260 | 0.259 | 0.220 | 0.247 | 0.309 | 0.222 | 0.278 | 0.283 | 0.145 |
| Peak 5 | 8.576 | 0.406 | 0.518 | 0.311 | 0.308 | 0.330 | 0.439 | 0.336 | 0.148 | 0.458 | 0.305 | 0.351 | 0.292 | 0.336 | 0.444 | 0.124 | 0.330 | 0.337 | 0.285 |
| Peak 6 | 9.807 | 0.310 | 0.323 | 0.261 | 0.259 | 0.274 | 0.096 | 0.098 | 0.073 | 0.285 | 0.110 | 0.114 | 0.168 | 0.129 | 0.165 | 0.067 | 0.146 | 0.177 | 0.475 |
| Peak 7 | 10.853 | 0.496 | 0.670 | 0.422 | 0.415 | 0.448 | 0.454 | 0.398 | 0.347 | 0.549 | 0.203 | 0.391 | 0.351 | 0.388 | 0.552 | 0.243 | 0.390 | 0.415 | 0.237 |
| Peak 8 | 12.546 | 0.313 | 0.402 | 0.349 | 0.278 | 0.349 | 0.436 | 0.236 | 0.274 | 0.305 | 0.065 | 0.279 | 0.226 | 0.261 | 0.389 | 0.141 | 0.366 | 0.291 | 0.331 |
| Peak 9 | 13.456 | 0.312 | 0.427 | 0.283 | 0.271 | 0.317 | 0.290 | 0.315 | 0.269 | 0.295 | 0.150 | 0.279 | 0.256 | 0.280 | 0.351 | 0.219 | 0.305 | 0.287 | 0.190 |
| Peak 10 | 13.769 | 0.386 | 0.275 | 0.325 | 0.300 | 0.380 | 0.253 | 0.140 | 0.193 | 0.255 | 0.123 | 0.215 | 0.156 | 0.154 | 0.237 | 0.175 | 0.316 | 0.242 | 0.350 |
| Peak 11 | 14.656 | 0.227 | 0.246 | 0.199 | 0.168 | 0.242 | 0.155 | 0.259 | 0.144 | 0.093 | 0.060 | 0.175 | 0.159 | 0.175 | 0.293 | 0.107 | 0.226 | 0.182 | 0.332 |
| Peak 12 | 15.074 | 0.177 | 0.197 | 0.150 | 0.145 | 0.195 | 0.266 | 0.199 | 0.196 | 0.141 | 0.085 | 0.190 | 0.166 | 0.181 | 0.246 | 0.075 | 0.155 | 0.172 | 0.296 |
| Peak 13 | 15.476 | 0.159 | 0.305 | 0.223 | 0.149 | 0.183 | 0.202 | 0.159 | 0.173 | 0.167 | 0.120 | 0.159 | 0.141 | 0.152 | 0.200 | 0.094 | 0.155 | 0.170 | 0.263 |
| Peak 14 | 15.726 | 0.187 | 0.129 | 0.126 | 0.180 | 0.215 | 0.182 | 0.151 | 0.195 | 0.192 | 0.088 | 0.148 | 0.138 | 0.141 | 0.190 | 0.071 | 0.156 | 0.155 | 0.250 |
| Peak 15 | 16.89 | 0.283 | 0.443 | 0.231 | 0.247 | 0.386 | 0.330 | 0.289 | 0.184 | 0.268 | 0.164 | 0.240 | 0.206 | 0.225 | 0.300 | 0.087 | 0.232 | 0.256 | 0.326 |
| Peak 16 | 18.218 | 0.216 | 0.267 | 0.202 | 0.161 | 0.265 | 0.242 | 0.182 | 0.116 | 0.190 | 0.144 | 0.178 | 0.145 | 0.160 | 0.169 | 0.014 | 0.190 | 0.177 | 0.358 |
| Peak 17 | 18.741 | 0.198 | 0.320 | 0.145 | 0.118 | 0.194 | 0.224 | 0.220 | 0.095 | 0.194 | 0.136 | 0.121 | 0.116 | 0.120 | 0.179 | 0.039 | 0.146 | 0.158 | 0.387 |
| Peak 18 | 22.089 | 0.097 | 0.194 | 0.101 | 0.045 | 0.211 | 0.090 | 0.139 | 0.065 | 0.085 | 0.087 | 0.083 | 0.056 | 0.061 | 0.099 | 0.057 | 0.089 | 0.097 | 0.484 |
| Peak 19 | 22.73 | 0.063 | 0.165 | 0.076 | 0.011 | 0.139 | 0.077 | 0.127 | 0.046 | 0.055 | 0.086 | 0.044 | 0.032 | 0.028 | 0.053 | 0.056 | 0.058 | 0.069 | 0.593 |
| Peak 20 | 23.9 | 0.061 | 0.142 | 0.065 | 0.030 | 0.171 | 0.060 | 0.092 | 0.046 | 0.030 | 0.084 | 0.037 | 0.035 | 0.033 | 0.045 | 0.068 | 0.050 | 0.066 | 0.620 |
| Peak 21 | 24.272 | 0.043 | 0.111 | 0.053 | 0.023 | 0.122 | 0.051 | 0.119 | 0.037 | 0.027 | 0.089 | 0.030 | 0.035 | 0.026 | 0.061 | 0.068 | 0.032 | 0.058 | 0.598 |
| Peak 22 | 26.673 | 0.055 | 0.087 | 0.067 | 0.050 | 0.140 | 0.050 | 0.068 | 0.098 | 0.045 | 0.049 | 0.071 | 0.061 | 0.055 | 0.028 | 0.107 | 0.063 | 0.069 | 0.431 |
| Peak 23 | 26.936 | 0.071 | 0.115 | 0.067 | 0.038 | 0.121 | 0.079 | 0.108 | 0.105 | 0.076 | 0.084 | 0.071 | 0.069 | 0.065 | 0.084 | 0.108 | 0.059 | 0.082 | 0.285 |
| Peak 24 | 27.532 | 0.088 | 0.120 | 0.074 | 0.028 | 0.095 | 0.089 | 0.082 | 0.080 | 0.086 | 0.071 | 0.054 | 0.074 | 0.064 | 0.071 | 0.069 | 0.067 | 0.075 | 0.247 |
| Peak 25 | 28.678 | 0.071 | 0.151 | 0.076 | 0.071 | 0.117 | 0.074 | 0.081 | 0.103 | 0.104 | 0.067 | 0.059 | 0.070 | 0.067 | 0.087 | 0.084 | 0.066 | 0.083 | 0.253 |
| Peak 26 | 29.802 | 0.029 | 0.061 | 0.030 | 0.026 | 0.061 | 0.039 | 0.050 | 0.049 | 0.048 | 0.028 | 0.040 | 0.033 | 0.030 | 0.047 | 0.036 | 0.032 | 0.040 | 0.277 |
| Peak 27 | 30.259 | 0.010 | 0.016 | 0.011 | 0.010 | 0.013 | 0.010 | 0.009 | 0.010 | 0.029 | 0.010 | 0.010 | 0.009 | 0.009 | 0.012 | 0.016 | 0.009 | 0.012 | 0.363 |
| Peak 28 | 32.388 | 0.017 | 0.012 | 0.016 | 0.015 | 0.009 | 0.018 | 0.018 | 0.024 | 0.027 | 0.042 | 0.022 | 0.018 | 0.006 | 0.030 | 0.009 | 0.015 | 0.018 | 0.446 |
tR is the retention time. Peak 1 (tR = 5.942) was selected as the reference peak because it was the maximum peak in this chromatogram.
RSD = Relative Standard Deviation.
Table 3.
Similarities of corn peptides from 16 origins.
| Batch no. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1 | |||||||||||||||
| S2 | 0.839 | 1 | ||||||||||||||
| S3 | 0.982 | 0.837 | 1 | |||||||||||||
| S4 | 0.981 | 0.834 | 0.986 | 1 | ||||||||||||
| S5 | 0.958 | 0.862 | 0.962 | 0.95 | 1 | |||||||||||
| S6 | 0.809 | 0.812 | 0.824 | 0.823 | 0.825 | 1 | ||||||||||
| S7 | 0.812 | 0.88 | 0.827 | 0.828 | 0.832 | 0.833 | 1 | |||||||||
| S8 | 0.831 | 0.808 | 0.853 | 0.858 | 0.841 | 0.805 | 0.867 | 1 | ||||||||
| S9 | 0.809 | 0.849 | 0.818 | 0.832 | 0.809 | 0.782 | 0.829 | 0.802 | 1 | |||||||
| S10 | 0.729 | 0.736 | 0.755 | 0.771 | 0.746 | 0.81 | 0.766 | 0.771 | 0.731 | 1 | ||||||
| S11 | 0.862 | 0.854 | 0.879 | 0.879 | 0.875 | 0.816 | 0.842 | 0.828 | 0.842 | 0.775 | 1 | |||||
| S12 | 0.863 | 0.857 | 0.885 | 0.889 | 0.877 | 0.823 | 0.864 | 0.87 | 0.852 | 0.787 | 0.974 | 1 | ||||
| S13 | 0.866 | 0.851 | 0.882 | 0.886 | 0.869 | 0.818 | 0.85 | 0.839 | 0.843 | 0.778 | 0.979 | 0.976 | 1 | |||
| S14 | 0.874 | 0.847 | 0.871 | 0.874 | 0.868 | 0.811 | 0.831 | 0.817 | 0.838 | 0.714 | 0.91 | 0.885 | 0.894 | 1 | ||
| S15 | 0.794 | 0.757 | 0.834 | 0.847 | 0.804 | 0.766 | 0.823 | 0.853 | 0.738 | 0.785 | 0.86 | 0.888 | 0.857 | 0.769 | 1 | |
| S16 | 0.964 | 0.844 | 0.968 | 0.963 | 0.966 | 0.843 | 0.842 | 0.849 | 0.829 | 0.755 | 0.896 | 0.898 | 0.895 | 0.904 | 0.832 | 1 |
| Reference chromatogram | 0.946 | 0.912 | 0.958 | 0.96 | 0.951 | 0.894 | 0.913 | 0.907 | 0.892 | 0.835 | 0.95 | 0.956 | 0.95 | 0.927 | 0.886 | 0.964 |
3.3. Primary structure identification of the CPs
According to previous research, the molecular weight of the CPs had effects on their bioactivities. The CPs with low Mw (<1 kDa) were better in facilitating alcohol metabolism [6], antioxidant activity [20], etc. Furthermore, the short chain peptides, which were mainly composed of two to seven amino acids, were easily digested and absorbed. Consequently, the scanning range of mass spectra was set in m/z 50–1100. Other mass spectrometric conditions, including capillary voltage, drying gas temperature, and dry gas flow rate, had been verified for the analysis of CPs in our former studies. Preliminarily, the ESI-MS and MS/MS analyses were carried out to identify the structure of CPs using the software of Agilent LCMSD-Trap Data Analysis. Then, the accurate structure of the CPs were determined and validated by searching the mass spectrometry database of SwissProt.2013.6.27 provided by the Regents of the University of California. Twenty-eight common peaks in the HPLC fingerprints (Fig. 1B) were elucidated. It was found that some of these peaks contained more than one peptide fragment in that CPs were complex compounds with hundreds of peptide fragments. The main ingredients of each peak were chosen and identified depending on the signal intensity. In total, 48 structures of the CPs were determined and are summarized in Table 4. Some typical peaks are discussed in detail to illustrate the identification process below.
Table 4.
Identification of corn peptides using HPLC–ESI-MS, MS/MS.
| Peak | tR(min) | Experimental mass (m/z) | Fragment ions (m/z) | Identification | Theoretical mass (m/z) |
|---|---|---|---|---|---|
| 1 | 5.924 | 374.4 | 242.8/131.8 | MHS | 374.1 |
| 388.4 | 242.0/146.8/129.8 | QLQ* | 388.2 | ||
| 542.5 | 453.0/355.9/241.9/186.8 | KLNPA | 542.3 | ||
| 416.3 | 288.0/242.1 | QIR* | 416.3 | ||
| 2 | 7.032 | 473.3 | 384.0/313.0/199.9 | QALAA* | 473.3 |
| 340.1 | 226.8/156 | LAH* | 340.2 | ||
| 3 | 7.729 | 485.6 | 396.0/371.2/298.9/211.9/186.9 | NPSPA | 485.2 |
| 4 | 8.246 | 423.8 | 276.8/146.7/129.9 | FEE | 424.2 |
| 531.3 | 441.9/344.9/230.9/186.8 | MVNPA* | 531.3 | ||
| 5 | 8.576 | 602.4 | 513.1/416.1/302.1/188.8 | STLNPA* | 602.3 |
| 6 | 9.807 | 446.3 | 299.8/228.8/129.8 | EVAQ | 446.2 |
| 316.3 | 228.8/132.1 | SLP | 316.2 | ||
| 622.6 | 476.0/347.9/234.8 | AYLQQ* | 622.3 | ||
| 7 | 10.853 | 494.3 | 347.9/234.9/146.8 | AYLQ* | 494.3 |
| 8 | 12.546 | 407.3 | 260.8/146.7 | FLQ* | 407.2 |
| 527.3 | 340.9/241.9/186.9 | QIVPA* | 527.3 | ||
| 9 | 13.456 | 359.2 | 227.7/116.9 | VQL* | 359.2 |
| 501.5 | 355.0/241.9/146.8 | LQLQ* | 501.3 | ||
| 10 | 13.769 | 460.4 | 355.0/241.9/128.8 | QLLS* | 460.3 |
| 585.4 | 419.9/291.8/180.9 | YQQF* | 585.3 | ||
| 11 | 14.656 | 495.4 | 363.8/200.9 | AEYL* | 495.2 |
| 593.5 | 411.9/365.9/314.9/278.8 | NLSPY* | 593.3 | ||
| 12 | 15.074 | 541.3 | 356.9/243.7 | AIIPQ* | 541.3 |
| 13 | 15.476 | 584.5 | 453.1/356.0/242.0 | KLNPI | 584.4 |
| 14 | 15.726 | 494.4 | 347.9/234.9/146.8 | AYLQ* | 494.3 |
| 447.5 | 314.8/186.7 | WKN | 447.2 | ||
| 15 | 16.89 | 605.4 | 391.0/243.8 | TIFPQ* | 605.3 |
| 16 | 18.218 | 654.5 | 522.2/409.2/310.1/213.0 | VLPVIN* | 654.4 |
| 575.4 | 389.1/276.1 | QFLPA* | 575.3 | ||
| 504.4 | 275.7/228.7 | QFLP* | 504.3 | ||
| 17 | 18.741 | 470.3 | 339.1/241.9/228.9 | QLPL* | 470.3 |
| 18 | 22.089 | 441.3 | 341.7/228.8 | VLPL* | 441.3 |
| 633.6 | 453.0/392.0/355.0/278.9 | QLLPY* | 633.4 | ||
| 19 | 22.73 | 504.4 | 262.8/165.8 | QLPF* | 504.3 |
| 20 | 23.9 | 697.6 | 566.2/469.2/356.0/243.0 | QLLNPL* | 697.4 |
| 21 | 24.272 | 377.6 | 280.0/228.8 | MPM | 378.2 |
| 22 | 26.673 | 459.0 | 362.0/326.0/262.9 | PVED | 459.2 |
| 561.9 | 428.1/376.0/262.8 | WLED | 562.3 | ||
| 23 | 26.936 | 437.4 | 305.9/262.8 | MMR | 437.2 |
| 24 | 27.532 | 512.4 | 342.4/228.8 | GILPL * | 512.3 |
| 745.7 | 483.2/370.2/263.1 | QQLLPF * | 745.4 | ||
| 25 | 28.678 | 617.4 | 375.9/355.0/263.0/242.1 | QLLPF * [6] | 617.4 |
| 489.4 | 262.7/229.0 | LLPF * [20] | 489.3 | ||
| 779.5 | 614.0/517.1/404.0/256.8 | QQFLPF * | 779.4 | ||
| 26 | 29.802 | 651.3 | 389.0/275.9/262.9 | QFLPF * | 651.4 |
| 523.3 | 357.8/262.8/165.8 | FLPF * [20] | 523.3 | ||
| 27 | 30.259 | 858.5 | 596.2/483.0/370.0/256.7 | QQILLPF * | 858.5 |
| 28 | 32.388 | 730.7 | 565.2/468.2/355.7/241.9 | QILLPF * | 730.4 |
The primary structures of the CPs were determined and validated by searching the mass spectrometry database of SwissProt.2013.6.27 with the Protein prospector engine provided by the Regents of the University of California, the searching taxonomy was Zea mays (ZEA).
tR is the retention time.
The peptides marked with an asterisk * could be found in the zein sequence frequently, which indicated these peptides were most probably hydrolyzed from the zein.
CP = corn peptides; HPLC–ESI-MS = high performance liquid chromatography-electrospray ionization mass spectrometry; MS/MS = tandem mass spectrometry; A (Ala), R (Arg), N (Asn), D (Asp), C (Cys), Q (Gln), E (Glu), G (Gly), H (His), I (Ile), L (Leu), K (Lys), M (Met), F (Phe), P (Pro), S (Ser), T (Thr), W (Trp), Y (Tyr), V (Val).
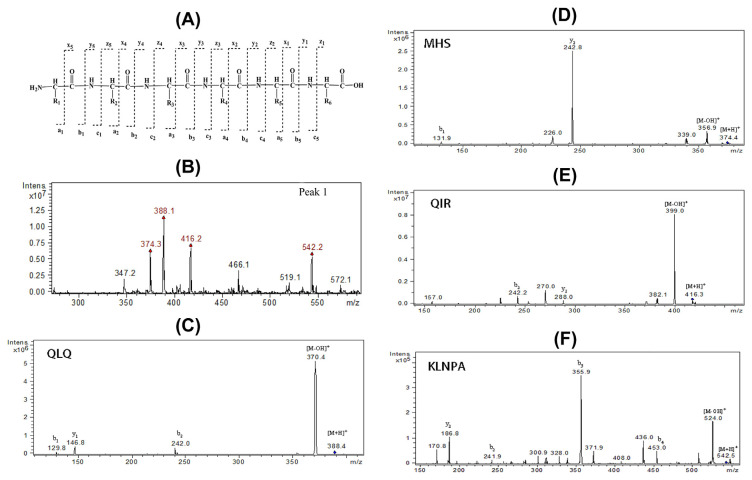
The cleavage pattern of peptide is shown in Fig. 2A. The fragment ions at the N-terminal and C-terminal were denoted by a, b, c and x, y, z, respectively. The patterns of y and b caused by the peptide bond breaking were the most common types in mass spectra and their signal intensity was relatively strong [21]. Peak 1 (tR = 5.942) with the strongest signal was detailed, and its ESI-MS spectrum, shown in Fig. 2B, indicated that Peak 1 was composed of four principal components (m/z 374.4, 388.4, 416.3, and 542.5). It could not be verified whether one of them came from the fracture of the others, thus, these four components were identified respectively, according to the MS2 spectra in Fig. 2C, 2D, 2E, and 2F. Take, for example, the fragment of m/z 542.5, which was the longest chain among them. As is shown in Fig. 2F, the ion at m/z 542.5 and 524.0 were the quasi-molecular ion [M+H]+ and [M-OH]+. The m/z 524.0 – 453.0 = 71.0, which indicated the first amino acid from the C-terminal was an Ala (A) residue and m/z 453.0 was the b4 ion. Similarly, m/z 453.0 (b4) – 355.9 (b3) = 96.9, thus we could infer that the second amino acid from the C-terminal was a Pro (P) residue. Next, b3 – b2 = m/z 355.9 – 241.9 = 114.0, which suggested that the third amino acid of the C-terminal was an Asn (N) residue. Because smaller ion signals were hardly found in the mass spectra, the form of the b2 ion could only be speculated in theory.
Fig. 2.
(A) The cleavage pattern of peptide; (B) HPLC–ESI-MS of Peak 1; (C,D,E,F) HPLC–ESI-MS/MS of the four major components in Peak 1. HPLC–ESI-MS = high performance liquid chromatography–electrospray ionization mass spectrometry; HPLC–ESI-MS/MS = high performance liquid chromatography–electrospray ionization tandem mass spectrometry.
The possible constitution of b2 (m/z 241.9) were Leu/Ile (L/I) and Gln/Lys (Q/K). L and I were isomers, and the molecular weight of Q and K were rather close (m/z 128.05858 and 128.09496, respectively), making it impossible to distinguish them under the instrument condition of this experiment. There were eight possible sequences in this situation, namely Q/K–L/I–N–P–A and L/I–Q/K–N–P–A. After comparison with the sequence of ZEA in SwissProt.2013.6.27 databases, K–L–N–P–A was the unique matched result. Therefore, the peptide with m/z 542.5 was determined to be K–L–N–P–A. Using this method, three other peptides were identified as M–H–S (m/z 374.4), Q–L–Q (m/z 388.4), and Q–I–R (m/z 416.3). The peptide of Q–L–Q, the leading component in Peak 1 (Fig. 2B), was found in abundance in the zein, which was the major protein fraction found in corn protein (representing about 68% in corn gluten meal [22]) by searching the database. There were 38 kinds of zein proteins containing this fraction, from which we could infer that the Q–L–Q was one of the important ingredients in CPs.
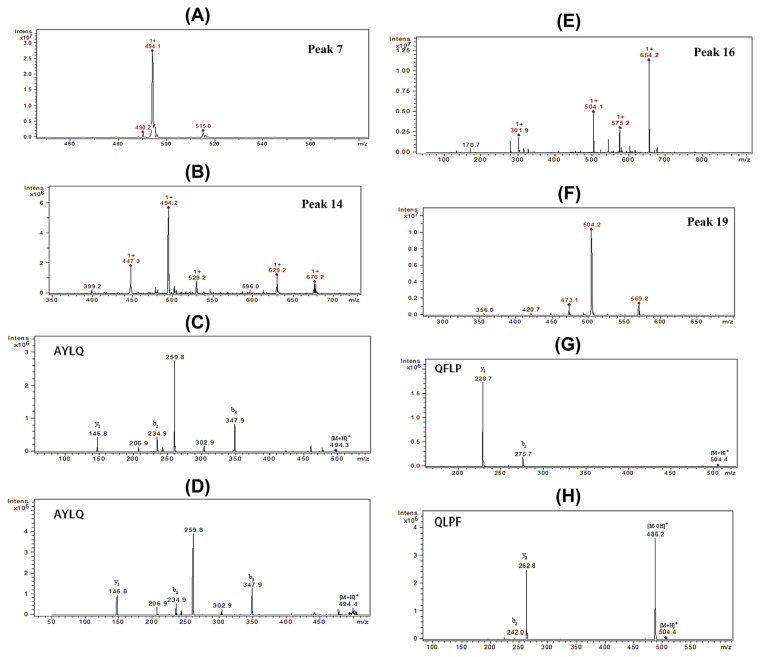
The ESI-MS spectra (Fig. 3A and 3B) showed that Peak 7 and Peak 14 had two ions with virtually the same m/z (494.1 and 494.2, respectively). Furthermore, their MS2 spectra (Fig. 3C and 3D) contained almost identical fragmental ions. The above suggested that they were the same peptide. In accordance with the MS2 data, it was identified as A–Y–L–Q. We supposed that this abnormal phenomenon was caused by the incomplete dissociation of the peptide in this HPLC condition: a portion of the peptide was eluted in the form of a molecule whereas the other part was in ion form. Ultimately, all of them could be detected using the UV and MS detectors. The incomplete dissociation, as a unique phenomenon in this experiment, had little influence on the qualitative analysis and the overall identification. The two ions in Peak 7 and Peak 14 were tentatively characterized as A–Y–L–Q. The other pair of ions with nearly equal m/z were m/z 504.4 from Peak 16 and m/z 504.4 from Peak 19 (Fig. 3E and 3F). Unlike the situation above, these two ions had distinct differences in the MS2, based on which their primary structures were determined to be Q–F–L–P and Q–L–P–F, respectively.
Fig. 3.
(A, B) HPLC–ESI-MS of Peak 7 and Peak 14, respectively; (C,D) HPLC–ESI-MS/MS of the two components of m/z 494.1 and 494.2 in Peak 7 and Peak 14, respectively, they were identified as the same peptide fraction (A–Y–L–Q) according to the identical MS/MS. (E,F) HPLC–ESI-MS of Peak 16 and Peak 19, respectively; (G,H) HPLC–ESI-MS/MS of the two components of m/z 504.1 and 504.2 in Peak 16 and Peak 19, respectively, which were identified as different peptide fractions (Q–F–L–P and Q–L–P–F). HPLC–ESI-MS = high performance liquid chromatography–electrospray ionization mass spectrometry; HPLC–ESI-MS/MS = high performance liquid chromatography–electrospray ionization tandem mass spectrometry; MS/ MS = tandem mass spectrometry.
Table 4 shows that 36 peptides fragments (marked with an *) among a total of 48 could be frequently found in the sequences of zein by searching the SwissProt. databases, which indicated the mass of these peptides, were most probably hydrolyzed from the zein. This result agreed with the abundance of zein in corn protein. In addition, the most common amino acids in all these peptides were Q (Gln) and L (Leu). Twenty six of the 48 peptides contained Q and 33 of 48 contained L. In our previous studies, L was the main amino acid component in CPs, representing approximately 17% of total amino acid content [23]. Q was also abundant in corn protein especially in zein. These facts were in accordance with the identification. It had been reported that L and A (Ala) contributed considerably to the bioactivity of facilitating alcohol metabolism, and L had better availability. Furthermore, L played an important role in skeletal muscle contraction and generated A as a source of pyruvic acid in muscle to sustain the tricarboxylic acid cycle which was broken by the excessive drinking [7]. Our laboratory had synthesized the peptide fraction of Q–L–L–P–F using solid phase peptide synthesis (SPPS) synthesizer (Sigma–Aldrich, St. Louis, Missouri, United States) in former studies. This peptide was certified to have a stronger ability to speed up alcohol metabolism than the mixed peptides [6]. Therefore, we inferred that the CPs, containing more L and A such as Q–A–L–A–A in Peak 2, Q–L–L–N–P–L in Peak 20, and Q–I–L–L–P–F in Peak 27, might have positive effects on alcohol metabolism. The antioxidant activity of peptides reported by Zhuang et al [24] was attributed to the high hydrophobic amino acid residue content (Leu, Pro, and Phe) which had great ability to scavenge free radicals. The L–L–P–F and F–L–P–F, which could also be found in Peak 25 and Peak 26, proved to exhibit activities of free radical scavenging and lipid peroxidation inhibition in his trial [25]. Meanwhile, these abilities of free radical scavenging and lipid peroxidation inhibition led to the prevention of hepatic fibrosis in our previous research [23]. In Table 4, many peptides with hydrophobic amino acid could be predicted to have similar activities to these two peptide fractions, and further studies should be carried out. In addition, the antihypertension effects of CPs was closely connected with their primary structure. Peptides, with aromatic amino acids (Tyr, Phe, Trp, and His), aliphatic hydrophobic amino acids (Ile, Ala, Leu, and Met), and Pro in the C-terminal, as well as branched chain amino acids (Leu, Ile, and Val) in N-terminal, were tested to be an inhibitor of angiotensin converting enzyme and exhibit activity of hypotension [26]. A considerable amount of peptide fractions in Table 4, such as L–A–H, N–P–S–P–A, and F–L–P–F in Peak 2, Peak 3, and Peak 26, respectively, had the potential ability of antihypertension, according to the above theory.
Although the CPs had been studied for decades, the objectives of previous research were mainly focused on the efficacy of the mixed peptide rather than the specific structure. In the present research on the structure of CPs, the most common method has been to identify one or two peaks in chromatograms after exploring the activities of some major ingredients. With this method, the identification of the peaks was solitary and accidental, without enough persuasion. Our experiment aimed to analyze the primary structures of CPs comprehensively and systematically to seek some connections among the peptide fractions in such a complex compound, tentatively explaining some relationships between the structures and functions. The other purpose of this study was to provide a reference for future research. In the future, the activities of many of the identified peptides could be tested both individually and cooperatively.
In summary, this study established a HPLC fingerprinting method to assess the integral quality of CPs, which laid a good foundation for further research, exploitations, and applications in industry. The primary structures of CPs were firstly analyzed systematically. Twenty-eight peaks including 48 major peptide fractions were identified. The majority of these peptides had not been reported before. Many of them contained the amino acids of Q, L, and A, which played an important role in the exhibition of the bioactivities of CPs. Furthermore, many peptides had a similar primary structure to the peptides which had been proved to be bioactive, such as facilitating alcohol metabolism, scavenging free radicals, and inhibiting lipid peroxidation. Future studies should be carried out to illuminate other connections between the structures and functions of CPs.
Acknowledgments
This research is supported by the Fundamental Research Funds for the Central Universities (No. 2013PY095).
Funding Statement
This research is supported by the Fundamental Research Funds for the Central Universities (No. 2013PY095).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1. Mellander O. The physiological importance of the casein phosphopeptides calcium salts. II. Per oral calcium dosage of infants. Acta Society Medicine Uppsala. 1950;55:247–55. [PubMed] [Google Scholar]
- 2. Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. 2014;54:171–9. doi: 10.1016/j.peptides.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 3. Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Tech. 2000;11:254–62. [Google Scholar]
- 4. Schaafsma G. Safety of protein hydrolysates, fractions thereof, and bioactive peptides in human nutrition. Eur J Clin Nutr. 2009;63:1161–8. doi: 10.1038/ejcn.2009.56. [DOI] [PubMed] [Google Scholar]
- 5. Hur SJ, Lim BO, Decker EA. In vitro human digestion models for food applications. Food Chem. 2011;125:1–12. [Google Scholar]
- 6. Ma ZL, Zhang WJ, Yu GC, He H, Zhang Y. The primary structure identification of a corn peptide facilitating alcohol metabolism by HPLC–MS/MS. Peptides. 2012;37(1):138–43. doi: 10.1016/j.peptides.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi M, Nishikiori F, Ito M, Furukawa Y. The effects of corn peptide ingestion on facilitating alcohol metabolism in healthy men. Biosci Biotech Biochem. 1997;61:1474–81. doi: 10.1271/bbb.61.1474. [DOI] [PubMed] [Google Scholar]
- 8. Yu GC, Li JT, He H, Huang WH, Zhang WJ. Ultrafiltration preparation of potent bioactive corn peptide as alcohol metabolism stimulator in vivo and study on its mechanism of action. J Food Biochem. 2013;37(2):161–7. [Google Scholar]
- 9. Zhou K, Sun S, Canning C. Production and functional characterisation of antioxidative hydrolysates from corn protein via enzymatic hydrolysis and ultrafiltration. Food Chem. 2012;135(3):1192–7. doi: 10.1016/j.foodchem.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 10. Li JT, Zhang JL, He H, Ma ZL, Nie ZK, Wang ZZ, Xu XG. Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food Chem Toxicol. 2013;51:297–305. doi: 10.1016/j.fct.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 11. Huang WH, Sun J, He H, Dong HW, Li JT. Antihypertensive effect of corn peptides, produced by a continuous production in enzymatic membrane reactor, in spontaneously hypertensive rats. Food Chem. 2011;128(4):968–73. [Google Scholar]
- 12. Lv J, Nie ZK, Zhang JL, Liu FY, Wang ZZ, Ma ZL, He H. Corn peptides protect against thioacetamide-induced hepatic fibrosis in rats. J Med Food. 2013;16(10):912–9. doi: 10.1089/jmf.2012.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu GC, Lv J, He H, Huang WH, Han Y. Hepatoprotective effects of corn peptides against carbon tetrachloride—induced liver injury in mice. J Food Biochem. 2012;36(4):458–64. [Google Scholar]
- 14. Guo H, Sun J, He H, Yu GC, Du J. Antihepatotoxic effect of corn peptides against Bacillus Calmette–Guerin/ lipopolysaccharide-induced liver injury in mice. Food Chem Toxicol. 2009;47(10):2431–5. doi: 10.1016/j.fct.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Liang J, Liu J, Zhao Y, Gao J, Sun W, Ito Y. Quality control and identification of steroid saponins from Dioscorea zingiberensis C.H. Wright by fingerprint with HPLC-ELSD and HPLC-ESI-Quadrupole/Time-of-fight tandem mass spectrometry. J Pharmaceut Biomed. 2014;91:46–59. doi: 10.1016/j.jpba.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yudthavorasit S, Wongravee K, Leepipatpiboon N. Characteristic fingerprint based on gingerol derivative analysis for discrimination of ginger (Zingiber officinale) according to geographical origin using HPLC-DAD combined with chemometrics. Food Chem. 2014;158:101–11. doi: 10.1016/j.foodchem.2014.02.086. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Li Q, Wang Q, Li Y, Ling J, Liu L, Chen X. Simultaneous determination of seven bioactive components in Oolong Tea Camellia sinensis: quality control by chemical composition and HPLC fingerprints. J Agr Food Chem. 2011;60(1):256–60. doi: 10.1021/jf204312w. [DOI] [PubMed] [Google Scholar]
- 18. Xie Z, Zhao Y, Chen P, Jing P, Yue J, Yu L. Chromatographic fingerprint analysis and rutin and quercetin compositions in the leaf and whole-plant samples of di-and tetraploid Gynostemma pentaphyllum. J Agr Food Chem. 2011;59(7):3042–9. doi: 10.1021/jf104329v. [DOI] [PubMed] [Google Scholar]
- 19. Losito I, Carbonara T, Domenia De Bari M, Gobbetti M, Palmisano F, Rizzello CG, Zambonin PG. Identification of peptides in antimicrobial fractions of cheese extracts by electrospray ionization ion trap mass spectrometry coupled to a two-dimensional liquid chromatographic separation. Rapid Commun Mass Spectrom. 2006;20(3):447–55. doi: 10.1002/rcm.2323. [DOI] [PubMed] [Google Scholar]
- 20. Li HM, Hu X, Guo P, Fu P, Xu L, Zhang XZ. Antioxidant properties and possible mode of action of corn protein peptides and zein peptides. J Food Biochem. 2010;34:44–60. [Google Scholar]
- 21.Yang PY, Qian XH, Sheng LS, editors. Biological mass spectrometry technology and the method. Beijing: Science Press; 2003. In Chinese. [Google Scholar]
- 22. Kim JM, Whang JH, Kim KM, Koh JH, Suh HJ. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process Biochem. 2004;39(8):989–94. [Google Scholar]
- 23.Lv J. MSD thesis. Wu Han: Huazhong Agricultural University; 2012. Preparation of corn peptides with antialcoholism and hepatoprotective effects by enzyme membrane reactor and their structures, activities. In Chinese. [Google Scholar]
- 24. Zhuang H, Tang N, Yuan Y. Purification and identification of antioxidant peptides from corn gluten meal. J Funct Foods. 2013;5(4):1810–21. [Google Scholar]
- 25. Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A placebo-controlled study of the sour milk on blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–30. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 26. Cheung HS, Wang FL, Ondetti MA, Sabo EF, Cushman DW. Binding of peptide substrate and inhibition of angiotensin I—converting enzyme importance of the COOH-terminal dipeptides sequence. J Biol Chem. 1980;255:401–7. [PubMed] [Google Scholar]