Abstract
Mycotoxins are toxic food contaminants that are naturally produced by certain fungi. They induce negative effects on human health by making food unsafe for consumption. In this study, analyses were performed to determine the levels and incidence of aflatoxins (AFs) in peanut products, tree nuts, spices, and Coix seeds; ochratoxin A (OTA) in wheat and roasted coffee, as well as OTA and AFs in rice; and citrinin (CIT) in red yeast rice (RYR) products. A total of 712 samples from nine different food categories were collected between 2012 and 2013. The samples were analyzed over 2 years for AFs, OTA, and CIT by methods recommended by the Ministry of Health and Welfare. These official analytical methods were extensively validated in-house and through interlaboratory trials. The analytical values of suspected contaminated specimens were confirmed by liquid chromatography – tandem mass spectrometry analysis to identify the specific mycotoxin present in the sample. We show that 689 samples (96.8%) complied with the regulations set by the Ministry of Health and Welfare. AFs were found in four peanut-candy products, one peanut-flour product, one pistachio product, one Sichuan-pepper product, and one Coix seed product. All had exceeded the maximum levels of 15 parts per billion for peanut and 10 parts per billion for other food products. Furthermore, 14 RYR samples contained CIT above 5 parts per million, and one RYR tablet exceeded the maximum amount allowed. Instances of AFs in substandard Sichuan pepper and Coix seeds were first detected in Taiwan. Measures were taken by the relevant authorities to remove substandard products from the market in order to decrease consumer exposure to mycotoxin. Border control measures were applied to importing food commodities with a higher risk of mycotoxin contamination, such as peanut, Sichuan pepper, and RYR products. Declining trends were observed in the noncompliance rate of AFs in peanut products, as well as that of CIT in RYR raw materials monitored from 2010 to 2013.
Keywords: aflatoxin, citrinin, ochratoxin A, Taiwan
1. Introduction
Mycotoxins are toxic metabolites produced by fungi. They are mostly found as natural contaminants of food products sold by supermarket chains and grocery markets, and are detrimental to human health [1,2]. Currently, more than 400 different types of mycotoxins have been identified. However, the most significant type presenting strong public-health concerns is aflatoxin (AF), followed by ochratoxin A (OTA), and other Fusarium toxins [3]. The occurrence of mycotoxins in food often corresponds to a geographical pattern. Worldwide food trading results in worldwide distribution of contaminated materials [4].
Aspergillus parasiticus and Aspergillus flavus represent the major proportion of AFs found in peanuts, nuts, seeds, spices, and various other crops and food products. Both of them contain AF-producing gene clusters. A. parasiticus produces AFB1, AFB2, AFG1, and AFG2. However, A. flavus only produces AFB1 and AFB2 [5,6]. AF production was observed in A. parasiticus, grown on media with glucose or lactose as the sole carbon source [7,8]. AF production was enhanced in the presence of sugar and unsaturated fatty acids in media [7,9]. AFB1 is one of the most potent natural carcinogens known [10]. Regardless of the ingested dose level, its cumulative effect is to increase cancer risk and heighten the probability of liver cancer in patients suffering from hepatitis B or hepatitis C. Interestingly, children display the greatest susceptibility to AFs [11]. The International Agency for Research on Cancer (IARC) classified AFs as a group 1 human carcinogen [10]. The CODEX Alimentarius Commission (CAC) recommended that intake should be reduced to levels as low as reasonably possible for AF B, G, and M [12].
OTA is a common contaminant of grain storage in temperate regions. The gene clusters responsible for the biosynthesis of OTA have been identified in Aspergillus and Penicillium genera [13]. The presence of OTA has also been reported in food categories, such as rice, wheat, coffee, dried fruit, and spices. OTA exhibits nephrotoxic, carcinogenic, and immunotoxic properties. Its main target is the renal proximal tubule, where it exerts cytotoxic and carcinogenic effects [14]. The IARC classified OTA as class 2B, indicating that it is a possible human carcinogen [15]. OTA has a half-life of up to 840 hours in the human bloodstream with cumulative effect in vivo [11]. The CAC set a provisional tolerable weekly intake (PTWI) of 0.0001 mg/kg body weight (BW) [12].
Red yeast rice (RYR) made from Monascus-fermented cooked rice is a traditional cuisine in Taiwan, and is often used as food coloring and preservative. The lactone form of monacolin K produced by Monascus purpureus, also known as lovastatin, has an inhibitory effect on cholesterol synthesis [16]. Since lovastatin became a patented prescription drug, monacolin K-containing Monascus products can only be used as food or nonprescription dietary supplements [17]. However, some strains of Monascus contain gene clusters responsible for the biosynthesis of monacolin K and citrinin (CIT) [18,19]. The toxicological effects of such strains have been shown to arise through interference with mitochondrial electron transport and calcium homeostasis, leading to kidney swelling or necrosis in animal studies [20,21]. The IARC has designated CIT as group 3, indicating that it is not classifiable as a human carcinogen [22]. In a 90-day study, the nonobservable adverse effect level of CIT was determined to be 20 μg/kg of BW/day in rats [23]. The European Union (EU) recommended the level of no concern of nephrotoxicity as 0.2 μg/kg of BW/day [17].
Mycotoxin legal limits were established in several countries and international organizations worldwide, specifying the maximum limits for mycotoxins [12,24–27]. Taiwan set a total AF limit of 15 parts per billion (ppb) for peanut and maize, and 10 ppb for other foods. The EU set the lowest limits at 4 ppb for total AFs and 2 ppb for AFB1. However, the United States regulates all foods at 20 ppb for AFs. CAC provisions stipulate 10 ppb for ready-to-eat nuts and 15 ppb for peanuts and nuts intended for further processing. For OTA, the limits were set at 5 ppb for coffee and wheat and rice categories, which is similar to the EU. As for CIT content requirements, RYR pigments, raw RYR, and RYR-based foods had limits set at 200 ppb, 5 parts per million (ppm), and 2 ppm, respectively, or below (Table 1).
Table 1.
Regulation limits of aflatoxins, ochratoxin A, and citrinin set by the CODEX Alimentarius Commission, European Commission, USA and Japan.
| Organizations and countries | Mycotoxins | Maximum limits (μg/kg) | Food commodities | Year of announcement |
|---|---|---|---|---|
| CAC | Total aflatoxins | 10 | Peanuts and pistachios (ready to eat) | 2013 |
| Ochratoxin A | 5 | Raw wheat, barley, and rye | ||
| EC | Total aflatoxins | 4.0 | Peanuts intended for direct human consumption | 2006 |
| 10 | Pistachios and almonds intended for direct human consumption | |||
| 10 | Capsicum spp. | |||
| 4.0 | All cereals and all products derived from cereals | |||
| Aflatoxin B1 | 2.0 | Peanuts intended for direct human consumption | ||
| 8.0 | Pistachios and almonds intended for direct human consumption | |||
| 5.0 | Capsicum spp. | |||
| 2.0 | All cereals and all products derived from cereals | |||
| Ochratoxin A | 3.0 | All products derived from unprocessed cereals intended for direct human consumption | ||
| 5.0 | Roasted coffee beans and ground roasted coffee | |||
| USA | Total aflatoxins | 20 | All foods | 2013 |
| Japan | Total aflatoxins | 10.0 | All foods | 2010 |
CAC = CODEX Alimentarius Commission; EC = European Commission.
It is difficult to remove mycotoxins from food products once contaminated. Previous studies have demonstrated higher incidences of AF contamination in peanuts, nuts, and spices, as well as CIT in RYR products [28,29]. In Taiwan, rice and wheat flour are staple foods, and the consumption of coffee has increased in the last decade. In order to protect the consumer’s food safety, a market-monitoring plan was designed to investigate the levels of AFs in peanuts, nuts, and spices; OTA in coffee and wheat products; AFs and OTA in rice-based products; and CIT in RYR-based products. The results of this investigation will provide health authorities with an assessment of mycotoxin contamination in circulating foods in domestic markets, and serve as a reference for food management.
2. Methods
2.1. Samples
A total of 712 samples, including peanut products, nuts, dried fruit, wheat and rice products, Coix seeds, coffee, and RYR products, were collected from supermarkets, traditional markets, and grain dealers by 22 local health bureaus within their jurisdictions, between March 2012 and September 2013. The samples were sent to the laboratory, where they were grounded with a grinding mill (ZM 200; Retsch, Haan, Germany) and stored at 4°C prior to further analysis. For capsule-supplement samples, the capsule walls were removed before testing.
2.2. Chemicals
The AFB1, AFB2, AFG1, AFG2, and OTA standards were purchased from Supelco (St. Louis, MO, USA). The CIT standards were purchased from Fermentek (Jerusalem, Israel). The AflaTest-P and OchraTest columns were both purchased from Waters (Milford, MA, USA). Mass spectrometry (MS) grade methanol and acetonitrile were purchased from J.T. Baker (Center Valley, PA, USA). Sodium bicarbonate, sodium chloride, anhydrous disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, sodium carbonate, formic acid, and hydrochloric acid were purchased from Merck (Darmstadt, Germany). Advantec filter papers of 150 mm in diameter were purchased from Toyo Roshi Kaisha (Tokyo, Japan). Syringe filters with a pore size of 0.22 μm (nylon, 47 mm) were purchased from ChromTech (Johannesburg, South Africa). A KRC 25-25 photoderivatization kit was purchased from Aura (New York, NY, USA), and the water-purification system was purchased from ELGA (Lowell, MA, USA).
2.3. Extraction of samples
The protocols for testing for AFs, OTA, and CIT in different food categories were performed according to official testing methods as described previously [28–30]. About 25 g of peanut samples was extracted with 5 g of sodium chloride and 125 mL of 60% methanol solution, and homogenized with a homogenizer (Nihon Seiki, Tokyo, Japan) at 15,000 rpm for 2 minutes. About 50 g of grain sample was homogenized with 5 g of sodium chloride and 100 mL of 80% methanol solution. The homogeneous mixture was filtered with filter paper, and then 20 mL of peanut filtrate or 10 mL of grain filtrate was diluted with 20 mL or 40 mL of deionized water, respectively, and filtered using a glass wool filter. Next, 10 mL of filtrate was passed through an AflaTest column and washed twice with 10 mL of deionized water. AFs were eluted from the column with 1 mL of methanol. Deionized water was added to the eluate to bring the volume to 2 mL. It was then filtered using a 0.22 μm syringe filter and collected in a brown glass tube. About 5 g of ground coffee was placed into a 50 mL centrifuge tube, and to which 25 mL of coffee-extraction solution was added. This mixture was shaken for 3 minutes, and then centrifuged at 2500g for 10 minutes. The supernatant was filtered through a filter paper. The filtrate (2 mL) was added to 48 mL of phosphate-buffered solution and filtered through a glass wool filter. For rice and wheat specimens, 25 g of sample was added to 100 mL of grain-extraction solution and shaken for 3 minutes, and then centrifuged at 2500g for 10 minutes. The supernatant was filtered through a filter paper, and 4 mL of filtrate was mixed with 44 mL of phosphate-buffered solution, and filtered through a glass wool filter. Then, 25 mL of coffee filtrate (equivalent to 0.2 g of specimen) or all of the grain filtrate was passed through the OchraTest column, washed twice with 10 mL of deionized water, and then eluted with 2 mL of methanol with a flow rate of 1 drop/s. The eluate was evaporated at 40°C with nitrogen until dryness was achieved. The residue was reconstituted in 50% acetonitrile solution at a volume of 1 mL, and then filtered using a 0.22 μm syringe filter. The filtrate was then used for analysis. About 1 g of ground RYR sample was added to 20 mL of methanol, and shaken for 1 minute. The mixture was then placed in a 70°C water bath for 30 minutes, and was then allowed to cool at room temperature. The supernatant was collected and filtered using a 0.22 μm syringe filter, and then used for analysis.
2.4. High-performance-liquid-chromatography analysis
The high-performance-liquid-chromatography (HPLC) system used in this study was a Hitachi L-2300 series (Schaumburg, IL, USA) equipped with a fluorescence detector. A 50 μL aliquot of the AF test solution was used for precolumn photoderivatization in a photochemical reactor, and then separated by a Cosmosil C18-AR column (5 μm, 4.6 mm × 250 mm) (NACALAI TESQUE, INC, Japan). The mobile phase consisted of methanol/water [45/55, volume/volume (v/v) %] at a flow rate of 1 mL/minute. AFs were detected using the fluorescence detector at an excitation wavelength (Ex) of 360 nm and emission wavelength (Em) of 440 nm. OTA was analyzed using a mobile phase of deionized water, acetonitrile, and acetic-acid solution at a 99:99:2 ratio (v/v), respectively, and a flow rate of 1 mL/min. The injection volume was 100 μL. OTA was separated by a reversed-phase C18 column (5 μm, inner diameter of 4.6 mm × 25 cm). The Ex was set at 333 nm and the Em at 460 nm. A 20 μL aliquot of the CIT test solution was injected into the HPLC system. The mobile phase consisted of acetonitrile, deionized water, and formic acid at a 49:49:2 ratio (v/v), respectively, and a flow rate of 1 mL/minute. The chromatographic column was Atlantis T3 (Waters) (5 μm, inner diameter of 4.6 mm × 25 cm). The Ex was set at 330 nm and the Em at 500 nm.
2.5. Quality assurance
The testing methods were validated in accordance with the guidelines of the validation for testing methods in food chemistry [31], and included linearity of calibration curve, limit of quantification (LOQ), accuracy, and precision. In validating the linear calibration curve, the AFB1 and AFG1 standards were prepared in the range of 0.2–50 ng/mL, the AFB2 and AFG2 standards were 0.1–15 ng/mL, while the OTA standard was diluted to 0.3–50 ng/mL. Each standard calibration-curve plot consisted of 6 points. The relative coefficient (r) was expected to be greater than 0.99 for standard curve validation. The CIT standard was spiked into blank RYR samples in the range of 0.0025–0.5 μg/mL. The r value of the CIT calibration curve was also expected to be greater than 0.99 for curve validation. LOQ was validated by spiking at the level of detection limits described in the official testing methods into blank specimens. The recommended procedures for extraction were followed and samples were analyzed in triplicate. An acceptable resulting signal/noise ratio was designated as greater than 10. The accuracy and precision were assessed by spiking the AF, OTA, and CIT standards at the levels of 1, 2, and 10 folds, respectively, of the detection limits of each official testing method in blank food samples. The samples were analyzed in five replicates, and the recommended procedures for extraction and HPLC analysis were performed. The recovery and coefficient of variation (%CV) were expected to be consistent with the published guidelines [31]. Furthermore, we evaluated the results of the international proficiency tests for satisfactory conformance to the recommendations.
2.6. Liquid chromatography/mass spectrometry/mass spectrometry analysis
Substandard specimens were confirmed by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) analysis, which was performed on a Xevo TQ system (Waters) equipped with Acquity LC pump (Waters), autosampler, and an electrospray ionization (ESI) interface. Data acquisition was performed using the MassLynx version 4.1 software (Waters). Chromatographic separation was achieved using a UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm, Waters). The injection volume was 10 μL, and the mobile phase was composed of A solution (0.5% formic acid in deionized water) and B solution (0.5% formic acid in methanol) at a flow rate of 0.3 mL/min. The solvent gradient was as follows: 0 minutes, 95% A; 0–5 minutes, 95–15% A; 5–5.8 minutes, 15–0% A; 5.8–7 minutes, 0% A; 7–7.1 minutes, 0–95% A; 7.1–9 minutes, 95% A. The ESI interface was operated in the positive-ion mode. The parameters for ESI operation were as follows: capillary voltage, 3.4 KV; ion-source temperature, 150°C; desolvation temperature, 500°C; desolvation gas flow, 1000 L/h. The quantitative and confirmative determination of AFs, OTA, and CIT was applied in the multiple-reaction-monitoring mode. Each toxin transition ion pair for AFB1 was m/z 313 → 245 and m/z 313 →285, AFB2: m/z 315 → 287 and m/z 315 → 259, AFG1: m/z 329 →200 and m/z 329 →243, AFG2: m/z 331 → 189 and m/z 331 →313, OTA: m/z 404 →239 and m/z 404 →102, and CIT: m/z 251 →233 and m/z 251 →205.
2.7. Data analysis
The AF, OTA, and CIT concentrations from the HPLC and LC/MS/MS analyses were determined using the EZChrom Elite software version 3.17 (Hitachi Co., Tokyo, Japan) and MassLynx software version 4.1, respectively. The data were exported to Microsoft Excel 2010 (Microsoft Co., Redmond, WA, USA) to calculate the mean, standard deviation, and %CV.
3. Results
3.1. Method performance
The LOQ for each mycotoxin was determined. The LOQ for AFB1 and AFG1 in peanuts was 0.2 μg/kg, the AFB2 and AFG2 in peanuts was 0.1 μg/kg, the OTA in rice was 0.3 μg/kg, the OTA in coffee was 0.5 μg/kg, and the CIT in RYR was 0.05 μg/kg. Good linearity (r > 0.995) was observed in AFB1 (0.2–50 ng/mL), AFB2 (0.1–15 ng/mL), AFG1 (0.2–50 ng/mL), AFG2 (0.1–15 ng/mL), OTA (0.3–50 ng/mL), and CIT (0.025–5.0 μg/mL). Table 2 shows the validation results of the testing methods for quantification of AFs, OTA, and CIT. Recoveries were ascertained by spiking 0.4, 1, and 2 μg/kg of AFB1; AFG1: 0.2, 0.5, and 1 μg/kg of AFB2; and AFG2: 0.6, 1.5, and 3 μg/kg of OTA in blank, peanut, and coffee samples, respectively. Recoveries of AFB1, AFG1, AFB2, and AFG2 from the peanut matrix ranged from 75.1% to 91.8%, with %CV between 3.37% and 7.93%. OTA recovery in coffee ranged between 78.2% and 81.9% with %CV at 2.63–4.91%. CIT recovery in RYR was 92.6–94.6% with %CV at 3.37–4.50%. These results are in line with food guidelines. The testing performance of the laboratory was verified by participating in a proficiency test of AFs in peanut flour (number 04211) and OTA in coffee powder (number 17119) under the Food Analysis Performance Assessment Scheme. Our obtained z scores for AFB1, AFB2, AFG1, AFG2, total AF, and OTA were all within the satisfactory limits.
Table 2.
Validation of the analytical methods used for determining the levels of aflatoxins, ochratoxin A, and citrinin in various foods.
| Product | Mycotoxins | Spiking levels (μg/kg) | Recovery % (n = 5) | Repeatability (n = 5) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Average | Requirement (%)a | %CV | Requirement (%)a | |||
| Peanut candy | Aflatoxin B1 | 0.2 | 89.5 ± 4.1 | 50–125 | 4.63 | <35 |
| 0.4 | 90.2 ± 5.2 | 50–125 | 4.50 | <35 | ||
| 2 | 88.5 ± 5.5 | 60–125 | 6.23 | <30 | ||
| Aflatoxin B2 | 0.1 | 90.5 ± 7.4 | 50–125 | 8.15 | <35 | |
| 0.2 | 88.3 ± 5.5 | 50–125 | 6.23 | <35 | ||
| 1 | 91.8 ± 5.0 | 60–125 | 5.47 | <30 | ||
| Aflatoxin G1 | 0.2 | 84.4 ± 4.7 | 50–125 | 6.81 | <35 | |
| 0.4 | 87.6 ± 4.4 | 50–125 | 7.93 | <35 | ||
| 2 | 86.3 ± 5.0 | 60–125 | 7.42 | <30 | ||
| Aflatoxin G2 | 0.1 | 75.1 ± 4.3 | 50–125 | 4.34 | <35 | |
| 0.2 | 77.9 ± 5.3 | 50–125 | 6.61 | <35 | ||
| 1 | 76.8 ± 6.4 | 60–125 | 6.03 | <30 | ||
| Coffee | Ochratoxin A | 0.5 | 78.2 ± 5.1 | 50–125 | 4.09 | <35 |
| 1 | 81.1 ± 4.3 | 60–125 | 2.63 | <30 | ||
| 5 | 81.9 ± 3.7 | 60–125 | 4.91 | <30 | ||
| Red yeast rice | Citrinin | 50 | 92.6 ± 4.7 | 70–120 | 3.54 | <20 |
| 100 | 93.4 ± 4.6 | 70–120 | 4.50 | <15 | ||
| 500 | 94.6 ± 3.2 | 70–120 | 3.37 | <15 | ||
%CV = coefficient of variation.
The validation guidelines for testing methods in food chemistry set by the Taiwan Food and Drug Administration.
3.2. Monitoring results
In this study, a total of 712 specimens were collected, and the recommended methods for testing AFs, OTA, and CIT were applied. Of these samples, 689 (96.8%) were qualifying samples and 23 were substandard specimens, as determined by the LC/MS/MS analysis. Eight samples had AF contamination levels that exceeded the regulatory limits, while 15 cases had CIT contamination that exceeded limits.
The incidence and occurrence levels of AFs are shown in Table 3. The results demonstrated that 25.5% of the samples, including peanut products, tree nuts, spices, Coix seeds, and raisins, were positive for AFs. However, no AF contamination was detected in rice. Foods having a higher incidence of AF contamination included Sichuan pepper (100%), peanut butter (60%), peanut flour (43.5%), Coix seeds (33.3%), and peanut candy (24.4%). Four peanut-candy cases (3.2%) and one peanut-flour case (6.2%) contained AF contamination that exceeded the limit for AFs in peanuts. Furthermore, there was one case (5.5%) of red Coix seeds, one case (20%) of Sichuan pepper, and one case (10%) of pistachios exceeding the AF limits for other foods (10 ppb). The highest AF contamination levels were found in pistachios at 245.6 ppb and peanut candy at 117 ppb. Among the substandard products, the incidence of AFB1 was the highest, followed by AFB2. There were two cases of substandard peanut candy, pistachios, Sichuan pepper, and Coix seeds that were only contaminated with the B-type AF, while there were two cases of peanut candy contaminated with type B and type G, but only with AFG2 and not AFG1 (Table 4). These results suggest that peanut candy bore the highest risk of AF contamination, followed by peanut flour, pistachios, Sichuan pepper, and Coix seeds.
Table 3.
Incidence and occurrence levels of total aflatoxins in various foods in Taiwan from 2012 to 2013.
| Sample | Year | No. of samples | No. of positive samples (%) | Mean concentration of positive samples (range of concentration) | No. of substandard samples (%) |
|---|---|---|---|---|---|
| Whole peanut | 2012 | 23 | 2 (8.7) | 1.5 (0.3–2.7) | 0 |
| 2013 | 12 | 0 | <LOQ | 0 | |
| Total | 35 | 2 (5.7) | 1.5 (0.3–2.7) | 0 | |
| Peanut butter | 2012 | 14 | 11 (78.6) | 1.9 (0.2–3.5) | 0 |
| 2013 | 16 | 7 (43.8) | 2.8 (0.3–5.6) | 0 | |
| Total | 30 | 18 (60.0) | 2.2 (0.2–5.6) | 0 | |
| Peanut candy | 2012 | 44 | 10 (22.7) | 18.4 (0.3–117) | 2 (4.5) |
| 2013 | 79 | 20 (25.3) | 6.0 (0.2–40.5) | 2 (2.5) | |
| Total | 123 | 30 (24.4) | 12.2 (0.2–117) | 4 (3.2) | |
| Peanut flour | 2012 | 30 | 12 (40.0) | 2.9 (0.2–8.2) | 0 |
| 2013 | 16 | 8 (50.0) | 6.1 (0.2–31.6) | 1 (6.2) | |
| Total | 46 | 20 (43.5) | 4.3 (0.2–31.6) | 1 (2.2) | |
| Overall total of peanut products | 234 | 72 (30.8) | 7.76 (0.2–117) | 5 (2.1) | |
| Nuts | 2012 | 40 | 6 (15) | 41.3 (0.2–245.6) | 1 (2.5) |
| 2013 | 16 | 1 (6.2) | 0.2 | 0 | |
| Total | 56 | 7 (12.5%) | 35.4 (0.2–245.6) | 1 (1.8) | |
| Spices | 2012 | 30 | 9 (30.0) | 3.3 (0.2–23.5) | 1 (3.3) |
| Coix seeds | 2013 | 18 | 6 (33.3) | 4.3 (0.5–15.7) | 1 (5.5) |
| Dried fruit | 2013 | 14 | 1 (7.1) | 0.1 | 0 |
| Rice | 2013 | 20a | 0 | <LOQ | 0 |
| Overall total of samples | 372 | 95 (25.5) | 9.07 (0.1–245.6) | 8 (2.2) |
AF = aflatoxin; LOQ = limit of quantification; OTA = ochratoxin A.
AFs and OTA were concurrently analyzed in 20 rice specimens.
Table 4.
The level of aflatoxin contamination and origin of substandard products in Taiwan from 2012 to 2013.
| Year | Product | Level of contamination (μg/kg) | Maximum level of total AFs (μg/kg) | Origin | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||||
| 2012 | Peanut candy | 35.2 | 7.0 | <LOQ | 0.8 | 42.2 | 15 | Vietnam |
| Peanut candy | 89.3 | 24.5 | <LOQ | 2.8 | 117 | 15 | Taiwan | |
| Pistachio | 233.6 | 12.0 | <LOQ | <LOQ | 245.6 | 10 | Iran | |
| Sichuan pepper | 20.2 | 3.3 | <LOQ | <LOQ | 23.5 | 10 | China | |
| 2013 | Peanut candy | 35.0 | 5.4 | <LOQ | <LOQ | 40.4 | 15 | Vietnam |
| Peanut candy | 34.0 | 6.5 | <LOQ | <LOQ | 40.5 | 15 | Vietnam | |
| Peanut flour | 26.4 | 5.2 | <LOQ | <LOQ | 31.6 | 15 | Taiwan | |
| Coix seeds | 14.3 | 0.9 | <LOQ | <LOQ | 15.2 | 10 | Thailand | |
AF = aflatoxin; LOQ = limit of quantification.
OTA and AFs were concurrently analyzed in 20 rice-product samples. There were no detectable levels in the rice products (Table 3). Two (10%) oat-product cases were found to be OTA positive (Table 5). In 28 cases of roasted coffee beans, no instance of OTA was detected. However, in 32 cases of ground coffee, three cases (9.4%) contained OTA (Table 5) with a range of 0.8–2.1 ppb, and the average contamination level was 1.47 ppb.
Table 5.
Incidence and levels of ochratoxin A contamination in various commercial cereals and coffee products in Taiwan from 2012 to 2013.
| Sample | No. of samples | No. of positive samples (%) | Mean of positive samples (range, ng/g) | No. of substandard samples (%) |
|---|---|---|---|---|
| Rice | 45 | 0 | <LOQ | 0 |
| Oat | 20 | 2 (10.0) | 2.95 (2.9–3.0) | 0 |
| Wheat and their products | 29 | 0 | <LOQ | 0 |
| Roasted coffee bean | 28 | 0 | <LOQ | 0 |
| Coffee powder | 32 | 3 (9.4) | 1.47 (0.8–2.1) | 0 |
| Total | 154 | 5 (3.2) | 2.06 (0.8–3.0) | 0 |
LOQ = limit of quantification.
Out of a total of 206 RYR product samples, there was a CIT-positive rate of 27.2%. In 15 of these cases (7.3%), the CIT contamination levels exceeded the maximum limits (Table 6). Higher incidences of CIT contamination were found in RYR raw material at 63.6% and RYR supplement at 24.1%. For the RYR raw material, there were 14 cases (42.4%) exhibiting CIT contamination levels exceeding the maximum limit of 5 ppm, and one case (1.7%) of RYR supplement showing a CIT contamination level exceeding the maximum limit of 2 ppm. The average CIT contamination levels were 13.0 mg/kg in RYR raw materials, 0.63 mg/kg in RYR dietary supplements, and 0.59 mg/kg for processed foods containing RYR raw materials. The highest level of CIT contamination was found in RYR raw materials at a level of 63.4 mg/kg, and 4.9 mg/kg for RYR tablets.
Table 6.
Incidence and levels of citrinin contamination in various commercial Monascus products in Taiwan in 2012–2013.
| Product | Year | No. of samples | No. of CIT-positive samples (%) | Mean concentrationa (range, mg/kg) | No. of substandard samples (%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Imported | Domestic | |||||
| Red yeast rice (raw material) | 2012 | 18 | 12 | 14.0 (1.45–31.43) | 7 (38.9) | 3 (16.7) |
| 2013 | 15 | 9 (60.0) | 11.7 (1.9–63.4) | 1 (6.7) | 3 (20) | |
| Total | 33 | 21 (63.6) | 13.0 (1.45–63.4) | 8 (24.2) | 6 (18.2) | |
| Dietary supplements | 2012 | 28 | 8 (28.6) | 0.42 (0.07–1.66) | 0 | 0 |
| 2013 | 30 | 6 (20.0) | 0.91 (0.07–4.9) | 0 | 1 (3.3) | |
| Total | 58 | 14 (24.1) | 0.63 (0.07–4.9) | 0 | 1 (1.7) | |
| Processed products | 2012 | 38 | 9 (23.7) | 0.48 (0.08–1.29) | 0 | 0 |
| 2013 | 77 | 12 (15.6) | 0.34 (0.07–1.27) | 0 | 0 | |
| Total | 115 | 21 (22.1) | 0.4 (0.07–1.29) | 0 | 0 | |
| Overall total | 206 | 56 (27.2) | 5.08 (0.07–63.4) | 8 (3.9) | 7 (3.4) | |
CIT = citrinin.
Average contaminated levels of positive samples.
4. Discussion
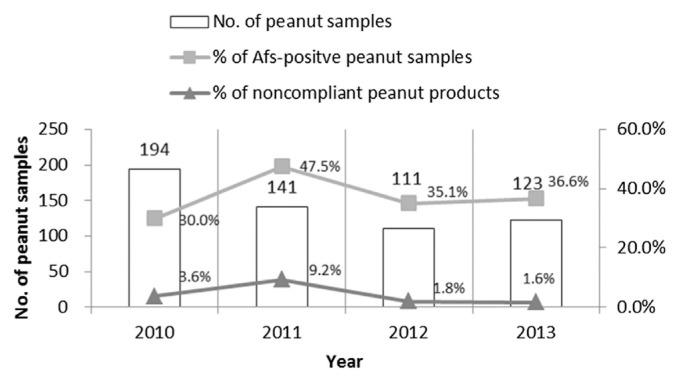
Fig. 1 shows the results of AF contamination in peanut products in Taiwan from 2010 to 2013 [28]. The AF-positive rate and noncompliance rate for peanuts dropped in 2011, from 47.5% and 9.2% to 36.6% and 1.6%, respectively, in 2013. In our analysis, there was a single instance of noncompliant pistachios (2.5%). Furthermore, this study represents the first official detection of AF contamination in Coix seeds and Sichuan pepper in the market with corresponding failure rates of 5.5% and 20%, respectively. Similar results were reported in a study conducted in Japan from 1982 to 1996 [32]. The author reported AF failure rates in peanut products, pistachios, and Coix seeds of 7.6%, 1.9% and 0.5%, respectively, with the highest concentration detected in pistachios at 1382 ppb. Of the eight substandard products contaminated with AFs, two peanut-product cases came from domestic manufacturers, three peanut cases were imported from Vietnam, pistachios were from Iran, Sichuan peppers were from China, and Coix seeds were from Thailand (Table 4). The health authorities in Taiwan ordered food businesses involved in the production of these substandard products to recall and destroy these products in accordance with their supervisory authorities under the law. The domestic manufacturers were ordered to make corrections to their production procedures and product-quality management. Follow-up sampling and testing were conducted by health authorities to confirm the completeness of their corrections. Actions were taken by the boundary control unit to raise the border batch-sampling probability on liability companies that import substandard products. AF contamination of peanuts and corn from countries in South and Southeast Asia has long since been a big concern [1,33]. In 2013, the batch-sampling probability of peanut products imported from Vietnam, Malaysia, the Philippines, Myanmar, Indonesia, and India increased to 20%, and 50% for peanut candy and Sichuan pepper. Since Iran’s food authorities took measures to reinforce sampling and testing at pistachio farms and the stage prior to export, the number of failed batches exported to the EU dropped from 457 batches in 2005 to 38 in 2011 [34]. Taiwan designates Coix seeds and Sichuan pepper as traditional Chinese medicine to be used as food. AF contamination of these products is highly regulated and should be consistent with the maximum limits set for foods. Thailand is the main source of Coix-seed importation. In 2006–2011, the average AF contamination level of Coix seeds in Thailand was 12.12 ppb, with the highest level at 95.9 ppb [33]. In Taiwan, 9.1% of Coix seeds designated for medicinal use were contaminated with AFs, with one sample having a level above 10 ppb [35]. Given the wide variety of food categories that are susceptible to AF contamination, active risk-management measures can help to reduce the failure rate of AFs on commercially available food. Additionally, market monitoring must be sustained.
Fig. 1.
The overall results of aflatoxin contamination in peanut products in Taiwan from 2010 to 2013.
AF = aflatoxin.
Rice constitutes a staple food in Taiwan. A previous study indicated that among domestic commercially available rice, there were no detectable levels of AFs and OTA [36]. However, a study conducted in Thailand, a major rice exporter, indicated the average AF contamination level was 3.01 μg/kg in Thai rice with a range of 0.8–12.6 μg/g [33]. In Taiwan, most of the wheat products and related raw materials are imported from abroad. In this study, two cases (10%) of oat samples were OTA positive. The European Food Safety Authority conducted a large-scale study in 2007–2012, and reported that cereals and cereal processed products exhibited AF-positive rates of 3.4% and 10%, respectively [37]. The incidence of OTA contamination was 30% and 28% in oats and wheat, respectively, for EU member states, with average contamination levels at 0.192 μg/kg and 0.269 μg/kg, respectively [38]. The importation of rice and wheat from countries with a higher incidence of AF and OTA contamination may result in the circulation of contaminated materials in the domestic market. Therefore, AF and OTA contamination in wheat and rice must still be monitored.
In a study conducted in 2004, no OTA-positive samples were found in roasted coffee beans collected in Taiwan, while ground coffee had a positive rate of 25% [36]. An EU study [37] reported OTA-positive rates of up to 36% and 46% for green coffee beans and roasted coffee products, respectively, among member states, with average contamination levels of 3.641 μg/kg and 1.092 μg/kg, respectively. OTA is a moderate heat-stable chemical. Light roasting causes reductions in OTA of 0–80%. Dark roasting may cause reductions of more than 90% [11]. Taiwan increased its import of coffee beans from 8680 tons in 2003 to 21,800 tons in 2013, while domestic production was only about 800 tons. This rapid increase in coffee consumption demonstrates the need for continuous monitoring of OTA contamination in coffee products.
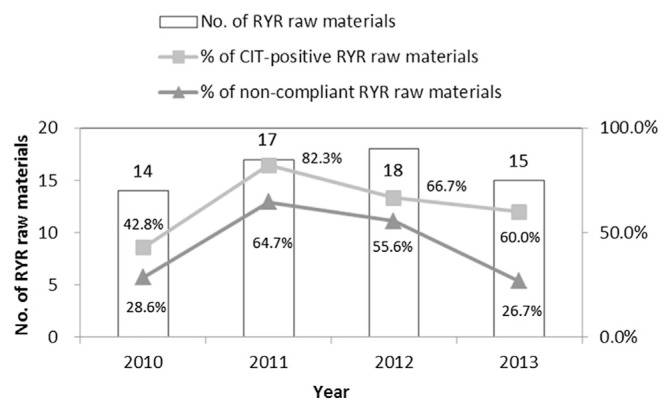
In 2009, 32 (62.7%) out of 51 samples of RYR raw materials collected from markets and ports were contaminated with CIT, and 23 samples (45.1%) contained CIT levels exceeding 5 ppm [39]. A similar result was found in a survey conducted in China. The study reported that 90% of Chinese RYR products were contaminated with CIT, with contamination levels in the range of 18.2–5253 μg/kg [40]. Considering the nephrotoxicity and high incidence of CIT contamination in RYR, the authority of boundary inspection has inspected each batch of RYR coming from the mainland of China since August 5, 2009. On December 4 of the same year, the CIT maximum limits were announced in Taiwan (Table 1). Up to 20 out of 26 substandard samples in 2011–2012 were traced to be imported from foreign countries, and were registered as “not to be used as food.” On September 1, 2012, Taiwan changed the import provisions for RYR raw materials from an F02 classification, which states that input goods, such as for food purposes, shall apply to the Taiwan Food and Drug Administration for import inspection, to an F01 classification, in which RYR raw materials under the product description should directly be handled upon import for food inspection. Fig. 2 shows the results of the market monitoring conducted after the legal CIT maximum limits were announced. In 2011, there were 10 cases (64.7%) of substandard RYR raw materials and 10 cases (55.6%) in 2012, but these then decreased to four cases (26.7%) in 2013, out of which there was only one RYR-related product from China, which was imported before the implementation of batch inspections. Boundary control measures effectively blocked substandard RYR from entering the domestic market. Most instances of domestic substandard RYR were manufactured by small-scale food manufacturers, and these industry participants were unable to undertake the selection of low-CIT-yielding strains and optimization of fermentation conditions. Aside from the continued implementation of border controls and market monitoring, guidelines should also be created to inform small food manufacturers of how to carefully select low-CIT-producing Monascus strains, optimize their fermentation parameters, and implement self-management, in order to reduce the high noncompliance rate among RYR products in Taiwan.
Fig. 2.
The overall results of citrinin contamination in red-yeast-rice raw materials in Taiwan from 2010 to 2013. CIT = citrinin; RYR = red yeast rice.
In Taiwan, the average daily domestic individual intake of peanuts and nuts is 4.17 g for males and 3.04 g for females [41], and the average BWs are 69 kg and 56.6 kg, respectively [42]. The probable mean daily intake (PDIM) values of AFs of 0.47 μg/kg for men and 0.42 μg for women were calculated using the mean average concentration (9.07 μg/kg) of all AF-positive samples and the aforementioned data. The estimated PDIM in the present study is lower than the PDIM values reported previously in the literature [33,43,44].
Among roasted-coffee products, this survey detected a maximum OTA level of 2.1 μg/kg. To attain the CODEX-specified PTWI value of 100 ng/kg BW/wk for OTA [14], men and women would need to consume the equivalent of 469 g and 385 g of coffee per day, respectively. Considering that 12 g of coffee is in a commercially available 150 mL cup of coffee, men and women would therefore have to drink 39 cups and 32 cups per day, respectively. Among the oat products, a maximum OTA level of 3.0 μg/kg was detected. To attain the PTWI value, men and women would need to consume the equivalent of 328 g and 270 g, respectively, of oat products per day. These results demonstrate that the risk was low regarding any OTA-related detrimental effects from drinking coffee or ingestion of oatmeal in Taiwan.
The market-monitoring program presented in this study evaluated 712 food products, and found that 689 test results (96.8%) were in compliance with provisions, while 23 test results (3.2%) were not. Of the latter, eight cases contained excessive levels of AFs (including four cases of peanut candy, one of peanut powder, one of pistachios, one of Sichuan pepper, and one of red Coix seeds). Another 15 cases had excessive levels of CIT (including 14 cases of RYR raw materials and one case of RYR supplements). The instances of substandard Sichuan pepper and Coix seeds containing AFs were first found detected in Taiwan. Information obtained from this survey and the study of mycotoxin occurrence was used to provide a sound scientific basis for establishing the priorities of a number of subsequent control activities, as well as mycotoxin regulations. Health authorities have already orchestrated the complete destruction of substandard goods that were recalled, and traced the sources of supply to prevent the associated businesses from further manufacturing substandard products. Imported RYR products with the identifying product description were directed for food inspection upon entry, and were subjected to batch testing. The implemented measures for managing domestic substandard products and importing food commodities have effectively decreased the AF noncompliance rate in peanut products and that of CIT in RYR products. The risk of AF contamination in peanut, Coix seeds, and Sichuan pepper, as well as CIT in RYR products, should be continuously investigated in further monitoring programs.
Acknowledgments
This study was funded by the Ministry of Health and Welfare, Taiwan, under the following grants: DOH101-FDA-81201 and DOH102-FDA-81003.
Funding Statement
This study was funded by the Ministry of Health and Welfare, Taiwan, under the following grants: DOH101-FDA-81201 and DOH102-FDA-81003.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 82. Lyon, France: World Health Organization; 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene; pp. 171–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration of the United States. Bad bug book, foodborne pathogenic microorganisms and natural toxins. 2nd ed. New Hampshire, United States: USFDA; 2012. pp. 231–6. [Google Scholar]
- 3. Binder EM, Tan LM, Chin LJ, Handl J, Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim Feed Sci Tech. 2007;137:265–82. [Google Scholar]
- 4.Food and Agriculture Organization. Food safety and quality—mycotoxins. Rome, Italy: Food and Agriculture Organization of the United Nations; 2014. [accessed 15, 06, 15]. Available at: http://www.fao.org/food/food-safety-quality/a-z-index/mycotoxins/en/ [Google Scholar]
- 5. Yu J. Current understanding on aflatoxin biosynthesis and future. Toxins (Basel) 2012;4(11):1024–57. doi: 10.3390/toxins4111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amare MG, Keller NP. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet Biol. 2014;66:11–8. doi: 10.1016/j.fgb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 7. Maggio-Hall LA, Wilson RA, Keller NP. Fundamental contribution of β-oxidation to polyketide mycotoxin production in planta. Mol Plant Microbe Interact. 2005;18:783–93. doi: 10.1094/MPMI-18-0783. [DOI] [PubMed] [Google Scholar]
- 8. Chanda A, Roze LV, Kang S, Artymovich KA, Hicks GR, Raikhel NV, Calvo AM, Linz JE. A key role for vesicles in fungal secondary metabolism. Proc Natl Acad Sci U S A. 2009;106:19533–8. doi: 10.1073/pnas.0907416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reverberi M, Punelli M, Smith CA, Jalic S, Scarpari M, Scala V, Cardinali G, Aspite N, Pinzari F, Payne GA, Fabbri AA, Fanelli C. How peroxisomes affect aflatoxin biosynthesis in Aspergillus flavus. PLoS One. 2012;7:e48097. doi: 10.1371/journal.pone.0048097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. Aflatoxins. IARC monographs on the review of human carcinogens. 100F. Lyon, France: World Health Organization; 2002. pp. 225–48. [Google Scholar]
- 11. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain additives and contaminants—aflatoxins. World Health Organ Tech Rep Ser. 2007;947:157–81. [Google Scholar]
- 12.CODEX Alimentarius Commission. CODEX general standard for contaminants and toxins in food and feed. Rome, Italy: CODEX Alimentarius Commission; 2013. [Google Scholar]
- 13. Khoury A, Atoui A, Ochratoxin A. General overview and actual molecular status. Toxins. 2010;2:461–93. doi: 10.3390/toxins2040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2006;365:1–56. [Google Scholar]
- 15. International Agency for Research on Cancer Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins IARC monographs 56 Lyon, France: World Health Organization; 1993. 489 521 8411629 [Google Scholar]
- 16. Bogsrud MP, Ose L, Langlet G. HypoCol (red yeast rice) lowers plasma cholesterol: a randomized placebo controlled study. Scand Cardiovasc J. 2010;44:197–200. doi: 10.3109/14017431003624123. [DOI] [PubMed] [Google Scholar]
- 17. European Food Safety Authority. Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012;10(3):2605. [Google Scholar]
- 18. Chen YP, Tseng CP, Liaw LL, Wang CL, Chen IC, Wu WJ, Wu MD, Yuan GF. Cloning and characterization of monacolin K biosynthetic gene cluster from Monascus pilosus. J Agric Food Chem. 2008;56:5639–46. doi: 10.1021/jf800595k. [DOI] [PubMed] [Google Scholar]
- 19. Shimizu T, Kinoshita H, Nihira T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol. 2007;73:5097–103. doi: 10.1128/AEM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aleo MD, Wyatt RD, Schnellmann RG. The role of altered mitochondrial function in citrinin-induced toxicity to rat renal proximal tubule suspensions. Toxicol Appl Pharmacol. 1991;109:455–63. doi: 10.1016/0041-008x(91)90008-3. [DOI] [PubMed] [Google Scholar]
- 21. Chagas GM, Oliveira MBM, Campello AP, Kluppel MLW. Mechanism of citrinin-induced dysfunction of mitochondria. IV: effect on Ca2+ transport. Cell Biochem Funct. 1995;13:53–9. doi: 10.1002/cbf.290130110. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer. IARC monographs. Vol. 40. Lyon, France: World Health Organization; 1987. Some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation; pp. 75–82. [Google Scholar]
- 23. Lee CH, Lee CL, Pan TM. A 90-d toxicity study of Monascus-fermented products including high citrinin level. J Food Sci. 2010;75:91–7. doi: 10.1111/j.1750-3841.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- 24.European Union. Commission regulation (EC) no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Brussels, Belgium: European Union; 2006. [Google Scholar]
- 25.World Trade Organization. Revision of indicator and sampling size of aflatoxins in food. Geneva, Switzerland: World Trade Organization; 2010. [Google Scholar]
- 26.Ministry of Health. National food safety standard: maximum levels of mycotoxins in foods. Beijing, People’s Republic of China: Ministry of Health of the People’s Republic of China; 2011. [Google Scholar]
- 27.Ministry of Health and Welfare. The maximum levels of mycotoxins in foods. Taipei, Taiwan, R.O.C: Ministry of Health and Welfare of the Republic of China; 2013. [Google Scholar]
- 28. Chen YC, Liao CD, Lin HY, Chiueh LC, Shih DYC. Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. J Food Drug Anal. 2013;21:247–52. [Google Scholar]
- 29. Liao CD, Chen YC, Lin HY, Shih DYC. Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012. Food Control. 2014;38:178–83. [Google Scholar]
- 30.Taiwan Food and Drug Administration. Method of test for mycotoxin in foods—test of ochratoxin A. Taipei, Taiwan, R.O.C: Ministry of Health and Welfare of the Republic of China; 2014. [accessed 24, 07, 15]. Available at: http://www.fda.gov.tw/TC/siteListContent.aspx?sid=103&id=9430&chk=95618aaa-d626-41b5-8eab-af4e0188b594¶m=pn%3d1%26sid%3d103%26classifyID%3d176#.VbGP_1IVjL0. [Google Scholar]
- 31.Taiwan Food and Drug Administration. Validation specification for testing methods on food chemistry. Taipei, Taiwan, R.O.C: Ministry of Health and Welfare of the Republic of China; 2014. [accessed 15, 06, 15]. Available at: http://www.fda.gov.tw/TC/siteContent.aspx?sid=1861. [Google Scholar]
- 32. Tabata S. Development of analytical methods for mycotoxins, and research for food safety. JSM Mycotoxins. 2012;62:63–75. [Google Scholar]
- 33. Anukul N, Vangnai K, Mahakarnchanakul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs in national level. J Food Drug Anal. 2013;21:227–41. [Google Scholar]
- 34. Dini A, Khazaeli P, Roohbakhsh A, Madadlou A, Pourenamdari M, Setoodeh L, Askarian A, Doraki N, Farrokhi H, Moradi H. Aflatoxin contamination level in Iran’s pistachio nut during years 2009–2011. Food Control. 2013;30:540–4. [Google Scholar]
- 35. Chin L, Chang-Chien IF, Cheng RB, Huang CY, Lin JH. An investigation on aflatoxin contamination in Chinese herb. Ann Rept BFDA. 2006;24:143–50. [Google Scholar]
- 36. Lin LC, Chen PC, Fu YM, Shih DYC. Ochratoxin A contamination in coffees, cereals, red wines and beers in Taiwan. J Food Drug Anal. 2005;13:84–92. [Google Scholar]
- 37. European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain related to aflatoxins in food (question no. EFSA-Q-2006-174) EFSA J. 2007;446:1–127. [Google Scholar]
- 38.Scientific Cooperation. Assessment of dietary intake of ochratoxin A by the population of EU member states Reports on tasks for scientific cooperation Rome, Italy. Brussels, Belgium: European Commission; 2002. [Google Scholar]
- 39. Liao CD, Lin PC, Lin HY, Shih DYC. Survey on citrinin in commercial Monascus products. Ann Rept Food Drug Res. 2010;1:109–16. [Google Scholar]
- 40. Li Y, Zhou YC, Yang MH, Ou-Yang Z. Natural occurrence of citrinin in widely consumed traditional Chinese food red yeast rice, medicinal plants and their related products. Food Chem. 2012;132:1040–5. [Google Scholar]
- 41.National Health Institute. Nutrition and health survey from 2005 to 2008 in Taiwan. Taipei, Taiwan: National Health Institute of the Republic of China; 2003. [accessed 15, 06, 15]. Available at: http://nahsit.nhri.org.tw/node/14. [Google Scholar]
- 42. Wu SJ, Chang YH, Fang CW. Food sources of weight, calories, and three macro-nutrients—NAHSIT 1993–1996. Nutr Sci J. 1999;24:41–58. [Google Scholar]
- 43. Oliveria CAF, Goncalves NB, Rosim RE. Determination of aflatoxins in peanut products in the northeastern region of Sao Paulo, Brazil. Int J Mol Sci. 2009;10:174–83. doi: 10.3390/ijms10010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ok HE, Kim HJ, Shim WB. Natural occurrence of aflatoxin B1 in marketed foods and risk estimates of dietary exposure in Koreans. J Food Prot. 2007;70:2824–8. doi: 10.4315/0362-028x-70.12.2824. [DOI] [PubMed] [Google Scholar]