Abstract
Tetrodotoxin (TTX) is a naturally occurring toxin in food, especially in puffer fish. TTX poisoning is observed frequently in South East Asian regions. In TTX-derived food poisoning outbreaks, the amount of TTX recovered from suspicious fish samples or leftovers, and residual levels from biological fluids of victims are typically trace. However, liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry methods have been demonstrated to qualitatively and quantitatively determine TTX in clinical samples from victims. Identification and validation of the TTX-originating seafood species responsible for a food poisoning incident is needed. A polymerase chain reaction-based method on mitochondrial DNA analysis is useful for identification of fish species. This review aims to collect pertinent information available on TTX-borne food poisoning incidents with a special emphasis on the analytical methods employed for TTX detection in clinical laboratories as well as for the identification of TTX-bearing species.
Keywords: identification, liquid chromatography-tandem, mass spectrometry, polymerase chain reaction method, coupled with restriction fragment, length polymorphism, tetrodotoxin, tetrodotoxin poisoning incident
1. Introduction
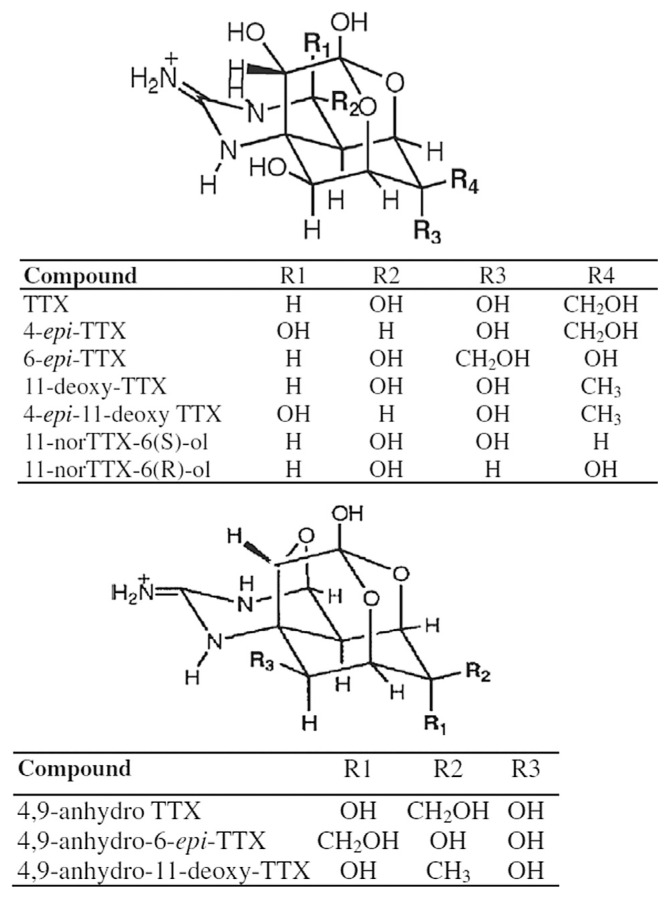
Tetrodotoxin (TTX) was first discovered in 1909 by Dr Yoshizumi Tahara from the ovaries of globefish, and was first isolated in 1950 by Dr Yokoo as a crystalline prism from toxic puffer fish. TTX is a naturally occurring neurotoxin of low molecular weight. The molecular formula of TTX is C11H17O8N3 (molecular weight = 319 Da), which has more than 10 analogs (Fig. 1). Among them, TTX has the highest toxicity. TTX consists of a positively charged guanidinium group and a pyrimidine ring that stabilize the TTX–sodium channel binding complex at the aqueous interface [1]. TTX prevents sodium currents in nerves and muscles by selectively binding to voltage-gated sodium channels for inhibiting the production of action potential and finally paralyzing nerve and muscle functions [2,3].
Fig. 1.
Structure of tetrodotoxin (TTX).
TTX is predominately isolated from the ovaries and liver of puffer fish; it is widely distributed in marine and some terrestrial organisms including newts, gastropods, trumpet shell, starfish, crabs, frogs, sea slugs, gobies, octopuses, flat-worms, ribbon worms, and bacteria [4–7]. The occurrence and distribution of TTX among a broad range of organisms gave rise to the speculation that TTX accumulation in organisms originated from symbiotic bacteria. Indeed, a number of bacteria have been shown to produce TTX, including the genera Aeromonas and Alteromonas, Escherichia coli, Otobacterium phosphoreum, Plesiomonas shigelloides, Pseudomonas sp., and some Vibrio sp. [8]. Furthermore, nontoxic puffer fish become toxic when they are administered a TTX-containing diet [9], and TTX transfer, accumulation, as well as elimination may be associated with the liver development of puffer fish [10]. Toxic puffer fish become nontoxic when they are fed on a TTX-free diet [11]. These lines of evidence demonstrated that the TTX accumulated in puffer fish is derived from the food chain that starts with marine symbiotic bacteria.
TTX poisoning cases have occurred in Asian countries, especially in Japan [7], Taiwan [12], China [7], Hong Kong [13], Thailand [14], and Bangladesh [15,16]. Cases of TTX poisoning have been reported mainly due to the ingestion of puffer fish in Taiwan and in other countries. However, recent studies demonstrated that TTX has spread to the Pacific, American, and Mediterranean regions [17,18]. TTX poisoning produces symptoms including perioral paresthesia, nausea, vomiting, diarrhea, ataxia, weakness of all limbs, paresthesia of the body, and respiration failure [19].
Several techniques are presently applied to analyze TTX. These include mouse bioassay [4–6], liquid chromatography–fluorescence detection [20], thin-layer chromatography [2], immunoassay [21], gas chromatography–mass spectrometry [22], enzyme-linked immunosorbent assay [16,23], liquid chromatography–mass spectrometry (LC–MS) [24,25], liquid chromatography–tandem mass spectrometry (LC–MS/MS) [26–31], ultraperformance liquid chromatography–MS/MS [32], and surface plasmon resonance [33,34]. Although there are various TTX determination assays, most of them are used for food tissue or leftover samples. Among them, LC–MS and LC–MS/MS are the most simple, powerful, and sensitive methods for qualitative and quantitative determination of TTX from human urine, blood, or other fluids [8,13].
Even when we obtain sufficient information to confirm TTX poisoning of a victim, the TTX-bearing species may still remain unknown. Currently, based on mitochondrial DNA analysis, it is possible to identify the toxic species consumed. Several articles described that a polymerase chain reaction (PCR) for analysis of the cytochrome b (Cytb) gene was useful for identification of fish species even after cooking [35–38].
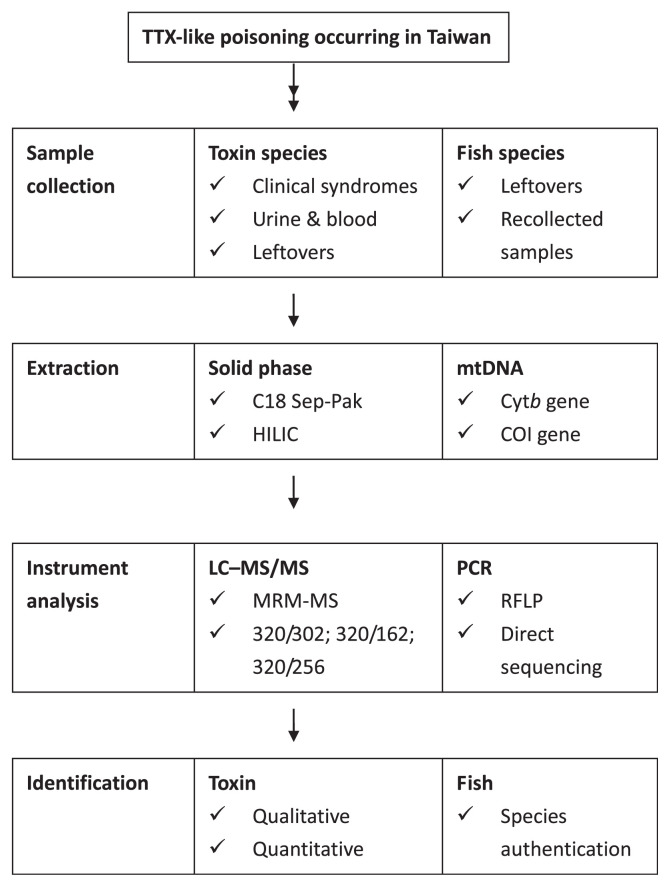
Therefore, we will review the LC–MS and LC–MS/MS methods used to detect the level and distribution of TTX in the urine and blood of victims. Meanwhile, the PCR-based method was used to amplify the partial Cytb gene in mitochondrial DNA and identify the marine species implicated in food poisoning incidents. Through a combination of identification of TTX-bearing species and biological fluids of the victim, better risk analysis, management, and control of TTX-borne disease may be achieved (Fig. 2).
Fig. 2.
Methodology validated in identifying toxins in clinical samples and fish species associated with TTX-borne poisoning incidents. COI = cytochrome c oxidase subunit I gene; HILIC = hydrophilic interaction liquid chromatography; LC–MS/MS = liquid chromatography–tandem mass spectrometry; MRM-MS = multiple reaction monitoring-mass spectrometry; mtDNA = mitochondrial DNA; PCR = polymerase chain reaction; RFLP = restriction fragment length polymorphism; TTX = tetrodotoxin.
2. Brief review of recently occurred TTX poisoning incidents
Four grades of TTX poisoning were described by Fukuda and Tani [19]:
Grade 1: perioral numbness and paresthesia (skin syndromes including tingling, tickling, prickling, or burning), may be accompanied by gastrointestinal symptoms;
Grade 2: lingual numbness (numbness of the face and related regions), early motor paralysis and incoordination, and slurred speech with normal reflexes;
Grade 3: generalized flaccid paralysis (muscle weakness), respiratory distress, aphonia (the inability to produce voice due to disruption of the recurrent laryngeal nerve), and fixed/dilated pupils (conscious patient); and
Grade 4: severe respiratory failure and hypoxia (inadequacy of oxygen), hypotension, bradycardia (resting heart rate <60 beats/min), cardiac dysrhythmias (irregular heartbeat), and possibility of unconsciousness
In Taiwan, 58 cases occurring from 1988 to 2011 for TTX poisoning were comprehensively reviewed by our team in 2012, resulting in 192 people intoxicated and 22 deaths [12]. Most of the TTX poisoning cases were caused by puffer fish, followed by gastropods and gobies. In addition, crabs and octopuses were also found to contain TTX and/or paralytic shellfish poisons [2,3,7].
More recently, a food poisoning incident due to ingestion of unknown octopus occurred in Taipei in December 2010 [39]. Victims were a 39-year-old and a 42-year-old man. After eating one specimen of octopus for 15 minutes, the first victim experienced acute numbness of the mouth, lips, fingers, and toes, followed by dimmed vision, muscle weakness, fatigue, headache, dizziness, and nausea/vomiting. Progressive acronumbness began in the ambulance, and then on admission, hyperesthesia of the upper limbs and a fluctuation of the heart rate were found. The other victim (the 42-year-old man) ate several specimens of octopuses and got severe respiratory symptoms, including mechanical ventilation and dopamine drip, upon reaching the intensive care unit. The symptoms subsided within 5 days and the patient recovered fully [39].
In 2008, large outbreaks of puffer fish poisoning occurred in Bangladesh, with 17 deaths out of 141 hospitalized patients [16]. The series of large outbreaks of puffer fish poisoning involving 82 males and 59 females was mainly due to the sudden availability of marine puffer fish in the local markets. The symptoms were similar to those of TTX intoxication, including initial lip and tongue paresthesia occurring in <30 minutes to several hours after the ingestion of puffer fish, followed by facial and limb paresthesia and numbness. Salivation, nausea, vomiting, and diarrhea with abdominal pain also developed early, as well as motor weakness and difficulty in speaking. In 131 cases, the most common symptom was perioral paresthesia (89%), followed by tingling sensation over the entire body (69%), nausea, vomiting, dizziness (60%), headache, abdominal pain, vertigo, and even death (11%). The higher death rate might have been caused by insufficient ventilatory or respiratory support when the victims suffered from respiratory muscle paralysis. Hence, in such severe cases of TTX-like poisoning, ventilation should be performed promptly. Even in less severe cases, victims should be kept under close observation for 1 day. Due to nearly identical initial clinical symptoms among all victims, it was difficult to evaluate early who would develop severe respiratory failure [16,40].
In Thailand, a total of 280 cases of TTX poisoning occurred following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda between 1994 and 2006 [14]. Of 245 available medical records, 100 cases were in Stage 1, 74 were in Stage 2, three were in Stage 3, and 68 were in Stage 4. The most common symptoms were circumoral and lingual numbness (98%), numbness of the hands and feet (94.7%), weakness (59.6%), dizziness and vertigo (54.3%), and nausea and vomiting (52.6%). All patients received symptomatic and supportive treatment. Among them, 239 patients showed complete recovery, five (2%) died, and one encountered anoxic brain damage. Seasonal variations of TTX poisoning following ingestion of the toxic eggs of C. rotundicauda were found to peak from December through March [14].
Thirteen patients presented TTX-like poisoning symptoms in Israel between 2005 and 2008 after ingestion of toxic puffer fish, Lagocephalus sceleratus [18]. The two most severely poisoned cases ate almost a whole fish liver. Their symptoms began within 10 minutes, and rapidly progressed within an hour with whole body paresthesia, vomiting, dyspnea, and hypertension. They required mechanical ventilation for 12–24 hours, and one patient received an intravenous injection of 0.4 mg naloxone. The length of hospital stay ranged from 1 day to 4 days; all patients were discharged asymptomatic. The authors stated that the research was limited due to the lack of identification of TTX in patients' serum or urine and in fish specimens, and due to the absence of electrophysiological studies [18].
3. Toxin identification from clinical samples of victims
In this section, we would not emphasize the methodology for identification of TTX from toxin-originating species [7]. Instead, we shift the focus to identification of TTX in patients' biological fluids, which is more difficult due to lower TTX levels. Several analytical methods for measuring TTX levels in urine and blood samples of victims have been demonstrated, including high-performance liquid chromatography (HPLC) with a UV detector (HPLC–UV), HPLC with postcolumn derivatization and fluorescence detection, liquid chromatography (LC) coupled with (tandem) mass spectrometry (MC) (LC–MS/MS), gas chromatography–mass spectrometry, immunoaffinity chromatography, and TTX-specific enzyme-linked immunoassay [8,13]. In a very first report in 2006, the authors used the sample cleanup procedure, in which the clinical samples were dissolved in 0.5M acetic acid and centrifuged. The supernatant was passed through a C18 Sep-Pak cartridge and then eluted by 0.3% acetic acid. The elution was filtered using a 3000-MW cutoff microcentrifuge filter. The filtrate was freeze dried and dissolved in water for LC–MS analysis. The combined LC–MS was performed using an Agilent model 1100 series (Agilent Technologies, Waldbronn, Germany) LC/MSD Trap system coupled to a mass spectrometer with a positive ion electrospray ionization interface. The mobile phase was 1% acetonitrite, 10mM trimethylamine, and 10mM ammonium formate (pH 4.0, flow rate 0.4 mL/min). The standard curve of TTX was in the range of 93.75–937.5nM. The recovery of spiked TTX in urine and blood was >88.9%, indicating that ion suppression from the matrix component could be ignored. The detection limit was 15.6nM of TTX. The concentration of TTX in the blood of victims was between 4.5nM and 40.6nM, and the prominent TTX concentration was between 47nM and 344nM in the urine [25].
The double solid-phase extraction, including C18 and hydrophilic interaction liquid chromatography, was developed to purify TTX from the victim's urine and blood samples [28,29]. Subsequent qualitative and quantitative analyses of TTX were conducted using HPLC coupled with tandem mass spectrometry. This study used 5mM heptafluorobutyric acid as an anionic ion-pairing reagent in mobile phase but could not prevent the ion suppression effect [30]. Either C18- hydrophilic interaction liquid chromatography (HILIC) or Sep-Pak-HILIC could increase the efficiency in removing matrix and ion suppression. Owing to the hydrophobic behavior of C18 and Sep-Pak cartridges, the hydrophobic interfering substances could be retained and removed. The combination of double solid-phase extraction and an ion-pairing reagent made isocratic elution possible, markedly reducing HPLC analysis time (to 5.5 minutes) and organic solvent amounts. In LC–MS/MS analysis, the precursor ion was selected as 320.1 m/z for TTX. The 320.1–302.3 m/z and 256.2 m/z mass transitions were used as qualitative ions for positive identification, and the shift of 320.1–162.3 m/z was used for quantitation. The limit of detection was determined at 0.13 ng/mL and the limit of quantification was 2.5 ng/mL for both urine and plasma. In all eight patients, TTX was detected only in the urine but not in the blood samples. After creatinine correction, the urine TTX ranged from 7.4 ng/mol to 41.1 ng/mol creatinine [31]. The patients with the highest levels of urine TTX appeared to have liver derangement, generalized muscle weakness, and even severe respiratory failure that required immediate ventilation.
In 2009, a lethal case of TTX poisoning occurred in Taiwan due to ingestion of suspicious puffer fish. The blood, urine, bile, head cerebrospinal fluid, spinal cord cerebrospinal fluid, pleural effusion, and pericardial effusion from the victim were collected and found to contain <0.10 ng/mL, 10.42 ng/mL, 5.02 ng/mL, <0.10 ng/mL, 4.66 ng/mL, 6.30 ng/mL, and 4.14 ng/mL TTX, respectively. All biological fluids collected from the victim contained TTX, except for the blood and cerebrospinal fluid from head (no detectable TTX) [41].
In 2010, two octopus species implicated in a food paralytic poisoning incident in Taipei was investigated. The remaining specimens of octopuses and urine and plasma from diseased victims were assayed for toxicity using LC–MS/MS. The levels of TTX in the two residues of unknown octopus were 31.8 μg/g and 94.3 μg/g. The corresponding levels of TTX in the urine of the victims were 39.1 ng/mL and 83.4 ng/mL. The TTX levels of the plasma samples of the victims were <0.1 ng/mL. In addition, the six octopus samples with the blue ring recollected from markets after the outbreak were shown to contain 106–127 μg/specimen [39].
4. Identification of toxin-bearing species from suspicious seafood consumed
The main limitation of several previous TTX-borne food poisoning incidents is the lack of identification and/or determination of TTX in the victims' biological fluids and in fish specimens, or the absence of identification in TTX-harboring species [14–18]. To identify toxic puffer fish in thermally processed fish products, a PCR method coupled with restriction fragment length polymorphism and sequence analysis has been well established using the conserved region of mitochondrial DNA [35–39]. Prolonged autoclaving protocols were used to validate the level of DNA damage. Severe thermal processing (121°C/90 minutes) hampers the extraction of fragments larger than 400–500 bp due to severe degradation of DNA samples [42]. The mitochondrial Cytb gene locus has been well characterized among different vertebrates and is therefore broadly used for authenticating related species [43,44]. Both direct sequencing and restriction enzymatic analysis on the Cytb gene are good strategies for identifying species. However, direct sequencing analysis is more expensive and time consuming, and also requires sophisticated equipment and skill. In 2010, 60 commercial roasted and/or dried–dressed fish products were collected from fishing markets in Taiwan [42]. The content of nontoxic puffer fish species, including mainly Lagocephalus gloveri and Lagocephalus wheeleri, was 82%. The content of toxic puffer fish, illegal to use for dried–dressed fish fillets, was 15%, consisting mostly of Lagocephalus lunaris and rarely Takifugu oblongus. This method uses a simple PCR amplification on Cytb gene, and then a further two-step restriction enzymatic reaction for successfully authenticating 17 puffer fish species within 9 hours. For example, the use of a pair of restriction enzymes (BsaJ I and Aci I) can differentiate L. gloveri from L. wheeleri due to their specific restriction fragment patterns. The use of BsaJ I, Hinf I, and Sap I can identify L. lunaris among all 17 puffer fish species (partial information adapted from Hsieh et al [42] in Table 1).
Table 1.
Lengths of restriction fragments generated by digestion of 376 bp cytochrome b gene with restriction enzymes (PCR–RFLP) from the common puffer fish species in Taiwan.
| Restriction fragment size (bp) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BsaJ I | Aci I | Bsa I | Hinf I | Sap I | Taq I | Mse I | |
| Lagocephalus gloveri | 162 + 214 | 137 + 239 | |||||
| Lagocephalus wheeleri | 119 + 257 | 376 | |||||
| Lagocephalus lunaris | 376 | 174 + 202 | 91 + 285 | ||||
| Lagocephalus sceleratus | 376 | 170 + 206 | 376 | ||||
| Lagocephalus inermis | 119 + 257 | 62 + 137 + 177 | 376 | 113 + 263 | |||
| Takifugu xanthopterus | 139 + 237 | 59 + 122 + 195 | 129 + 247 | ||||
| Takifugu oblongus | 376 | 376 | 139 + 237 | ||||
| Takifugu rubripes | 139 + 237 | 59 + 122 + 195 | 376 | 376 | |||
The most common puffer fish species implicated in poisoning in Taiwan is L. lunaris [35–38], however, Takifugu nipholes [45] and Chelonodon patoca [41] have been found to be associated with TTX-borne food poisoning incidents. The consumed poisonous species were chiefly puffer fish, gastropods, and gobies, and the edible portions include the liver, viscera, and roe [2,3,8]. The blue-ring octopus-implicated food poisoning incidents are sporadic. The first food poisoning incident due to ingestion of TTX-bearing octopus was reported in Taiwan. In 2010, two unknown species of toxic octopuses were consumed by two victims in Taipei [38]. The octopus residues and urine samples from the victims were found to contain TTX using LC–MS/MS. The partial Cytb gene and cytochrome c oxidase subunit I gene (COI) of the octopuses were determined by PCR amplification using primer pairs OCT1F/OCT1R and LCOI1490/HCO2198, respectively. Direct sequencing on the amplicons of Cytb and COI genes from residues and recollected samples from markets was performed. The residue with the blue ring in the skin was identified as the toxic octopus Hapalochlaena fasciata, and the other residue without the blue ring in the skin was identified as the nontoxic octopus Octopus aegina [39].
5. Conclusion
To control outbreaks of TTX-like poisoning, a standard and robust protocol needs to be established and validated for identifying food poisoning incidents. For clinical samples such as the urine and blood of victims, LC–MS/MS is a particularly appropriate methodology due to its speed and sensitivity. For TTX-bearing fish species, PCR coupled with direct sequencing or restriction fragment length polymorphism techniques, according to the partial sequence of mtDNA gene products, is a validated platform. Integration with TTX-bearing species and biological fluids identifications could help improve risk analysis, management, and control of TTX-borne disease.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Jen HC, Nguyen TAT, Wu YJ, Hoang T, Arakawa O, Lin WF, Hwang DF. Tetrodotoxin and paralytic shellfish poisons in gastropod species from Vietnam analyzed by high-performance liquid chromatography and liquid chromatography–tandem mass spectrometry. J. Food Drug Anal. 2014;22:178–88. [Google Scholar]
- 2. Hwang DF, Noguchi T. Tetrodotoxin poisoning. Adv Food Nutr Res. 2007;52:141–236. doi: 10.1016/S1043-4526(06)52004-2. [DOI] [PubMed] [Google Scholar]
- 3. Noguchi T, Arakawa O. Tetrodotoxin—distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar Drugs. 2008;6:220–42. doi: 10.3390/md20080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hwang DF, Shiu YC, Hwang PA, Lu YH. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J Food Prot. 2002;65:1341–4. doi: 10.4315/0362-028x-65.8.1341. [DOI] [PubMed] [Google Scholar]
- 5. Hwang PA, Tsai YH, Lu YH, Hwang DF. Paralytic toxins in three new gastropod (Olividae) species implicated in food poisoning in southern Taiwan. Toxicon. 2003;41:529–33. doi: 10.1016/s0041-0101(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 6. Hwang PA, Tsai YH, Deng JF, Cheng CA, Ho PH, Hwang DF. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J Food Prot. 2005;68:1696–701. doi: 10.4315/0362-028x-68.8.1696. [DOI] [PubMed] [Google Scholar]
- 7. Noguchi T, Onuki K, Arakawa O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011;2011:1–10. doi: 10.5402/2011/276939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A. Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kono M, Matsui T, Furukawa K, Yotsu-Yamashita M, Yamamori K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon. 2008;51:1269–73. doi: 10.1016/j.toxicon.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 10. Tatsuno R, Shikina M, Shirai Y, Wang J, Soyano K, Nishihara GN, Takatani T, Arakawa O. Change in the transfer profile of orally administered tetrodotoxin to non-toxic cultured pufferfish Takifugu rubripes depending of its development stage. Toxicon. 2013;65:76–80. doi: 10.1016/j.toxicon.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 11. Noguchi T, Arakawa O, Takatani T. Toxicity of pufferfish Takifugu rubripes cultured in net cages at sea or aquaria on land. Comp Biochem Phys D. 2006;1:153–7. doi: 10.1016/j.cbd.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12. Lin WF, Hwang DF. Analysis of poisoning cases, monitoring and risk warning for marine toxins (TTX, PSP and CTXs) in Taiwan. J Food Drug Anal. 2012;20:764–71. [Google Scholar]
- 13. Leung KSY, Fong BMW, Tsoi YK. Analytical challenges: determination of tetrodotoxin in human urine and plasma by LC-MS/MS. Mar Drugs. 2011;9:2291–303. doi: 10.3390/md9112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanchanapongkul J. Tetrodotoxin poisoning following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda, a case series from 1994 through 2006. Southeast Asian J Trop Med Public Health. 2008;39:303–6. [PubMed] [Google Scholar]
- 15. Ahasan HAMN, Mamun AA, Karim SR, Bakar MA, Gazi EA, Bala CS. Paralytic complications of puffer fish (tetrodotoxin) poisoning. Singapore Med J. 2004;45:73–4. [PubMed] [Google Scholar]
- 16. Islam QT, Razzak MA, Islam MA, Bari MI, Basher A, Chowdhury FR, Sayeduzzaman AB, Ahasan HA, Faiz MA, Arakawa O, Yotsu-Yamashita M, Kuch U, Mebs D. Puffer fish poisoning in Bangladesh: clinical and toxicological results from large outbreaks in 2008. Trans R Soc Trop Med Hyg. 2011;105:74–80. doi: 10.1016/j.trstmh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17. Silva M, Azevedo J, Rodriguez P, Alfonso A, Botana LM, Vasconcelos V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar Drugs. 2012;10:712–26. doi: 10.3390/md10040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bentur Y, Ashkar J, Lurie Y, Levy Y, Azzam ZS, Litmanovich M, Golik M, Gurevych B, Golani D, Eisenman A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon. 2008;52:964–8. doi: 10.1016/j.toxicon.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19. Fukuda A, Tani A. Records of puffer poisoning report 3. Nippo Igaku Oyobi Kenko Hoken. 1941;3528:7–13. [Google Scholar]
- 20. Yu CH, Yu CF, Tam S, Yu PH. Rapid screening of tetrodotoxin in urine and plasma of patients with puffer fish poisoning by HPLC with creatinine correction. Food Addit Contam. 2010;27:89–96. doi: 10.1080/02652030903207250. [DOI] [PubMed] [Google Scholar]
- 21. Kawatsu K, Shibata T, Hamano Y. Application of immunoaffinity chromatography for detection of tetrodotoxin from urine samples of poisoned patients. Toxicon. 1999;97:325–33. doi: 10.1016/s0041-0101(98)00116-0. [DOI] [PubMed] [Google Scholar]
- 22. Kurono S, Hattori H, Suzuki O, Yamada T, Seno H. Sensitive analysis of tetrodotoxin in human plasma by solid-phase extraction and gas chromatography/mass spectrometry. Anal Lett. 2011;34:2439–46. [Google Scholar]
- 23. Kawatsu K, Hamano Y, Yoda T, Terano Y, Shibata T. Rapid and highly sensitive enzyme immunoassay for quantitative determination of tetrodotoxin. Jpn J Med Sci Biol. 1997;50:133–50. doi: 10.7883/yoken1952.50.133. [DOI] [PubMed] [Google Scholar]
- 24. Horie M, Kobayashi S, Shimizu N, Nakazawa H. Determination of tetrodotoxin in puffer-fish by liquid chromatography–electrospray ionization mass spectrometry. Analyst. 2002;127:755–9. doi: 10.1039/b201128j. [DOI] [PubMed] [Google Scholar]
- 25. Tsai YH, Hwang DF, Cheng CA, Hwang CC, Deng JF. Determination of tetrodotoxin in human urine and blood using C18 cartridge column, ultrafiltration and LC–MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:75–80. doi: 10.1016/j.jchromb.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 26. Jen HC, Lin SJ, Lin SY, Huang YW, Liao IC, Arakawa O, Hwang DF. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit Contam. 2007;8:902–9. doi: 10.1080/02652030701245171. [DOI] [PubMed] [Google Scholar]
- 27. Jen HC, Lin SJ, Tsai YH, Chen CH, Lin ZC, Hwang DF. Tetrodotoxin poisoning evidenced by solid-phase extraction combining with liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:95–100. doi: 10.1016/j.jchromb.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 28. Cho HE, Ahn SY, Son IS, In S, Hong RS, Kim DW, Woo SH, Moon DC, Kim S. Determination and validation of tetrodotoxin in human whole blood using hydrophilic interaction liquid chromatography–tandem mass spectroscopy and its application. Forensic Sci Int. 2012;217:76–80. doi: 10.1016/j.forsciint.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 29. Yotsu-Yamashita M, Jang JH, Cho Y, Konoki K. Optimization of simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2011;29:61–4. [Google Scholar]
- 30. Fong BMW, Tam S, Tsui SH, Leung KS. Development and validation of a high-throughput double solid phase extraction-liquid chromatography–tandem mass spectrometry method for the determination of tetrodotoxin in human urine and plasma. Talanta. 2011;83:1030–6. doi: 10.1016/j.talanta.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 31. Akaki K, Hatano K. Determination of tetrodotoxin in puffer-fish tissues, and in serum and urine of intoxicated humans by liquid chromatography with tandem mass spectrometry. Shokuhin Eiseigaku Zasshi. 2006;47:46–50. doi: 10.3358/shokueishi.47.46. [DOI] [PubMed] [Google Scholar]
- 32. Nzoughet JK, Campbell K, Barnes P, Cooper KM, Chevallier OP, Elliott CT. Comparison of sample preparation methods, validation of an UPLC-MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013;136:1584–9. doi: 10.1016/j.foodchem.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 33. Taylor AD, Vaisocherová H, Deeds J, DeGrasse S, Jiang S. Tetrodotoxin detection by a surface plasmon resonance sensor in pufferfish matrices and urine. J Sens. 2011;2011:1–10. [Google Scholar]
- 34. Yakes BJ, Kanyuck KM, DeGrasse SL. First report of a direct surface Plasmon resonance immunosensor for a small molecule seafood toxin. Anal Chem. 2014;86:9251–5. doi: 10.1021/ac502271y. [DOI] [PubMed] [Google Scholar]
- 35. Chen TY, Hsieh YW, Tsai YH, Shiau CY, Hwang DF. Identification of species and measurement of tetrodotoxin in dried dressed fillets of the puffer fish, Lagocephalus lunaris. J Food Prot. 2002;65:1670–3. doi: 10.4315/0362-028x-65.10.1670. [DOI] [PubMed] [Google Scholar]
- 36. Hsieh YW, Hwang PA, Pan HH, Chen JB, Hwang DF. Identification of tetrodotoxin and fish species in an adulterated dried mullet roe implicated in food poisoning. J Food Sci. 2003;68:142–6. [Google Scholar]
- 37. Hwang DF, Hwang PA, Tsai YH, Chen SK, Cheng CA. Identification of tetrodotoxin and fish species in dried dressed fish fillets implicated in food poisoning. J Food Prot. 2002;685:389–92. doi: 10.4315/0362-028x-65.2.389. [DOI] [PubMed] [Google Scholar]
- 38. Wu YJ, Jen HC, Deng JF, Hwang DF. Identification of toxin and species of causative fish roe implicated into a food poisoning incident. J Fish Soc Taiwan. 2008;35:359–67. [Google Scholar]
- 39. Wu YJ, Lin CL, Chen CH, Hsieh CH, Jen HC, Jian SJ, Hwang DF. Toxin and species identification of toxic octopus implicated into food poisoning in Taiwan. Toxicon. 2014;91:96–102. doi: 10.1016/j.toxicon.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 40. Homaira N, Rahman M, Luby SP, Rahman M, Haider MS, Faruque LI, Khan D, Parveen S, Gurley ES. Multiple outbreaks of puffer fish intoxication in Bangladesh, 2008. Am J Trop Med Hyg. 2010;83:440–4. doi: 10.4269/ajtmh.2010.10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu YJ, Cheng YJ, Jen HC, Pan CH, Lin TC, Lin SJ, Hwang DF. Liquid chromatography–tandem mass spectrometry determination of the toxicity and identification of fish species in a suspected tetrodotoxin fish poisoning. J Food Prot. 2011;74:789–95. doi: 10.4315/0362-028X.JFP-10-435. [DOI] [PubMed] [Google Scholar]
- 42. Hsieh CH, Chang WT, Chang HC, Hsieh HS, Chung YL, Hwang DF. Puffer fish-based commercial fraud identification in a segment of cytochrome b region by PCR–RFLP analysis. Food Chem. 2010;121:1305–11. [Google Scholar]
- 43. Sebastio P, Zanelli P, Neri TM. Identification of anchovy (Engraulis encrasicholus L.) and gilt sardine (Sardinella aurita) by polymerase chain reaction, sequence of their mitochondrial cytochrome b gene, and restriction analysis of polymerase chain reaction products in semipreserves. J Agric Food Chem. 2001;49:1194–9. doi: 10.1021/jf000875x. [DOI] [PubMed] [Google Scholar]
- 44. Jerome M, Lemaire C, Verrez-Bagnis V, Etienne M. Direct sequencing method for species identification of canned sardine and sardine-type products. J Agric Food Chem. 2003;51:7326–32. doi: 10.1021/jf034652t. [DOI] [PubMed] [Google Scholar]
- 45. Hsieh YW, Shiu YC, Cheng CA, Chen SK, Hwang DF. Identification of toxin and fish species in cooked fish liver implicated in food poisoning. J Food Sci. 2002;67:948–52. doi: 10.4315/0362-028x-65.2.389. [DOI] [PubMed] [Google Scholar]