Abstract
Diospyros blancoi A. DC. is an evergreen tree species of high-quality wood. Mabolo, the fruit of this plant, is popular among the natives in Taiwan, but its potential in economic use has not been fully explored. Mabolo has a rich aroma. Of the 39 different volatile compounds isolated, its intact fruit and peel were found to both contain 24 compounds, whereas the pulp contained 28 compounds. The most important aroma compounds were esters and α-farnesene. Our data show that mabolo is rich in dietary fiber (3.2%), and the contents of other nutrients such as malic acid, vitamin B2, vitamin B3, folic acid, pantothenic acid, and choline chloride were 227.1 mg/100 g, 0.075 mg/100 g, 0.157 mg/100 g, 0.623 mg/100 g, 0.19 mg/100 g, and 62.52 mg/100 g, respectively. Moreover, it is rich in calcium and zinc; the contents of which were found to be 42.8 mg/100 g and 3.6 mg/100 g, respectively. Our results show that D. blancoi has the potential to be bred for a novel fruit.
Keywords: Diospyros discolor, Diospyros philippensis, ethnobotany, novel fruit, velvet apple
1. Introduction
Diospyros blancoi, the subject of this study, is an Ebenaceae plant that can grow up to 20 m high or more. There are scientific names of different origins referring to this evergreen plant, with the three most commonly used being Diospyros discolor Willd., Diospyros philippensis (Desr.) Gürke and D. blancoi A. DC. Knapp and Gibert [1] see D. discolor as the single correct name for the plant, whereas in the book Flora of Taiwan [2], it is referred to as D. philippensis. However, as early as in 1971, Howard [3] had already legitimized D. blancoi as the plant’s scientific name, and thus it is the one that we use in this study.
In the Filipino language Tagalog, D. blancoi is called kamagong, and the fruit is known as mabolo, meaning “hairy” [4]. The wood of this plant is extremely dense, hard, and dark in color, consequently in Taiwan it is known as “Taiwan’s black ebony” and considered a valuable timber species. In the Philippines, kamagong timber is also known as “iron wood” since it is durable and considered unbreakable. It is widely distributed in the Philippines [5], and is native to eastern and southern Taiwan [2,6]. On a northeastern Taiwanese island known as Turtle Island, a tree with a diameter at breast height (DBH) of 210 cm was discovered, and this may be the northernmost part of the world where this plant is found. The species, known as maoshi in Taiwan [2], produces a fruit with a fluffy exterior, which is the same reference as mabolo in the Philippines.
D. blancoi is an important ethnobotanical plant in the Austronesian society. In China, the earliest mentioning of D. blancoi was in “Dong Fan Jì” by Chen [7] in 1603 during the Ming Dynasty. A travel report recorded in Taiwan, the article describes the western coast of Taiwan and the customs of Siraya tribal life, highlighting the consumption of important edible fruits and vegetables such as coconut, bergamot, sugarcane, as well as mabolo (Fig. 1). Taiwanese ethnobotanist Han-Wen Zheng [8] mentioned that mabolo is commonly found in the eastern and southern Taiwanese residential courtyards of Kebalan, Pangcah, Payuan, and Pinuyumayan. Tao people plant mabolo in private lands and taro fields. In Pinuyumayan culture, the lifecycle of mabolo is used as an indicator for the annual work calendar. This includes instructions stating that weeding should be done while the plant is flowering, millet should be collected when its fruit begins to form, or upland rice should be collected when fruits are harvested. Moreover, the elderly or sick people are honored with the mature fruit [8].
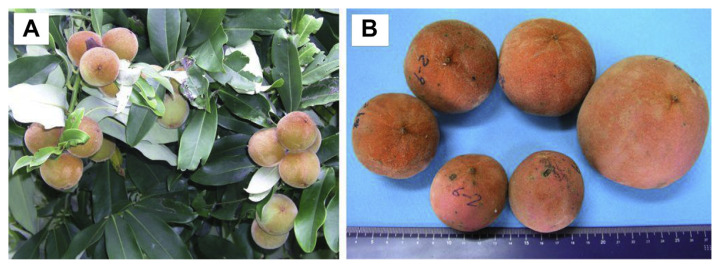
Fig. 1.
The Diospyros blancoi A. DC. (A) bears abundant fruit (mabolo), and (B) varies greatly in size within populations. The ruler units are centimeters.
Seedless mabolo has occasionally been found in the field. With only the female plant found on Turtle Island, it suggests that a lack of pollen causes approximately 90% of the fruit to be seedless. Seeded fruits contain an average of three seeds, and the relatively low number of seeds is indicative of the existence of parthenocarpic varieties of D. blancoi. Our recent studies show that the seedless mabolo was also a result of artificially induced production [9].
D. blancoi maintains high diversity of fruit size within populations (Fig. 1B) and can be considered a good breeding resource. However, as it exhibits a low level of domestication, selecting elite trees and establishing initial lines that can be used for a potential traits screen becomes very important. The aims of this study are to highlight the importance of mabolo and promote the fact that D. blancoi can potentially be an important economic fruit [9].
2. Methods
2.1. Analysis of aroma compounds
Fresh ripe fruits that had fallen on the ground (random samples) were collected from the Kenting area. The fruit was subjected to three types of analysis. For the first analysis, the intact fruit was used; for the second analysis, the fruit peel (approximately 50 g) was used; and for the third analysis, the pulp of the fruit was used after the peel and seeds were removed. Each analysis was performed in triplicate. The samples were placed in the sample cylinder to extract aroma compounds using the headspace solid-phase micro-extraction (SPME) method for 30 minutes, which used polydimethylsiloxane/divinylbenzene (PDMS/DVB; 65 μm) fiber for adsorption. Gas chromatography-mass spectrometry (GC-MS) was performed with the Thermo GC Focus Series and Trace DSQ-MS (Thermo Fisher Scientific Inc., Waltham, MA, USA). A BPX5 (30 m length, 0.25 mm inside diameter, 0.25 μm film thickness) column (Thomas Scientific Inc., Swedesboro, NJ, USA) was used with helium (99.999%) as carrier gas at a flow rate of 1.0 mL/min, and the injector was set in the splitless mode. The injection port was set at 220°C, the MS interface was maintained at 210°C, and the ion trap was set at 200°C. The operating temperature range was 55–130°C, increased at a rate of 3°C/min; 130–210°C, increased at a rate of 2°C/min; and finally held at 210°C for an additional 2 minutes.
The ionization energy of electron impact (EI) was 70 eV and the scanning range was between 33 m/z and 400 m/z. The mass spectra obtained were compared with the mass spectra in the NIST 02 version mass spectral database (National Institute of Standards and Technology, Washington, DC, USA). The match of the mass spectra fragmentation was over 850, and 10% or more probability was the basis for the identification of compounds. The concentrations of compounds were calculated based on the waveform area of the chromatogram. The process was repeated, and if the variation was more than 10%, then the samples were resampled, and the data were analyzed until consistent results were obtained.
2.2. Analysis of nutrient contents
Thirty fruits were collected as samples from the Kenting area (Taiwan), and the pulp of each fruit was weighed after peeling and the removal of seeds. The dry weight of the pulp was determined after freeze-drying for 1 week before the pulp was powdered and stored at −80°C. The process was repeated after conducting an analysis. If the variation between the two data was within 10%, the average of the two datasets was considered as the result. If the variation was more than 10%, the process was continued until a consistent result was obtained. The following analyses were performed according to the following protocols/methods after some modifications to the mentioned methods: moisture content = (fresh weight – dry weight) * 100%; calorie = 9 (crude fat) + 4 (crude protein) + 4 (carbohydrate); carbohydrate = 100 – moisture – ash – crude protein – crude fat; and calories from fat = 9 (crude fat).
Analysis of ash by ashing, was carried out according to Chinese National Standard (CNS) 5034-N6115 [10]. Dietary fiber analysis was carried out by enzymatic gravimetry, according to the Association of Analytical Communities (AOAC) official method 985.29 [11]. Crude protein analysis was carried out by digestion-titrimetry, according to CNS 5035-N6116 [12]. Crude fat analysis was carried out by ether extract, according to CNS 5036-N6117 [13], trans fat by GC, according to the AOAC official method 985.21 [11], and saturated fatty acid by GC, according to the AOAC official method 996.06 [11]. Glucose, fructose, sucrose, lactose, and maltose analysis was performed by high-performance liquid chromatography (HPLC), according to CNS12634-N6223 [14]. Cholesterol analysis was performed by GC, according to the AOAC official method 994.10 [11]. Vitamin A analysis was performed by HPLC, according to CNS12725-N6230 [15], vitamin C by HPLC, according to Odriozola-Serrano et al [16], vitamin E by LC, according to the AOAC official method 992.03 [11], β-carotene by HPLC, according to Chen et al [17], vitaminD3byHPLC, accordingtoKlejdus etal[18], vitamin K1by HPLC, according to Jakob and Elmadfa [19], vitamin B1 by fluorometry, according to the AOAC official method 953.17 [11], vitamin B2 by fluorometry, according to Hoffmann-LaRoche [20], vitamin B3 by colorimetry, according to the AOAC official method 961.14 [11], vitamin B6, according to the AOAC official method 985.32 [11], vitamin B12 by titrimetry, according to the AOAC official method 952.20 [11], vitamin H by HPLC, according to BS EN15607 [21], folic acid according to the AOAC official method 944.12 [11], pantothenic acid according to the AOAC official method 945.74 [11], and inositol by GC-flame ionization detector (FID), according to International Classification for Standards (ICS) 67.040 C53 [22]. Choline chloride was analyzed by HPLC, according to Chen et al [23], citric acid, malic acid, fumaric acid, propionic acid, acetic acid, tartaric acid, succinic acid, and formic acid by LC, according to Hyun et al [24]. Sodium was analysed by atomic absorption (AA)-spectrophotometry, according to CNS12869-N6231 [25], calcium, iron, chromium, potassium, magnesium, manganese, nickel, phosphorus, zinc, cobalt, molybdenum, titanium, selenium by AA-spectrophotometry, according to the AOAC official method 985.35 [11]. Total tannin content was analysed by Folin–Ciocalteu colorimetry, according to Li et al [26].
3. Results and discussion
3.1. Analysis of aroma compounds
We were able to detect 39 different volatile aroma compounds (Table 1). Twenty-four compounds were detected in the intact fruit. The five major compounds (each constituting > 5% of the total content of volatile compounds) detected in the intact fruit were butyl benzoate (25.57%), hexyl butyrate (16.46%), benzyl butyrate (14.71%), hexyl benzoate (13.78%), and α-farnesene (9.60%). Collectively, these compounds constituted 80.12% of the total content of volatile compounds. The volatile compound composition of the peel was similar to that of the intact fruit, with only minor differences observed. Twenty-four different compounds were detected in the peel, of which the seven major compounds (each constituting > 5% of the total content of volatile compounds) were butyl benzoate (31.54%), hexyl butyrate (16.98%), α-farnesene (11.73%), benzyl butyrate (11.50%), butyl 2-methylbutyrate (7.48%), hexyl benzoate (6.02%), and butanoic acid, 2-methyl-hexyl ester (5.02%). Collectively, these compounds constituted 90.27% of the total content of volatile compounds. Twenty-eight volatile compounds were detected in the pulp, of which the six major compounds (each constituting > 5% of the total content of volatile compounds) were hexyl butyrate (29.37%), benzyl butyrate (15.08%), α-farnesene (13.62%), phenylethyl butyrate (9.24%), hexyl hexanoate (8.33%), and butyl benzoate (6.39%). Collectively, these compounds constituted 82.03% of the total content of volatile compounds. The main aroma compounds in the intact fruit, pulp, and peel were esters and α-farnesene. Not only for mabolo, the most important volatile compounds of many fruits are esters. For example, esters accounted for 78–92% of whole volatile compounds of apples [27], and there were 33 kinds of esters in the total 58 kinds of identified volatile compounds of passion fruit juice [28].
Table 1.
Volatile constituents of fruit, pulp, and peel at room temperature.
| Compounds | Mw (Da) | Retention time (min) | Relative content (%) | ||
|---|---|---|---|---|---|
|
| |||||
| Fruit | Pulp | Peel | |||
| Hexyl acetate | 144 | 0.45 | 0.24 | 0.90 | ND |
| Butanoic acid, 1-methylbutyl ester | 158 | 1.79 | 0.61 | ND | 0.84 |
| Benzyl alcohol | 108 | 3.65 | ND | ND | 0.58 |
| Butyl 2-methylbutyrate | 158 | 3.95 | 2.56 | 0.96 | 7.48 |
| Butanoic acid, 3-methylbutyl ester | 158 | 5.68 | ND | 0.45 | ND |
| Butanoic acid, 2-methylbutyl ester | 158 | 5.86 | ND | 0.17 | ND |
| Linalool | 154 | 9.45 | ND | 0.24 | ND |
| Benzyl nitrile | 117 | 12.78 | ND | 0.43 | ND |
| Benzyl acetate | 150 | 14.17 | 2.28 | 1.54 | 0.21 |
| Benzoic acid, ethyl ester | 150 | 14.57 | 0.33 | 0.57 | 0.29 |
| Butanoic acid, 3-hexenyl ester, (Z)- | 170 | 15.43 | 0.24 | 0.22 | 2.45 |
| Hexyl butyrate | 172 | 15.83 | 16.46 | 29.37 | 16.98 |
| Acetic acid, octyl ester | 172 | 16.93 | 0.10 | 0.27 | ND |
| cis-3-Hexenyl valerate | 184 | 17.98 | ND | ND | 0.87 |
| Butanoic acid, 2-methyl-, hexyl ester | 186 | 18.24 | 2.98 | 1.23 | 5.02 |
| Hexyl crotonate | 170 | 18.79 | ND | 0.28 | ND |
| Isopentyl hexanoate | 186 | 19.09 | ND | 0.58 | ND |
| Phenethyl acetate | 164 | 19.48 | ND | 0.78 | ND |
| Propyl benzoate | 164 | 20.27 | 0.44 | 0.21 | 0.41 |
| Cinnamyl alcohol | 134 | 22.33 | 0.10 | 0.24 | 0.23 |
| Benzyl butyrate | 178 | 24.10 | 14.71 | 15.08 | 11.50 |
| Eugenol | 164 | 24.37 | ND | ND | 0.15 |
| Copaene | 204 | 25.11 | 0.10 | ND | 0.60 |
| Butyl benzoate | 178 | 25.43 | 25.57 | 6.39 | 31.54 |
| Hexyl hexanoate | 200 | 25.73 | 1.25 | 8.33 | 0.69 |
| Eugenol methyl ether | 178 | 26.72 | ND | ND | 0.32 |
| Phenylethyl butyrate | 192 | 28.50 | 0.62 | 9.24 | 1.20 |
| Cinnamyl acetate | 176 | 28.90 | 1.19 | 0.83 | ND |
| α-Farnesene | 204 | 31.39 | 9.60 | 13.62 | 11.73 |
| Cadinene | 204 | 32.10 | 0.09 | ND | 0.36 |
| Hexanoic acid, phenylmethyl ester | 206 | 33.69 | 4.91 | 4.21 | ND |
| Elemicin | 208 | 33.86 | ND | ND | 0.36 |
| Phenylpropyl iso-butyrate | 206 | 34.25 | ND | 0.44 | ND |
| 3-Hexen-1-ol, benzoate, (Z)- | 204 | 35.03 | ND | ND | 0.82 |
| Hexyl benzoate | 206 | 35.41 | 13.78 | 1.76 | 6.02 |
| Butyric acid, cinnamyl ester | 204 | 38.33 | 1.61 | 0.82 | 0.23 |
| Phenethyl hexanoate | 220 | 38.48 | ND | 1.00 | ND |
| Cinnamyl isovalerate | 218 | 40.36 | 0.31 | ND | ND |
| Benzyl benzoate | 212 | 45.04 | 0.17 | ND | ND |
Da = daltons; Mw = molecular weight; ND = not detected.
Mabolo has a rich aroma [29–35]. The strong aroma, which is due to the peel, is described by Smith and Oliveros-Belardo [29] as a smell that can increase appetite, while Morton [4] describes it as a cheese-like odor. As the strong aroma is not preferred by some people, it is recommended that the skin is peeled and the fruit is placed in the refrigerator for several hours before consumption. Selection of a line with a less intense aroma is of importance in order to cater to public taste. It has been suggested by Morton [4] that strains producing fruit with purple skin had better flavor. The fresh sweet aroma of the ripe mabolo soon after the fruit has fallen to the ground turns unpleasant as time progresses. Smith and Oliveros-Belardo [29] suggest that free butyric acid is the source of this unpleasant smell.
Collins and Halim [33] utilized steam distillation to obtain essential oils from mabolo, and after analyzing the oils by GC, infrared spectroscopy (IR), and MS, they identified 24 different volatile compounds. Smith and Oliveros-Belardo [29] analyzed the petroleum ether extract of mabolo and identified five main constituents, including benzyl salicylate (26.9%), benzyl benzoate (19.2%), cinnamyl benzoate (10.3%), butyl benzoate (6.0%), and benzyl butyrate (4.1%). Wong et al [34] analyzed the dichloromethane extract of mabolo and identified 67 compounds by GC and GC-MS analysis [34]. Esters constituted 88.6% of the total volatile content, wherein the most abundant esters were methyl butyrate (32.9%), ethyl butyrate (10.7%), butyl butyrate (10.2%), and benzyl butyrate (10.0%). When these results are compared with those from Smith and Oliveros-Belardo [29], they are found to vary depending on the analysis method used. Utilizing GC and GC-MS analysis, Pino et al [35] identified 96 different aroma components by distillation of pulp, which included benzyl butyrate (33.9% of the total composition), butyl butyrate (12.5%) and (E)-cinnamyl butyrate (6.8%). This result was similar to that obtained by Wong et al [34]. The main constituents, butyl benzoate and benzyl butyrate, were the same while compared to the result from Smith and Oliveros-Belardo [29] where only benzyl butyrate was the same. In this study, we used fiber at room temperature with headspace SPME and combined this with GC-MS for analysis. The four important aroma components in the intact fruit, pulp, and peel were butyl benzoate, hexyl butyrate, benzyl butyrate, and α-farnesene. Hexyl butyrate and α-farnesene have not been reported previously, which indicates variability in the results due to the different extraction methods used. The detection of hexyl butyrate and benzyl butyrate indicates that mabolo contains butyric acid esters, whereas Smith and Oliveros-Belardo [29] have suggested that the degradation of free butyric acid is the source of the unpleasant smell [29]. This needs to be confirmed by further studies.
3.2. Analysis of nutrient contents
The nutrients quantified from mabolo are shown in Table 2. The undetected compounds were transfat, saturated fatty acid, sucrose, lactose, maltose, cholesterol, vitamin A, β-carotene, vitamin D3, vitamin K1, vitamin B1, vitamin B6, vitamin B12, vitamin H, inositol, citric acid, cobalt, molybdenum, titanium, selenium, iron, chromium, manganese, nickel, propionic acid, acetic acid, tartaric acid, succinic acid, and formic acid.
Table 2.
Nutrient contents of mabolo and comparison with other representative fruits.
| Composition | Content | Relative nutrition content of representative fruitsa | Remark/postscript | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Persimmon PCA | Persimmon PCNA | Apple “Red-Delicious” | Apple “Fuji” | |||
| Moisture (g/100 g) | 84.4 | 81 | 85 | 86 | 87 | |
| Ash (g/100 g) | 0.8 | 0.3 | 0.4 | 0.3 | 0.4 | |
| Calorie (kcal/100 g) | 62 | 68 | 52 | 50 | 46 | |
| Carbohydrate (g/100 g) | 13.8 | 18 | 13.8 | 13.4 | 12.1 | |
| Dietary fiber (g/100 g) | 3.2 | 4.7 | 1.3 | 1.6 | 1.2 | |
| Crude protein (g/100 g) | 0.4 | 0.5 | 0.5 | 0.1 | 0.3 | |
| Calories from fat (kcal/100 g) | 5.4 | — | — | — | — | |
| Crude fat (g/100 g) | 0.6 | 0.2 | 0.1 | 0.1 | 0.2 | |
| Glucose (g/100 g) | 1.9 | — | — | — | — | |
| Fructose (g/100 g) | 2.4 | — | — | — | — | |
| Vitamin C (mg/100 g) | 2.2 | 46 | 79 | 2.1 | 2 | |
| Vitamin E (mg/100 g) | 0.59 | — | — | — | — | DL-α-tocopherol |
| Vitamin B2 (mg/100 g) | 0.075 | 0 | 0.03 | 0 | 0.01 | |
| Vitamin B3 (mg/100 g) | 0.157 | — | — | — | — | Niacin |
| Folic acid (mg/100 g) | 0.623 | — | — | — | — | Vitamin B9 |
| Pantothenic acid (mg/100 g) | 0.19 | — | — | — | — | Vitamin B5 |
| Choline chloride (mg/100 g) | 62.52 | — | — | — | — | |
| Malic acid (mg/100 g) | 227.1 | EU regulations require apple juice contains > 3 g/L [36] = 300 | ||||
| Fumaric acid (mg/100 g) | 4.5 | — | — | — | — | |
| Sodium (mg/100 g) | 2.0 | 6 | 10 | 4 | 3 | |
| Calcium (mg/100 g) | 42.8 | 10 | 9 | 3 | 5 | High-quality milk 95 |
| Potassium (mg/100 g) | 19.6 | 150 | 150 | 100 | 110 | |
| Magnesium (mg/100 g) | 7.7 | 8 | 6 | 4 | 4 | |
| Phosphorus (mg/100 g) | 17 | 14 | 19 | 11 | 11 | |
| Zinc (mg/100 g) | 3.6 | 0.4 | 0.1 | 0 | 0.1 | Oyster 7.1 |
| Tannin acid (mg/100 g) | 69.2 | — | — | — | — | light green |
| Tannin acid (mg/100 g) | 155.3 | — | — | — | — | dark green |
| Tannin acid (mg/100 g) | 213.3 | — | — | — | — | ripe |
PCA = pollination constant and astringent; PCNA = pollination constant and nonastringent.
Data from the Food and Drug Administration, Ministry of Health and Welfare, Taiwan [37].
The nutrients in mabolo have previously been analyzed in the Philippines and in India, and mabolo was found to be an excellent source of iron, calcium, and vitamin B complex [4]. In our study, we found that the calcium content was 42.8 mg/100 g, which is nearly half of what is obtained from milk (95.0 mg/100 g; Table 2). We could not detect iron in our analysis as the iron content was below the detection limit (0.2 ppm). The fact that mabolo is considered as a source of iron-nutrition could simply be folklore of the Philippines or India. In other words, the author of that cited literature may have only used a record of ethnobotany. If excluding the possibility of the above, the differences between our results and those from literature review may be attributed to sample contamination, difference of detection methods, different soil conditions for plant growth, or even difference of strains. This needs to be further studied for confirmation. Vitamin B2 (0.075 mg/100 g), vitamin B3 (0.157 mg/100 g), folic acid (0.623 mg/100 g), pantothenic acid (0.19 mg/100 g), and choline chloride (62.52 mg/100 g) were all detected in mabolo, proving the fruit to be an excellent source of vitamin B complex. Many B vitamins are involved in homocysteine metabolism, and hyperhomocysteinemia has been associated with cardiovascular disease and other age-related diseases [38]. Moreover, mabolo is rich in malic acid and zinc. Malic acid can be directly absorbed by the body to provide immediate energy. Malic acid also aids recovery from fatigue, and is often used to relieve muscle aches, enhance strength, and protect the liver, heart, and skin [39]. Our data show that mabolo contains high levels of zinc (3.6 mg/100 g), hence 340–420 g of mabolo can provide the recommended daily allowance of zinc [40]. Zinc is helpful for health, as it is required for the functioning of many enzymes and is an essential nutrient for the maintenance of normal gonadal function and for neurogenesis, synaptogenesis and neuronal growth [41,42]. Taking these results into account and considering the contents of vitamin B complex, malic acid, calcium and zinc in mabolo, we can now appreciate why the aboriginal Pinuyumayan honor the elderly with mabolo or offer it to patients. Moreover, we found that the dietary fiber content of mabolo was 3.2 g/100 g, which was higher than that of the “Red-Delicious” apple (1.6 g/100 g), “Fuji” apple (1.2 g/100 g), and pollination constant and non-astringent (PCNA)-persimmon (1.3 g/100 g; Table 2).
In this study, we measured tannins by Folin-Ciocalteu colorimetry. The data obtained can also be interpreted as the total polyphenol content. While tannins in mature mabolo are condensed and insoluble, they can still be measured. The total polyphenol content increased from 69.2 mg/100 g to 155.3 mg/100 g as the fruit matured and turned from light green to dark green, and the content in ripe mabolo was found to be 213.3 mg/100 g. Phenolic compounds are potent natural antioxidants, which decrease the generation of reactive oxygen species and scavenge free radicals. Phenolic compounds (phenolic acids, polyphenols, and flavonoids) have been used as antioxidants by humans [43]. They also possess anti-inflammatory and anti-cancer properties, thus aiding the prevention of many diseases [44].
4. Conclusion
Many ethnobotanical plants or wild plants without domestication have high value for human use in that they can provide new food sources such as an excellent edible oil [45] or vegetable for nutritional supplement [46] and so on. Many functional foods or medicinal ingredients are also developed from such plants. In some recent studies, a number of plant species with antioxidants such as polyphenols and flavonoids have been reported [47–53], and plants with other effects such as antitumor [48,54], antibacterial [48], anti-inflammation [46,52], liver protection [53,55], immunomodulatory effects [47] or even containing antiacetylcholinesterase activity [50] have been screened out in succession. Such plants with potential of development should undergo considerable research on their economic use, including mabolo as it contains good nutrients for health functions and it can be directly eaten as a vegetable or fruit. For vegetables and fruits of further use, it is important that we clearly know their chemical composition and potential biological properties [46]. Compared to the general persimmon, mabolo has a rich aroma and a high nutritious value that is good for human health. We start this research project and publicize the preliminary results at the initial stage to engender the interest of researchers around the world and speed up the usage of D. blancoi as an important economic fruit tree.
Acknowledgments
We extend our sincere gratitude to Dr Jung-Tai Chao, the former deputy director of Taiwan Forestry Research Institute, for his support in this project. This project was funded (97AgrSci-11.4.1-For-G2 to S.F.H.) by The Council of Agriculture, Executive Yuan, Taiwan, R.O.C.
Funding Statement
This project was funded (97AgrSci-11.4.1-For-G2 to S.F.H.) by The Council of Agriculture, Executive Yuan, Taiwan, R.O.C.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Knapp S, Gibert MG. Proposal to conserve the name Diospyros discolor against Cavanillea philippensis (Ebenaceae) Taxon. 2002;51:579–80. [Google Scholar]
- 2.Li HL, Lu SY, Yang YP. Flora of Taiwan. 2nd ed. Second Edition. Vol. 4. Taipei, Taiwan: Editorial Committee of the Flora of Taiwan; 1998. Diospyros philippensis (Desr.) Gürke; p. 92. [Google Scholar]
- 3. Howard RA. The correct name of the mabolo or velvet persimmon. Baileya. 1971;18:26. [Google Scholar]
- 4.Morton JF. Mabolo. In: Morton JF, editor. Fruits of warm climates. Miami: Creative Resource Systems, Inc; 1987. pp. 418–9. [Google Scholar]
- 5.Coronel RE. Diospyros blancoi A. DC. In: Coronel RE, Verheij EWM, editors. Plant resources of South-East Asia. no 2: Edible fruits and nuts. Bogor, Indonesia: Prosea Foundation; 1992. pp. 151–2. [Google Scholar]
- 6.Lee SK, Gilbert MG, White F. Flora of China. Vol. 15. Beijing, China: Science Press; 1996. Diospyros philippensis (Desrousseaux) Gürke; p. 232. [Google Scholar]
- 7. Chen D. Dong Fan Ji. Chinese ancient writings. 1603 In Chinese. [Google Scholar]
- 8.Zheng HW. Diospyros discolor Willd. Forestry Bureau; 2001. [accessed 17, 05, 15]. Available at: http://trail.forest.gov.tw/lib/lib_3_4_1.aspx?bio_id=201. [In Chinese]. [Google Scholar]
- 9. Hung SF, Chen IZ, Roan SF. Preliminary results of fruit selection and induced parthenocarpy of mabolo (Diospyros blancoi A. DC.) Genet Resour Crop Evol. 2015;62:1127–34. [Google Scholar]
- 10.Bureau of Standards, Metrology & Inspection, MOEA, ROC. CNS 5034 N6115. Method of test for ash in food. Taipei, Taiwan: Chinese National Standards; 1984. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 11.Association of Analytical Communities (AOAC) Official methods of analysis. Rev. 2. 18th ed. AOAC International; Gaithersburg, MD, USA: 2007. [Google Scholar]
- 12.Bureau of Standards Metrology & Inspection, MOEA, ROC. CNS 5035 N6116. Method of test for crude protein in food. Taipei, Taiwan: Chinese National Standards; 1986. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 13.Bureau of Standards Metrology & Inspection, MOEA, ROC. CNS 5036, N6117, Methods of test for crude fat in food. Taipei, Taiwan: Chinese National Standards; 1984. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 14.Bureau of Standards, Metrology & Inspection, MOEA, ROC. CNS 12634, N6223 Method of test for fruit and vegetable juice products–Determination of sugars by HPLC. Taipei, Taiwan: Chinese National Standards; 2004. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 15.Bureau of Standards Metrology & Inspection, MOEA, ROC. CNS 12725, N6230 Method of test for determining of vitamin A content for infant formula. Taipei, Taiwan: Chinese National Standards; 1990. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 16. Odriozola-Serrano I, Hernandez-Jover T, Martin-Belloso O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007;105:1151–8. [Google Scholar]
- 17. Chen BH, Chuang JR, Lin JH, Chiu CP. Quantification of provitamin A compounds in Chinese vegetables by high-performance liquid chromatography. J Food Prot. 1993;56:51–4. doi: 10.4315/0362-028X-56.1.51. [DOI] [PubMed] [Google Scholar]
- 18. Klejdus B, Petrlova J, Potesil D, Adam V, Mikelova R, Vacek J, Kizek R, Kuban V. Simultaneous determination of water-and fat-soluble vitamins in pharmaceutical preparations by high-performance liquid chromatography coupled with diode array detection. Anal Chim Acta. 2004;520:57–67. [Google Scholar]
- 19. Jakob E, Elmadfa I. Rapid and simple HPLC analysis of vitamin K in food, tissues and blood. Food Chem. 2000;68:219–21. [Google Scholar]
- 20.Hoffmann-LaRoche F. Analytical procedures for the determination of vitamins in multivitamin preparations: fluorometric determination of vitamin B2. Basle: F. Hoffmann-LaRoche & Co; 1970. pp. 39–40. [Google Scholar]
- 21.BS EN 15607, 2009 Foodstuffs–Determination of d-biotin by HPLC. London: British standard; 2009. [Google Scholar]
- 22.GB/T 5009, 196 Determination of inositol in health foods. Ministry of Health, China Standardization Administration of China; 2003. pp. 549–54. [In Chinese]. [Google Scholar]
- 23. Chen S, Soneji V, Webster J. Determination of choline in pharmaceutical formulations by reversed-phase high-performance liquid chromatography and postcolumn suppression conductivity detection. J Chromatogr A. 1996;739:351–7. doi: 10.1016/0021-9673(96)00215-4. [DOI] [PubMed] [Google Scholar]
- 24. Hyun TK, Song SC, Song CK, Kim JS. Nutritional and nutraceutical characteristics of Sageretia theezans fruit. J Food Drug Anal. 2015;23:742–9. doi: 10.1016/j.jfda.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bureau of Standards Metrology & Inspection, MOEA, ROC. CNS 12869, N6231 Methods of test for minerals in infant formula–Test of copper, iron, magnesium, manganese, potassium, sodium and zinc. Taipei, Taiwan: Chinese National Standards; 1991. [accessed 17, 05, 15]. Available at: http://www.cnsonline.com.tw/?node=result&typeof=common&locale=zh_TW. [Google Scholar]
- 26. Li WX, Yu D, Lin L, Sun J, Lan ZW, Huo JS. Study on the determination of total polyphenols content in vegetables and fruits by Folin-Ciocalteu colorimetry. Ying Yang Xue Bao. 2011;33:302–7. [In Chinese, English abstract] [Google Scholar]
- 27. Dixon J, Hewett EW. Factors affecting apple aroma/flavor volatile concentration: a review. N Z J Crop Hortic Sci. 2000;28:155–73. [Google Scholar]
- 28. Chen CC, Kuo MC, Hwang LS, Wu JSB, Wu CM. Headspace components of passion fruit juice. J Agric Food Chem. 1982;30:1211–5. [Google Scholar]
- 29. Smith RM, Oliveros-Belardo L. Volatile compounds from the fruit peelings of Diospyros discolor Willd. JEOR. 1992;4:287–9. [Google Scholar]
- 30. Hung SF, Chang TL, Lai YT, Tsai CT, Chen IZ. Graftings of Diospyros discolor Willd. J Taiwan Soc Hort Sci. 2007;53:409–18. [In Chinese, English abstract] [Google Scholar]
- 31. Hung SF, Chang TL, Chen IZ. An improved grafting technique for mabolo (Diospyros discolor Willd.) J Taiwan Soc Hort Sci. 2009;55:179–89. [In Chinese, English abstract] [Google Scholar]
- 32. Pan FJ, Hung SF, Kuo HC, Sen YC. Chemical compounds of Diospyros discolor Willd. from flowers. Quart J Chinese For. 2008;41:93–104. [In Chinese, English abstract] [Google Scholar]
- 33. Collins RP, Halim AF. Characterization of the volatile compounds of Diospyros blancoi. Econ Bot. 1976;30:313–6. [Google Scholar]
- 34. Wong KC, Zul Rusdi NHN, Chee SG. Volatile constituents of Diospyros blancoi DC. fruit. JEOR. 1997;9:699–702. [Google Scholar]
- 35. Pino JA, Cuevas-Glory L, Fuentes V. Volatile Components of Mabolo (Diospyros blancoi A. DC.) Grown in Cuba. JEOR. 2008;20:506–8. [Google Scholar]
- 36. European Fruit Juice Association (AIJN) Reference guideline for apple. AIJN Rev. 2007;080227:6.3. [Google Scholar]
- 37.Food and Drug Administration. Food composition table. 2010. [Accessed 27, 05, 15]. Available at: https://consumer.fda.gov.tw/FoodAnalysis/ingredients.htm?nodeID=640. [In Chinese].
- 38. Chen KJ, Pan WH, Yang FL, Wei IL, Shaw NS, Lin BF. Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14:250–5. [PubMed] [Google Scholar]
- 39. Wu JL, Wu QP, Zhang JM. Research progress of physiological functions of L-malate. Food Sci. 2008;29:692–5. [In Chinese, English abstract] [Google Scholar]
- 40.Health promotion administration. Dietary reference intakes. 2011. [accessed 27, 05, 15]. Available at: http://www.hpa.gov.tw/BHPNet/Portal/File/ThemeDocFile/201308300213031785/%E5%9C%8B%E4%BA%BA%E7%87%9F%E9%A4%8A%E7%B4%A0%E5%8F%83%E8%80%83%E6%94%9D%E5%8F%96%E9%87%8F(DRIs)%E6%9F%A5%E8%A9%A2.pdf. [In Chinese].
- 41. Wang X, Du Y, Liu H. Preparation, characterization and antimicrobial activity of chitosan-Zn complex. Carbohydr Polym. 2004;56:21–6. [Google Scholar]
- 42. Liu F, Pei F, Mariga AM, Gao L, Chen G, Zhao L. Separation and speciation analysis of zinc from Flammulina velutipes. J Food Drug Anal. 2015;23:630–5. doi: 10.1016/j.jfda.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–61. [Google Scholar]
- 45. Janporn S, Ho CT, Chavasit V, Pan MH, Chittrakorn S, Ruttarattanamongkol K, Weerawatanakorn M. Physicochemical properties of Terminalia catappa seed oil as a novel dietary lipid source. J Food Drug Anal. 2015;23:201–9. doi: 10.1016/j.jfda.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ponnusamy R, Thangaraj P. Total nutritional capacity and inflammation inhibition effect of Acalypha alnifolia Klein ex wild–An unexplored wild leafy vegetable. J Food Drug Anal. 2014;22:439–47. doi: 10.1016/j.jfda.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao DY, Chai YC, Wang SH, Chen CW, Tsai MS. Antioxidant activities and contents of flavonoids and phenolic acids of Talinum triangulare extracts and their immunomodulatory effects. J Food Drug Anal. 2015;23:294–302. doi: 10.1016/j.jfda.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J Food Drug Anal. 2015;23:109–15. doi: 10.1016/j.jfda.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang YS, Li WZ, Ning JM, Hong RH, Wu HP. Major flavonoid constituents and short-term effects of Chun Mee tea in rats. J Food Drug Anal. 2015;23:93–8. doi: 10.1016/j.jfda.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hasbal G, Yilmaz-Ozden T, Can A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. J Food Drug Anal. 2015;23:57–62. doi: 10.1016/j.jfda.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei JB, Li X, Song H, Liang YH, Pan YZ, Ruan JX, Qin X, Chen YX, Nong CL, Su ZH. Characterization and determination of antioxidant components in the leaves of Camellia chrysantha (Hu) Tuyama based on composition–activity relationship approach. J Food Drug Anal. 2015;23:40–8. doi: 10.1016/j.jfda.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mathew LE, Sindhu G, Helen A. Dolichos biflorus exhibits anti-inflammatory and antioxidant properties in an acute inflammatory model. J Food Drug Anal. 2014;22:455–62. doi: 10.1016/j.jfda.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 54. Yiu CY, Chen SY, Chen YP, Lin TP. Inhibition of the ethanolic extract from Polygonum cuspidatum root on the functions of Epstein-Barr Virus latent membrane protein 1. J Food Drug Anal. 2013;21:20–6. [Google Scholar]
- 55. Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 2015;23:310–7. doi: 10.1016/j.jfda.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]