Abstract
Ligusticum chuanxiong (LC)–Gastrodia elata (GE) compatibility is widely used in the clinic for the treatment of migraine. It has been shown that the changes of neurotransmitters in the central nervous system are closely related to the pathogenesis of migraine; whether LC–GE compatibility might affect the neurotransmitters in migraine rats has not yet been studied. In this study, high performance liquid chromatography-fluorescence detector methods for quantification of serotonin (5-hydroxytryptamine, 5-HT) and excitatory amino acids (EAAs) in rat brain were developed. The 5-HT was measured directly, while EAAs were determined by using dansyl chloride as precolumn derivative reagent. The validation of the methods, including selectivity, linearity, sensitivity, precision, accuracy, recoveries, and stability were carried out and demonstrated to meet the requirements of quantitative analysis. Compared with the model group, the expression of 5-HT in migraine rat brain was enhanced from 30 minutes to 120 minutes and glutamate (L-Glu) was suppressed from 30 minutes to 60 minutes in an LC–GE (4:3) group compared with the model group (p < 0.05, p < 0.01, respectively). These findings showed that the analytical methods were simple, sensitive, selective, and low cost, and LC–GE 4:3 compatibility could have better efficacy for treating migraine through upregulating 5-HT levels and downregulating L-Glu levels.
Keywords: excitatory amino acids, HPLC-FLD, Ligusticum chuanxiong, Gastrodia, elata compatibility, migraine, serotonin
1. Introduction
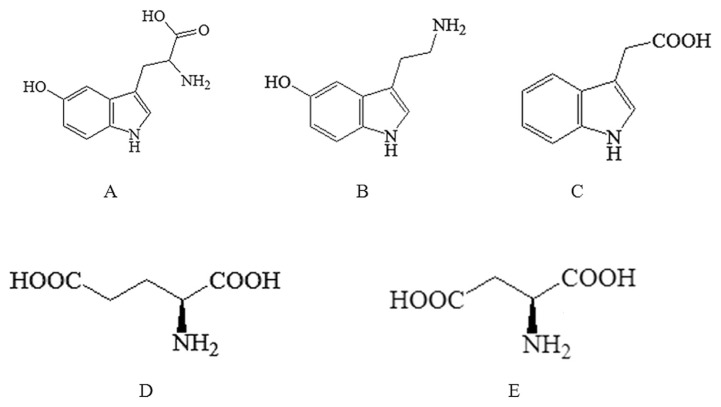
Migraine is a chronic trigeminal pain condition that affects the daily lives of a large part of our population [1]. Recent clinical and experimental evidence has shown that the changes of serotonin (5-hydroxytryptamine, 5-HT) and excitatory amino acids (EAAs) in the central nervous system (CNS) might be critical mediators in the pathogenesis of migraine [2]. 5-HT is involved in the regulation of both physiological and pathophysiological conditions in tissues throughout the body; and the other two neurotransmitters namely 5-hydroxytryptophan (5-HTP) and 5-hydroxyindoleacetic acid (5-HIAA; Fig. 1) are its direct precursor and metabolite, respectively [3,4]. It is confirmed that cerebral vasodilation and low serotonin levels are the possible pathogenesis of migraine, meanwhile, several receptors including 5-HT1, 5-HT2 and 5-HT3 are associated with migraine [5]. 5-HT and related compounds could inhibit N-methyl D-aspartate (NMDA) receptors and attenuate glutamate (L-Glu)-mediated excitatory synaptic responses in a concentration dependent manner, which may play a role in the protection of the blood–brain barrier (BBB) [6]. In addition, the amino acids L-Glu and aspartate (L-Asp; Fig. 1) are recognized as the major excitatory neurotransmitters in the CNS. They may activate NMDA receptors and decrease the phosphorylation of insulin-like growth factors-1 (IGF-1) receptors, and amplify and reinforce pain transmission and brain damage. Therefore, NMDA antagonists are important neuroprotectors that could reduce excitotoxicity, and restrain the excessive release of EAAs and their intracellular effects [7]. It has been proven that the changes of neurotransmitters in the CNS are closely related to the pathogenesis of migraine, and the determination of 5-HT and EAAs in the brain could provide evidence to explore the pathological mechanism of migraine.
Fig. 1.
Chemical structures of all the analytes. (A) 5-hydroxytryptophan (5-HTP); (B) 5-hydroxytryptamine (5-HT); (C) 5-hydroxyindoleacetic acid (5-HIAA); (D) glutamate (L-Glu); and (E) aspartate (L-Asp).
Currently many bioanalytical techniques have been developed for the analysis of 5-HT and EAAs. gas chromatography (GC) [8,9], capillary electrophoresis (CE) [10,11] and liquid chromatography-electrochemical [12] have been reported to determine neurotransmitters levels. However, most of these methods are complex and require higher costs. Amino acid levels are determined using an automatic amino acid analyzer traditionally [13]. In recent years, the majority of methods that have been reported for determination of amino acids are based on precolumn derivatization, and derivatization reagents such as O-phthalaldehydes [14], phenyl isothiocyanate [15], 9-fluorenylmethyl chloroformate [16], and dansyl chloride (DNS-Cl) [17], etc. are abundant. However, some derivatization reagents would shorten the life of the analytical column, could not be detected by fluorescence detection, react with secondary amino acids, and their derivatives are not stable either. Therefore, DNS-Cl derivatization is chosen for its simplicity, selectivity, sensitivity, accuracy, and economy. Thus, simple, economical, and rapid methods will be established to determine 5-HT and EAAs in rat brain.
Ligusticum chuanxiong (LC)–Gastrodia elata (GE) 1:3, 2:3 and 4:3 compatibility are commonly used for treating migraine from clinical application and ancient books. Preliminary studies show that LC and GE ethanol extracts are the main therapeutically effective parts; on this basis, active components of LC and GE are obtained [18]. It has been demonstrated that ligustilide (i.e., senkyunolide I) and organic acids (i.e., ferulic acid) from LC as well as gastrodin from GE are the main active components for the treatment of migraine, an antimigraine effect of which has also been suggested by previous studies [19–22]. However, whether different dose-ratios of LC–GE compatibility could affect the neurotransmitters in migraine rats has not yet been studied. In the current study, different dose-ratios of LC and GE were given to 6-week-old Sprague–Dawley rats orally. The effects of different dose-ratios of LC and GE on the content of 5-HT and EEAs in migraine rats were systematically profiled. Thus, the possible compatibility mechanism of the two herbs may be found.
2. Materials and methods
2.1. Chemicals and reagents
Active components of LC and GE were both extracted with ethanol and further purified with macroporous resin and pH precipitation, respectively. The yield of the LC extract was 1.44%, which contained 6.20% of ferulic acid and 10.74% of senkyunolide I. The yield of the GE extract was 5.68%, which contained 14.85% of gastrodin. The codergocrine mesylate (CDM) tablets and nitroglycerin injection were purchased from Tianjin Huajin Pharmaceutical Co. Ltd. (Tianjin, China) and Beijing Yimin Pharmaceutical Co. Ltd. (Peking, China), respectively. 5-HTP, 5-HIAA and 5-HT were obtained from Sigma-Aldrich (Shanghai, China). L-Glu and L-Asp were from Sinopharm Chemical Reagents (Shanghai, China). DNS-Cl was from Tokyo Chemical Industry (Tokyo, Japan). High performance liquid chromatography (HPLC) grade acetonitrile and methanol were purchased from Merck, Inc. (Darmstadt, Germany). All other chemical solvents were of analytical grade from Sinopharm Chemical Reagents.
2.2. Instrument and analytical conditions
2.2.1. HPLC analysis for 5-HTP, 5-HT, and 5-HIAA
HPLC analysis for 5-HTP, 5-HT, and 5-HIAA was performed on an Agilent 1200 HPLC apparatus, equipped with fluorescence detector (Agilent technologies, Santa Clara, CA, USA). The column used was ZORBAX SB-C18, 5 μm, 4.6 mm × 250 mm (Agilent technologies). The mobile phase was composed of methanol:acetate buffer (pH 5.1; 0.1M; 8:92%, v/v) at a flow rate of 1.0 mL/min. Acetate buffer was prepared by dissolving 8.20 g of sodium acetate and 33.62 mg of sodium-EDTA in 1 L of triple-distilled water, and its pH was adjusted to 5.1 with acetic acid. The column was heated to 25°C and the three neurotransmitters were detected by their fluorescence at an emission wavelength of 330 nm and excitation at 290 nm.
2.2.2. HPLC analysis for L-Glu and L-Asp
The chromatographic system and the column for measuring L-Glu and L-Asp were of the same composition as described above. The flow rate was 0.8 mL/min and the temperature of the column oven was maintained at 30°C. The derivatized DNS-amino acids were detected by their fluorescence at an emission wavelength of 515 nm and excitation at 340 nm. Acetonitrile (A) and ammonium acetate buffer (B: (pH 3.5; 5mM) were used for gradient elution; the ammonium acetate buffer was prepared by dissolving 0.39 g of ammonium acetate, 0.96 g of citric acid, 64.47 mg of tetrabutyl ammonium bromide, and 16.81 mg of sodium-EDTA in 1 L of triple-distilled water, and its pH was adjusted to 3.5 with formic acid. The initial elution condition was A:B (20:80, v/v), then linearly changed to A:B (25:75, v/v) at 3 minutes, A:B (30:70, v/v) at 10 minutes, A:B (32:68, v/v) at 16 minutes, A:B (33:67, v/v) at 25 minutes, and A:B (20:80, v/v) at 28 minutes.
2.3. Preparation of reference standards solutions
Aqueous stock solutions of 5-HTP (124.00 μg/mL), 5-HT (40.00 μg/mL), 5-HIAA (110.00 μg/mL), L-Glu (182.40 μg/mL), and L-Asp (102.20 μg/mL) were prepared in 0.1M hydrochloric acid and aliquoted to a brown volumetric flask. These solutions were diluted daily to working concentrations with 0.1M hydrochloric acid. All solutions were stored at 4°C in the dark.
2.4. Preparation of sample solutions
The CDM tablets (each tablet contains 1 mg codergocrine) prepared in physiological saline and diluted to 0.1 mg/mL CDM solution were used as the positive drug. The extracts of LC and GE were mixed with the weight ratios of 1:3, 2:3, and 4:3, the mixture was dissolved in physiological saline and diluted to form the solutions of 16.40 mg/mL, 32.60 mg/mL, and 65.20 mg/mL extracts of LC (equivalent to 1.01 mg/mL, 2.02 mg/mL, and 4.04 mg/mL ferulic acid, and 1.75 mg/mL, 3.50 mg/mL, and 7.00 mg/mL senkyunolide I) and 48.40 mg/mL extracts of GE (equivalent to 7.19 mg/mL gastrodin), respectively. All drug solutions were stored at 4°C and shaken well before use.
The DNS-Cl solution was prepared just before derivatization by dissolving 80.00 mg of DNS-Cl in 10 mL of acetonitrile. The borate buffer (pH 9.0; 0.2M) was prepared by dissolving 1.25 g of boric acid and 1.50 g sodium chloride in 100 mL of ultrapure water, and 0.2M sodium hydroxide was added to adjust the pH to 9.0.
2.5. Animals, drug administration, and sampling
Male Sprague–Dawley rats (180–220 g) were supplied by the Lab Animal Center of Shanghai University of Traditional Chinese Medicine (SYXK (hu) 2008-0016), and were randomly divided into six groups, named normal group, model group, positive group, LC–GE (1:3) group, LC–GE (2:3) group, and LC–GE (4:3) group, respectively. They were kept in an environmentally controlled breeding room for 1 week before starting the experiments, and fed with standard laboratory food and water ad libitum. The animal facilities and protocols were approved by the Institutional Animal Care and Use Committee, Shanghai University of Traditional Chinese Medicine. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animal (The National Academies Press, revised edition 2010). Excepting the normal group, other groups of rats were subcutaneously injected with 10 mg/kg of nitroglycerin to establish an experimental rat migraine model as reported by Tassorelli et al [23]. Then, all the corresponding drug solutions were orally administered to rats in each group at a dosage of 10 mL/kg after 30 minutes, respectively. The rats in each group were anesthetized at 15 minutes, 30 minutes, 60 minutes, and 120 minutes, respectively. Brains were rapidly removed after collecting blood, and the brain samples were then stored at −80°C.
The brains were homogenized in one volume of cold physiological saline. An equal volume of 0.4 M perchloric acid was added in an appropriate amount of homogenate, followed by centrifugation (10,000 r/min for 10 minutes at 4°C) to remove the protein precipitate. Twenty microliters of the supernatant was injected into the HPLC system for determination of 5-HT.
Another 50 μL of the supernatant was aspirated precisely and put into a centrifuge tube; 1.0M sodium hydroxide (10 μL), 0.2M borate buffer (50 μL), and 8.00 mg/mL DNS-Cl solution (50 μL) were added, and after mixing vigorously for 10 seconds, the mixture was reacted in the dark at 30°C in a water bath for 80 minutes. Then, an aliquot (40 μL) of acetic acid was added into the tube to stop the reaction. The mixture was centrifuged at 4000 r/min for 5 minutes. The supernatant (10 μL) of the reaction mixture was injected into the HPLC system for determination of EAAs.
2.6. Optimization of the procedure for DNS-Cl derivatization
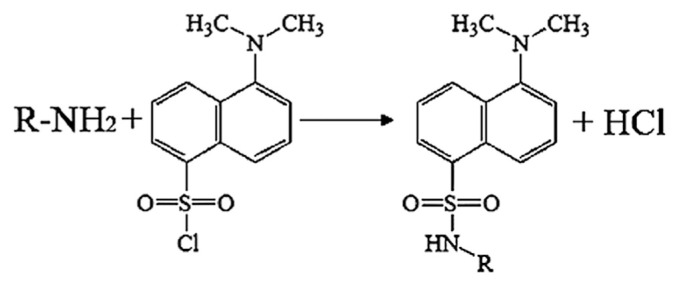
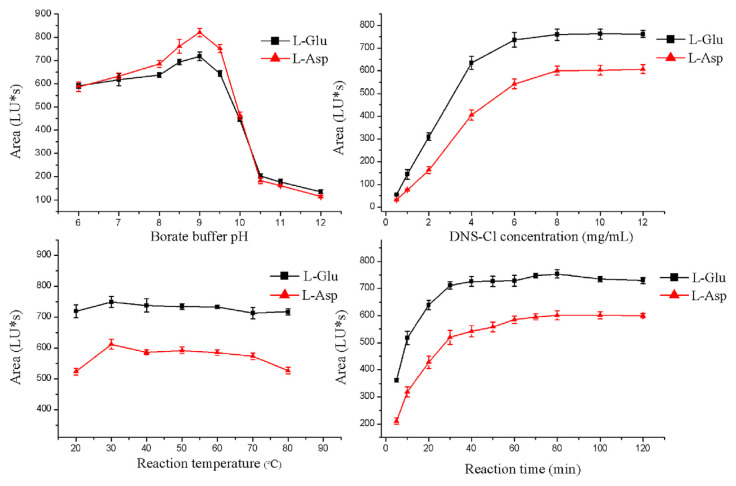
Since both the 5-HT and EAAs have amino groups, the two neurotransmitters in the brain tissues seem to be simultaneously determined by the derivative methods. However, the 5-HT itself has fluorescence, and there are large differences in polarity between DNS-5-HT and DNS-L-Glu (or DNS-L-Asp), which cost much time. Therefore, 5-HT and EAAs were determined separately. The reaction of EAAs with DNS-Cl (Fig. 2) was optimized by using blank brain samples. Several parameters were assayed: the borate buffer pH (pH 6.0–12.0); the DNS-Cl concentration (0.5–12.0 mg/mL); temperature (20–80°C); and reaction time (5–120 min). After the derivatization reaction, the peak area was recorded to evaluate the optimal conditions.
Fig. 2.
Simplified reaction scheme for the derivatization of excitatory amino acids (EAAs) with dansyl chloride (DNS-Cl).
2.7. Analytical method validation
2.7.1. Specificity
Interferences of endogenous and exogenous substances in rat brain were investigated. The specificity of the method was tested by comparing the mixed reference standards and blank brain samples for 5-HT, while for EAAs it occurred after the derivatization reaction.
2.7.2. Linearity, limit of detection and limit of quantification
A series of standard solutions were obtained by further dilution of stock solution, through which the concentration for serotonin and EAAs were in the range of 0.07–1.50 μg/mL and 1.25–480.00 μg/mL, respectively. The limit of detection (LOD) and the limit of quantification (LOQ) of the method were calculated from the signal-to-noise ratio, corresponding to threefold and 10-fold of the noise level, respectively.
2.7.3. Precision, accuracy, recovery, and stability
The intrabatch precision was determined by analyzing six replicates of each reference standard at concentrations of 0.10 μg/mL, 0.42 μg/mL, and 1.50 μg/mL for 5-HT and 2.50 μg/mL, 16.00 μg/mL, and 240.00 μg/mL for EAAs which were added to the blank brain samples on the same day. The interbatch precision was determined by assaying six replicates daily over 3 consecutive days. The accuracy was assessed by expressing the mean calculated concentration as a percentage of the nominal concentration, reflecting the difference between the measured concentration and spiked concentration. Absolute recoveries of 5-HT and EAAs were determined by comparing the peak area ratio after extraction with those of unextracted solutions containing the same concentrations of spiked 5-HT and EAAs standards in rat brain. Stabilities of 5-HT and EAAs in rat brain were examined after being kept at room temperature for 24 hours, or undergoing one and two freeze–thaw cycles, six replicates were analyzed in one analytical batch.
2.8. Statistical analysis
Data were expressed as the mean ± standard deviation. For the comparisons of different groups sampled at same timeline, one-way analysis of variance and further Least-Significant Difference (LSD) t multiple comparisons were employed using Statistical Product and Service Solutions (SPSS) for Windows, version 15.0 (SPSS Inc., Chicago, Illinois, USA) A p value < 0.05 or p < 0.01 was taken to indicate significant difference between data means.
3. Results and discussion
3.1. Optimization of the procedure for DNS-Cl derivatization
This reaction of EAAs with DNS-Cl was optimized by assaying the borate buffer pH, the DNS-Cl concentration, temperature, and reaction time. As can be seen from Fig. 3, the optimal conditions for the derivatization reaction were: borate buffer (pH 9.0; 0.2M), 8.0 mg/mL DNS-Cl, reaction temperature 30°C, and reaction time 80 minutes.
Fig. 3.
Effect of pH value of borate buffer solution, DNS-Cl concentration, reaction temperature, and reaction time on derivation reaction. L-Asp = aspartate; DNS-Cl = dansyl chloride; L-Glu = glutamate; LU = Luminous.
3.2. Analytical method validation
3.2.1. Specificity
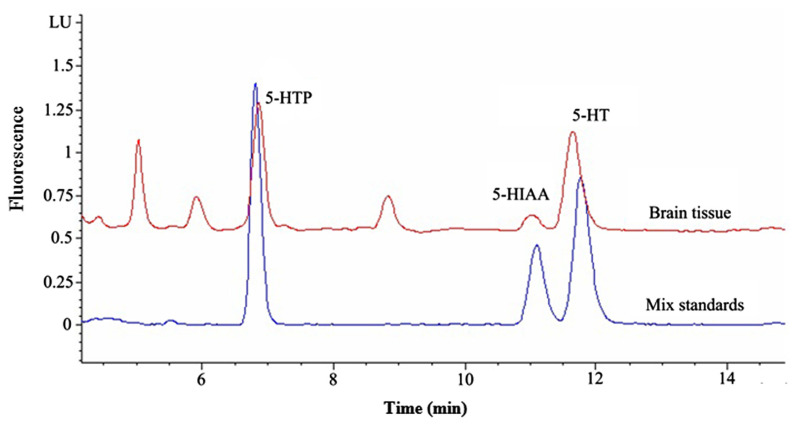
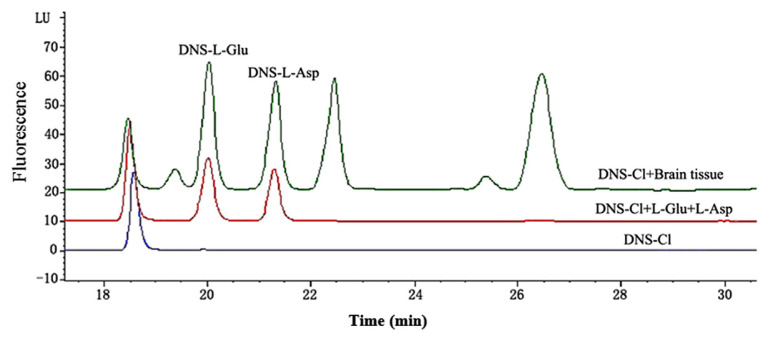
Representative chromatograms for the determination of the 5-HT and EAAs in rat brain had showed that 5-HT and EAAs were well separated, no interferences were detected from endogenous substances, and the typical retention time for 5-HTP, 5-HIAA, 5-HT were approximately 6.82 minutes, 11.18 minutes, and 11.86 minutes (Fig. 4); while for DNS-L-Glu and DNS-L-Asp they were approximately 20.06 minutes and 21.45 minutes (Fig. 5).
Fig. 4.
Representative chromatograms for the determination of serotonin with the mixed reference substances and brain sample. 5-HIAA =5-hydroxyindoleacetic acid; 5-HT =5-hydroxytryptamine; 5-HTP =5-hydroxytryptophan; LU =Luminous.
Fig. 5.
Representative chromatograms for the determination of the excitatory amino acids (EAAs) with the mixed reference substances and brain sample after the derivatization reaction. DNS = dansyl; DNS-Cl = dansyl chloride; L-Asp = aspartate; L-Glu = glutamate; LU = Luminous.
The carboxyl in 5-HTP and 5-HIAA might cause baseline instability and peak tailing, therefore, the mobile phase was adjusted to pH 5.1 to inhibit dissociation. Due to the existence of free carboxyl in the DNS-Cl derivatives, the two amino acids were not separated well when the mobile phase was adjusted to below pH 2.5. However, because 0.2mM tetrabutyl ammonium bromide was added into the ammonium acetate buffer, the DNS-Cl derivative formed a new molecular-type compound by combining the nitrogen positive ions, the two amino acids were separated much better, and the column was unaffected by these ion-pairing reagents. Moreover, although the hydrophobicity of L-Glu can be larger than that of L-Asp, DNS-L-Glu was eluted earlier than DNS-L-Asp.
3.2.2. Linearity, LOD and LOQ
Good correlations were found between the peak areas (Y) and the concentrations (X) using 1 or 1/X weighted linear least squares regression model (Table 1). The LODs for 5-HTP, 5-HT, and 5-HIAA were 0.85–7.78 ng/mL (Signal/Noise (S/N) = 3; injection: 20 μL), while the LODs for L-Glu and L-Asp were 94.38–108.57 ng/mL (S/N = 3; injection: 10 μL), and the LOQs were 2.84–25.94 ng/mL (S/N = 10; injection: 20 μL) and 314.61–361.90 ng/mL (S/N = 10; injection: 10 μL), respectively, which indicated that the methods were sensitive (Table 1).
Table 1.
Linearity and sensitivity of detection for serotonin and excitatory amino acids.
| Analyte | Regression equation | Correlation coefficient | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|
| 5-HTP | Y = 0.1999 X − 7.435 | 0.9998 | 0.8502 | 2.844 |
| 5-HIAA | Y = 0.1271 X − 12.66 | 0.9992 | 7.781 | 25.94 |
| 5-HT | Y = 0.1518 X − 11.71 | 0.9996 | 2.642 | 8.812 |
| L-Glu | Y = 3.909 X + 27.11 | 0.9998 | 108.6 | 361.9 |
| L-Asp | Y = 3.522 X − 2.612 | 0.9999 | 94.38 | 314.6 |
5-HIAA = 5-hydroxyindoleacetic acid; 5-HT = 5-hydroxytryptamine; 5-HTP = 5-hydroxytryptophan; L-Asp = aspartate; L-Glu = glutamate; LOD = limit of detection; LOQ = limit of quantification.
3.2.3. Precision, accuracy, recovery, and stability
The accuracy and precision of the methods were examined by using known amounts of reference standards. The results, which are summarized in Table 2, meet the requirements of accuracy within 95.00–105.00%, and precision relative standard deviation (RSD) < 5.00%. These results suggest that the methods were accurate and precise. The recoveries of 5-HTP, 5-HT, and 5-HIAA were 93.41–107.12% with an RSD <2.56%; meanwhile, L-Glu and L-Asp were 97.85–107.63% with an RSD <4.18%. The results described above showed that the proposed methods were reliable for the quality control of 5-HT and EAAs. The stability RSD value for 5-HTP, 5-HT, and 5-HIAA was approximately 2.63–7.50%, and that for L-Glu and L-Asp was approximately 0.37–2.27% at room temperature after 24 hours, first freeze–thaw, and second freeze–thaw, respectively. It indicated that all five compounds were considered stable in rat brain under different conditions.
Table 2.
Precision and accuracy of the method (n = 6).
| Analyte | Spiked conc. (μg/mL) | Intrabatch (n = 6) | Interbatch (n = 3) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Measured conc.a (μg/mL) | Accuracy (%) | Precision RSD (%) | Measured conc.a (μg/mL) | Accuracy (%) | Precision RSD (%) | ||
| 5-HTP | 0.10 | 0.10 ± 0.00 | 96.32 | 0.52 | 0.10 ± 0.00 | 95.12 | 4.23 |
| 0.42 | 0.41 ± 0.01 | 97.62 | 1.35 | 0.41 ± 0.02 | 96.53 | 4.88 | |
| 1.50 | 1.44 ± 0.02 | 96.17 | 1.39 | 1.46 ± 0.04 | 97.33 | 2.74 | |
| 5-HIAA | 0.10 | 0.10 ± 0.00 | 98.20 | 2.24 | 0.10 ± 0.00 | 97.03 | 2.83 |
| 0.42 | 0.40 ± 0.01 | 95.24 | 2.38 | 0.41 ± 0.02 | 97.62 | 4.88 | |
| 1.50 | 1.48 ± 0.02 | 98.67 | 1.35 | 1.46 ± 0.03 | 97.33 | 2.05 | |
| 5-HT | 0.10 | 0.10 ± 0.00 | 98.78 | 1.55 | 0.10 ± 0.00 | 96.64 | 3.66 |
| 0.42 | 0.41 ± 0.02 | 97.62 | 4.88 | 0.44 ± 0.02 | 104.76 | 4.55 | |
| 1.50 | 1.50 ± 0.02 | 99.87 | 1.33 | 1.53 ± 0.07 | 102.00 | 4.58 | |
| L-Glu | 2.50 | 2.47 ± 0.03 | 98.80 | 1.21 | 2.42 ± 0.08 | 96.80 | 3.31 |
| 16.00 | 15.96 ± 0.08 | 99.75 | 0.50 | 15.67 ± 0.22 | 97.94 | 1.40 | |
| 240.00 | 234.92 ± 0.87 | 97.88 | 0.37 | 234.08 ± 5.21 | 97.53 | 2.23 | |
| L-Asp | 2.50 | 2.44 ± 0.01 | 97.60 | 0.41 | 2.43 ± 0.06 | 97.20 | 2.45 |
| 16.00 | 15.39 ± 0.04 | 96.19 | 0.26 | 15.50 ± 0.11 | 96.88 | 0.71 | |
| 240.00 | 237.43 ± 0.88 | 98.93 | 0.37 | 248.21 ± 1.35 | 103.42 | 0.54 | |
Conc. = concentration; 5-HIAA = 5-hydroxyindoleacetic acid; 5-HT = 5-hydroxytryptamine; 5-HTP = 5-hydroxytryptophan; L-Asp = aspartate; L-Glu = glutamate: RSD = relative standard deviation.
Data expressed as mean ± standard deviation unless otherwise indicated.
3.3. Quantitative analysis of 5-HT in rat brain
The developed method for measuring 5-HT was applied to the brain samples from rats. The rat brain 5-HT amounts measured are shown in Tables 3–5. The results showed the amounts of 5-HTP in LC–GE (1:3), (2:3), and (4:3) groups were significantly higher than that of the model group at all sampling times (p < 0.05 or p < 0.01). Similarly, the 5-HT measured from the LC–GE (4:3) group was significantly higher than that of the model group at all sampling times except 15 minutes (p < 0.05 or p < 0.01), and the 5-HT measured from LC–GE (1:3) and (2:3) groups were significantly higher than that of the model group at 120 minutes (p < 0.05). However, as for 5-HIAA, there was no significant difference among LC–GE (1:3), (2:3), and (4:3) groups and the model group at all sampling times. Moreover, the amounts of 5-HT in the model group was significantly lower than that of the normal group at all sampling times (p < 0.01).
Table 3.
Amounts of 5-hydroxytryptophan (5-HTP) in rat brain (n = 6).
| Group | 5-HTP | |||
|---|---|---|---|---|
|
| ||||
| 15 min | 30 min | 60 min | 120 min | |
| Normal group | 641.72 ± 75.64a,b,c,* | 690.12 ± 82.87a,b,c,** | 619.87 ± 47.17a,b,c,* | 624.36 ± 46.85a,b,c,* |
| Model group | 639.78 ± 32.18a,b,c,* | 693.42 ± 62.60a,b,c,** | 619.63 ± 84.04a,b,c,* | 652.40 ± 106.03a,b,*,c,** |
| Positive group | 712.84 ± 58.08b,* | 708.35 ± 42.79 | 677.16 ± 18.09a,**,b,c,* | 683.96 ± 93.86a,b,** |
| LC–GE (1:3) group | 779.35 ± 73.35d,e,* | 781.29 ± 104.96d,e,** | 764.06 ± 84.25d,e,*,f,** | 793.75 ± 93.74d,e,*,f,** |
| LC–GE (2:3) group | 852.53 ± 133.68d,e,f,* | 792.94 ± 45.01d,e,** | 817.82 ± 44.03d,e,f,* | 798.53 ± 91.29d,e,*,f,** |
| LC–GE (4:3) group | 799.25 ± 42.27d,e,* | 780.20 ± 52.98d,e,** | 804.72 ± 62.98d,e,f,* | 772.80 ± 59.16d,*,e,f,** |
Data are presented as (ng/g), mean ± standard deviation.
p < 0.01.
p < 0.05.
5-HTP = 5-hydroxytryptophan; LC–GE = Ligusticum chuanxiong–Gastrodia elata.
Statistically significant, other groups versus LC-GE (1:3) group.
Statistically significant, other groups versus LC-GE (2:3) group.
Statistically significant, other groups versus LC-GE (4:3) group.
Statistically significant, other groups versus normal group.
Statistically significant, other groups versus model group.
Statistically significant, other groups versus positive group.
Table 4.
Amounts of 5-hydroxytryptamine (5-HT) in rat brain (n = 6).
| Group | 5-HT | |||
|---|---|---|---|---|
|
| ||||
| 15 min | 30 min | 60 min | 120 min | |
| Normal group | 405.14 ± 47.76a–e,* | 377.31 ± 70.00a,b,**,d,e,* | 397.69 ± 44.62a–e,* | 415.04 ± 64.10a–e,* |
| Model group | 286.61 ± 39.56f,* | 263.87 ± 16.27c,**,f,* | 249.37 ± 46.33f,* | 243.67 ± 22.50a,b,c,**,f,* |
| Positive group | 301.07 ± 33.50f,* | 296.47 ± 39.31f,* | 280.92 ± 33.00f,* | 296.49 ± 40.15f,* |
| LC–GE (1:3) group | 276.97 ± 46.80f,* | 308.52 ± 52.42f,** | 276.75 ± 45.33f,* | 306.55 ± 64.07d,**,f,* |
| LC–GE (2:3) group | 295.81 ± 78.51f,* | 307.78 ± 29.49f,** | 294.50 ± 49.86f,* | 307.86 ± 55.61d,**,f,* |
| LC–GE (4:3) group | 291.65 ± 17.05f,* | 324.57 ± 29.01d,** | 312.25 ± 41.71d,**,f,* | 308.58 ± 33.68d,**,f,* |
Data are presented as (ng/g), mean ± standard deviation.
p < 0.01.
p < 0.05.
5-HT = 5-hydroxytryptamine; LC–GE = Ligusticum chuanxiong–Gastrodia elata.
Statistically significant, other groups vs. LC-GE (1:3) group.
Statistically significant, other groups vs. LC-GE (2:3) group.
Statistically significant, other groups vs. LC-GE (4:3) group.
Statistically significant, other groups vs. model group.
Statistically significant, other groups vs. positive group.
Statistically significant, other groups vs. normal group.
Table 5.
Amounts of 5-hydroxyindoleacetic acid (5-HIAA) in rat brain (n = 6).
| Group | 5-HIAA | |||
|---|---|---|---|---|
|
| ||||
| 15 min | 30 min | 60 min | 120 min | |
| Normal group | 578.33 ± 22.97 | 560.48 ± 25.25 | 547.36 ± 19.05 | 545.26 ± 22.25 |
| Model group | 576.44 ± 24.56 | 558.17 ± 14.01 | 541.16 ± 11.22 | 539.91 ± 16.27 |
| Positive group | 577.81 ± 23.60 | 557.86 ± 21.98 | 544.21 ± 23.86 | 521.64 ± 16.06* |
| LC–GE (1:3) group | 587.25 ± 45.53 | 576.23 ± 27.58 | 557.33 ± 27.36 | 559.95 ± 22.92** |
| LC–GE (2:3) group | 603.53 ± 29.61 | 573.61 ± 25.72 | 542.63 ± 28.01 | 546.83 ± 32.24 |
| LC–GE (4:3) group | 582.53 ± 28.70 | 566.28 ± 12.66 | 560.48 ± 40.42 | 549.46 ± 24.67 |
Data are presented as (ng/g), mean ± standard deviation.
p < 0.01, other groups vs. LC–GE (1:3) group.
p < 0.01, other groups vs. positive group.
5-HIAA = 5-hydroxyindoleacetic acid; LC–GE = Ligusticum chuanxiong–Gastrodia elata.
5-HT is one of the main transmitters in the nervous system. Signaling of 5-HT is transmitted via receptors located on neurons and many other cell types, and 5-HT1 receptor is the one mainly expressed in the CNS, therefore, the 5-HT1D receptor agonist could suppress cerebral vasodilation and have an effect on the treatment of migraine [24]. In this paper, it was found that the experimental rat migraine model was successfully established from this aspect by injecting nitroglycerin subcutaneously. Meanwhile, LC–GE 4:3 compatibility could increase 5-HTP and 5-HT levels in migraine rat brain. As the agonist of 5-HT1D receptor, the increase of 5-HT levels was conducive to migraine treatment. However, it could relieve brain damage and decrease BBB permeability through inhibiting NMDA receptors. In addition, some drugs often had timeliness when modulating the physiological function. It was known that migraine occurred rapidly, and the main active ingredients of LC and GE, namely senkyunolide I, ferulic acid, and gastrodin, were absorbed quickly as well as eliminated fast, therefore, 120 minutes was the longest sampling time to research timeliness. The results showed that, compared with the pathological rat, the LC–GE 4:3 compatibility could increase 5-HT levels in migraine rat brain from 30 minutes to 120 minutes, while LC–GE 1:3 and 2:3 compatibility could increase 5-HT levels at 120 minutes. It suggested that LC-GE 4:3 compatibility may have better efficacy on upregulating 5-HT levels than 1:3 and 2:3 compatibility.
3.4. Quantitative analysis of EAAs in rat brain
The amounts of the EAAs found in rat brain are presented in Tables 6 and 7; the amounts of L-Glu in the LC–GE (4:3) group was significantly lower than that of the model group at 30 minutes and 60 minutes (p < 0.05 or p < 0.01), and the L-Glu measured from the LC–GE (4:3) group was significantly lower than that of the LC–GE (2:3) group at 60 minutes (p < 0.01). Meanwhile, the L-Asp measured from the LC–GE (4:3) group was significantly higher than that of LC–GE (1:3) and (2:3) groups at 15 minutes (p < 0.01). Moreover, the amounts of L-Glu in normal and positive groups were significantly lower than that of model group at 60 minutes (p < 0.05).
Table 6.
The amounts of L-Glu in rat brain (n = 6).
| L-Glu | ||||
|---|---|---|---|---|
|
| ||||
| 15 min | 30 min | 60 min | 120 min | |
| Normal group | 770.42 ± 77.93 | 771.77 ± 83.96 | 778.01 ± 82.05a* | 778.02 ± 66.14 |
| Model group | 873.25 ± 184.16 | 921.47 ± 83.88b,* | 963.74 ± 136.67b,**,c,d,* | 865.99 ± 88.24 |
| Positive group | 802.37 ± 94.89 | 797.07 ± 121.20 | 768.07 ± 92.84a,* | 772.92 ± 122.42 |
| LC–GE (1:3) group | 778.11 ± 172.86 | 843.32 ± 222.96 | 816.33 ± 221.72 | 837.92 ± 140.43 |
| LC–GE (2:3) group | 818.99 ± 202.19 | 825.49 ± 168.18 | 884.01 ± 199.18b,** | 878.43 ± 131.56 |
| LC–GE (4:3) group | 720.85 ± 278.16 | 725.14 ± 211.54a,* | 626.25 ± 128.63a,e,** | 720.00 ± 216.89 |
Data are presented as (μg/g), mean ± standard deviation.
p < 0.05.
p < 0.01.
LC–GE = Ligusticum chuanxiong–Gastrodia elata; L-Glu = glutamate.
Statistically significant, other groups vs. model group.
Statistically significant, other groups vs. LC-GE (4:3) group.
Statistically significant, other groups vs. normal group.
Statistically significant, other groups vs. positive group.
Statistically significant, other groups vs. LC-GE (2:3) group.
Table 7.
The amounts of L-Asp in rat brain (n = 6).
| L-Asp | ||||
|---|---|---|---|---|
|
| ||||
| 15 min | 30 min | 60 min | 120 min | |
| Normal group | 718.05 ± 130.62 | 719.41 ± 163.98 | 748.48 ± 120.56 | 750.50 ± 174.20 |
| Model group | 803.55 ± 187.25a,* | 725.28 ± 134.16 | 755.03 ± 109.54 | 762.75 ± 158.45 |
| Positive group | 751.72 ± 104.76 | 757.60 ± 151.57 | 730.05 ± 162.30 | 770.89 ± 161.58 |
| LC–GE (1:3) group | 665.85 ± 154.91b,* | 661.84 ± 109.75 | 711.72 ± 178.39 | 664.90 ± 130.95 |
| LC–GE (2:3) group | 625.01 ± 100.31b,*,c,* | 677.75 ± 81.89 | 710.26 ± 152.72 | 697.40 ± 119.51 |
| LC–GE (4:3) group | 849.95 ± 131.31a,**,d,* | 749.60 ± 116.38 | 783.80 ± 105.64 | 837.41 ± 60.34 |
Data are presented as μg/g, mean ± standard deviation.
p < 0.05.
p < 0.01.
L-Asp = aspartate; LC–GE = Ligusticum chuanxiong–Gastrodia elata.
Statistically significant, other groups vs. LC-GE (2:3) group.
Statistically significant, other groups vs. LC-GE (4:3) group.
Statistically significant, other groups vs. model group.
Statistically significant, other groups vs. LC-GE (1:3) group.
Glutamate receptors, activated by L-Glu and L-Asp, are divided into two types including ionic and metabolic. Glutamate ionotropic receptors of the NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid/kainate type are abundantly expressed on neurons and participate in the regulation of both motor and secretory functions of the whole body [25], while the metabotropic glutamate receptors are involved in many developmental processes and immune and synaptic functions, but the mode of their signal transduction is unclear [26]. When the EAAs activate NMDA receptors, brain damage can be caused. This paper showed that the experimental rat migraine model was successfully established from the L-Glu angle. Meanwhile, LC–GE 4:3 compatibility had better efficacy on downregulating L-Glu levels in migraine rat brain than 1:3 and 2:3 compatibility from 30 minutes to 60 minutes, and it had the same efficacy as the positive drug.
4. Conclusion
In this paper, HPLC-fluorescence detector methods were developed for the determination of two types of neurotransmitters, which were closely related to migraine. The results indicated that the methods were simple, sensitive, selective, low cost, and could successfully measure five major neurotransmitters in rat brain, and that LC–GE 4:3 compatibility could upregulate 5-HT levels from 30 minutes to 120 minutes and downregulate L-Glu levels from 30 minutes to 60 minutes in migraine rat brain. These findings provided further evidence that there are possible pathways for Chinese medicine to relieve brain damage and have an effect for treating migraine.
Acknowledgments
The work was supported by grants from the Shanghai Municipal Education Committee (Grant No. 12ZZ124), the Shanghai Science and Technology Committee (Grant No. 12401900402 and 11ZR1434500), the Macao Science and Technology Development Fund (Grant No. 102/2012/A3), and the Research Fund of the University of Macau (Grant No. MRG005/CMW/2014/ICMS).
Funding Statement
The work was supported by grants from the Shanghai Municipal Education Committee (Grant No. 12ZZ124), the Shanghai Science and Technology Committee (Grant No. 12401900402 and 11ZR1434500), the Macao Science and Technology Development Fund (Grant No. 102/2012/A3), and the Research Fund of the University of Macau (Grant No. MRG005/CMW/2014/ICMS).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Holmes WF, MacGregor EA, Dodick D. Migraine-related disability: impact and implications for sufferers’ lives and clinical issues. Neurology. 2001;56:S13–9. doi: 10.1212/wnl.56.suppl_1.s13. [DOI] [PubMed] [Google Scholar]
- 2. Charles A. The evolution of a migraine attack—a review of recent evidence. Headache. 2013;53:413–9. doi: 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- 3. Lynn-Bullock CP, Welshhans K, Pallas SL, Katz PS. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J Chem Neuroanat. 2004;27:129–38. doi: 10.1016/j.jchemneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4. Lorenzi V, Carpenter RE, Summers CH, Earley RL, Grober MS. Serotonin, social status and sex change in the bluebanded goby Lythrypnus dalli. Physiol Behav. 2009;97:476–83. doi: 10.1016/j.physbeh.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakai Y, Dobson C, Diksic M, Aubé M, Hamel E. Sumatriptan normalizes the migraine attack-related increase in brain serotonin synthesis. Neurology. 2008;70:431–9. doi: 10.1212/01.wnl.0000299095.65331.6f. [DOI] [PubMed] [Google Scholar]
- 6. Kloda A, Adams DJ. Voltage-dependent inhibition of recombinant NMDA receptor-mediated currents by 5-hydroxytryptamine. Br J Pharmacol. 2005;144:323–30. doi: 10.1038/sj.bjp.0706049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun C, Meng Q, Zhang L, Wang H, Quirion R, Zheng W. Glutamate attenuates IGF-1 receptor tyrosine phosphorylation in mouse brain: possible significance in ischemic brain damage. Neurosci Res. 2012;74:290–7. doi: 10.1016/j.neures.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 8. Naccarato A, Gionfriddo E, Sindona G, Tagarelli A. Development of a simple and rapid solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry method for the analysis of dopamine, serotonin and norepinephrine in human urine. Anal Chim Acta. 2014;810:17–24. doi: 10.1016/j.aca.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 9. Sano M, Ferchaud-Roucher V, Nael C, Aguesse A, Poupeau G, Castellano B, Darmaun D. Simultaneous detection of stable isotope-labeled and unlabeled L-tryptophan and of its main metabolites, L-kynurenine, serotonin and quinolinic acid, by gas chromatography/negative ion chemical ionization mass spectrometry. J Mass Spectrom. 2014;49:128–35. doi: 10.1002/jms.3313. [DOI] [PubMed] [Google Scholar]
- 10. Claude B, Nehm R, Morin P. Analysis of urinary neurotransmitters by capillary electrophoresis: sensitivity enhancement using field-amplified sample injection and molecular imprinted polymer solid phase extraction. Anal Chim Acta. 2011;699:242–8. doi: 10.1016/j.aca.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 11. Li MD, Tseng WL, Cheng TL. Ultrasensitive detection of indoleamines by combination of nanoparticle-based extraction with capillary electrophoresis/laser-induced native fluorescence. J Chromatogr A. 2009;1216:6451–8. doi: 10.1016/j.chroma.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 12. Guan LP, Tang LM, Pan CY, Zhao SL, Wang SH. Evaluation of potential antidepressant-like activity of chalcone-1203 in various murine experimental depressant models. Neurochem Res. 2014;39:313–20. doi: 10.1007/s11064-013-1224-8. [DOI] [PubMed] [Google Scholar]
- 13. Wu L, Li L, Meng S, Qi R, Mao Z, Lin M. Expression of argininosuccinate synthetase in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:365–8. doi: 10.1111/jgh.12043. [DOI] [PubMed] [Google Scholar]
- 14. Chan SW, Lin G, Yamamoto K, Yew DT, Rudd JA. Simultaneous determination of amino acids in discrete brain areas in Suncus murinus by high performance liquid chromatography with electrochemical detection. J Pharm Biomed Anal. 2010;53:705–9. doi: 10.1016/j.jpba.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15. Dube S, Khumalo MT, Torto N, Nyati JA. Characterization of amino acids in silk sericin protein from Gonometa rufobrunnae by MEKC with phenyl isothiocyanate derivatization. J Sep Sci. 2006;29:1245–50. doi: 10.1002/jssc.200600045. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Liu C, Kang J. A highly sensitive method for enantioseparation of fenoprofen and amino acid derivatives by capillary electrophoresis with online sample preconcentration. J Chromatogr A. 2011;1218:1775–9. doi: 10.1016/j.chroma.2011.01.081. [DOI] [PubMed] [Google Scholar]
- 17. Qi L, Yang G. On-column labeling technique and chiral ligand-exchange CE with zinc(II)-L-arginine complex as a chiral selector for assay of dansylated D, L-amino acids. Electrophoresis. 2009;30:2882–9. doi: 10.1002/elps.200800753. [DOI] [PubMed] [Google Scholar]
- 18. Hong YL, Feng Y, Xu DS, Liu HJ. Study on extraction and purification of active parts from Da ChuanXiong Fang for treatment of migraine. Chin Med Mat. 2007;30:721–3. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 19. Wan LH, Fan ZP, Wang ZR. The effect of Da Chuanxiong Fang extract on 5-HTlD receptor expression of neurogila. Med J West China. 2003;1:1–2. [In Chinese, English abstract] [Google Scholar]
- 20. Wang YH, Liang S, Xu DS, Lin X, He CY, Feng Y, Hong YL. Effect and mechanism of senkyunolide I as an anti-migraine compound from Ligusticum Chuanxiong. J Pharm Pharmacol. 2011;63:261–6. doi: 10.1111/j.2042-7158.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 21. Lin TY, Lu CW, Huang SK, Wang SJ. Ferulic acid suppresses glutamate release through inhibition of voltage-dependent calcium entry in rat cerebrocortical nerve terminals. J Med Food. 2013;16:112–9. doi: 10.1089/jmf.2012.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X, Lu Y, Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007;73:650–4. doi: 10.1055/s-2007-981523. [DOI] [PubMed] [Google Scholar]
- 23. Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats—a time-course study. Eur J Pharmacol. 2003;464:159–62. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- 24. Ahn AH, Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain. J Neurosci. 2006;26:8332–8. doi: 10.1523/JNEUROSCI.1989-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szegedi V, Juhász G, Budai D, Penke B. Divergent effects of Abeta1-42 on ionotropic glutamate receptor-mediated responses in CA1 neurons in vivo. Brain Res. 2005;1062:120–6. doi: 10.1016/j.brainres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26. Tambuyzer T, Ahmed T, Taylor CJ, Berckmans D, Balschun D, Aerts JM. System identification of mGluR-dependent long-term depression. Neural Comput. 2013;25:650–70. doi: 10.1162/NECO_a_00408. [DOI] [PubMed] [Google Scholar]