Abstract
This study investigates how long-term (40 mg/kg b.wt) consumption of aspartame can alter the antioxidant status, stress pathway genes, and apoptotic changes in the liver of Wistar albino rats. Numerous controversial reports are available on the use of aspartame as it releases methanol as one of its metabolites during metabolism. To mimic the human methanol metabolism the methotrexate treated rats were included to study the aspartame effects. The aspartame treated methotrexate (MTX animals showed a marked significant increase in the superoxide dismutase (SOD), catalase (CAT), lipid peroxidation (LPO), and Glutathione peroxidase (GPx) activity in the liver from control and MTX control animals, and showed a significant decrease in reduced glutathione (GSH) and protein thiol in aspartame treated animals. The aspartame treated MTX animals showed a marked significant decrease in the body weight, brain, and liver weight. The aspartame treated MTX animals showed a marked increase in the inducible nitric oxide (iNOS), neuronal nitric oxide (nNOS), c-fos, Heat shock protein (Hsp) 70 Tumour necrosis Factor (TNF)α, caspase 8, c-jun N terminal kinases (JNK) 3 and Nuclear factor kappa B (NFkB) gene expression in the liver from control and MTX control animals. The aspartame treated MTX animals showed a marked increase in the c-fos, Hsp 70, iNOS Caspase 8, and JNK 3 protein expression in the liver from control and MTX control animals indicating the enhancement of stress and apoptosis. The aspartame treated MTX animals showed a streak of marked DNA fragmentation in the liver. On immunohistochemical analysis aspartame treated animals showed brown colored positive hepatocytes indicating the stress specific and apoptotic protein expression. Since aspartame consumption is on the rise among people, it is essential to create awareness regarding the usage of this artificial sweetener.
Keywords: apoptosis, aspartame, hepatocyte, hepatotoxicity, stress specific genes
1. Introduction
In this modern world people are used to having carbonated beverages along with food intake which contains the artificial sweetener aspartame (N-alpha-aspartyl-L-phenylalanine), and it is known that prolonged aspartame consumption leads to risk due to the formation of metabolites, especially methanol (10%), aspartic acid (40%), and phenylalanine (50%), [1,2]. There are not many studies describing the misuse and intake of aspartame on health, that exceeds the recommended maximum daily intake of aspartame 40 mg/kg bodyweight in Europe and 50 mg/kg bodyweight in the United States [3]. But children and adults consume aspartame unintentionally to a larger amount in excess than the Food and Drugs Administration (FDA) approval [4] which leads to serious health complications because of its metabolites. Aspartame is also present in puddings, fillings, and chewing gum [5]. Approximately 375 million people consume 2000 tons of aspartame per year in Europe. Upon consumption of aspartame, its metabolites increase in the blood [6] and are mainly metabolized by the liver, since the liver is the chief organ in the breakdown, where xenobiotics of drugs and chemicals metabolism takes place to a large extent. Astonishingly, sometimes the derivatives of those breakdown substances are more toxic than the original substance itself [7]. Methanol is being documented as a substance that harms the liver cells when it gets oxidized to formaldehyde and formate [8]. Oxidation of methanol causes development of peroxides which leads to denaturation, fragmentation of proteins, and alteration of enzymatic properties [9], even organisms evolve in such a way with effective antioxidant defense mechanism [10]. One of these is Hsp70, which protect cells against a variety of stresses [11] that induces the production of ROS that act in response over the cellular molecules such as lipids, proteins, and DNA. Nitric oxide (NO), is a molecule, naturally synthesized in all cells, since it has a short half-life and is very difficult to measure but it has been found that it can be measured by Nitric oxide synthase (NOS) activity [12]. It facilitates many of the physiological functions [13] and protects the body from superoxide radicals, also involved in the regulation of apoptosis [14], it has three isoforms, namely neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). Of these nNOS and eNOS are structural enzymes, which are found in many cells. Nitric oxide production is mediated by the stimulation of nitric oxide synthases, its production is either toxic or protective and it depends on the expression, in the liver which has both cytoprotective and cytotoxic effects [13]. The prolonged consumption of aspartame resulted in increased methanol and its metabolites which are responsible for the generation of oxidative stress [15]. Body cells guard themselves against Reactive oxygen species (ROS) damage through antioxidants under regular conditions, but in excess it deranges our antioxidant system from balance and increases production of NO resulting in damage, because NO reacts in a diffusion controlled process to produce peroxynitrite. Also, it stimulates one of the main inflammatory markers NF-κB [16] and results in inflammation. Soffritti et al, [17] reported that aspartame consumption leads to a carcinogenic effect on the system which has a deleterious effect causing the whole body damage, including mainly liver damage. There is a scarcity of studies on the evidence of stress specific genes and extrinsic apoptotic effect of aspartame in the liver and the effect may be due to the generation of peroxynitrile free radicals that produce chromatin DNA clumping and apoptosis. Aspartame has a negative effect on the liver indirectly, mainly because of its metabolites. In this study we have added a folate deficient group to specifically find the methanol and formaldehyde effects which are metabolites of aspartame, the rodents do not develop metabolic acidosis during methanol poisoning, owing to their high liver folate content and in order to create similar results in humans only folate deficient rodents are required to accumulate formate in order to develop acidosis [18,19]. Hence, in this study, in order to mimic the human situation, a folate deficiency status is induced by administering methotrexate. Beyond the aspartame toxicity studies in the enzymatic level, its effect on the molecular stress and apoptotic pathways are scarce. In this present investigation, an attempt has been made to study whether the long term consumption of aspartame can alter the antioxidant status and stress pathway genes iNOS, nNOS, c-fos, and Hsp 70 followed by apoptotic changes in TNFα, caspase 8, JNK 3, and NFkB in the liver of Wistar albino rats.
2. Materials and methods
2.1. Animals
Wistar strain male albino rats (200–220 g) were maintained under standard laboratory conditions with water and food. For the folate-deficient group, a folate-deficient diet was provided for 45 days prior to the experiment and methotrexate (MTX) was administered for 1 week prior to the experiment. The animals were handled according to the principles of laboratory care framed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. Prior to the experimentation, proper approval was obtained from the Institutional Animal Ethical Committee (No: 01/032/2010/Aug-11), Dr. ALM Post Graduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai-113, TN, India.
2.2. Chemicals
Aspartame and methotrexate were purchased from Sigma chemical company, St. Louis, MO, USA. Nitric oxide assay kit was purchased from Biovision Inc, Milpitas, CA, USA. Taq-Polymerase, DNTPs from (Genet Bio Inc, Yuseong-gu Daejeon, Korea) China, reverse transcription (RT) enzyme kit from (Thermo Fisher Scientific Inc, MA, USA), and other molecular grade chemicals from Merck Bangalore, India. All other chemicals were of analar grade obtained from Sisco research Laboratory, Bombay, India.
2.3. Experimental design
The European Food Safety Authority recently confirmed an acceptable daily intake (ADI) for aspartame of 40 mg/kg b.wt./ d. In order to confine within the human permitted exposure limit, this dose was selected. Aspartame mixed in sterile saline was administered orally (40 mg/kg body weight) and this dosage was based on the FDA approved ADI limit.
The rats were divided into three groups, namely, saline control, MTX-treated control, and MTX-treated aspartame administered groups. Each group consisted of six animals. MTX in sterile saline was administered (0.2 mg/kg/d) subcutaneously for 7 days to folate-deficient control as well as to folate-deficient aspartame treated groups [20]. One week after treatment with MTX, folate deficiency was confirmed by estimating the urinary excretion of formiminoglutamic acid (FIGLU) [21]. From the 8th day, only the MTX-treated aspartame group received the aspartame, whereas the other two groups received equivalent volumes of saline as an oral dose and all animals were handled similarly. The chronic dose of aspartame was given for 90 days and all the animals were fed a folate-deficient diet except the control animals for 90 days.
2.4. Sample collections
The animals were sacrificed using a higher dose of long acting pentothal sodium (100 mg/kg.b.wt). The blood samples and isolation of liver was performed between 8 am and 10 am to avoid circadian rhythm induced changes. The liver was immediately removed and washed with ice-cold phosphate buffered saline (PBS). Further dissection was made on ice-cold glass plates. The homogenate (10% w/v) of the individual regions were prepared in a Teflon-glass tissue homogenizer, using ice-cold PBS (100 mm, pH 7.4) buffer and centrifuged separately in a refrigerated centrifuge at 10,000 g for 15 minutes. The supernatant was used for analyzing the parameters in this study.
2.5. Body weight and organ weight ratio
Body weight was checked between the groups and the organ weight of the liver and brain were studied.
2.6. Free radical scavenging enzymes
Interference of free radicals in auto-oxidation of pyrogallol is used as a convenient assay for superoxide dismutase (SOD; EC.1.15.1.1) and expressed as units/min/mg protein [22]. For catalase (EC.1.11.1.6) assay, standard hydrogen peroxide (0.2M) was used as a substrate and the catalase activity was terminated at intervals of 0-, 15-, 30-, and 60-seconds by addition of potassium dichromate–acetic acid reagent and is expressed as units/min/mg protein [23].
2.7. Nonenzymatic antioxidant estimations
The level of lipid peroxidation (LPO) was expressed as nanomoles of malondialdehyde (an intermediary product of lipid peroxidation, using thiobarbituric acid)/mg protein [24]. Reduced glutathione (GSH) was measured by its reaction with 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB), to form a compound that absorbs at 412 nm [25]. The level of GSH is expressed as μg of GSH/mg of protein. Protein bound (i.e., in the membrane plus the soluble fraction) sulfhydryl concentration was determined by the method of Sedlak and Lindsay [26] by subtracting the nonprotein sulfhydryl content from the total sulfhydryl content. Tissues were homogenized in 0.02M EDTA solution and analyzed for protein and sulfhydryl concentration.
2.8. Isolation of total RNA and reverse transcription-polymerized chain reaction (RT-PCR)
Total RNA was isolated from cells using Trizol reagent following the method of Chomczynski and Sacchi [27]. The total RNA obtained was free from protein and DNA contamination. The reverse transcription step was performed by using the RT enzyme kit. Each 20 μL reaction mixture contained 5 μL OligodT (10μM), 1 μL dNTP(10μM), 4 μL First Strand buffer (5×), 1 μl DTT (0.1 M), 0.2 μL super script III reverse transcriptase (200U/μL) varied quantity of RNA template (dependent on RNA concentration), and RNase free water to make up the volume. Thermal cycling conditions for the first strand reaction consisted of 25°C for 5 minutes, 50°C for 45 minutes, 70°C for 15 minutes, and finally maintained at 4°C for 5 minutes. PCR amplification was performed using Taq DNA Polymerase. Each 20 μL of sample contained 10 μL Master mix (2μM), 1 μL forward primer, and 1 μL reverse primer for both genes of interest and internal control consecutively, 2 μL RT sample, and 4 μL sterile water. The mixture was kept at thermocycler and amplified for 35 cycles. Each thermocycling consisted of 94°C for 30 seconds, varied annealing temperature for each gene of interest for 30 seconds, and 72°C for 30 seconds. β-Actin gene was coamplified with the gene of interest using the same procedures. The sense and antisense primer for the study is tabulated in Table 1. Ten microliters of each PCR product was analyzed by gel electrophoresis on 2% agarose gel.
Table 1.
The sense and antisense primer sequences of the gene of interest for PCR amplification.
| Gene | Sequence | Amplified product (bp) | Annealing temp/cycles |
|---|---|---|---|
| TNF-α | Sense: CTCCCAGAAAAGCAAGCAAC | 210 | 55°C/35 |
| Antisense: CGAGCAGGAATGAGAAGAGG | |||
| NFkB | Sense: CATCTTCAACATGGCAGACGACGA | 130 | 55°C/35 |
| Antisense: TGGGCCATCTGTTGACAGTGGTAT | |||
| JNK3 | Sense: AACAATCGCTACACCTCCAAAGAC | 330 | 56°C/35 |
| Antisense: GGCAATAGATGACACATCCACG | |||
| Casp8 | Sense: GCGACAGGTTACAGCTCTCC | 180 | 55°C/35 |
| Antisense: GCAGCCTCTGAAATAGCACC | |||
| β-actin | Sense: TCATGCCATCCTGCGTCTGGACCT | 598 | 55°C/35 |
| Antisense: CGGACTCATCGTACTCCTGCTTG | |||
| Hsp70 | Sense: GAGTCCTACGCCTTCAATATGAAG | 347 | 55°C/35 |
| Antisense: CATCAAGAGTCTGTCTCTAGCCAA | |||
| iNOS | Sense: TCTGTGCCTTTGCTCATGAC | 305 | 55°C/35 |
| Antisense: CATGGTGAACACGTTCTTGG | |||
| nNOS | Sense: CCTTCCGAAGCTTCTGGCAACAGC | 474 | 66°C/35 |
| Antisense: TGGACTCAGATCTAAGGCGGTTGG | |||
| c-fos | Sense: AGTGGTGAAGACCATGTCAGG | 296 | 55°C/35 |
| Antisense: CATTGGGGATCTTGCAGG |
Agarose gel electrophoresis is an effective method for the identification of purified DNA molecules [28]. Amplified product was analyzed by agarose gel electrophoresis with ethidium bromide staining. Then the gel containing cDNA was visualized with the help of fluorescent imager (Bio-Rad Laboratories, Hercules, CA). The band intensity was quantified by Quantity One Software. The band intensification for each enzyme mRNA was normalized with that of the internal control β-actin using Quantity One Software.
2.9. Immunoblotting
Tissue lysate was prepared with radio immuno assay buffer (RIPA; Sigma aldrich, St louis, USA) and protease inhibitor. Equal amounts of protein (60 μg) were electrophoresed on 10% SDS–PAGE. Following electrophoresis, separated proteins on SDS–PAGE gels were transferred on to a Polyvinylidene difluoride (PVDF) membrane (Millipore, USA). To block the nonspecific binding, the membranes were incubated in a blocking buffer with 5% skimmed milk for 2 hours. Membranes were probed with primary antibodies (Biovision). Blots were incubated with horse radish peroxidase-conjugated secondary antibodies (1:10,000; Merck). The bands were developed using ECL kit (Millipore) in Chemi Doc image scanner from Bio-Rad. The band intensity was quantified by Quantity One software (Bio-Rad). The membranes were stripped and reprobed for β-actin (Sigma; 1:5000) as an internal control.
2.10. Agarose gel electrophoresis for DNA fragmentation
The isolation of DNA from animal tissue was done according to the method of Iwasa et al [29] with some modifications. The tissue was homogenized in TE buffer and the suspension was treated with the lysis buffer and kept in a water bath for 12 hours at 37°C. DNA was extracted twice with equal volumes of a phenol, chloroform, and isoamylalcohol. To the aqueous phase 1/10 volume of 3M sodium acetate was added and DNA was extracted with 0.7 volume of chilled isopropanol. Following an addition of 70% ethanol the precipitated DNA was resuspended in 50ml of Tris EDTA buffer. DNA samples were electrophoresed in 0.8% agarose gel containing ethidium bromide and the gel was examined under UV light. A 1 kb ladder and 100 bp ladder was used as the molecular weight marker.
2.11. Histopathology
Animals were deeply anesthetized with ketamine hydrochloride. Rats were then perfused transcardially with phosphate-buffered saline, followed by buffered 10% formalin. The kidney was removed, and preserved in formalin until processed for histology. Then running water was used to remove formalin pigments and dehydrated with ascending grades of alcohol. After impregnation with paraffin wax, the paraffin blocks were made. They were processed and sections were cut into 6 μm thickness using “Spencer Lens”, rotatory microtome (Spencers Laboratory, No. 820, New York, NY, USA) and then stained with haematoxylin and eosin stain as followed for the kidney.
2.12. Immunohistochemical analysis
Immunohistochemical analysis was carried out using the 3,3'-diaminobenzidine tetrahydrochloride (DAB) universal staining kit (Merck Genie, Bengaluru, India). The sections were deparaffinized in xylene and dehydrated in ethanol. After washing with PBS, slides were incubated with 3% H2O2 at room temperature for 15 minutes to quench endogenous peroxidase activity. After antigen retrieval (15 minutes of autoclaving in 10mM citrate buffer, pH 6.0), the slides were incubated with blocking solution (10% normal goat serum) for 5 minutes at room temperature. Then, the sections were incubated overnight with a primary antibody. Subsequently, the sections were incubated with Horse radish peroxidase (HRP) secondary link antibody for 30 minutes at room temperature, and washed with PBS. Then, the sections were treated with DAB chromogen for 15 minutes. Finally, the sections were washed with deionized water, counter stained with haematoxylin, and mounted. Photographs were taken using a Nikon microscope (Nikon Japan DS-Fi1).
2.13. Statistical analysis
Statistical analysis was carried out using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as mean ± SD and the data were analyzed by the one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests when there is a significant “F” test ratio. The level of significance was fixed at p ≤ 0.05.
3. Results
The data from various groups for the individual parameters are presented as a bar diagram and a table with mean ± SD.
3.1. Body and organ weight
The results are given in Table 2. The body weight, brain, and liver weight in the MTX treated animals did not significantly differ from the controls. However, the aspartame treated MTX animals showed a marked significant decrease in the body weight, brain, and liver weight from the control as well as from the MTX treated control animals.
Table 2.
Effect of aspartame (40 mg/kg b.wt.) on body weight and organ weight (g).a
| Parameter | Control | MTX control | Asp + MTX treated* |
|---|---|---|---|
| Body weight (g) | 303.83 ± 27.06 | 295.16 ± 13.10 | 270.5 ± 10.13b,c |
| Liver weight (g) | 3.66 ± 0.24 | 3.45 ± 0.10 | 3.08 ± 0.07b,c |
| Brain weight (g) | 2.05 ± 0.06 | 1.85 ± 0.10 | 1.50 ± 0.06b,c |
The data from various groups for the individual parameters are presented as a bar diagram with mean ± SD.
Significance fixed at p < 0.05.
Comparison and analysis were done by the one-way analysis of variance (ANOVA), (n = 6) control group was compared with the MTX group and aspartame MTX group, the MTX group was compared with the aspartame MTX group. Control, MTX control-methotrexate treated group, Asp + MTX-aspartame + methotrexate treated group.
Aspartame treated group when compared to control significance.
MTX treated group significance when compared to control significance.
3.2. Free radical scavenging enzymes
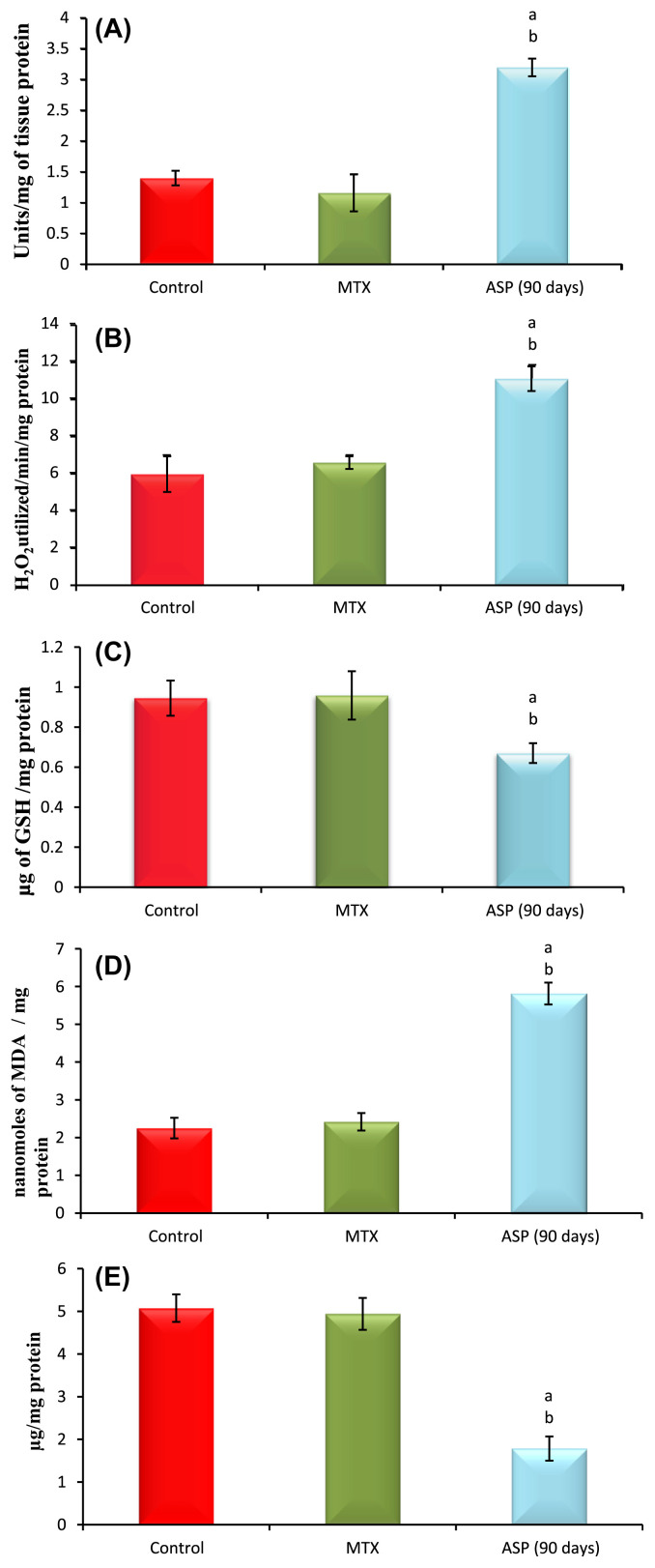
The data from various groups are presented as a bar diagram with mean ± SD (Fig. 1A). Activity of superoxide dismutase (SOD) was increased significantly in aspartame treated MTX animals respectively in the liver when compared to the control and MTX treated control. However, the control did not significantly deviate from the MTX treated control animals. The activity of catalase (CAT; Fig. 1B) was increased markedly in the liver of aspartame treated MTX animals with respect to the control and MTX treated control. However, the control did not significantly deviate from the MTX treated control animals.
Fig. 1.
(A) Effect of aspartame (40 mg/kg b.w) on superoxide dismutase (SOD) activity (units/mg of tissue protein) in rat liver. (B) Effect of aspartame (40 mg/kg b.w) on catalase (CAT) activity (H2O2 utilized/min/mg protein) in rat liver. (C) Effect of aspartame (40 mg/kg b.w) on reduced glutathione (GSH) concentration (μg of GSH/mg of protein) in rat liver. (D) Effect of aspartame (40 mg/kg b.w) on lipid peroxidation (LPO) (Moles of MDA/mg tissue) in rat liver. (E) Effect of aspartame (40 mg/kg b.w) on protein thiol (μg/mg protein) in rat liver. Comparison and analysis were done by the one-way analysis of variance, (n = 6) control group was compared with the MTX control group and aspartame MTX group. Significance fixed at p < 0.05. The data from various groups for the individual parameters are presented as bar diagram with mean ± SD. aAspartame treated group when compared to control significance. bMTX treated groups when compared to control significance. Control = MTX control–methotrexate treated group; MTX + Asp = methotrexate + aspartame treated group.
3.3. Nonenzymatic antioxidants
The results are given in Fig. 1C. The level of reduced glutathione (GSH) was decreased in the liver of aspartame treated MTX animals when compared to the control and MTX treated control. The control and the MTX treated controls did not significantly differ from each other. The level of lipid peroxidation (LPO; Fig. 1D) was increased markedly in the liver of aspartame treated MTX animals when compared to the control and MTX treated control. The control as well as MTX treated controls showed similar lipid per oxidation levels. The sulfhydryl (thiol; Fig. 1E) content of membrane proteins was decreased markedly in the aspartame treated MTX animals when compared to the control and MTX treated control. The control as well as MTX treated animals show similar results.
3.4. Stress specific gene expression
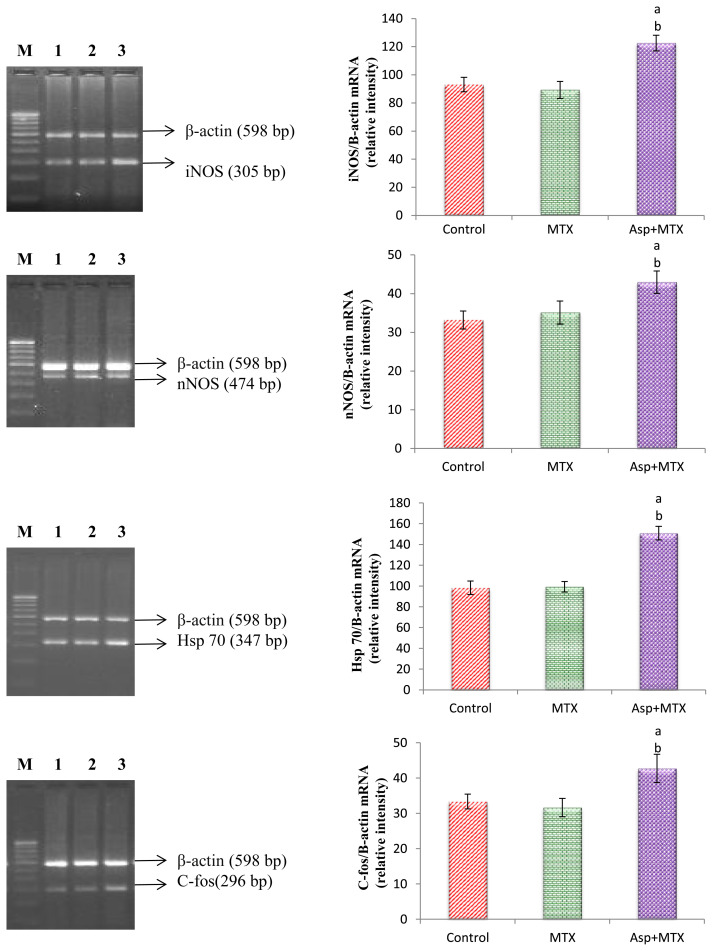
The results are given in Fig. 2. Using β-Actin as an internal control the stress specific genes, such as iNOS, nNOS, c-fos, and Hsp 70, gene expression was studied. The iNOS, nNOS, c-fos, and Hsp 70 gene expression in the MTX control animals did not significantly (p < 0.05) differ from the controls. However, the aspartame treated MTX animals showed a marked increase in the iNOS, nNOS, c-fos, and Hsp 70 gene expression in the liver from the control and MTX control animals indicating the enhancement of stress.
Fig. 2.
Effect of long term aspartame on iNOS, nNOS, Hsp 70, and C-fos mRNA expression in liver of Wistar albino rats. aAspartame treated group when compared to the control significance. bMTX treated groups significance. Lane 1 = control; Lane 2 = MTX control; Lane 3 = Aspartame + MTX treated; Lane M = 100 bp marker.
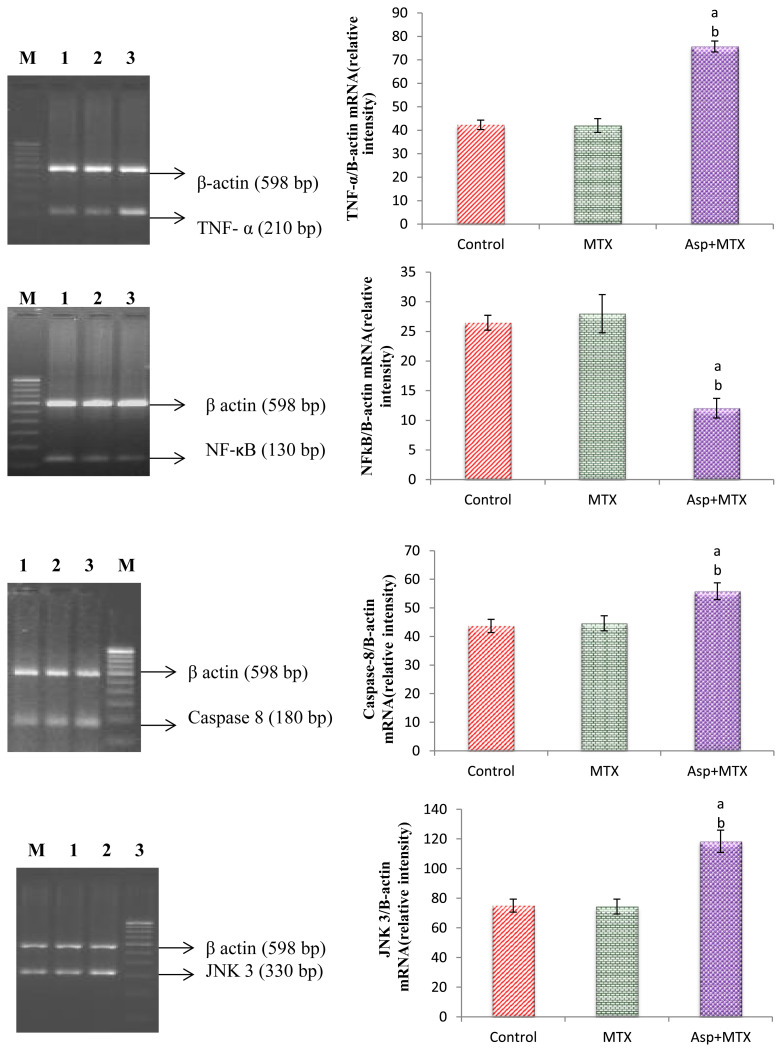
The results are given in Fig. 3. The TNFα, caspase 8, JNK 3, and NF-κB gene expression was studied by using β-Actin as an internal control. The TNFα, caspase 8, JNK 3, and NF-κB gene expression in the MTX control animals did not differ from the controls. However, the aspartame treated MTX animals (p < 0.05) showed a marked increase in the TNFα, caspase 8, JNK 3, and NF-κB gene expression in the liver from the control and MTX control animals indicating the enhancement of apoptosis.
Fig. 3.
Effect of long term aspartame on TNFα, NF-κB, Caspase 8 and JNK3 mRNA expression in liver of Wistar albino rats. aAspartame treated group when compared to the control significance. bMTX treated groups significance. Lane 1 = control; Lane 2 = MTX control; Lane 3 = aspartame + MTX treated; Lane M = 100 bp marker.
3.5. Protein expression by immunoblotting
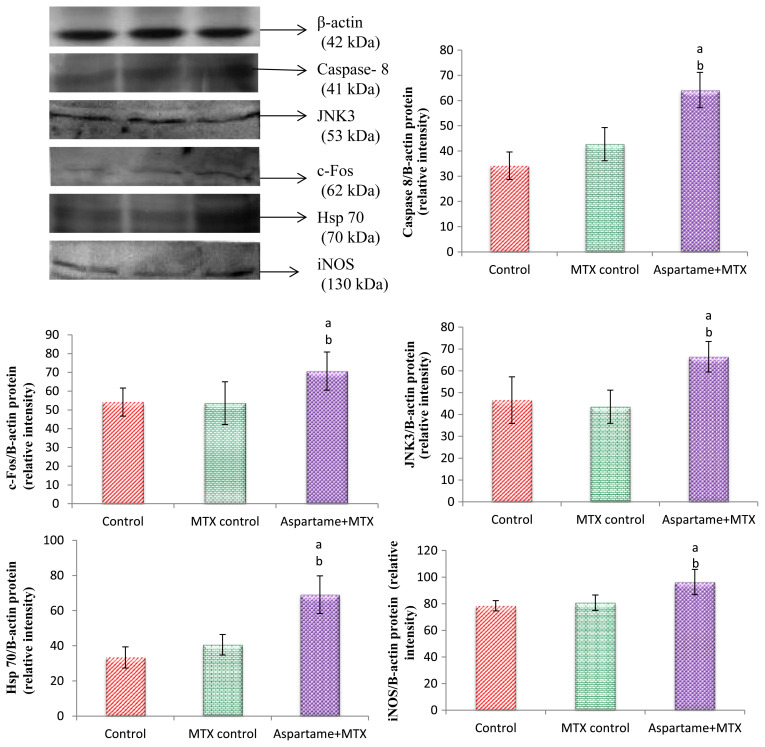
The results are given in Fig. 4. Using β-Actin as an internal control the c-fos, Hsp 70, iNOS Caspase 8, and JNK 3 protein expression was studied. The c-fos, Hsp 70, iNOS Caspase 8, and JNK 3 protein expression in the MTX control animals did not significantly (p < 0.05) differ from the controls. However, the aspartame treated MTX animals showed a marked increase in the c-fos, Hsp 70, iNOS, Caspase 8, and JNK 3 protein expression in the liver from the control and MTX control animals indicating the enhancement of stress and apoptosis.
Fig. 4.
Effect of long term aspartame on Caspase-8, JNK3, c-Fos, Hsp 70, and iNOS protein expression in liver of Wistar albino rats. Comparison and analysis were done by the one-way analysis of variance, (n = 6) control group was compared with the MTX control group and aspartame MTX group; the MTX control group was compared with the aspartame MTX group. aAspartame treated group when compared to the control significance. bMTX treated groups significance. Lane 1 = control; Lane 2 = MTX control; Lane 3 = aspartame + MTX treated.
3.6. DNA fragmentation by agarose gel electrophoresis
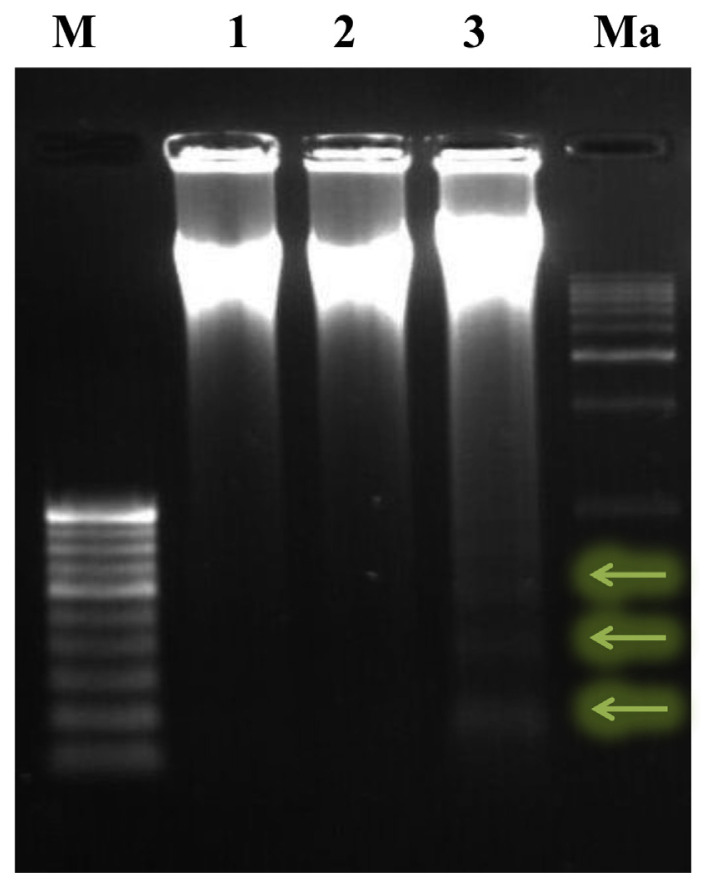
The results are given in Fig. 5. The effect of 90 days aspartame (40 mg/kg b.wt.) administration to Wistar albino rats on DNA fragmentation by agarose gel electrophoresis in the liver was assessed. The DNA was found intact in the MTX treated animals and the controls. However, the aspartame treated MTX animals showed a streak of marked DNA fragmentation in the liver when compared to the control and MTX controls.
Fig. 5.
DNA fragmentation assay by agarose gel electrophoresis. Effect of 90 days aspartame (40 mg/ kg b.wt.) administration to Wistar albino rats on DNA fragmentation by agarose gel electrophoresis in liver. Lane 1 = control; Lane 2 = MTX control; Lane 3 = MTX + aspartame; M = 100 bp marker; Ma = 1 kb marker.
3.7. Histopathology
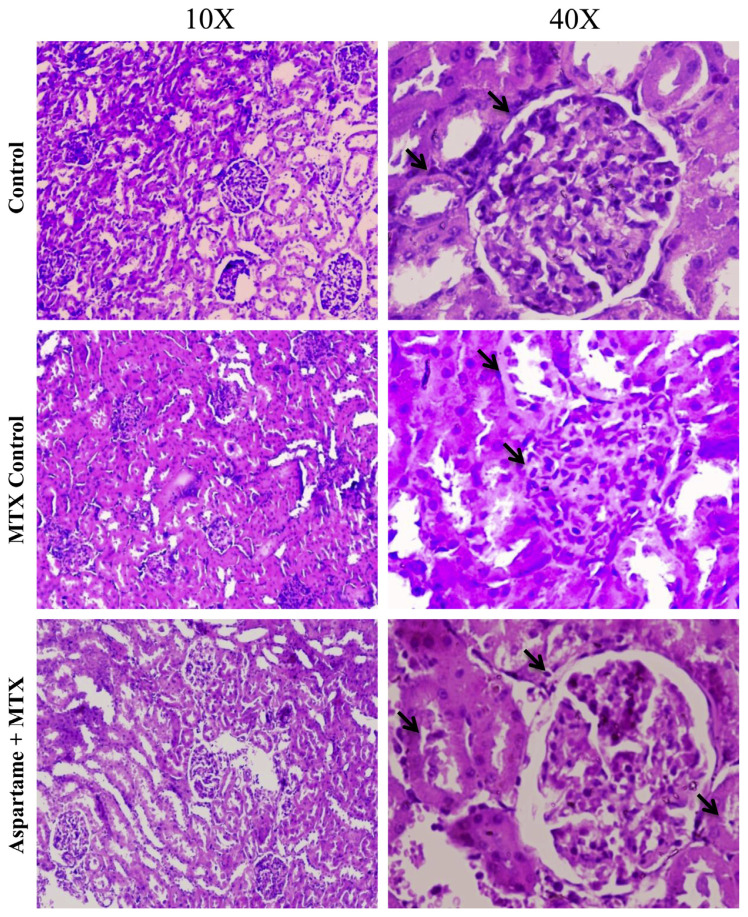
The results are given in Fig. 6. There was a significant change in the kidney of aspartame treated MTX animals when compared to the control and MTX control animals. In the renal cortex, marked glomerular damages were noted including the loss of normal architecture and reduction in their normal sizes. The overall results indicate that aspartame is effective in bringing about changes at cellular levels.
Fig. 6.
Effect of long term aspartame consumption (40 mg / kg bwt) on kidney in Wistar albino rats, the histomicrograph of kidney stained by hematoxylin and eosin. Arrows indicate marked glomerular damage and the loss of normal architecture.
3.8. Immunohistochemistry
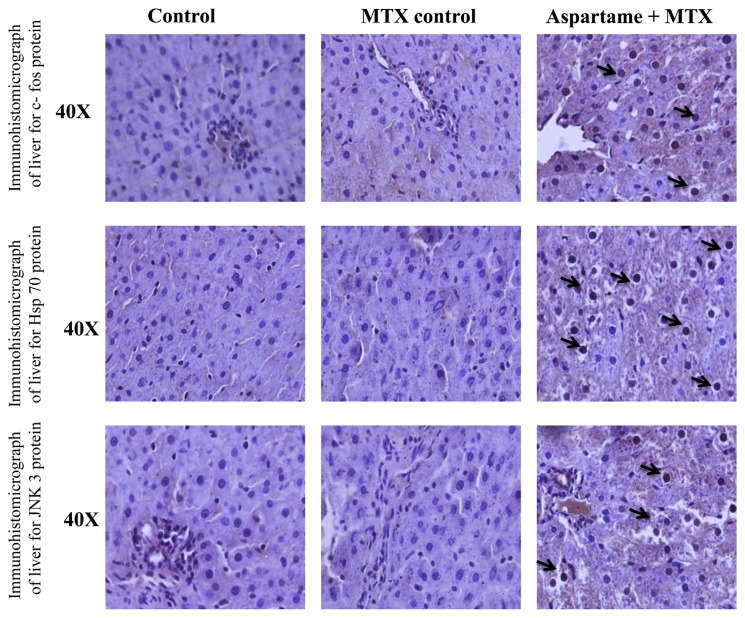
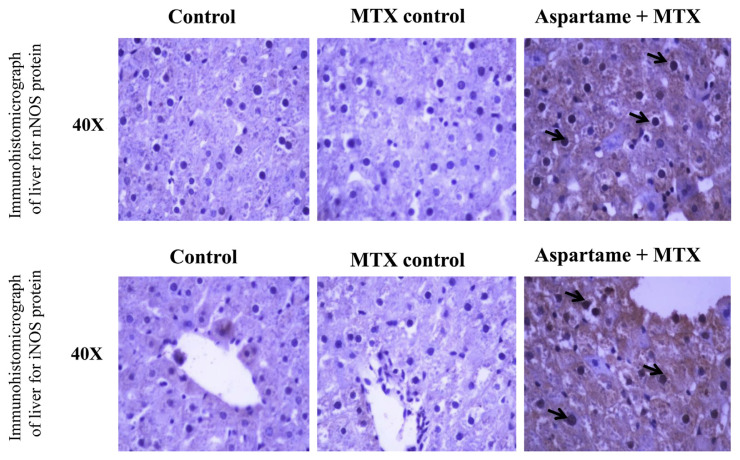
The results are given in Figs. 7 and 8. There was a significant change in the protein expression (immunohistochemistry) of Hsp 70, c-fos, iNOS, nNOS, and JNK 3 protein in the liver of aspartame treated MTX animals when compared to the control and MTX control animals. The photomicrograph shows a significant amount of hepatocytes which underwent immuno reactions and brown colored positive hepatocytes which clearly indicates the apoptotic protein expression in the liver. The liver of aspartame treated animals showed a marked significant increase in the immune-reaction when compared to the control and MTX control liver. The overall results indicate that aspartame is effective in bringing about changes at the cellular level.
Fig. 7.
Effect of long term aspartame on Hsp 70, c-fos, and JNK3 protein expression in liver of Wistar albino rats by immunohistochemistry.
Fig. 8.
Effect of long term aspartame on nNOS and iNOS protein expression in the liver of Wistar albino rats by immunohistochemistry.
4. Discussion
Besides the confusion and studies about the deleterious effect of aspartame on health, its consumption is growing day by day mainly because of beverages, since 70% of aspartame exists in soft drinks. People with diabetes consume aspartame as one of the sugar substitutes [30] because of its low calorie sweetener property [31], an unplanned increase in the intake of aspartame is above the norm. The present hypothesis is to analyze the effect of long term consumption of aspartame on stress and apoptotic pathways in the liver. After the administration of aspartame in human participants, the blood methanol concentration exceeded 2 mg/dL [32]. Even a minor quantity of aspartame significantly raises the methanol level in the plasma [33]. The prolonged consumption of aspartame results in increased methanol and its metabolites which are responsible for the generation of oxidative stress [15]. Methanol has its own effect on the liver mainly [34], and this is reliable with our observation which shows a decrease in the weight of the liver in aspartame treated MTX animals compared to the control and MTX treated animals. The photomicrograph of the liver in the present study shows a significant quantity of hepatocytes underwent immune reactions displaying brown colored positive hepatocytes which clearly indicate the apoptotic consequence on the liver. Only the liver of aspartame treated animals showed a marked significant increase in the brown colored positive hepatocytes when compared to the control and MTX control. This histological change probably indicates by altering the antioxidant status by the formation of peroxides and free radicals, since aspartame induces oxidative stress in the liver and kidney [35–37]. It has many deranging functions in our body because of the formation of peroxides and free radicals. Normally the system has its own protection from free radicals by using its endogenous antioxidants like superoxide dismutase (SOD), catalase (CAT), and GSH. Increased oxidative stress is accompanied with the increased level of superoxides and free radicals [38]. Depletion of GSH increases cell susceptibility to oxidative stress [4]. Here oxidative stress occurs as a result of aspartame metabolites, these altered status of both enzymatic and nonenzymatic antioxidants in the system that includes SOD activity, CAT activity, and LPO (lipid peroxidation) increased significantly and GSH (reduced glutathione) and sulfhydryl (thiol) was markedly decreased in the aspartame treated MTX animals when compared to the control and MTX treated control in the liver. However, the control did not significantly deviate from the MTX treated control animals. The oxidative stress followed by prolonged intake of aspartame has been well demonstrated in this study by Hsp 70, iNOS, and nNOS expressions in the liver. Hsp 70 (which normally protect the cells against a variety of stresses) even if the heat shock response is protective, most of this protective response is induced by deleterious stimuli and causes cellular injury followed by apoptosis with significant alterations in metabolism [39], the aspartame induced oxidative stress in turn stimulates nitric oxide and peroxynitrate production. In long term exposure of oxygen radicals and nitrogen oxide derivatives DNA undergoes damage [40], and the results obtained in this study too shows DNA fragmentation in the liver which further supports the consequences followed by the long term consumption of aspartame, because nitric oxide is a highly reactive molecule it interacts with iron and inhibits mitochondrial respiration, DNA synthesis, and cytotoxicity [41]. Due to its short half-life and unstable nature, iNOS and nNOS expression are the forms of NOS studied in the liver [42] which shows increased gene and protein expression in the aspartame treated MTX animals when compared to the control and MTX control animals clearly demonstrating the hepatocellular stress response to oxidative stress. Conversely the iNOS and nNOS gene expression in the MTX control animals did not significantly (p < 0.05) differ from the controls. Oxidative stress increased the generation of reactive oxygen mediating apoptosis by activating the translocation of nuclear factor-kappa B (NF-κB). TNF which acts as the sensor of oxidative stress [43] plays a vital role in liver homeostasis either in cell survival or death [44] but its action on liver cells mainly depends on the NF-κB and JNK signaling [16] which mediates caspase dependent cell death. Once the signaling gets activated by the oxidative stress it leads the TNF release [45]. TNF binds to TNF receptor 1 which leads to the activation of cleaving procaspase 8 to caspase 8 which directly triggers caspase 3 and initiates apoptosis [44], which is well demonstrated in this study by the increased expression of gene and protein expression of caspase 8 in aspartame treated MTX animals when compared to the control and MTX control animals. Hence our overall findings of this study is evidence that long term consumption of aspartame induces apoptosis and damage to the hepatocytes by oxidative stress via the JNK-NF signaling. Still aspartame usage is unavoidable because of its involvement in our daily food items in several forms, hence its consumption has to be controlled and the FDA has to bring awareness and firm rules against the chronic consumption and usage among people.
5. Conclusion
The observed results support the toxic nature of aspartame when consumed repeatedly for a prolonged period. The present study reveals that aspartame administration alters the enzyme activity in the liver by probably elevating the free radical levels. The observed changes may be due to the methanol or aspartame metabolite for the generation of free radicals in the liver. Moreover, the long term aspartame administration distorted the stress markers and proteins leading to hepatotoxicity. Since aspartame consumption is on the rise among people, it is essential to create awareness regarding the usage of this artificial sweetener. Further studies are required to evaluate the effect of aspartame in the future.
Acknowledgments
The author is grateful to the suggestion offered by Dr. NJ Parthasarathy and coauthors. The author is grateful to the help given by the Molecular Laboratory, Department of Genetics and Endocrinology, Dr. ALM Post Graduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai-113, TN, India. The financial assistance provided by the Indian Council of Medical Research (ICMR) File. No.3/1/2/ 29/Nut./2012/Dated 29-09-2013 for Senior Research Fellow is gratefully acknowledged. The University of Madras, Chennai, India is acknowledged for providing the resources to conduct the research.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
REFERENCES
- 1. Ranney RE, Opperman JA, Maldoon E, McMahon FG. Comparative metabolism of aspartame in experimental animals and humans. J Toxicol Environ Health. 1976;2:441–51. doi: 10.1080/15287397609529445. [DOI] [PubMed] [Google Scholar]
- 2. Humphries P, Pretorius E, Naude H. Direct and indirect cellular effects of aspartame on the brain. Eur J Clin Nutr. 2008;62:451–62. doi: 10.1038/sj.ejcn.1602866. [DOI] [PubMed] [Google Scholar]
- 3. Butchko HH, Stargel WW, Comer CP, Mayhew DA, Benninger C. Aspartame: review of Safety. Regul Toxicol Pharm. 2002:11–93. doi: 10.1006/rtph.2002.1542. [DOI] [PubMed] [Google Scholar]
- 4. Oyama Y, Sakai H, Arata T, Okano Y, Akaike N, Sakai K, Noda K. Cytotoxic effects of methanol, formaldehyde, and formate on dissociated rat thymocytes: a possibility of aspartame toxicity. Cell Biol Toxicol. 2002;18:43–50. doi: 10.1023/a:1014419229301. [DOI] [PubMed] [Google Scholar]
- 5. Rencuzogullari E, Tuylu BA, Topaktas M, Ila HB, Kayraldiz A, Arslan M, Diler SB. Genotoxicity of aspartame. Drug Chem Toxicol. 2004;27:257–68. doi: 10.1081/dct-120037506. [DOI] [PubMed] [Google Scholar]
- 6. Stegink LD. The aspartame story: a model for the clinical testing of a food additive. Am J Clin Nutr. 1987;46:204–15. doi: 10.1093/ajcn/46.1.204. [DOI] [PubMed] [Google Scholar]
- 7. Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic, and clinical aspects. Alcohol Clin Exp Res. 1991;15:45–66. doi: 10.1111/j.1530-0277.1991.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 8. Trocho C, Pardo R, Rafecas I, Virgili J, Remesar X, Fernández-López JA, Alemany M. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 1998;63:337–49. doi: 10.1016/s0024-3205(98)00282-3. [DOI] [PubMed] [Google Scholar]
- 9. Skrzydlewska E, Elas M, Farbiszewski R, Roszkowska A. Effect of methanol intoxication on free-radical induced protein oxidation. J Appl Toxicol. 2000;20:239–43. doi: 10.1002/(sici)1099-1263(200005/06)20:3<239::aid-jat654>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10. Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicol. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 11. Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–86. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 12. Rosselli M, Dubey RK, Rosselli MA, Macas E, Fink D, Lauper U, Keller PJ, lmthurn B. Identification of nitric oxide synthase in human and bovine oviduct. Mol Hum Reprod. 1996;2:607–12. doi: 10.1093/molehr/2.8.607. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Billiar TR. The antiapoptotic actions of nitric oxide in hepatocytes. Cell Death Differ. 1999;6:952–5. doi: 10.1038/sj.cdd.4400579. [DOI] [PubMed] [Google Scholar]
- 14. Shen KL, Harn HJ, Ho LL, Yu CP, Chiu SC, Lee WH. The extent of proliferative activity and apoptotic activity in intraductal and invasive ductal breast carcinomas detected by Ki-67 labeling and terminal deoxy nucleotidyl transferase mediated digoxigenin-11-dUTP nick end labeling. Cancer. 1998;82:2373–81. doi: 10.1002/(sici)1097-0142(19980615)82:12<2373::aid-cncr11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15. Ashok I, Sheeladevi R. Effect of chronic exposure to aspartame on oxidative stress in brain discrete regions of albino rats. J Biosci. 2012;37:1–10. doi: 10.1007/s12038-012-9236-0. [DOI] [PubMed] [Google Scholar]
- 16. Chang YF, Chi CW, Chern YT, Wang JJ. Effects of 1,6-Bis[4-(4-amino-3-hydroxyphenoxy)phenyl]diamantane (DPD), a reactive oxygen species and apoptosis inducing agent, on human leukemia cells in vitro and in vivo. Toxicol Appl Pharmacol. 2005;202:1–12. doi: 10.1016/j.taap.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 17. Soffritti M, Belpoggi F, Degli Esposti D, Falcioni L, Bua L. Consequences of exposure to carcinogens beginning during developmental life. Basic Clin Pharmacol Toxicol. 2008;102:118–24. doi: 10.1111/j.1742-7843.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 18. Lee EW, Garner CD, Terzo TS. Animal model for the study of methanol toxicity: comparison of folate-reduced rat responses with published monkey data. J Toxicol Environ Health. 1994;41:71–82. doi: 10.1080/15287399409531827. [DOI] [PubMed] [Google Scholar]
- 19. Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG. Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology. 2000;21:321–30. [PubMed] [Google Scholar]
- 20. Gonzalez-Quevedo A, Obregon F, Urbina M, Rousso T, Lima L. Effects of chronic methanol administration on amino acid and monoamines in retina, optic nerve, and brain of the rat. Toxicol Appl Pharmacol. 2002;185:77–84. doi: 10.1006/taap.2002.9477. [DOI] [PubMed] [Google Scholar]
- 21. Tabor H, Wyngarden L. A method for determination of formiminoglutamic acid in urine. J Clin Invest. 1958;37:824–8. doi: 10.1172/JCI103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 23. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 24. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1970;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25. Moron MA, DePierre JW, Mannervick B. Levels of glutathione, glutathione reductase, and glutathione-S-transferase activities in rat liver. Biochimt Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 26. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;24(25):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step RNA isolation from cultured cells or tissues. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York: Greene and Wiley Interscience; 1990. pp. 4.2.4–4.2.8. [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbour Laboratory Press; 1989. [Google Scholar]
- 29. Iwasa M, Maeno Y, Inoue H, Koyama H, Matoba R. Induction of apoptotic cell death in the rat thymus and spleen after a bolus injection of methamphetamine. Int J Legal Med. 1996;109:23–8. doi: 10.1007/BF01369597. [DOI] [PubMed] [Google Scholar]
- 30. Puica C, Craciun C, Rusu M, Cristescu M, Borsa M, Roman I, Cluj-napoca VM. Ultrastructural aspects concerning the hypothalamus-pituitary complex reactivity following chronic administration of aspartame in juvenile rabbits. Bulletin USAMV- CN. 2008:65. [Google Scholar]
- 31. Bandyopadhyay S, Kelley R, Krogan NJ, Ideker T. Functional maps of protein complexes from quantitative genetic interaction data. PLoS Comput Biol. 2008;4:e1000065. 1. doi: 10.1371/journal.pcbi.1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stegink LD, Freeman JB, Don Barsten L, Filer L. Maillard reaction products in parenteral nutrition. Food Sci. 1981:5265–78. [PubMed] [Google Scholar]
- 33. Davoli E, Cappellini L, Airoldi L, Fanelli R. Serum methanol concentrations in rats and in men after a single dose of aspartame. Food Chem Toxicol. 1986;24:187–9. doi: 10.1016/0278-6915(86)90227-9. [DOI] [PubMed] [Google Scholar]
- 34. Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sazevari O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol. 2003;22:205–11. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- 35. Mourad IM, Noor NA. Aspartame (a widely used artificial sweetener) and oxidative stress in the rat cerebral cortex. Int J Pharm Biomed Sci. 2011;2:4–10. [Google Scholar]
- 36. Ashok I, Sheeladevi R, Wankhar D. Long term effect of aspartame (artificial sweetener) on membrane homeostatic imbalance and histopathology in the rat brain. Free Radical Antioxidant. 2013;3:S42–9. [Google Scholar]
- 37. Ashok I, Sheeladevi R, Dapkupar W. Effect of long-term aspartame (artificial sweetener) on anxiety, locomotor activity, and emotionality behavior in Wistar Albino rats. Biomed Prev Nutr. 2014;4:39–43. [Google Scholar]
- 38. Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–8. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 39. Meng X, Harken AH. The interaction between Hsp70 and TNF-alpha expression: a novel mechanism for protection of the myocardium against postinjury depression. Shock. 2002;17:345–53. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 40. Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry. 1998;63:854–65. [PubMed] [Google Scholar]
- 41. Hibbs JB, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 42. Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29:173–8. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43. Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999;13:1137–43. [PubMed] [Google Scholar]
- 44. Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kB: effects on liver homeostasis and beyond. Endocr Rev. 2007;28:365–86. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 45. Berthonneche C, Sulpice T, Boucher F, Gouraud L, De Leiris J, O'Connor SE, Herbert JM, Janiak P. New insights into the pathological role of TNF-alpha in early cardiac dysfunction and subsequent heart failure after infarction in rats. Am J Physiol Heart Circ Physiol. 2004;287:340–50. doi: 10.1152/ajpheart.01210.2003. [DOI] [PubMed] [Google Scholar]